WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging
Abstract
1. Introduction
2. Results
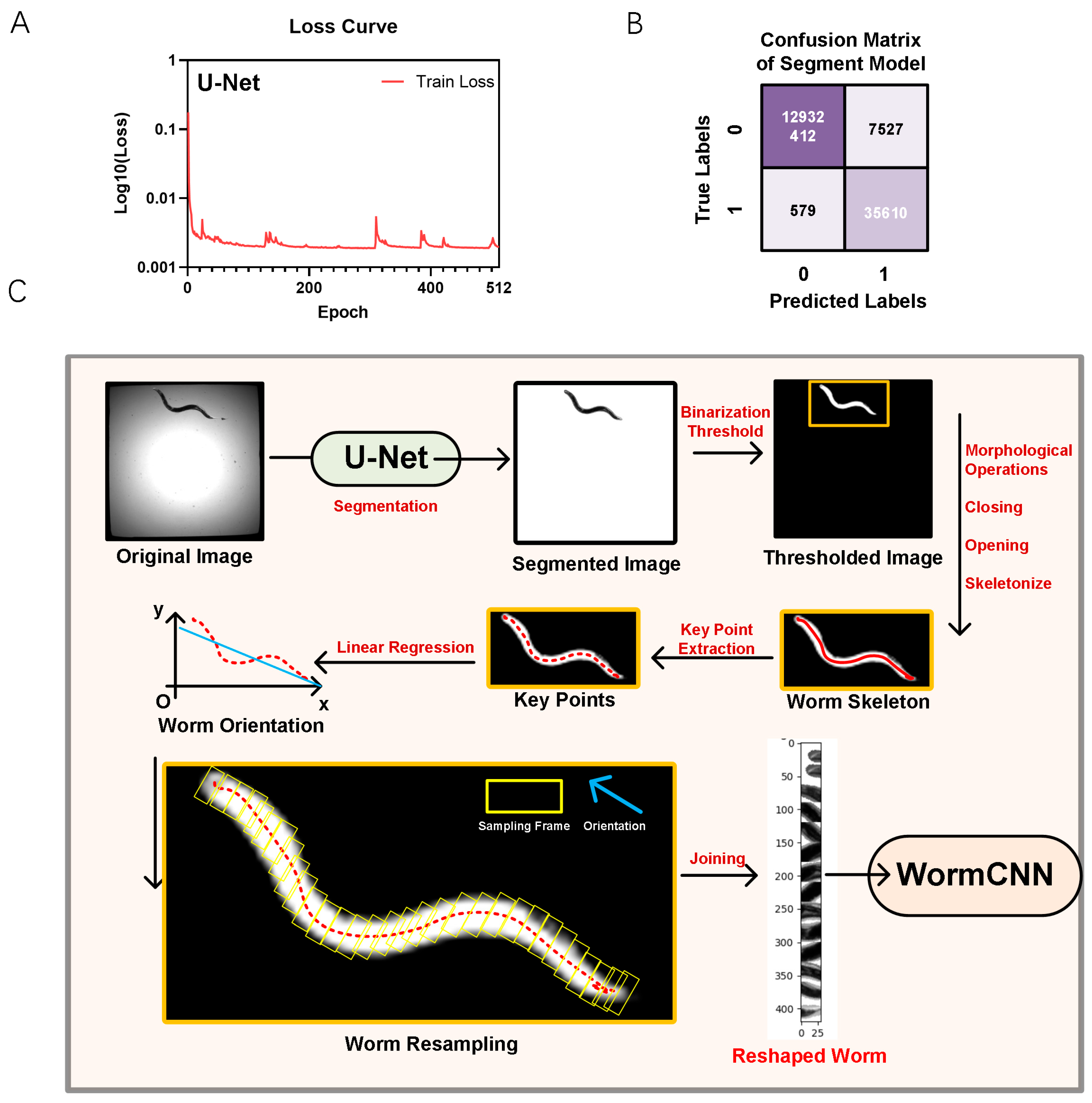
2.1. Lifespan Dataset Collection and Image Reshaping of C. elegans in a 384-Well Plate
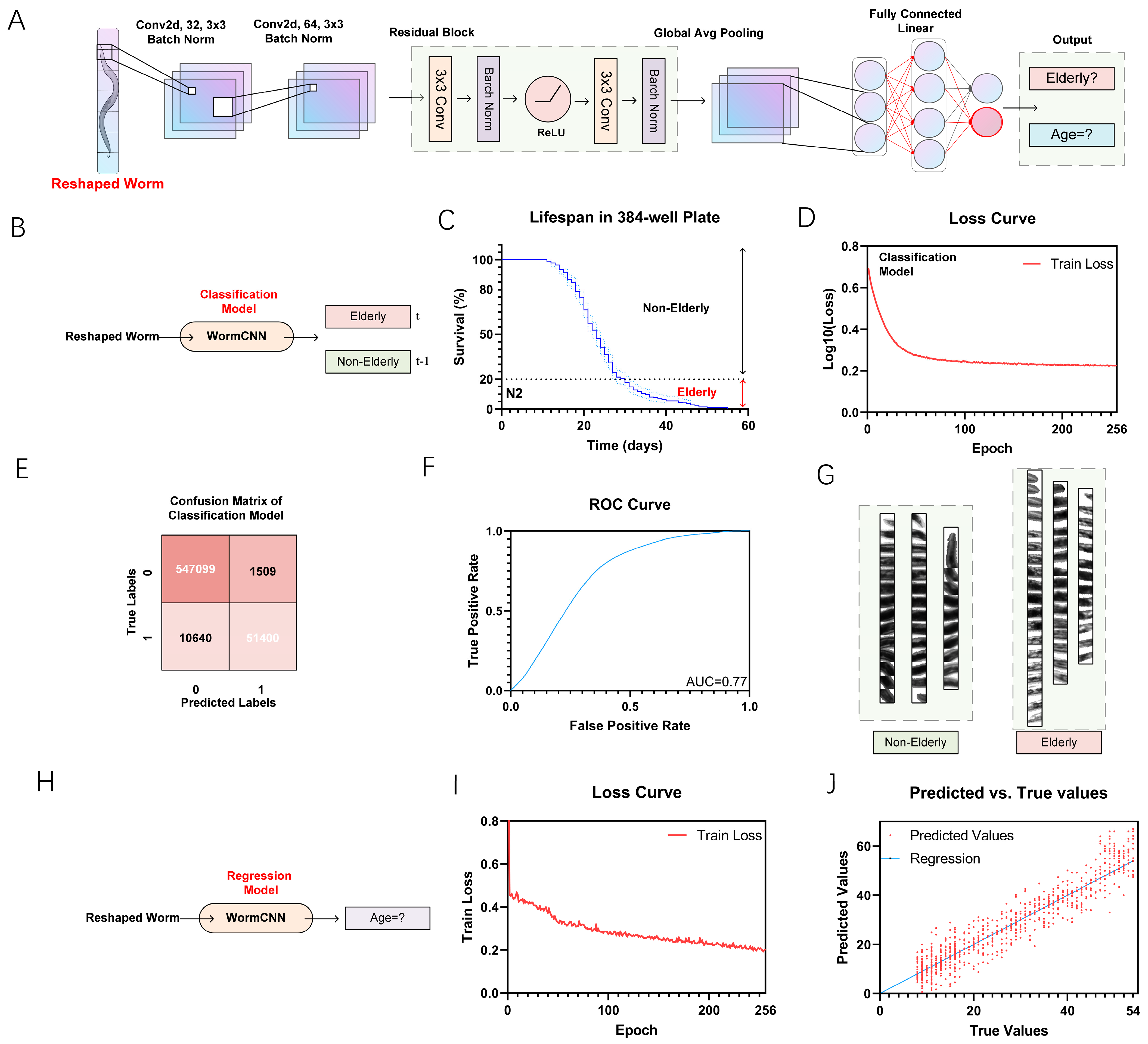
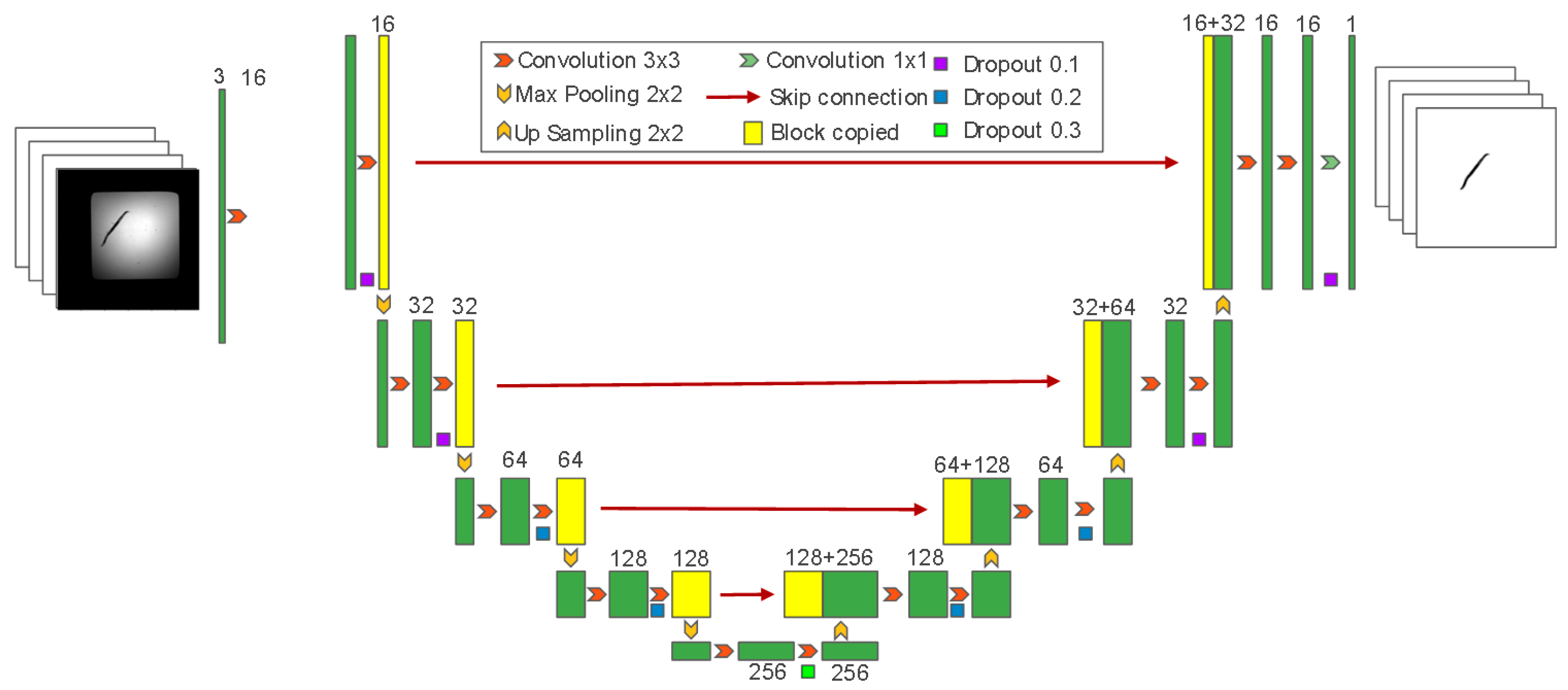
2.2. Elderly Classification and Regression Model with WormCNN
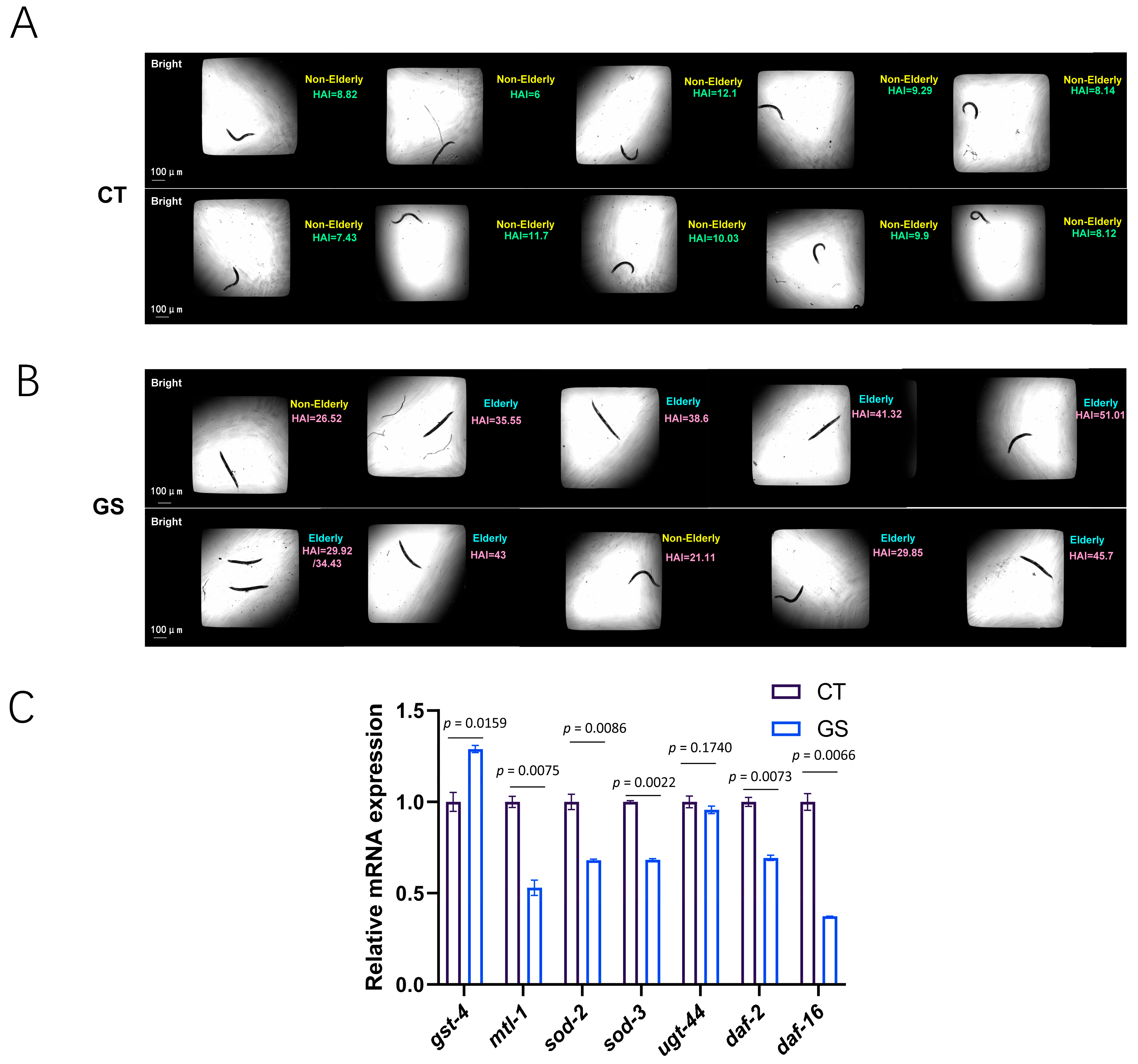
