The Unexplored Role of Mitochondria-Related Oxidative Stress in Diverticular Disease
Abstract
1. Introduction
2. Results
2.1. Human Tissue Collection
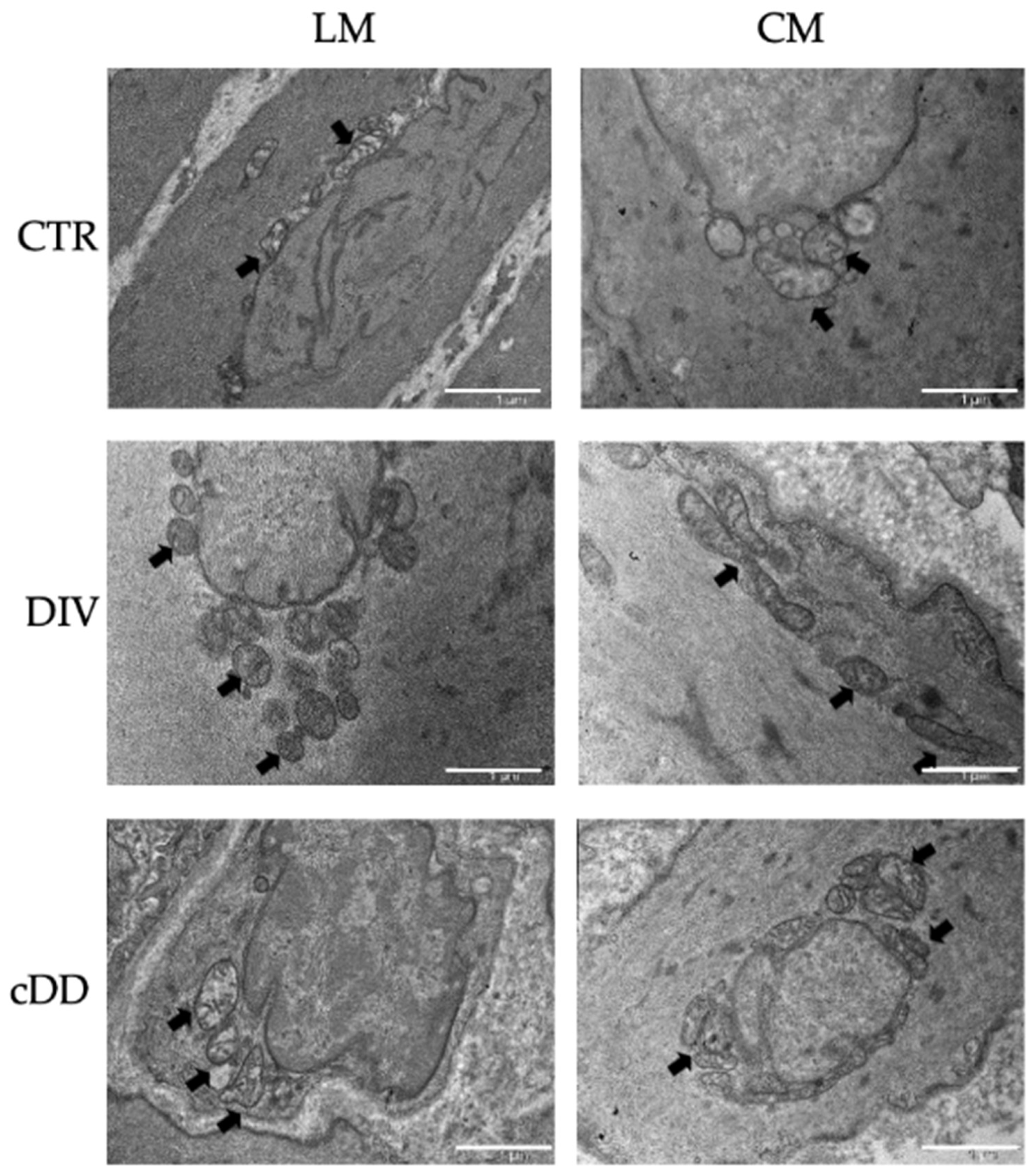
2.2. Ultrastructural Analyses of Mitochondria Smooth Muscle Tissues
2.3. Mitochondrial Respiratory Chain
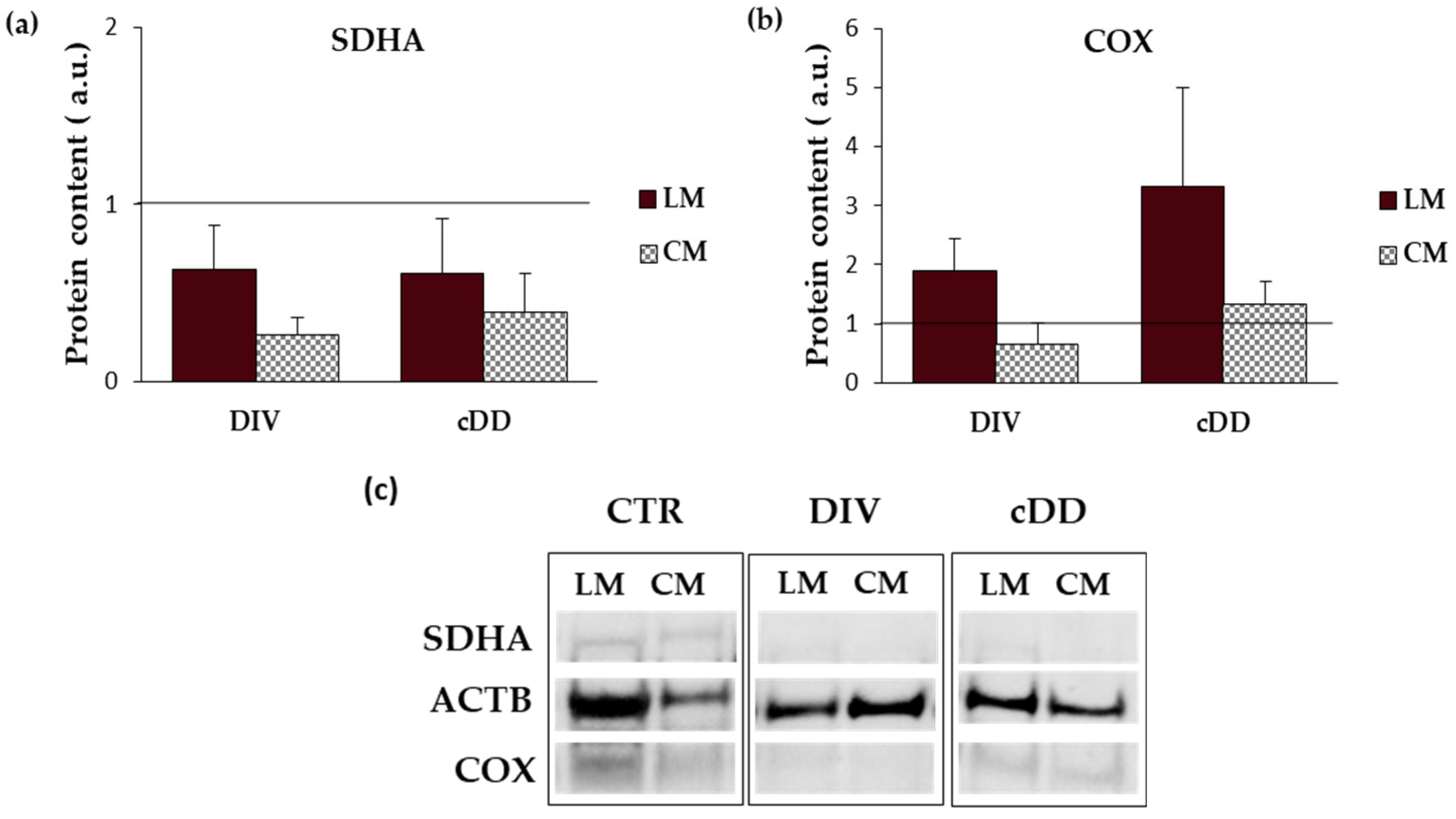
2.3.1. Mitochondrial Respiratory Chain-Complex II (SDHA)
2.3.2. Mitochondrial Respiratory Chain—Complex IV (COX)
2.4. Mitochondrial Alterations and Antioxidant Status Evaluation in Isolated Smooth Muscle Cells
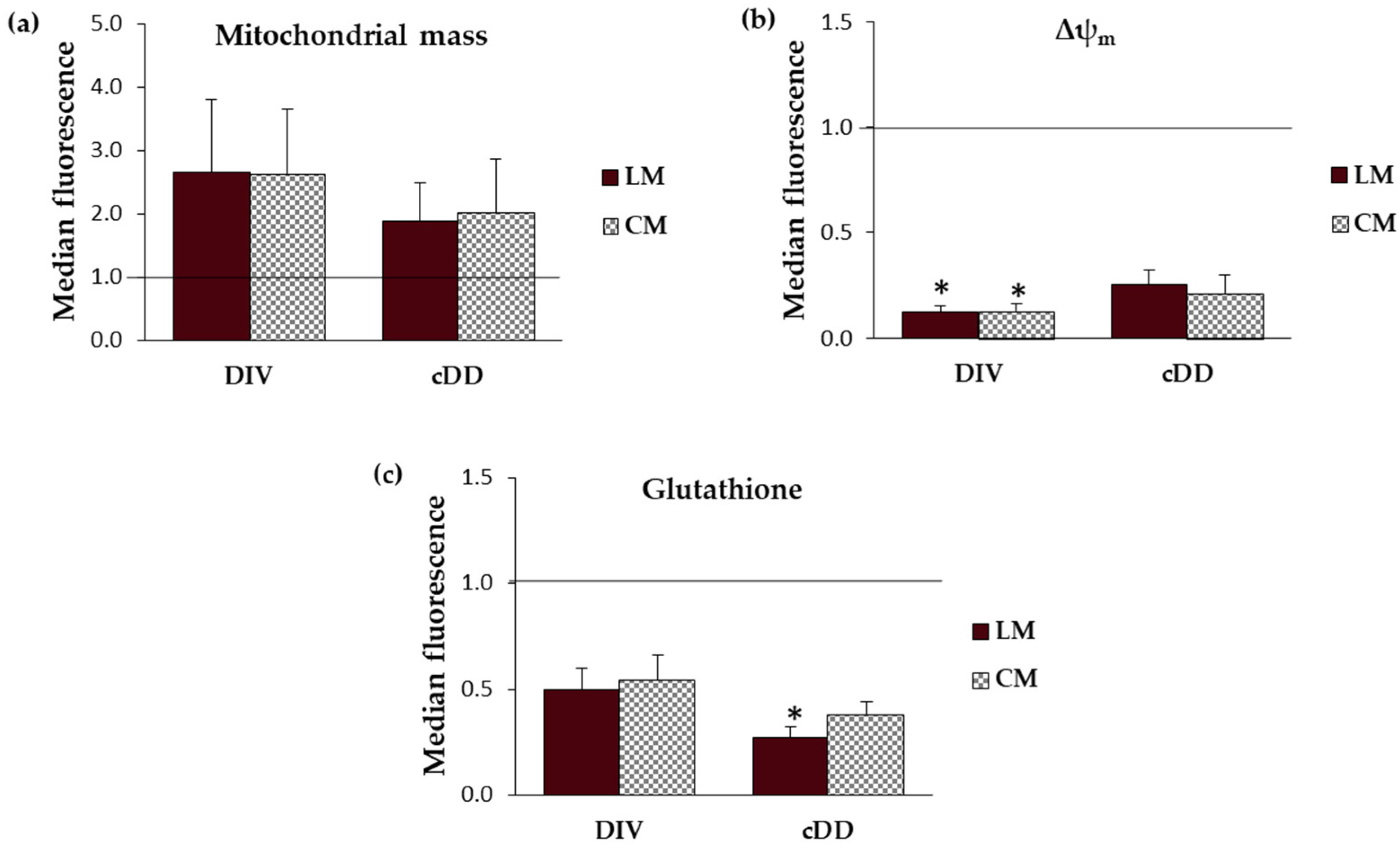
2.4.1. Mitochondrial Mass
2.4.2. Mitochondrial Membrane Potential (Δψm)
2.4.3. Glutathione Intracellular Content
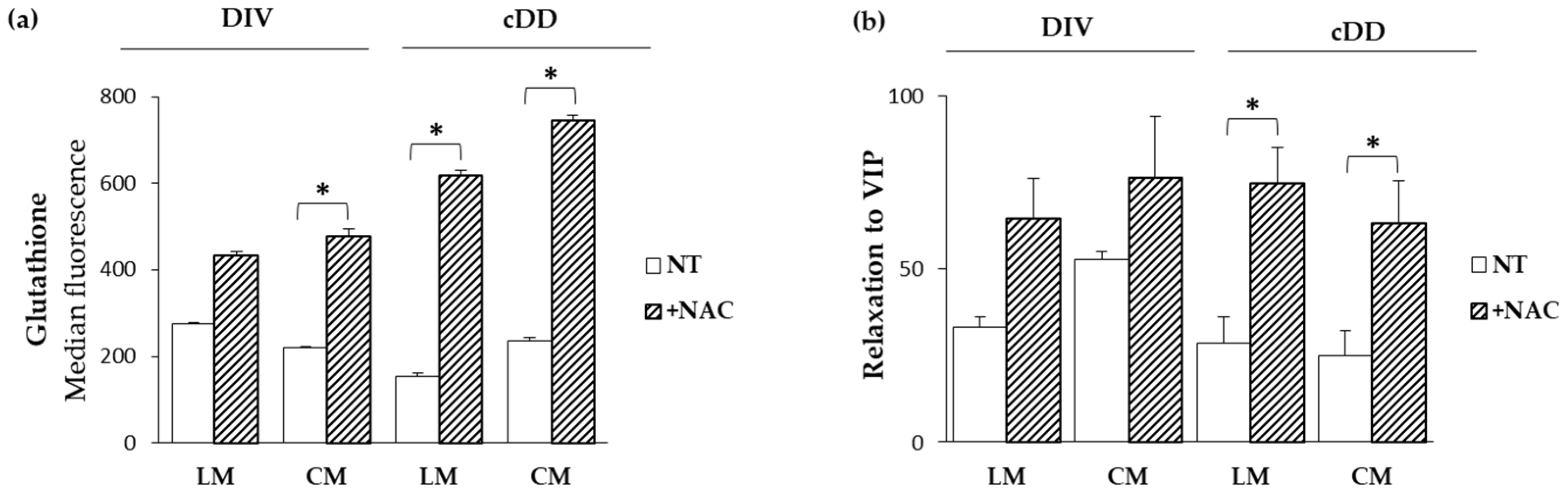
2.5. Functional Activity of Isolated Smooth Muscle Cells
2.6. Functional Effects of N-Acetylcysteine
3. Discussion
4. Materials and Methods
4.1. Patients and Colonic Specimens
4.2. Cellular Ex Vivo Mode
4.3. Transmission Electron Microscopy (TEM)
4.4. Western Blot
