Docetaxel-Induced Cell Death Is Regulated by a Fatty Acid-Binding Protein 12-Slug-Survivin Pathway in Prostate Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Fatty Acid-Binding Protein 12 (FABP12) Suppresses Docetaxel-Induced Cell Growth Inhibition
2.2. The Slug-Survivin Pathway Underlies the Role of Fatty Acid-Binding Protein 12 (FABP12) in Cell Growth
2.3. Depletion of Slug Restores Docetaxel Sensitivity in Fatty Acid-Binding Protein 12+ (FABP12+) Cells
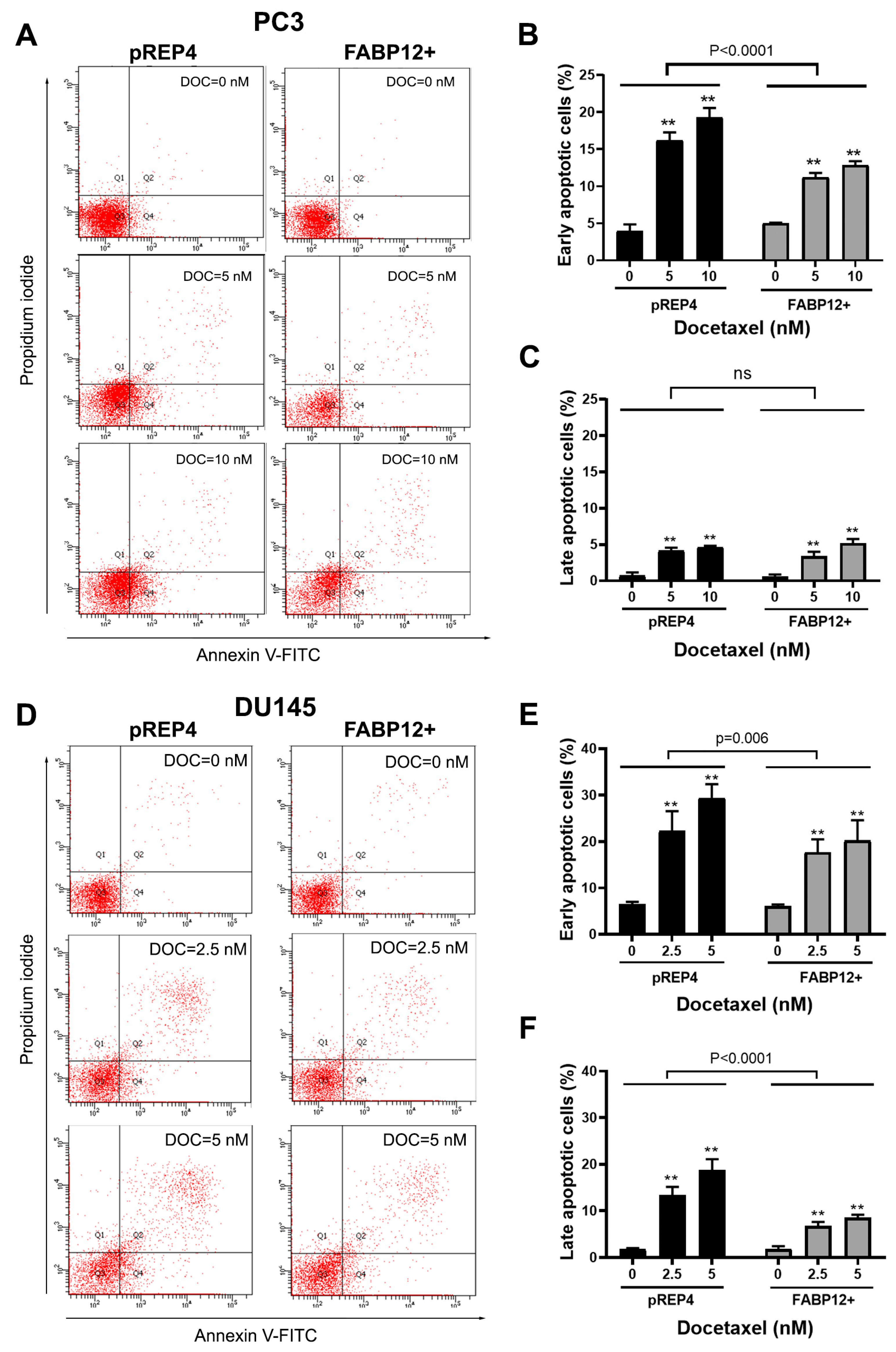
2.4. Docetaxel Induces Fatty Acid-Binding Protein 12 (FABP12)-Dependent Prostate Cancer (PCa) Cell Apoptosis
2.5. Survivin Is Associated with Fatty Acid-Binding Protein 12 (FABP12) and Patient Prognosis in Prostate Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Docetaxel Treatment
4.2. Stable Transfection of Fatty Acid-Binding Protein 12 (FABP12) in Prostate Cancer Cells
4.3. siRNA Transfection
4.4. Cell Growth and Viability Assays
4.5. Western Blotting
4.6. RT-PCR
4.7. Annexin V-PI Staining and Flow Cytometry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asmane, I.; Ceraline, J.; Duclos, B.; Rob, L.; Litique, V.; Barthelemy, P.; Bergerat, J.P.; Dufour, P.; Kurtz, J.E. New strategies for medical management of castration-resistant prostate cancer. Oncology 2011, 80, 1–11. [Google Scholar] [CrossRef]
- Crown, J.; O’Leary, M. The taxanes: An update. Lancet 2000, 355, 1176–1178. [Google Scholar] [CrossRef]
- Yvon, A.M.; Wadsworth, P.; Jordan, M.A. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 1999, 10, 947–959. [Google Scholar] [CrossRef]
- Shelley, M.; Harrison, C.; Coles, B.; Staffurth, J.; Wilt, T.J.; Mason, M.D. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst. Rev. 2006, 2006, CD005247. [Google Scholar] [CrossRef]
- Sekino, Y.; Teishima, J. Molecular mechanisms of docetaxel resistance in prostate cancer. Cancer Drug Resist. 2020, 3, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.; Kooijman, S.; Cho, N.J.; Storm, G.; van der Pluijm, G. Improving Taxane-Based Chemotherapy in Castration-Resistant Prostate Cancer. Trends Pharmacol. Sci. 2016, 37, 451–462. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Li, Z.; Xue, W.; Hu, S.; Kong, X. Lipid metabolism as a target for cancer drug resistance: Progress and prospects. Front. Pharmacol. 2023, 14, 1274335. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef] [PubMed]
- Marin-Aguilera, M.; Codony-Servat, J.; Reig, O.; Lozano, J.J.; Fernandez, P.L.; Pereira, M.V.; Jimenez, N.; Donovan, M.; Puig, P.; Mengual, L.; et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol. Cancer Ther. 2014, 13, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, T.; Mak, B.; Butler, L.; Selth, L.; Horvath, L.G. Targeting lipid metabolism in metastatic prostate cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231152839. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef]
- Vishwa, R.; BharathwajChetty, B.; Girisa, S.; Aswani, B.S.; Alqahtani, M.S.; Abbas, M.; Hegde, M.; Kunnumakkara, A.B. Lipid metabolism and its implications in tumor cell plasticity and drug resistance: What we learned thus far? Cancer Metastasis Rev. 2024, 43, 293–319. [Google Scholar] [CrossRef]