2.3. GS Modeling in C. elegans
2.4. GS Induces Premature Aging in C. elegans
3. Discussion
4. Materials and Methods
4.1. Maintenance and Experimental Setup for C. elegans
4.1.1. Culturing Worms
4.1.2. Glycation Induction in Bacterial Cultures
4.1.3. Detection of Advanced Glycation End-Products (AGEs)
4.1.4. GS Model in C. elegans
4.1.5. RT-qPCR Analysis
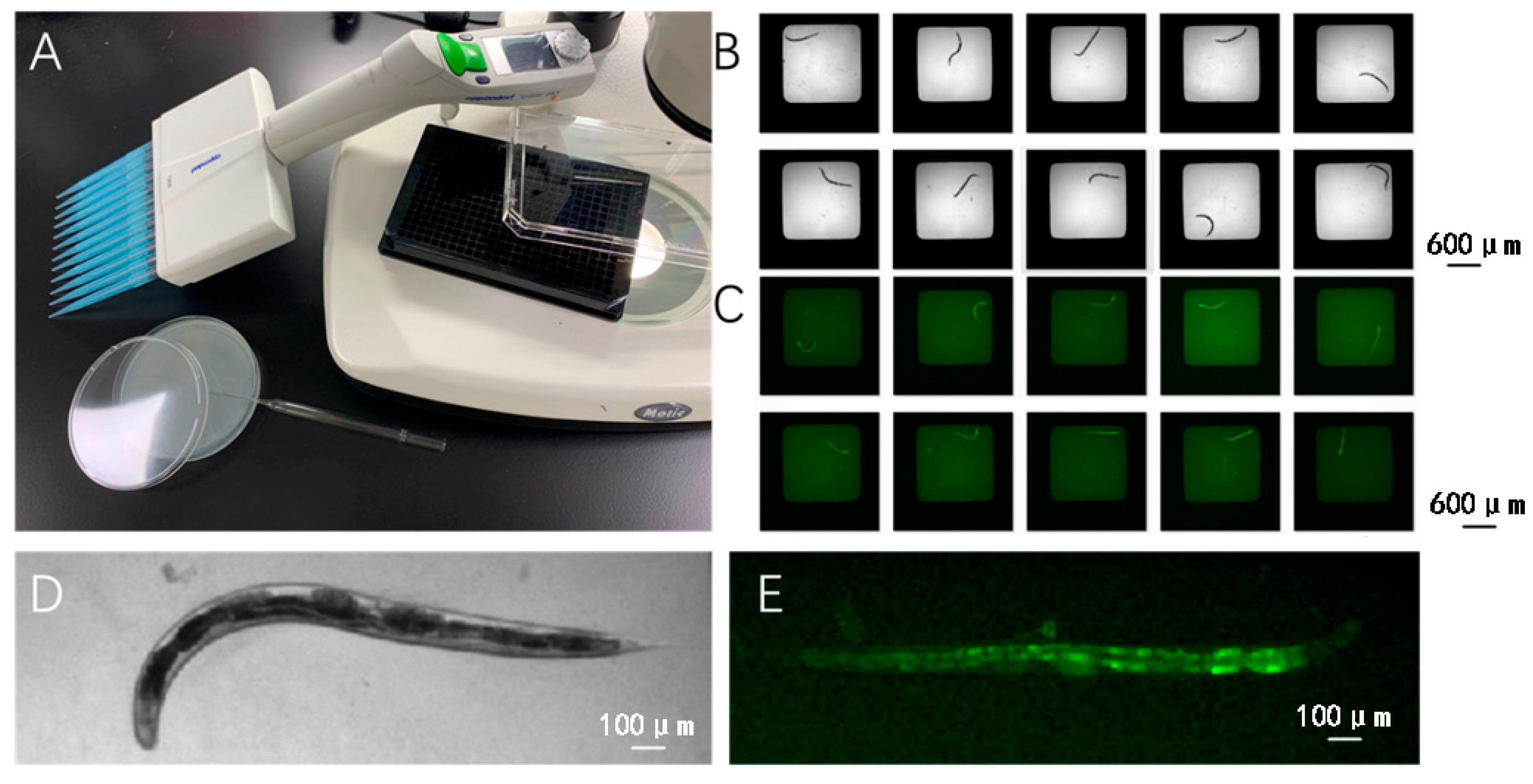