4.5. Flow Cytometry Analysis
4.6. Functional Study
4.7. Antioxidant In Vitro Model
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, J.K.; Azhar, N.; Binda, G.A.; Barbara, G.; Biondo, S.; Boermeester, M.A.; Chabok, A.; Consten, E.C.J.; van Dijk, S.T.; Johanssen, A.; et al. European Society of Coloproctology: Guidelines for the Management of Diverticular Disease of the Colon. Color. Dis. 2020, 22, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Annibale, B. Treatment of Diverticular Disease: An Update on Latest Evidence and Clinical Implications. Drugs Context 2018, 7, 212526. [Google Scholar] [CrossRef]
- Camilleri, M.; Sandler, R.S.; Peery, A.F. Etiopathogenetic Mechanisms in Diverticular Disease of the Colon. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 15–32. [Google Scholar] [CrossRef]
- Stollman, N.; Raskin, J.B. Diverticular disease of the colon. Lancet 2004, 363, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, L.; Pisano, A.; Vona, R.; Cappelletti, M.; Pignataro, M.G.; Tattoli, I.; Maselli, M.A.; Tarallo, M.; Casella, G.; Caronna, R.; et al. From Diverticulosis to Complicated Diverticular Disease: Progression of Myogenic Alterations and Oxidative Imbalance. Neurogastroenterol. Motil. 2024, 36, e14850. [Google Scholar] [CrossRef]
- Bassotti, G.; Battaglia, E.; Spinozzi, F.; Pelli, M.A.; Tonini, M. Twenty-four hour recordings of colonic motility in patients with diverticular disease: Evidence for abnormal motility and propulsive activity. Dis. Colon Rectum 2001, 44, 1814–1820. [Google Scholar] [CrossRef]
- Gallego, D.; Espín, F.; Mikulka, J.; Šmirg, O.; Gil, V.; Faundez-Zanuy, M.; Jiménez, M.; Clavé, P. In vitro motor patterns and electrophysiological changes in patients with colonic diverticular disease. Int. J. Color. Dis. 2013, 28, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Xiao, H.-H. The Role of Oxidative Stress and Natural Products in Maintaining Human Health. Nutrients 2024, 16, 1268. