The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity of hLf γ-Core-Containing Peptides
2.1.1. Microbicidal Efficacy
2.1.2. Cytoplasmic Membrane Permeabilization
2.1.3. Structure-Activity Relationships
2.2. Effect of hLf γ-Core-Containing Peptides on Cellular K+ Homeostasis
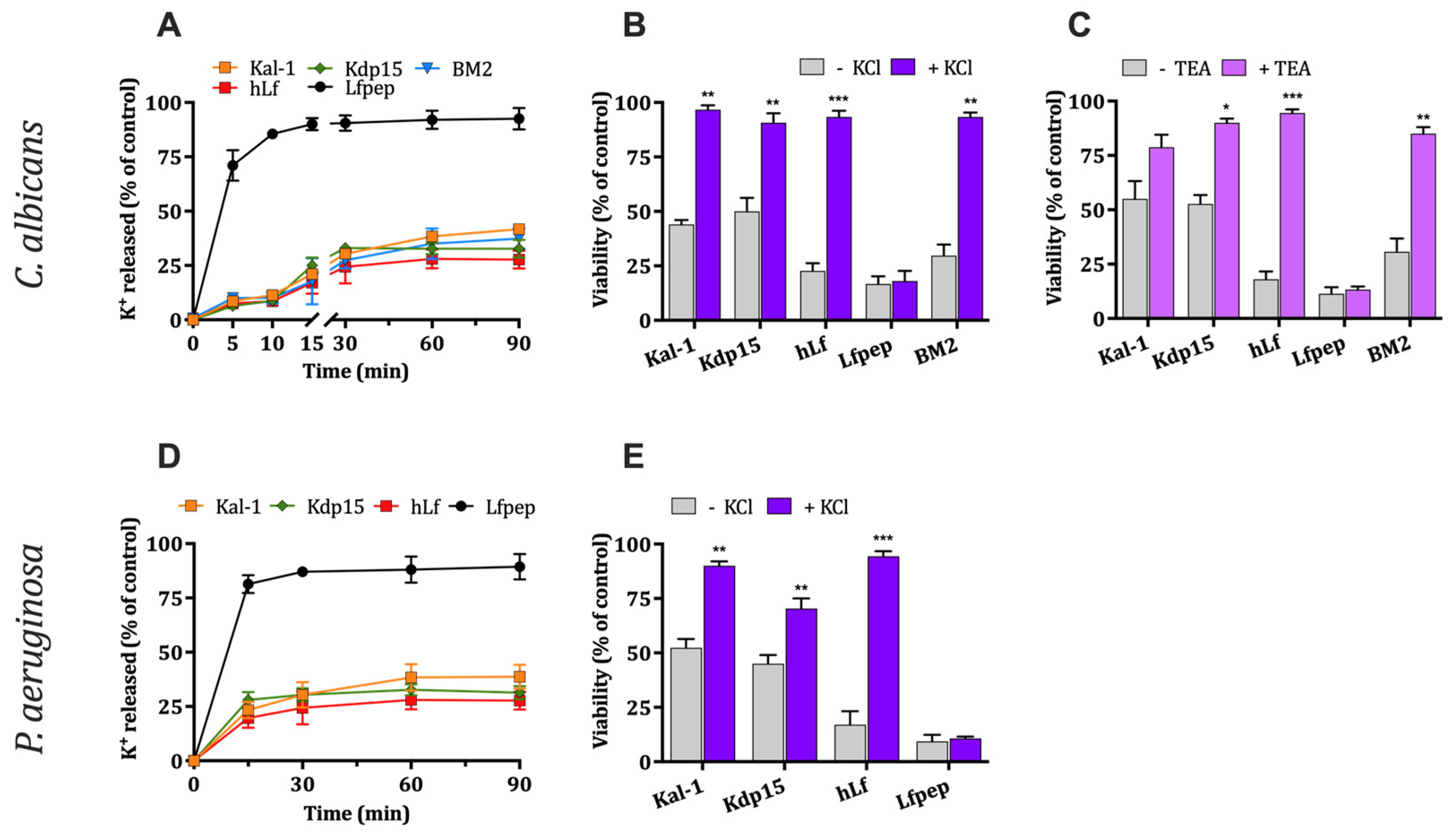
2.2.1. Evaluation of K+ Efflux Induced by hLf γ-Core-Containing Peptides
2.2.2. Effect of High External K+ Concentration on Antimicrobial Activity
2.2.3. Effect of the Inhibition of Fungal K+ Channel Tok1p on Antimicrobial Activity
2.3. Influence of Cellular Respiration on Microbicidal Activity of hLf γ-Core-Containing Peptides
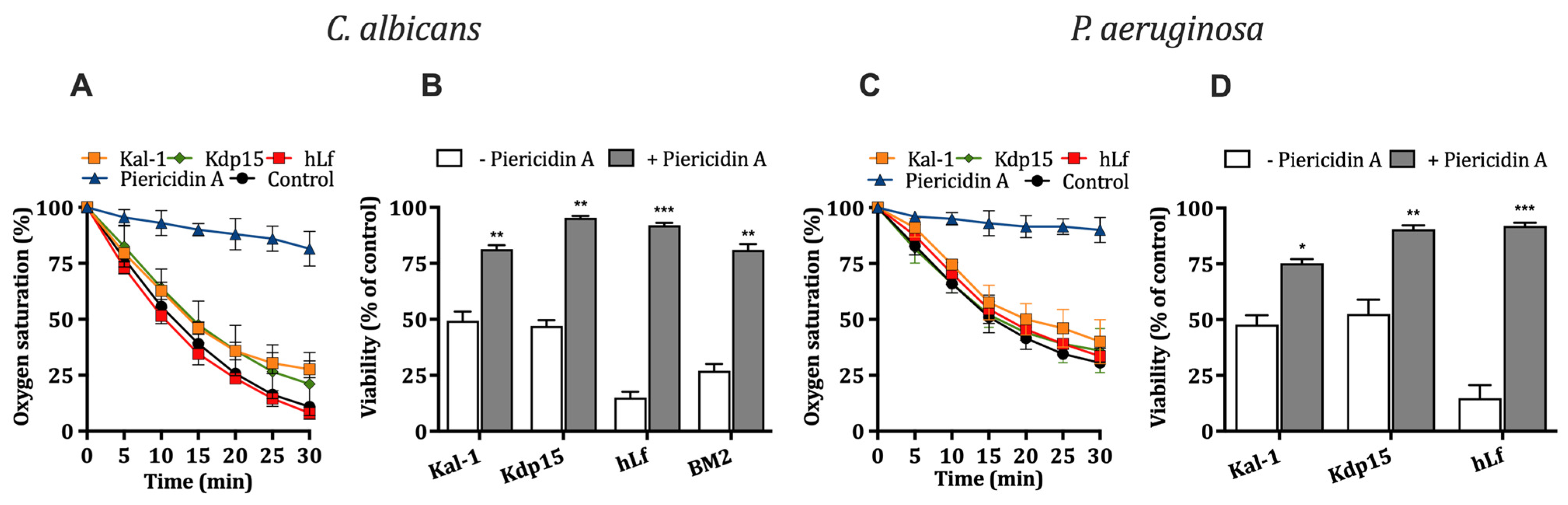
2.3.1. Effect of Kaliocin-1 and Kdp15 on Cellular Respiration
2.3.2. Influence of Cellular Respiration on Microbicidal Activity of Kaliocin-1 and Kdp15
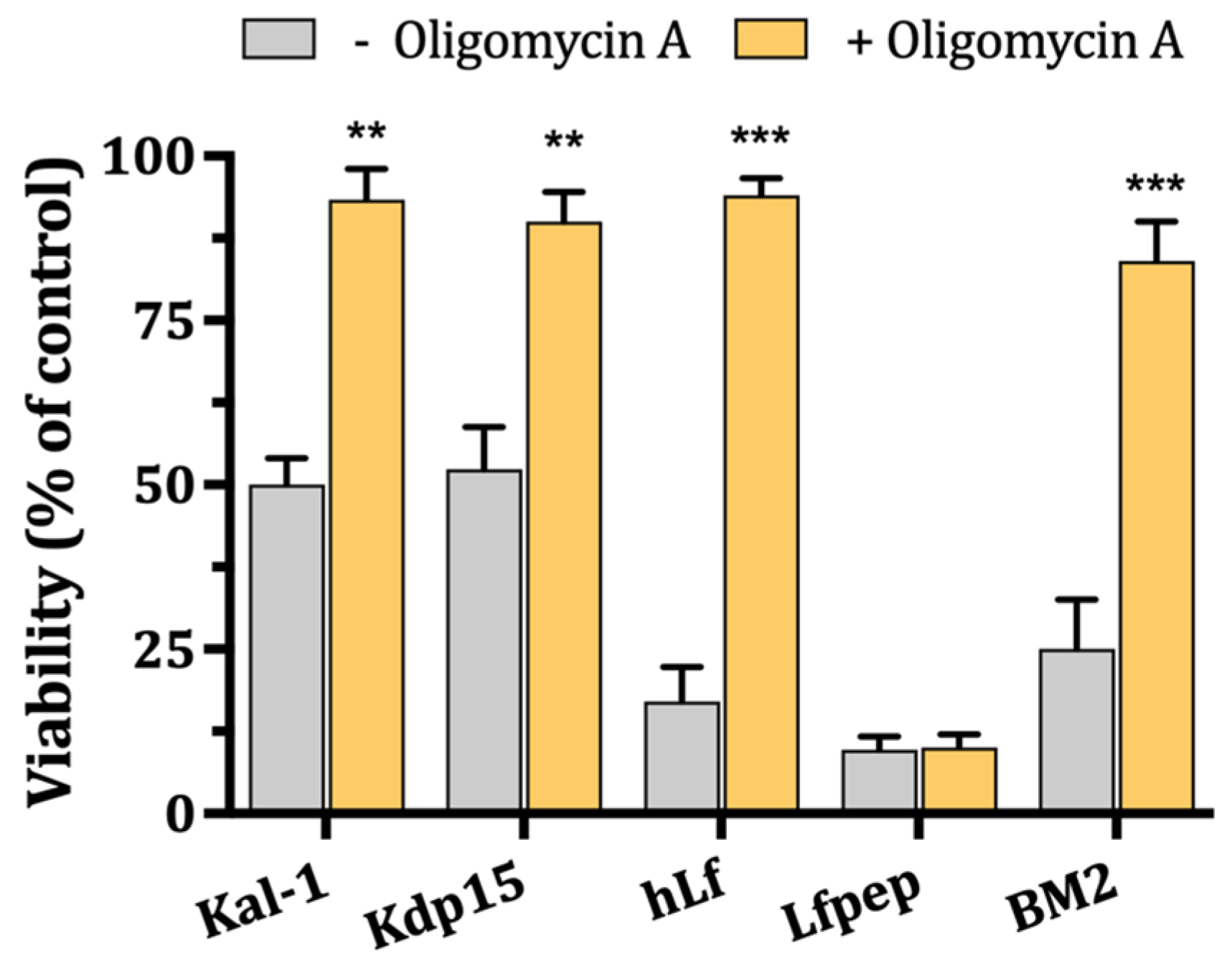
2.4. Role of Mitochondrial H+-ATPase on Candidacidal Activity of hLf γ-Core-Containing Peptides
2.5. Lactoferrin γ-Core-Containing Peptide Inhibition of Pma1p H+-ATPase
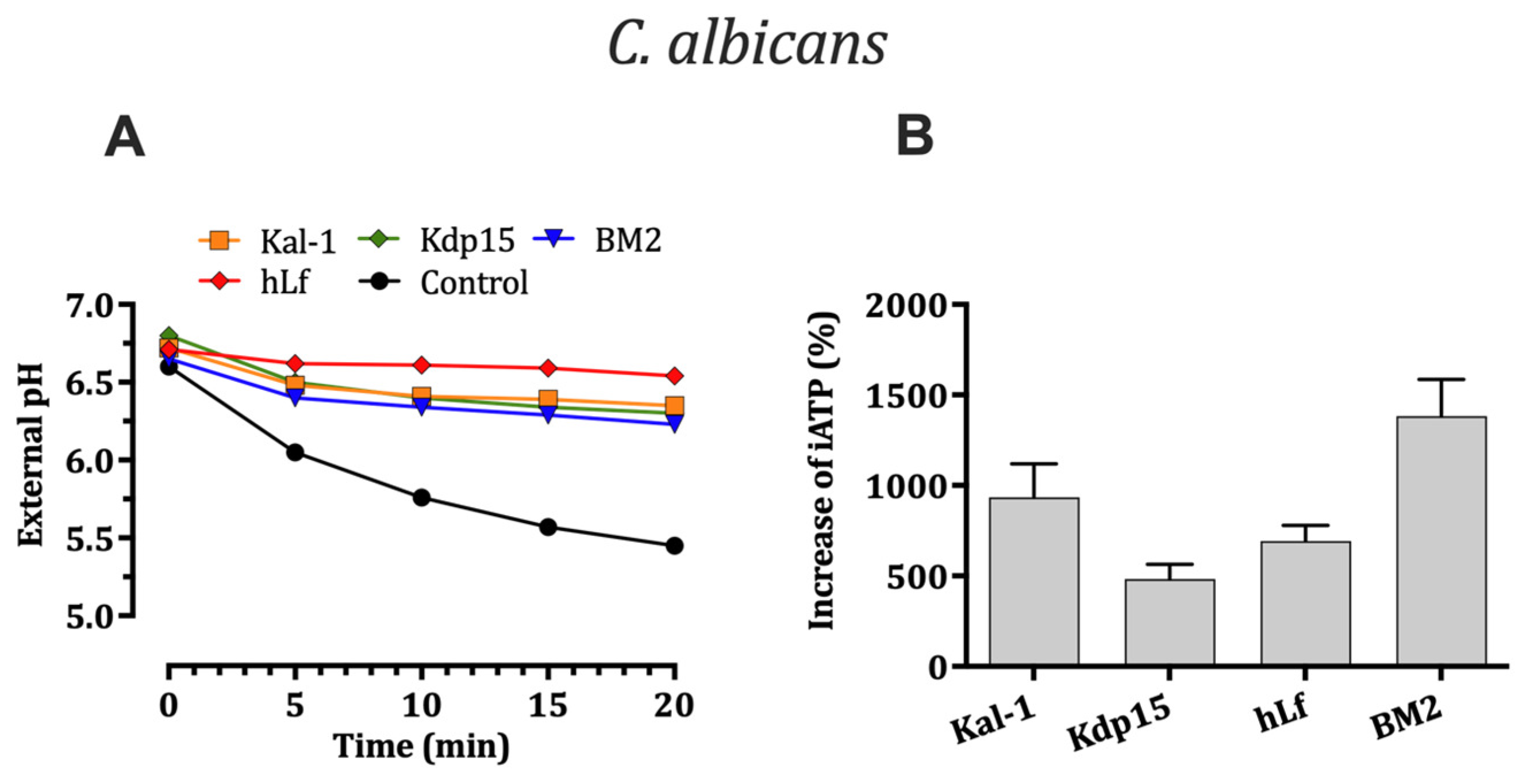
2.5.1. Effect on H+-Extrusion via Pma1p H+-ATPase
2.5.2. Evaluation of Intracellular ATP Levels Associated with Candidacidal Activity
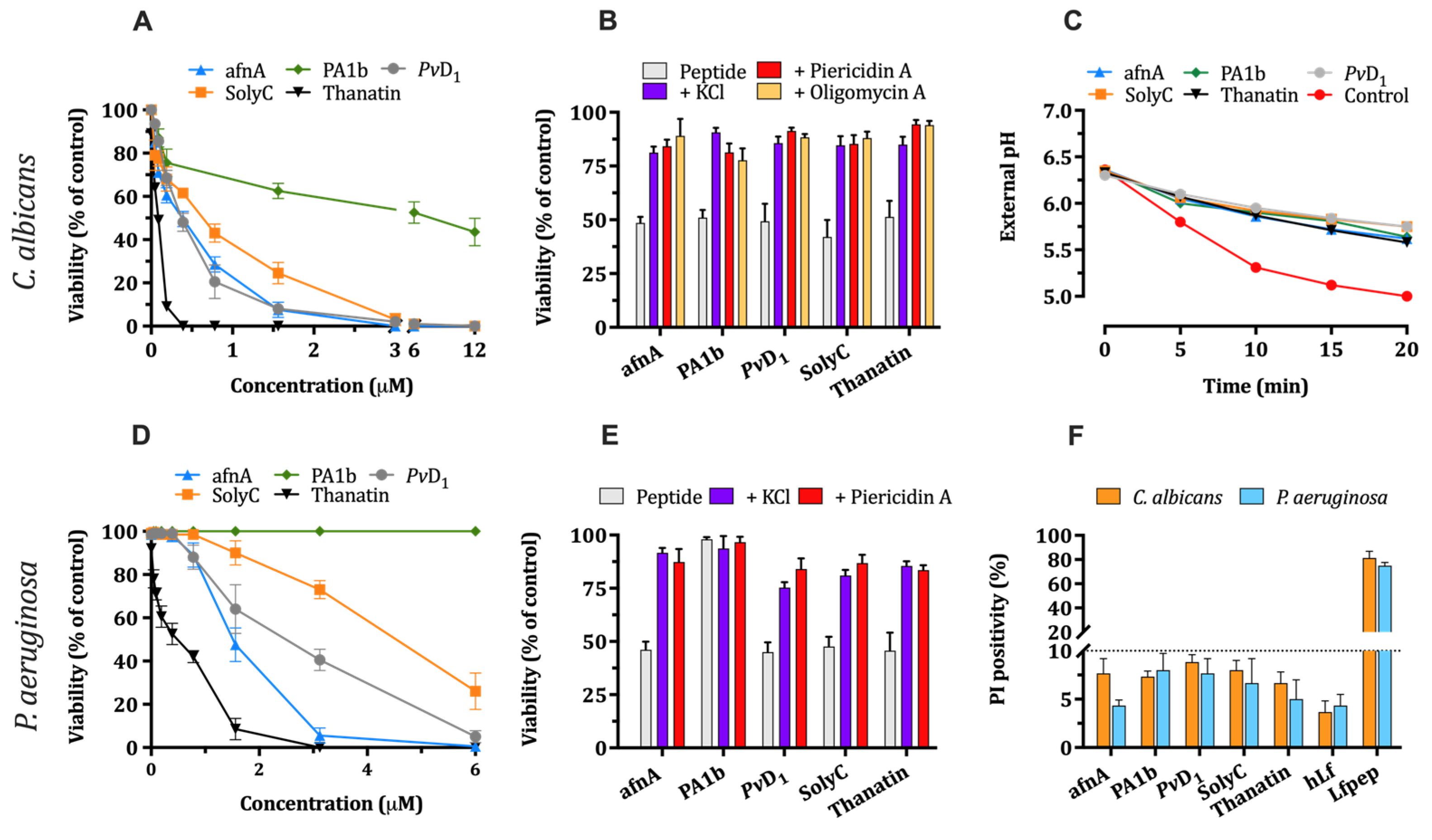
2.6. Evaluation of Microbicidal Activity of Phylogenetically Different γ-Core Motifs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis
4.3. Strains and Growth Conditions
4.4. Antimicrobial Activity Assays and Determination of Cell Viability
4.5. Cell Membrane Permeability Assay
4.6. Extracellular Potassium Measurement
4.7. Oxygen Consumption Measurement
4.8. Glucose-Induced Medium Acidification
4.9. Measurement of ATP
4.10. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, L.A. Molecular evolution of the transferrin family and associated receptors. Biochim. Biophys. Acta 2012, 1820, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.J.; Rogers, H.J.; Griffiths, E. Role of iron in bacterial infection. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 1978; Volume 80, pp. 1–35. [Google Scholar] [CrossRef]
- Arnold, R.R.; Cole, M.F.; McGhee, J.R. A bactericidal effect for human lactoferrin. Science 1977, 197, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.R.; Russell, J.E.; Champion, W.J.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Differentiation from the stasis of iron deprivation. Infect. Immun. 1982, 35, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Dalmastri, C.; Valenti, P.; Visca, P.; Vittorioso, P.; Orsi, N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica 1988, 11, 225–230. [Google Scholar] [PubMed]