4.2. Worm Imaging Collection and Analysis
4.2.1. Worm Image Collection
4.2.2. Segmentation of Worms
4.2.3. Worms Image Extraction and Reconstruction
4.3. WormCNN Architecture
4.3.1. Convolutional Layers
4.3.2. Residual Block
4.3.3. Global Average Pooling
4.3.4. Fully Connected Layer
4.4. Training Procedure
4.5. Model Validation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samanta, S.; Akhter, F.; Xue, R.; Sosunov, A.A.; Wu, L.; Chen, D.; Arancio, O.; Yan, S.F.; Yan, S.S. Synaptic Mitochondria Gly-cation Contributes to Mitochondrial Stress and Cognitive Dysfunction. Available online: https://doi.org/10.1093/brain/awae229 (accessed on 23 July 2024).
- Kumari, N.; Vaishnav, M.S.; Srikanta, S.; Krishnaswamy, P.R.; Bhat, N. Exploring glycated sites in human serum albumin: Impact of sample processing techniques on detection and analysis. Anal. Methods Adv. Methods Appl. 2024, 16, 5239–5247. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Huang, X.; Li, H.; Lin, S.; Huang, J.; Yang, W.; Ouyang, M.; Fang, J.; Xu, Q. YTHDF2 Regulates Advanced Glycation End Products-Induced Melanogenesis through Inhibiting A20 Expression in Human Dermal Fibroblasts. Available online: https://pubmed.ncbi.nlm.nih.gov/39009810/ (accessed on 23 July 2024).
