Impaired Hippocampal Long-Term Potentiation and Memory Deficits upon Haploinsufficiency of MDGA1 Can Be Rescued by Acute Administration of D-Cycloserine
Abstract
:1. Introduction
2. Results
2.1. Mdga1+/− Mice Exhibit Learning and Memory Deficits
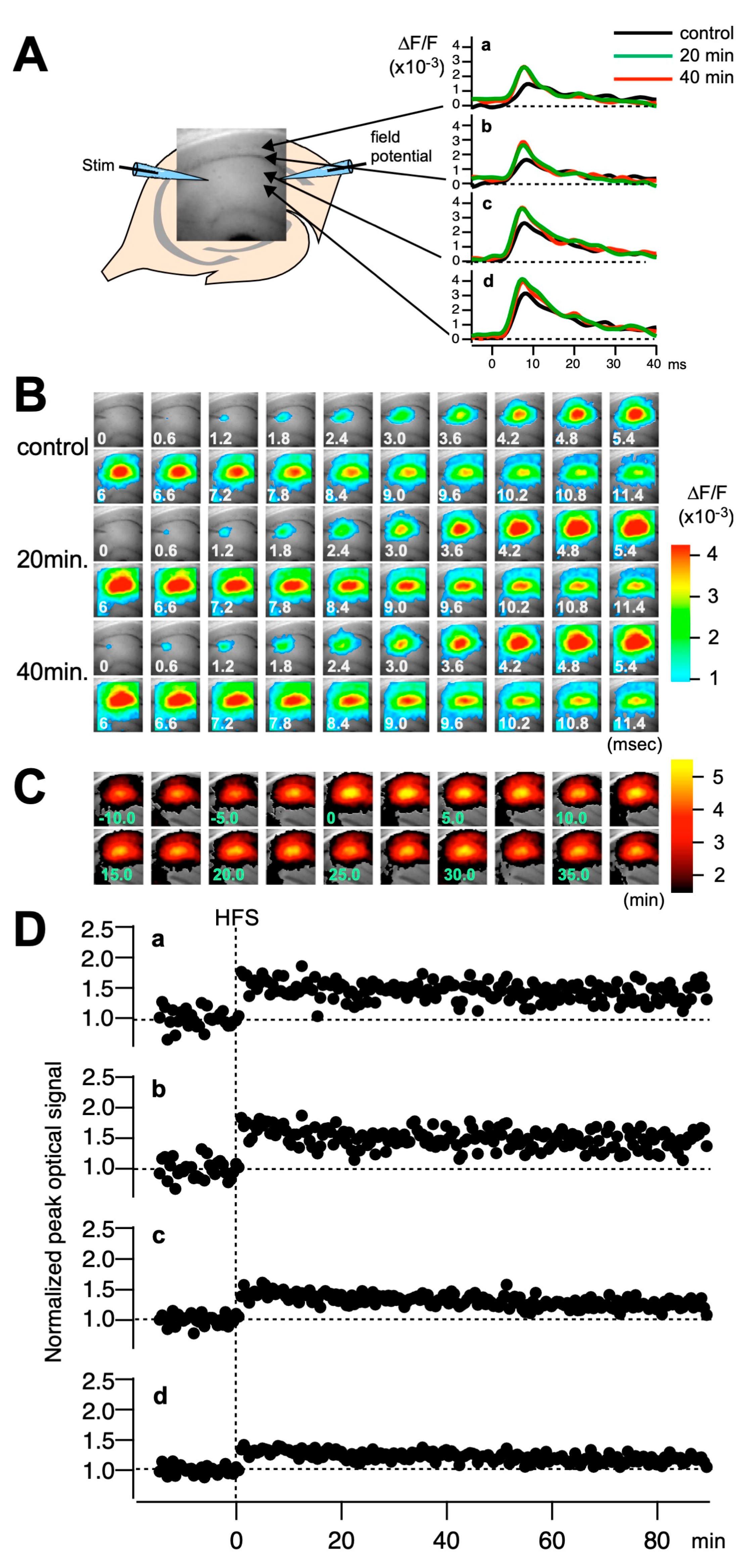
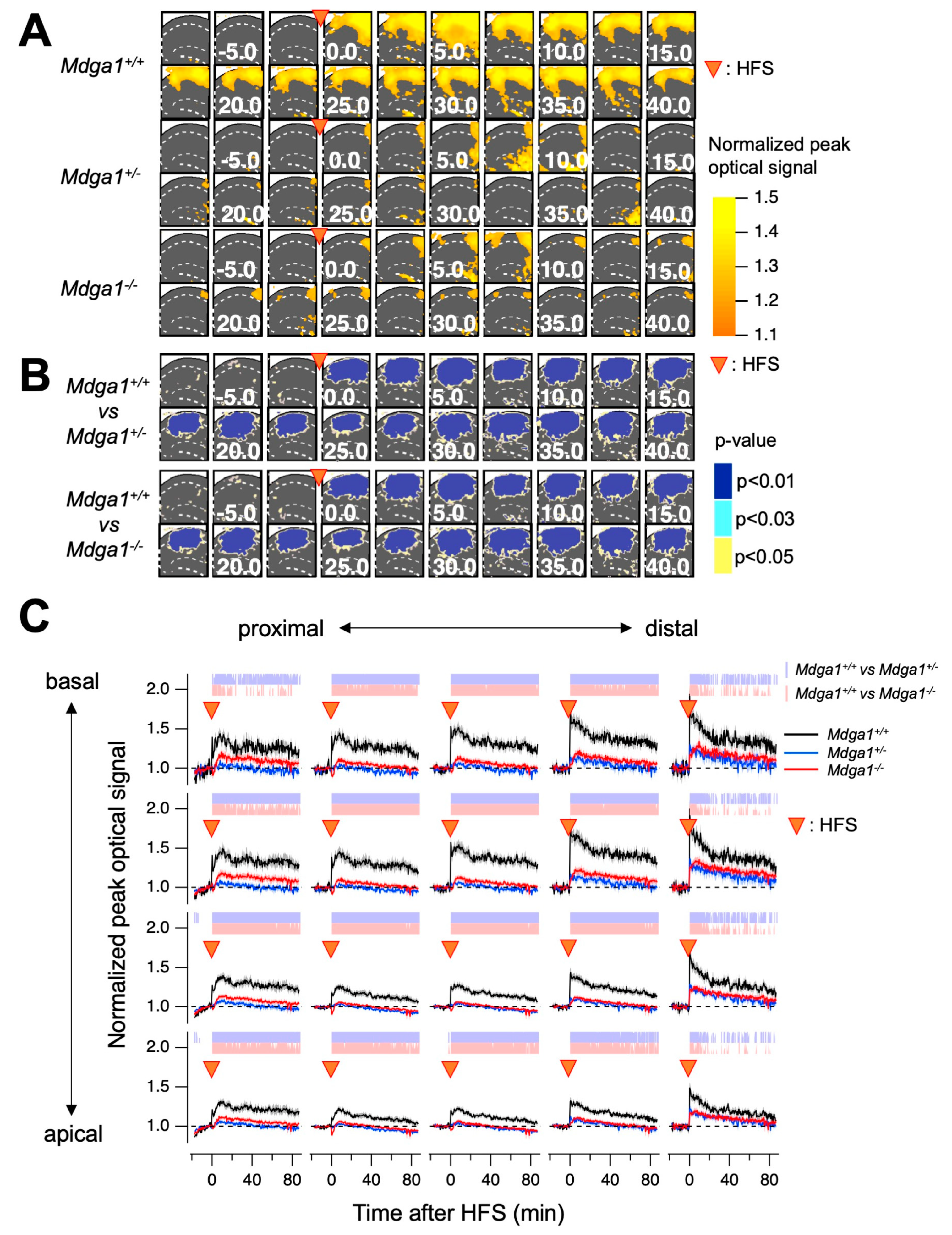
2.2. Mdga1+/− Mice Exhibit Deficit in LTP
2.3. Acute Administration of D-Cycloserine Ameliorates Memory Deficits in Mdga1+/− Mice
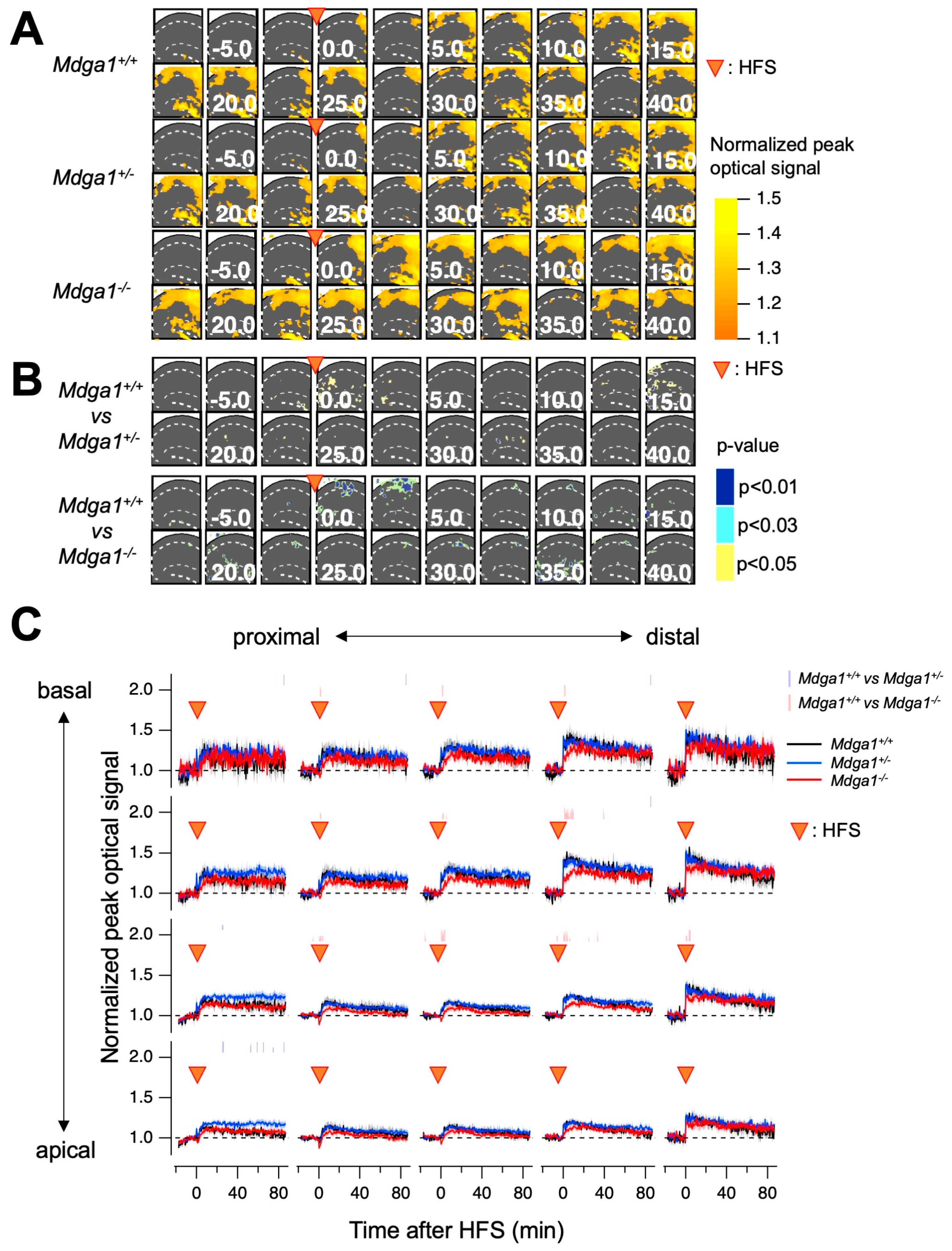
2.4. D-Cycloserine Restored LTP Deficit in Mdga1+/− Mice Brain Slices
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Morris Water Maze (MWM)
4.3. Contextual Fear Conditioning (CFC)
4.4. Electrophysiology
4.5. Voltage-Sensitive Dye (VSD) Imaging
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Südhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Verpoort, B.; de Wit, J. Cell Adhesion Molecule Signaling at the Synapse: Beyond the Scaffold. Cold Spring Harb. Perspect. Biol. 2024, 16, a041501. [Google Scholar] [CrossRef] [PubMed]
- Bemben, M.A.; Shipman, S.L.; Nicoll, R.A.; Roche, K.W. The cellular and molecular landscape of neuroligins. Trends Neurosci. 2015, 38, 496–505. [Google Scholar] [CrossRef]
- Südhof, T.C. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 2017, 171, 745–769. [Google Scholar] [CrossRef]
- Rudenko, G. Neurexins—Versatile molecular platforms in the synaptic cleft. Curr. Opin. Struct. Biol. 2019, 54, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Traunmüller, L.; Scheiffele, P. Neurexins: Molecular codes for shaping neuronal synapses. Nat. Rev. Neurosci. 2021, 22, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Boxer, E.E.; Aoto, J. Neurexins and their ligands at inhibitory synapses. Front. Synaptic Neurosci. 2022, 14, 1087238. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Singh, S.K.; Eroglu, C. Neuroligins Provide Molecular Links Between Syndromic and Nonsyndromic Autism. Sci. Signal. 2013, 6, re4. [Google Scholar] [CrossRef]