- Visca, P.; Dalmastri, C.; Verzili, D.; Antonini, G.; Chiancone, E.; Valenti, P. Interaction of lactoferrin with Escherichia coli cells and correlation with antibacterial activity. Med. Microbiol. Immunol. 1990, 179, 323–333. [Google Scholar] [CrossRef]
- Ellison, R.T., 3rd; Giehl, T.J.; LaForce, F.M. Damage of the outer membrane of enteric Gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar] [CrossRef]
- Viejo-Diaz, M.; Andrés, M.T.; Fierro, J.F. Effects of human lactoferrin on the cytoplasmic membrane of Candida albicans cells related with its candidacidal activity. FEMS Immunol. Med. Microbiol. 2004, 42, 181–185. [Google Scholar] [CrossRef]
- Andrés, M.T.; Fierro, J.F. Antimicrobial mechanism of action of transferrins: Selective inhibition of H+-ATPase. Antimicrob. Agents Chemother. 2010, 54, 4335–4342. [Google Scholar] [CrossRef]
- Andrés, M.T.; Acosta-Zaldívar, M.; Fierro, J.F. Antifungal mechanism of action of lactoferrin: Identification of H+-ATPase (P3A-Type) as a new apoptotic-cell membrane receptor. Antimicrob. Agents Chemother. 2016, 60, 4206–4216. [Google Scholar] [CrossRef]
- Andrés, M.T.; Viejo-Diaz, M.; Fierro, J.F. Human lactoferrin induces apoptosis-like cell death in Candida albicans: Critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008, 52, 4081–4088. [Google Scholar] [CrossRef]
- Andrés, M.T.; Acosta-Zaldívar, M.; González-Seisdedos, J.; Fierro, J.F. Cytosolic acidification is the first transduction signal of lactoferrin-induced regulated cell death pathway. Int. J. Mol. Sci. 2019, 20, 5838. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, C.; Rocha, J.F.; Fernandes, H.S.; Rodrigues, L.R.; Côrte-Real, M.; Sousa, S.F. The milk derived lactoferrin inhibits V-ATPase activity by targeting its V1 domain. Int. J. Biol. Macromol. 2021, 186, 54–70. [Google Scholar] [CrossRef]
- Santos-Pereira, C.; Guedes, J.P.; Ferreira, D.; Rodrigues, L.R.; Côrte-Real, M. Lactoferrin perturbs intracellular trafficking, disrupts cholesterol-rich lipid rafts and inhibits glycolysis of highly metastatic cancer cells harbouring plasmalemmal V-ATPase. Int. J. Biol. Macromol. 2022, 220, 1589–1604. [Google Scholar] [CrossRef]