- Awan, U.N.; Waraich, R.S.; Nangrejo, R.; Noor, S.S.; Siddiqui, I.A.; Ikram, K. RAGE Signalling Contributes to Oxidative Stress and Inflammation in Knee Osteoarthritis Patients with Metabolic Syndrome. Available online: https://pubmed.ncbi.nlm.nih.gov/39008290/ (accessed on 23 July 2024).
- Takata, T.; Inoue, S.; Masauji, T.; Miyazawa, K.; Motoo, Y. Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 7319. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, N.; Karimpour Reyhan, S.; Qahremani, R.; Seifouri, K.; Tavakoli, M.; Seyedi, S.A.; Ghaemi, F.; Abbaszadeh, M.; Esteghamati, A.; Nakhjavani, M.; et al. Vitamin D in Type 2 Diabetes and Its Correlation With Heat Shock Protein 70, Ferric Reducing Ability of Plasma, Advanced Oxidation Protein Products and Advanced Glycation End Products. Endocrinol. Diabetes Metab. 2024, 7, e508. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.; Solis, G.M.; Petrascheck, M. C. elegans as Model for Drug Discovery. Curr. Top. Med. Chem. 2017, 17, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hou, B.-H.; Xie, G.-L.; Shao, Y.-T.; Yang, J.; Xu, C. Transient inhibition of mitochondrial function by chrysin and apigenin prolong longevity via mitohormesis in C. elegans. Free Radic. Biol. Med. 2023, 203, 24–33. [Google Scholar] [CrossRef]
- Gusarov, I.; Shamovsky, I.; Pani, B.; Gautier, L.; Eremina, S.; Katkova-Zhukotskaya, O.; Mironov, A.; Makarov, A.A.; Nudler, E. Dietary thiols accelerate aging of C. elegans. Nat. Commun. 2021, 12, 4336. [Google Scholar] [CrossRef]
- Carrara, M.; Richaud, M.; Cuq, P.; Galas, S.; Margout-Jantac, D. Influence of Oleacein, an Olive Oil and Olive Mill Wastewater Phenolic Compound, on Caenorhabditis elegans Longevity and Stress Resistance. Foods Basel Switz. 2024, 13, 2146. [Google Scholar] [CrossRef]
- Cooper, J.F.; Van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Park. Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef]
- Ezcurra, M.; Benedetto, A.; Sornda, T.; Gilliat, A.F.; Au, C.; Zhang, Q.; Van Schelt, S.; Petrache, A.L.; Wang, H.; De La Guardia, Y.; et al. C. elegans Eats Its Own Intestine to Make Yolk Leading to Multiple Senescent Pathologies. Curr. Biol. 2018, 28, 2544–2556.e5. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Swain, S.C.; Keusekotten, K.; Baumeister, R.; Stürzenbaum, S.R. C. elegans Metallothioneins: New Insights into the Phenotypic Effects of Cadmium Toxicosis. J. Mol. Biol. 2004, 341, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, M.; Lai, W.; Dong, X.; Zhang, S.; Zhang, L.; Chen, G. ShengqingJiangzhuo capsule ameliorates diabetic nephropathy by improving Keap1/Nrf2 signaling pathway. J. Pharm. Pharmacol. 2024, 76, 1149–1159. [Google Scholar] [CrossRef]
- Kopp, W. Aging and “Age-Related” Diseases—What Is the Relation? Available online: https://pubmed.ncbi.nlm.nih.gov/39012663/ (accessed on 23 July 2024).