- Guilmatre, A.; Huguet, G.; Delorme, R.; Bourgeron, T. The emerging role of SHANK genes in neuropsychiatric disorders. Dev. Neurobiol. 2013, 74, 113–122. [Google Scholar] [CrossRef]
- Ali, H.; Marth, L.; Krueger-Burg, D. Neuroligin-2 as a central organizer of inhibitory synapses in health and disease. Sci. Signal. 2020, 13, eabd8379. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.A.; Elegheert, J.; Xie, Y.; Craig, A.M. Pumping the brakes: Suppression of synapse development by MDGA–neuroligin interactions. Curr. Opin. Neurobiol. 2019, 57, 71–80. [Google Scholar] [CrossRef]
- Connor, S.A.; Siddiqui, T.J. Synapse organizers as molecular codes for synaptic plasticity. Trends Neurosci. 2023, 46, 971–985. [Google Scholar] [CrossRef]
- De Juan, C.; Iniesta, P.; González-Quevedo, R.; Morán, A.; Sánchez-Pernaute, A.; Torres, A.J.; Balibrea, J.L.; Díaz-Rubio, E.; Cruces, J.; Benito, M. Genomic organization of a novel glycosylphosphatidylinositol MAM gene expressed in human tissues and tumors. Oncogene 2002, 21, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Litwack, E.; Babey, R.; Buser, R.; Gesemann, M.; O’Leary, D.D. Identification and characterization of two novel brain-derived immunoglobulin superfamily members with a unique structural organization. Mol. Cell. Neurosci. 2004, 25, 263–274. [Google Scholar] [CrossRef]
- Díaz-López, A.; Rivas, C.; Iniesta, P.; Morán, A.; García-Aranda, C.; Megías, D.; Sánchez-Pernaute, A.; Torres, A.; Díaz-Rubio, E.; Benito, M.; et al. Characterization of MDGA1, a novel human glycosylphosphatidylinositol-anchored protein localized in lipid rafts. Exp. Cell Res. 2005, 307, 91–99. [Google Scholar] [CrossRef]