- Santos-Pereira, C.; Andrés, M.T.; Fierro, J.F.; Rodrigues, L.R.; Côrte-Real, M. A review on lactoferrin as a proton pump inhibitor. Int. J. Biol. Macromol. 2022, 202, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, M.I.; Groenink, J.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Hiratani, T.; Uchida, K.; Yamaguchi, H. Antifungal spectrum and fungicidal mechanism of an N-terminal peptide of bovine lactoferrin. J. Infect. Chemother. 1996, 1, 185–189. [Google Scholar] [CrossRef]
- Sánchez-Gómez, S.; Lamata, M.; Leiva, J.; Blondelle, S.E.; Jerala, R.; Andrä, J.; Brandenburg, K.; Lohner, K.; Moriyón, I.; Martínez-de-Tejada, G. Comparative analysis of selected methods for the assessment of antimicrobial and membrane-permeabilizing activity: A case study for lactoferricin derived peptides. BMC Microbiol. 2008, 8, 196. [Google Scholar] [CrossRef]
- van der Kraan, M.I.; van Marle, J.; Nazmi, K.; Groenink, J.; van ‘t Hof, W.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Ultrastructural effects of antimicrobial peptides from bovine lactoferrin on the membranes of Candida albicans and Escherichia coli. Peptides 2005, 26, 1537–1542. [Google Scholar] [CrossRef]
- Viejo-Diaz, M.; Andrés, M.T.; Pérez-Gil, J.; Sánchez, M.; Fierro, J.F. Potassium efflux induced by a new lactoferrin-derived peptide mimicking the effect of native human lactoferrin on the bacterial cytoplasmic membrane. Biochemistry 2003, 68, 217–227. [Google Scholar] [CrossRef]
- Viejo-Diaz, M.; Andrés, M.T.; Fierro, J.F. Different anti-Candida activities of two human lactoferrin derived peptides, Lfpep and kaliocin-1. Antimicrob. Agents Chemother. 2005, 49, 2583–2588. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Andrés, M.T.; Fierro, J.F.; Yeaman, M.R. The gamma-core motif correlates with antimicrobial activity in cysteine-containing kaliocin-1 originating from transferrins. Biochim. Biophys. Acta 2007, 1768, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 2007, 5, 727–740. [Google Scholar] [CrossRef]
- Nigro, E.; Colavita, I.; Sarnataro, D.; Scudiero, O.; Zambrano, G.; Granata, V.; Daniele, A.; Carotenuto, A.; Galdiero, S.; Folliero, V.; et al. An ancestral host defence peptide within human β-defensin 3 recapitulates the antibacterial and antiviral activity of the full-length molecule. Sci. Rep. 2015, 5, 18450. [Google Scholar] [CrossRef]