- Muthaiyan Shanmugam, M.; Chaudhuri, J.; Sellegounder, D.; Sahu, A.K.; Guha, S.; Chamoli, M.; Hodge, B.; Bose, N.; Amber, C.; Farrera, D.O.; et al. Methylglyoxal-derived hydroimidazolone, MG-H1, increases food intake by altering tyramine signaling via the GATA transcription factor ELT-3 in Caenorhabditis elegans. eLife 2023, 12, e82446. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Litke, R.; Rianha, S.; Paul-Constant, C.; Lo Guidice, J.-M.; Taront, S.; Tessier, F.J.; Boulanger, E.; Fradin, C. Exposure of Caenorhabditis elegans to Dietary Nε-Carboxymethyllysine Emphasizes Endocytosis as a New Route for Intestinal Absorption of Advanced Glycation End Products. Nutrients 2021, 13, 4398. [Google Scholar] [CrossRef] [PubMed]
- Uppuganti, S.; Creecy, A.; Fernandes, D.; Garrett, K.; Donovan, K.; Ahmed, R.; Voziyan, P.; Rendina-Ruedy, E.; Nyman, J.S. Bone Fragility in High Fat Diet-induced Obesity is Partially Independent of Type 2 Diabetes in Mice. Calcif. Tissue Int. 2024, 115, 298–314. [Google Scholar] [CrossRef]
- Clarke, D.M.; Koutnik, A.P.; Johnson, R.J.; DeBlasi, J.M.; Bikman, B.T.; Arroyo, J.A.; Reynolds, P.R. Differential Rates of Glycation Following Exposure to Unique Monosaccharides. Int. J. Mol. Sci. 2024, 25, 6921. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Yuan, H.; Yong, R.; Duan, S.; Li, Y.; Spencer, J.; Lim, E.G.; Yu, L.; Song, P. Deep Learning for Microfluidic-Assisted Caenorhabditis elegans Multi-Parameter Identification Using YOLOv7. Micromachines 2023, 14, 1339. [Google Scholar] [CrossRef]
- Zabardast, A.; Tamer, E.G.; Son, Y.A.; Yılmaz, A. An automated framework for evaluation of deep learning models for splice site predictions. Sci. Rep. 2023, 13, 10221. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Yin, Y.; Tang, J. Estimation of Caenorhabditis Elegans Lifespan Stages Using a Dual-Path Network Combining Biomarkers and Physiological Changes. Bioengineering 2022, 9, 689. [Google Scholar] [CrossRef]
- García-Garví, A.; Layana-Castro, P.E.; Puchalt, J.C.; Sánchez-Salmerón, A.-J. Automation of Caenorhabditis elegans lifespan assay using a simplified domain synthetic image-based neural network training strategy. Comput. Struct. Biotechnol. J. 2023, 21, 5049–5065. [Google Scholar] [CrossRef]
- McClanahan, P.D.; Golinelli, L.; Le, T.A.; Temmerman, L. Automated scoring of nematode nictation on a textured background. PLoS ONE 2023, 18, e0289326. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-J.; Cha, H.-J. Deep Learning Approach Based on Transcriptome Profile for Data Driven Drug Discovery. Mol. Cells 2023, 46, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.C.; Khan, K.A.; Sharma, R. Deep-Worm-Tracker: Deep Learning Methods for Accurate Detection and Tracking for Behavioral Studies in C. elegans. Appl. Anim. Behav. Sci. 2023, 266, 106024. [Google Scholar] [CrossRef]
- Bates, K.; Le, K.N.; Lu, H. Deep learning for robust and flexible tracking in behavioral studies for C. elegans. PLoS Comput. Biol. 2022, 18, e1009942. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.-J.V. Recent Progress in Regulation of Aging by Insulin/IGF-1 Signaling in Caenorhabditis elegans. Mol. Cells 2022, 45, 763–770. [Google Scholar] [CrossRef]
- Fabrizio, P.; Alcolei, A.; Solari, F. Considering Caenorhabditis elegans Aging on a Temporal and Tissue Scale: The Case of Insulin/IGF-1 Signaling. Cells 2024, 13, 288. [Google Scholar] [CrossRef]
- Delligatti, C.E.; Kirk, J.A. Glycation in the cardiomyocyte. Vitam. Horm. 2024, 125, 47–88. [Google Scholar] [CrossRef]