- Liu, R.Z.; Li, X.; Godbout, R. A novel fatty acid-binding protein (FABP) gene resulting from tandem gene duplication in mammals: Transcription in rat retina and testis. Genomics 2008, 92, 436–445. [Google Scholar] [CrossRef]
- Liu, R.Z.; Choi, W.S.; Jain, S.; Dinakaran, D.; Xu, X.; Han, W.H.; Yang, X.H.; Glubrecht, D.D.; Moore, R.B.; Lemieux, H.; et al. The FABP12/PPARgamma pathway promotes metastatic transformation by inducing epithelial-to-mesenchymal transition and lipid-derived energy production in prostate cancer cells. Mol. Oncol. 2020, 14, 3100–3120. [Google Scholar] [CrossRef]
- Ahmad, I.; Mui, E.; Galbraith, L.; Patel, R.; Tan, E.H.; Salji, M.; Rust, A.G.; Repiscak, P.; Hedley, A.; Markert, E.; et al. Sleeping Beauty screen reveals Pparg activation in metastatic prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 8290–8295. [Google Scholar] [CrossRef]
- Hartley, A.; Ahmad, I. The role of PPARgamma in prostate cancer development and progression. Br. J. Cancer 2023, 128, 940–945. [Google Scholar] [CrossRef]
- Warrier, N.M.; Agarwal, P.; Kumar, P. Emerging Importance of Survivin in Stem Cells and Cancer: The Development of New Cancer Therapeutics. Stem Cell Rev. Rep. 2020, 16, 828–852. [Google Scholar] [CrossRef]
- Wheatley, S.P.; Altieri, D.C. Survivin at a glance. J. Cell Sci. 2019, 132, jcs223826. [Google Scholar] [CrossRef]
- Han, T.L.; Sha, H.; Ji, J.; Li, Y.T.; Wu, D.S.; Lin, H.; Hu, B.; Jiang, Z.X. Depletion of Survivin suppresses docetaxel-induced apoptosis in HeLa cells by facilitating mitotic slippage. Sci. Rep. 2021, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, Y.; Chen, H.; Liu, Y.; Tang, Q.; Liu, J.; Chen, H. Survivin, Ki-67 and tumor grade as predictors of response to docetaxel-based neoadjuvant chemotherapy in locally advanced breast cancer. Mol. Clin. Oncol. 2013, 1, 839–844. [Google Scholar] [CrossRef][Green Version]
- Ye, X.; Tam, W.L.; Shibue, T.; Kaygusuz, Y.; Reinhardt, F.; Ng Eaton, E.; Weinberg, R.A. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 2015, 525, 256–260. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Kwak, L.; Xie, W.; Eastham, J.A.; James, N.D.; Sandler, H.M.; Feng, F.Y.; Brihoum, M.; Fizazi, K.; Sweeney, C.; et al. Mortality Risk for Docetaxel-Treated, High-Grade Prostate Cancer With Low PSA Levels: A Meta-Analysis. JAMA Netw. Open 2023, 6, e2340787. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Celia-Terrassa, T.; Kang, Y. How important is EMT for cancer metastasis? PLoS Biol. 2024, 22, e3002487. [Google Scholar] [CrossRef]
- Grant, C.M.; Kyprianou, N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl. Androl. Urol. 2013, 2, 202–211. [Google Scholar]
- Hashemi, M.; Zandieh, M.A.; Talebi, Y.; Rahmanian, P.; Shafiee, S.S.; Nejad, M.M.; Babaei, R.; Sadi, F.H.; Rajabi, R.; Abkenar, Z.O.; et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies. Biomed. Pharmacother. 2023, 160, 114392. [Google Scholar] [CrossRef]