- Viejo-Diaz, M.; Andrés, M.T.; Fierro, J.F. Modulation of in vitro fungicidal activity of human lactoferrin against Candida albicans by extracellular cation concentration and target cell metabolic activity. Antimicrob. Agents Chemother. 2004, 48, 1242–1248. [Google Scholar] [CrossRef]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Büttner, S.; Thevissen, K. Regulated cell death as a therapeutic target for novel antifungal peptides and biologics. Oxid. Med. Cell Longev. 2018, 2018, 5473817. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, J.E.; Lee, B.J. Antimicrobial peptides from the skin of a korean frog, Rana rugosa. Biochem. Biophys. Res. Comm. 1994, 205, 948–954. [Google Scholar] [CrossRef]
- Monk, B.C.; Niimi, K.; Lin, S.; Knight, A.; Kardos, T.B.; Cannon, R.D.; Parshot, R.; King, A.; Lun, D.; Harding, D.R. Surface-active fungicidal D-peptide inhibitors of the plasma membrane proton pump that block azole resistance. Antimicrob. Agents Chemother. 2005, 49, 57–70. [Google Scholar] [CrossRef]
- Kjellerup, L.; Gordon, S.; Cohrt, K.O.; Brown, W.D.; Fuglsang, A.T.; Winther, A.M.-L. Identification of antifungal H+-ATPase inhibitors with effect on plasma membrane potential. Antimicrob. Agents Chemother. 2017, 61, e00032-17. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.L.; Heremans, J.F.; Dive, C.H. An iron-binding protein common to many external secretions. Clin. Chim. Acta 1966, 14, 735–739. [Google Scholar] [CrossRef]
- Thevissen, K.; Marchand, A.; Chaltin, P.; Meert, M.K.; Cammue, B.P.A. Antifungal carbazoles. Curr. Med. Chem. 2009, 16, 2205–2211. [Google Scholar] [CrossRef]
- Clausen, J.D.; Kjellerup, L.; Cohrt, K.O.; Hansen, J.B.; Dalby-Brown, W.; Winther, A.L. Elucidation of antimicrobial activity and mechanism of action by N-substituted carbazole derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 4564–4570. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, M.; Kjellerup, L.; Cohrt, K.O.; Gordon, S.; Mortensen, A.L.; Clausen, J.D.; Pallin, T.D.; Hansen, J.B.; Fuglsang, A.T.; Dalby-Brown, W.; et al. Tetrahydrocarbazoles are a novel class of potent P-type ATPase inhibitors with antifungal activity. PLoS ONE 2018, 13, e0188620. [Google Scholar] [CrossRef]