- Fujimura, Y.; Iwashita, M.; Matsuzaki, F.; Yamamoto, T. MDGA1, an IgSF molecule containing a MAM domain, heterophilically associates with axon- and muscle-associated binding partners through distinct structural domains. Brain Res. 2006, 1101, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Takashima, S.; Niwa, H.; Yokoi, H.; Shimada, A.; Arenz, A.; Wittbrodt, J.; Takeda, H. Characterization of teleost Mdga1 using a gene-trap approach in medaka (Oryzias latipes). Genesis 2009, 47, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Gotoh, N.; Murayama, C.; Abe, T.; Iwashita, M.; Matsuzaki, F.; Suzuki, T.; Yamamoto, T. IgSF molecule MDGA1 is involved in radial migration and positioning of a subset of cortical upper-layer neurons. Dev. Dyn. 2010, 240, 96–107. [Google Scholar] [CrossRef]
- Connor, S.A.; Ammendrup-Johnsen, I.; Chan, A.W.; Kishimoto, Y.; Murayama, C.; Kurihara, N.; Tada, A.; Ge, Y.; Lu, H.; Yan, R.; et al. Altered Cortical Dynamics and Cognitive Function upon Haploinsufficiency of the Autism-Linked Excitatory Synaptic Suppressor MDGA2. Neuron 2016, 91, 1052–1068. [Google Scholar] [CrossRef]
- Pettem, K.L.; Yokomaku, D.; Takahashi, H.; Ge, Y.; Craig, A.M. Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J. Cell Biol. 2013, 200, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, Y.; Lee, S.-J.; Qiang, Y.; Lee, D.; Lee, H.W.; Kim, H.; Je, H.S.; Südhof, T.C.; Ko, J. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc. Natl. Acad. Sci. USA 2013, 110, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, D.; Won, S.Y.; Han, K.A.; Park, D.; Cho, E.; Yun, N.; An, H.J.; Um, J.W.; Kim, E.; et al. Structural Insights into Modulation of Neurexin-Neuroligin Trans-synaptic Adhesion by MDGA1/Neuroligin-2 Complex. Neuron 2017, 94, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, S.P.; Zhong, X.; Seshadrinathan, S.; Chen, H.; Machius, M.; Rudenko, G. Molecular Mechanism of MDGA1: Regulation of Neuroligin 2: Neurexin Trans-synaptic Bridges. Neuron 2017, 94, 1132–1141. [Google Scholar] [CrossRef]