- Ozawa, Y.; Takegami, Y.; Seki, T.; Osawa, Y.; Iida, H.; Okamoto, M.; Nakashima, H.; Ishizuka, S.; Hasegawa, Y.; Imagama, S. Relationship between locomotive syndrome and advanced glycation end products measured by skin autofluorescence in community-dwelling patients: The Yakumo Study. Nagoya J. Med. Sci. 2024, 86, 314–325. [Google Scholar] [CrossRef]
- Ueno, K.; Nohara, I.; Miyashita, M.; Itokawa, M.; Okado, H.; Arai, M.; Saitoe, M. Behavioral Dysfunctions Caused by Pyridoxamine Deficiency in Drosophila melanogaster. J. Nutr. Sci. Vitaminol. 2024, 70, 252–261. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, Z.; Zhao, Y.; Yin, D. Obesogenic potentials of environmental artificial sweeteners with disturbances on both lipid metabolism and neural responses. Sci. Total Environ. 2024, 919, 170755. [Google Scholar] [CrossRef]
- Selvarathinam, H.; Elkhalil, A.; Schargel, W.E.; Ghose, P. Neurodegeneration-related genes influence C. elegans pharyngeal activity. MicroPublication Biol. 2024. [Google Scholar] [CrossRef]
- Weng, Y.; Zhou, S.; Morillo, K.; Kaletsky, R.; Lin, S.; Murphy, C.T. The neuron-specific IIS/FOXO transcriptome in aged animals reveals regulatory mechanisms of cognitive aging. eLife 2024, 13, RP95621. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardo, M.; León Guerrero, V.L.; Sutoski, J.C.; Hardy, W.R.; MacNeil, L.T. SHC-3: A Previously Unidentified C. elegans Shc Family Member Functions in the Insulin-like Signaling Pathway to Enhance Survival During L1 Arrest. Available online: https://pubmed.ncbi.nlm.nih.gov/38861412/ (accessed on 23 July 2024).
- Dello Russo, M.; Sirangelo, I.; Lauria, F.; Formisano, A.; Iannuzzi, C.; Hebestreit, A.; Pala, V.; Siani, A.; Russo, P. Dietary Advanced Glycation End Products (AGEs) and Urinary Fluorescent AGEs in Children and Adolescents: Findings from the Italian I. Family Project. Nutrients 2024, 16, 1831. [Google Scholar] [CrossRef] [PubMed]

| Metric | Value |
|---|---|
| True Positive (TP) | 35,610 |
| False Positive (FP) | 7527 |
| True Negative (TN) | 12,932,412 |
| False Negative (FN) | 579 |
| Sensitivity (TPR) | 98.40% |
| Specificity (TNR) | 99.94% |
| Overall Accuracy | 99.94% |
| Precision (PPV) | 82.55% |
| F1-Score | 89.78% |
| Metric | Value |
|---|---|
| True Positives (TP) | 54,100 |
| False Positives (FP) | 1509 |
| True Negatives (TN) | 547,099 |
| False Negatives (FN) | 10,640 |
| True Positive Rate (TPR) | 83.6% |
| Precision (PPV) | 97.3% |
| Specificity (TNR) | 99.72% |
| Overall Accuracy | 98.0% |
| F1-Score | 89.91% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Huang, Z.; Cai, H.; Li, Z.; Zhu, J.; Wu, D.; Xu, W.; Qiu, H.; Zhang, N.; Li, G.; et al. WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging. Int. J. Mol. Sci. 2024, 25, 9675. https://doi.org/10.3390/ijms25179675
Pan Y, Huang Z, Cai H, Li Z, Zhu J, Wu D, Xu W, Qiu H, Zhang N, Li G, et al. WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging. International Journal of Molecular Sciences. 2024; 25(17):9675. https://doi.org/10.3390/ijms25179675
Chicago/Turabian StylePan, Yan, Zhihang Huang, Hongxia Cai, Zhiru Li, Jingyuan Zhu, Dan Wu, Wentao Xu, Hexiang Qiu, Nan Zhang, Guojun Li, and et al. 2024. "WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging" International Journal of Molecular Sciences 25, no. 17: 9675. https://doi.org/10.3390/ijms25179675
APA StylePan, Y., Huang, Z., Cai, H., Li, Z., Zhu, J., Wu, D., Xu, W., Qiu, H., Zhang, N., Li, G., Gao, S., & Xian, B. (2024). WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging. International Journal of Molecular Sciences, 25(17), 9675. https://doi.org/10.3390/ijms25179675