- Lee, M.K.; Cha, L.; Lee, S.-H.; Hahm, K. -S. Role of amino acid residues within the disulfide loop of thanatin, a potent antibiotic peptide. J. Biochem. Mol. Biol. 2002, 35, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Yamamoto, T.; Tamura, A.; Murabayashi, S.; Hashimoto, S.; Shimada, H.; Taguchi, S. NMR based structure–activity relationship analysis of an antimicrobial peptide thanatin, engineered by sitespecific chemical modification: Activity improvement and spectrum alteration. Biochem. Biophys. Res. Commun. 2008, 369, 609–615. [Google Scholar] [CrossRef]
- Sonderegger, C.; Váradi, G.; Galgóczy, L.; Kocsubé, S.; Posch, W.; Borics, A.; Dubrac, S.; Tóth, G.K.; Wilflingseder, D.; Marx, F. The evolutionary conserved γ-core motif influences the anti-Candida activity of the Penicillium chrysogenum antifungal protein PAF. Front. Microbiol. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Nehls, C.; Böhling, A.; Podschun, R.; Schubert, S.; Grötzinger, J.; Schromm, A.; Fedders, H.; Leippe, M.; Harder, J.; Kaconis, Y.; et al. Influence of disulfide bonds in human beta defensin-3 on its strain specific activity against Gram-negative bacteria. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183273. [Google Scholar] [CrossRef]
- Chaili, S.; Cheung, A.L.; Bayer, A.S.; Xiong, Y.Q.; Waring, A.J.; Memmi, G.; Donegan, N.; Yang, S.J.; Yeaman, M.R. The GraS Sensor in Staphylococcus aureus mediates resistance to host defense peptides differing in mechanisms of action. Infect. Immun. 2015, 84, 459–466. [Google Scholar] [CrossRef]
- Avitabile, C.; Capparelli, R.; Rigano, M.M.; Fulgione, A.; Barone, A.; Pedone, C.; Romanelli, A. Antimicrobial peptides from plants: Stabilization of the γ core of a tomato defensin by intramolecular disulfide bond. J. Pept. Sci. 2013, 19, 240–245. [Google Scholar] [CrossRef]
- Mello, É.O.; Taveira, G.B.; Carvalho, A.O.; Gomes, V.M. Improved smallest peptides based on positive charge increase of the γ-core motif from PvD1 and their mechanism of action against Candida species. Int. J. Nanomed. 2019, 14, 407–420. [Google Scholar] [CrossRef]
- Fernández, A.; Colombo, M.L.; Curto, L.M.; Gómez, G.E.; Delfino, J.M.; Guzmán, F.; Bakás, L.; Malbrán, I.; Vairo-Cavalli, S.E. Peptides derived from the α-core and γ-core regions of a putative Silybum marianum flower defensin show antifungal activity against Fusarium graminearum. Front. Microbiol. 2021, 12, 632008. [Google Scholar] [CrossRef] [PubMed]