- Elegheert, J.; Cvetkovska, V.; Clayton, A.J.; Heroven, C.; Vennekens, K.M.; Smukowski, S.N.; Regan, M.C.; Jia, W.; Smith, A.C.; Furukawa, H.; et al. Structural Mechanism for Modulation of Synaptic Neuroligin-Neurexin Signaling by MDGA Proteins. Neuron 2017, 95, 896–913.e10. [Google Scholar] [CrossRef]
- Connor, S.A.; Ammendrup-Johnsen, I.; Kishimoto, Y.; Tari, P.K.; Cvetkovska, V.; Harada, T.; Ojima, D.; Yamamoto, T.; Wang, Y.T.; Craig, A.M. Loss of Synapse Repressor MDGA1 Enhances Perisomatic Inhibition, Confers Resistance to Network Excitation, and Impairs Cognitive Function. Cell Rep. 2017, 21, 3637–3645. [Google Scholar] [CrossRef]
- Kuboyama, K.; Shirakawa, Y.; Kawada, K.; Fujii, N.; Ojima, D.; Kishimoto, Y.; Yamamoto, T.; Yamada, M.K. Visually cued fear conditioning test for memory impairment related to cortical function. Neuropsychopharmacol. Rep. 2020, 40, 371–375. [Google Scholar] [CrossRef]
- Hossain, R.; Jamal, M.; Tanoue, Y.; Ojima, D.; Takahashi, H.; Kubota, T.; Ansary, T.M.; Ito, A.; Tanaka, N.; Kinoshita, H.; et al. MDGA1-deficiency attenuates prepulse inhibition with alterations of dopamine and serotonin metabolism: An ex vivo HPLC-ECD analysis. Neurosci. Lett. 2020, 716, 134677. [Google Scholar] [CrossRef]
- Tominaga, T.; Tominaga, Y.; Yamada, H.; Matsumoto, G.; Ichikawa, M. Quantification of optical signals with electrophysiological signals in neural activities of Di-4-ANEPPS stained rat hippocampal slices. J. Neurosci. Methods 2000, 102, 11–23. [Google Scholar] [CrossRef]