- Hanrahan, K.; O’Neill, A.; Prencipe, M.; Bugler, J.; Murphy, L.; Fabre, A.; Puhr, M.; Culig, Z.; Murphy, K.; Watson, R.W. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol. Oncol. 2017, 11, 251–265. [Google Scholar] [CrossRef]
- Shariat, S.F.; Lotan, Y.; Saboorian, H.; Khoddami, S.M.; Roehrborn, C.G.; Slawin, K.M.; Ashfaq, R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 2004, 100, 751–757. [Google Scholar] [CrossRef]
- Castellon, E.A.; Indo, S.; Contreras, H.R. Cancer Stemness/Epithelial-Mesenchymal Transition Axis Influences Metastasis and Castration Resistance in Prostate Cancer: Potential Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 14917. [Google Scholar] [CrossRef]
- Siragusa, G.; Tomasello, L.; Giordano, C.; Pizzolanti, G. Survivin (BIRC5): Implications in cancer therapy. Life Sci. 2024, 350, 122788. [Google Scholar] [CrossRef] [PubMed]
- Cartharius, K.; Frech, K.; Grote, K.; Klocke, B.; Haltmeier, M.; Klingenhoff, A.; Frisch, M.; Bayerlein, M.; Werner, T. MatInspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics 2005, 21, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Storch, J.; Corsico, B. The Multifunctional Family of Mammalian Fatty Acid-Binding Proteins. Annu. Rev. Nutr. 2023, 43, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, M.E.; Emara, M.; Godbout, R. Interaction of brain fatty acid-binding protein with the polyunsaturated fatty acid environment as a potential determinant of poor prognosis in malignant glioma. Prog. Lipid Res. 2013, 52, 562–570. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol. Ther. 2021, 218, 107670. [Google Scholar] [CrossRef]
- Liu, R.Z.; Graham, K.; Glubrecht, D.D.; Germain, D.R.; Mackey, J.R.; Godbout, R. Association of FABP5 expression with poor survival in triple-negative breast cancer: Implication for retinoic acid therapy. Am. J. Pathol. 2011, 178, 997–1008. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.-Z.; Garg, M.; Yang, X.-H.; Godbout, R. Docetaxel-Induced Cell Death Is Regulated by a Fatty Acid-Binding Protein 12-Slug-Survivin Pathway in Prostate Cancer Cells. Int. J. Mol. Sci. 2024, 25, 9669. https://doi.org/10.3390/ijms25179669
Liu R-Z, Garg M, Yang X-H, Godbout R. Docetaxel-Induced Cell Death Is Regulated by a Fatty Acid-Binding Protein 12-Slug-Survivin Pathway in Prostate Cancer Cells. International Journal of Molecular Sciences. 2024; 25(17):9669. https://doi.org/10.3390/ijms25179669
Chicago/Turabian StyleLiu, Rong-Zong, Mansi Garg, Xiao-Hong Yang, and Roseline Godbout. 2024. "Docetaxel-Induced Cell Death Is Regulated by a Fatty Acid-Binding Protein 12-Slug-Survivin Pathway in Prostate Cancer Cells" International Journal of Molecular Sciences 25, no. 17: 9669. https://doi.org/10.3390/ijms25179669
APA StyleLiu, R.-Z., Garg, M., Yang, X.-H., & Godbout, R. (2024). Docetaxel-Induced Cell Death Is Regulated by a Fatty Acid-Binding Protein 12-Slug-Survivin Pathway in Prostate Cancer Cells. International Journal of Molecular Sciences, 25(17), 9669. https://doi.org/10.3390/ijms25179669