- Slezina, M.P.; Istomina, E.A.; Kulakovskaya, E.V.; Abashina, T.N.; Odintsova, T.I. Synthetic oligopeptides mimicking γ-core regions of cysteine-rich peptides of Solanum lycopersicum possess antimicrobial activity agains human and plant pathogens. Curr. Issues Mol. Biol. 2021, 43, 1226–1242. [Google Scholar] [CrossRef]
- Leiter, E.; Szappanos, H.; Oberparleiter, C.; Kaiserer, L.; Csernoch, L.; Pusztahelyi, T.; Emri, T.; Pócsi, I.; Salvenmoser, W.; Marx, F. Antifungal protein PAF severely affects the integrity of the plasma membrane of Aspergillus nidulans and induces an apoptosis-like phenotype. Antimicrob. Agents Chemother. 2005, 49, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; Carmona-Gutierrez, D.; Lefevre, S.; Govaert, G.; François, I.E.J.A.; Madeo, F.; Santos, R.; Cammue, B.P.A.; Thevissen, K. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 2009, 583, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; Bammens, L.; Govaert, G.; Carmona-Gutierrez, D.; Madeo, F.; Cammue, B.P.A.; Thevissen, K. The antifungal plant defensin HsAFP1 from Heuchera sanguinea induces apoptosis in Candida albicans. Front. Microbiol. 2011, 2, 47. [Google Scholar] [CrossRef]
- Mello, É.O.; Ribeiro, S.F.; Carvalho, A.O.; Santos, I.S.; Da Cunha, M.; Santa-Catarina, C.; Gomes, V.M. Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr. Microbiol. 2011, 62, 1209–1217. [Google Scholar] [CrossRef]
- Eyraud, V.; Balmand, S.; Karaki, L.; Rahioui, I.; Sivignon, C.; Delmas, A.F.; Royer, C.; Rahbé, Y.; Da Silva, P.; Gressent, F. The interaction of the bioinsecticide PA1b (Pea Albumin 1 subunit b) with the insect V-ATPase triggers apoptosis. Sci. Rep. 2017, 7, 4902. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Antifungal activity of human antimicrobial peptides targeting apoptosis in Candida auris. J. Med. Microbiol. 2024, 73, 001835. [Google Scholar] [CrossRef]
- Chouabe, C.; Eyraud, V.; Da Silva, P.; Rahioui, I.; Royer, C.; Soulage, C.; Bonvallet, R.; Huss, M.; Gressent, F. New mode of action for a knottin protein bioinsecticide: Pea albumin 1 subunit b (PA1b) is the first peptidic inhibitor of V-ATPase. J. Biol. Chem. 2011, 286, 36291–36296. [Google Scholar] [CrossRef] [PubMed]