- Tominaga, Y.; Taketoshi, M.; Tominaga, T. Overall Assay of Neuronal Signal Propagation Pattern With Long-Term Potentiation (LTP) in Hippocampal Slices From the CA1 Area With Fast Voltage-Sensitive Dye Imaging. Front. Cell. Neurosci. 2018, 12, 389. [Google Scholar] [CrossRef]
- Emmett, M.R.; Mick, S.J.; Cler, J.A.; Rao, T.S.; Iyengar, S.; Wood, P.L. Actions of D-cycloserine at the N-methyl-D-aspartate-associated glycine receptor site in vivo. Neuropharmacology 1991, 30, 1167–1171. [Google Scholar] [CrossRef]
- Toledo, A.; Letellier, M.; Bimbi, G.; Tessier, B.; Daburon, S.; Favereaux, A.; Chamma, I.; Vennekens, K.; Vanderlinden, J.; Sainlos, M.; et al. MDGAs are fast-diffusing molecules that delay excitatory synapse development by altering neuroligin behavior. eLife 2022, 11, e75233. [Google Scholar] [CrossRef]
- Topolnik, L.; Tamboli, S. The role of inhibitory circuits in hippocampal memory processing. Nat. Rev. Neurosci. 2022, 23, 476–492. [Google Scholar] [CrossRef]
- Tzilivaki, A.; Tukker, J.J.; Maier, N.; Poirazi, P.; Sammons, R.P.; Schmitz, D. Hippocampal GABAergic interneurons and memory. Neuron 2023, 111, 3154–3175. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Kim, H.; Hwang, I.-W.; Bae, S.; Karki, S.; Kim, D.; Ogelman, R.; Bang, G.; Kim, J.Y.; et al. MDGA1 negatively regulates amyloid precursor protein–mediated synapse inhibition in the hippocampus. Proc. Natl. Acad. Sci. USA 2022, 119, e2115326119. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental disorders: From genetics to functional pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Saganich, M.J.; Schroeder, B.E.; Galvan, V.; Bredesen, D.E.; Koo, E.H.; Heinemann, S.F. Deficits in Synaptic Transmission and Learning in Amyloid Precursor Protein (APP) Transgenic Mice Require C-Terminal Cleavage of APP. J. Neurosci. 2006, 26, 13428–13436. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Higashihara, E.; Fukuta, A.; Nagao, A.; Kirino, Y. Early impairment in a water-finding test in a longitudinal study of the Tg2576 mouse model of Alzheimer’s disease. Brain Res. 2013, 26, 117–126. [Google Scholar] [CrossRef]