- Gressent, F.; Duport, G.; Rahioui, I.; Pauchet, Y.; Bolland, P.; Specty, O.; Rahbé, Y. Biological activity, and binding site characteristics of the PA1b entomotoxin on insects from different orders. J. Insect. Sci. 2007, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.; Strzepa, A.; Jouvensal, L.; Rahioui, I.; Gressent, F.; Delmas, A.F. A folded and functional synthetic PA1b: An interlocked entomotoxic miniprotein. Biopolymers 2009, 92, 436–444. [Google Scholar] [CrossRef]
- Gressent, F.; Da Silva, P.; Eyraud, V.; Karaki, L.; Royer, C. Pea Albumin 1 subunit b (PA1b), a promising bioinsecticide of plant origin. Toxins 2011, 3, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Rahioui, I.; Eyraud, V.; Karaki, L.; Sasse, F.; Carre-Pierrat, M.; Qin, A.; Zheng, M.H.; Toepfer, S.; Sivignon, C.; Royer, C.; et al. Host range of the potential biopesticide Pea Albumin 1b (PA1b) is limited to insects. Toxicon 2014, 89, 67–76. [Google Scholar] [CrossRef]
- Bontems, F.; Roumestand, C.; Gilquin, B.; Ménez, A.; Toma, F. Refined structure of charybdotoxin: Common motifs in scorpion toxins and insect defensins. Science 1991, 254, 1521–1523. [Google Scholar] [CrossRef]
- Vriens, K.; Peigneur, S.; De Coninck, B.; Tytgat, J.; Cammue, B.P.; Thevissen, K. The antifungal plant defensin AtPDF2.3 from Arabidopsis thaliana blocks potassium channels. Sci. Rep. 2016, 6, 32121. [Google Scholar] [CrossRef]
- Sugrue, I.; O’Connor, P.M.; Hill, C.; Stanton, C.; Ross, R.P. Actinomyces pruduces defensin-like bacteriocins (Actifensins) with a highly degenerate structure and broad antimicrobial activity. J. Bacteriol. 2020, 202, e00529-19. [Google Scholar] [CrossRef]
- Gbala, I.D.; Macharia, R.W.; Bargul, J.L.; Magoma, G. Membrane permeabilization and antimicrobial activity of recombinant defensin-d2 and actifensin against multidrug-resistant Pseudomonas aeruginosa and Candida albicans. Molecules 2022, 27, 4325. [Google Scholar] [CrossRef]
- Gbala, I.D.; Macharia, R.W.; Bargul, J.L.; Magoma, G. Recombinant actifensin and defensin-d2 induce critical changes in the proteomes of multidrug-resistant Pseudomonas aeruginosa and Candida albicans. Microbiol Spectr. 2022, 10, e0206222. [Google Scholar] [CrossRef]
- Dash, R.; Bhattacharjya, S. Thanatin: An emerging host defense antimicrobial peptide with multiple modes of action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef]
- Andrés, M.T.; Fierro, P.; Antuña, V.; Fierro, J.F. The antimicrobial activity of human defensins at physiological non-permeabilizing concentrations is caused by the inhibition of the plasma membrane H+-ATPases. Int. J. Mol. Sci. 2024, 25, 7335. [Google Scholar] [CrossRef]

| Peptide | Amino Acid Sequence | Lactoferrin Sequence | Aa (No.) | mav | Z | HR | Refs. |
|---|---|---|---|---|---|---|---|
| Kaliocin-1 | FFSASCVPGADKGQFPNLCRLCAGTGENKCA | 153–183 | 31 | 3193 | +1 | 45% | [21,22,23] |
| Kdp15 | NLCRLCAGTGENKCA | 169–183 | 15 | 1553 | +1 | 40% | This work |
| Kdp15-G3 | NLGRLCAGTGENKCA | 169–183 | 15 | 1507 | +1 | 40% | This work |
| Kdp15-G6 | NLCRLGAGTGENKCA | 169–183 | 15 | 1507 | +1 | 40% | This work |
| Kdp15-G14 | NLCRLCAGTGENKGA | 169–183 | 15 | 1507 | +1 | 40% | This work |
| Kdp9 | CAGTGENKC | 174–182 | 9 | 882 | 0 | 33% | This work |
| Lfpep | TKCFQWQRNMRKVRGPPVSCIKR | 37–59 | 23 | 2819 | +7 | 35% | [21,22] |
| Peptide (Origin) | Amino Acid Sequence 1 | Aa (No.) 2 | γ-Core Motif (Isoform) 3 | Disulfide Bridge 4 | UniProtKB Access. No. |
|---|---|---|---|---|---|
| Thanatin (insect) | GSKKPVPIIYCNRRTGKCQRM | 21 (C) | L-2 | C11–C18 | P55788 |
| PvD1 (plant) | HCKNKEHLRSGRCRDDFRCWCT | 22 (F) | L-2 | C24–C43 | V7BTW4 |
| SolyC (plant) | VCETERFSGGNCRGFRRRCFCT | 22 (F) | L-2 | C55–C74 | A0A3Q7H3Y0 |
| PA1b (plant) | ASCNGVCSPFEMPPCGTSACRCIPVGLVIGYCRNPSG | 37 (C) | D, L-2 | C3–C20/C7–C22 | P62927 |
| afnA (bacteria) | HCKSVGYRGGYCKLRTVCTCY | 21 (F) | L-2 | C18–C36 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, M.T.; Yount, N.Y.; Acosta-Zaldívar, M.; Yeaman, M.R.; Fierro, J.F. The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens. Int. J. Mol. Sci. 2024, 25, 9672. https://doi.org/10.3390/ijms25179672
Andrés MT, Yount NY, Acosta-Zaldívar M, Yeaman MR, Fierro JF. The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens. International Journal of Molecular Sciences. 2024; 25(17):9672. https://doi.org/10.3390/ijms25179672
Chicago/Turabian StyleAndrés, María T., Nannette Y. Yount, Maikel Acosta-Zaldívar, Michael R. Yeaman, and José F. Fierro. 2024. "The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens" International Journal of Molecular Sciences 25, no. 17: 9672. https://doi.org/10.3390/ijms25179672
APA StyleAndrés, M. T., Yount, N. Y., Acosta-Zaldívar, M., Yeaman, M. R., & Fierro, J. F. (2024). The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens. International Journal of Molecular Sciences, 25(17), 9672. https://doi.org/10.3390/ijms25179672