- Utsumi, Y.; Taketoshi, M.; Miwa, M.; Tominaga, Y.; Tominaga, T. Assessing seizure liability in vitro with voltage-sensitive dye imaging in mouse hippocampal slices. Front. Cell. Neurosci. 2023, 17, 1217368. [Google Scholar] [CrossRef]
- Tominaga, Y.; Taketoshi, M.; Maeda, N.; Tominaga, T. Wide-field Single-photon Optical Recording in Brain Slices Using Voltage-sensitive Dye. J. Vis. Exp. 2019, 46, e59692. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojima, D.; Tominaga, Y.; Kubota, T.; Tada, A.; Takahashi, H.; Kishimoto, Y.; Tominaga, T.; Yamamoto, T. Impaired Hippocampal Long-Term Potentiation and Memory Deficits upon Haploinsufficiency of MDGA1 Can Be Rescued by Acute Administration of D-Cycloserine. Int. J. Mol. Sci. 2024, 25, 9674. https://doi.org/10.3390/ijms25179674
Ojima D, Tominaga Y, Kubota T, Tada A, Takahashi H, Kishimoto Y, Tominaga T, Yamamoto T. Impaired Hippocampal Long-Term Potentiation and Memory Deficits upon Haploinsufficiency of MDGA1 Can Be Rescued by Acute Administration of D-Cycloserine. International Journal of Molecular Sciences. 2024; 25(17):9674. https://doi.org/10.3390/ijms25179674
Chicago/Turabian StyleOjima, Daiki, Yoko Tominaga, Takashi Kubota, Atsushi Tada, Hiroo Takahashi, Yasushi Kishimoto, Takashi Tominaga, and Tohru Yamamoto. 2024. "Impaired Hippocampal Long-Term Potentiation and Memory Deficits upon Haploinsufficiency of MDGA1 Can Be Rescued by Acute Administration of D-Cycloserine" International Journal of Molecular Sciences 25, no. 17: 9674. https://doi.org/10.3390/ijms25179674