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial Dynamics in the Regulation of Nutrient Utilization and Energy Expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Perli, E.; Pisano, A.; Glasgow, R.I.C.; Carbo, M.; Hardy, S.A.; Falkous, G.; He, L.; Cerbelli, B.; Pignataro, M.G.; Zacara, E.; et al. Novel Compound Mutations in the Mitochondrial Translation Elongation Factor (TSFM) Gene Cause Severe Cardiomyopathy with Myocardial Fibro-Adipose Replacement. Sci. Rep. 2019, 9, 5108. [Google Scholar] [CrossRef] [PubMed]
- Popov, L. Mitochondrial Biogenesis: An Update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Zeng, J.W.; Chen, B.Y.; Lv, X.F.; Sun, L.; Zeng, X.L.; Zheng, H.Q.; Du, Y.H.; Wang, G.L.; Ma, M.M.; Guan, Y.Y. Transmembrane member 16A participates in hydrogen peroxide-induced apoptosis by facilitating mitochondria-dependent pathway in vascular smooth muscle cells. Br. J. Pharmacol. 2018, 175, 3669–3684. [Google Scholar] [CrossRef]
- Wredenberg, A.; Wibom, R.; Wilhelmsson, H.; Graff, C.; Wiener, H.H.; Burden, S.J.; Oldfors, A.; Westerblad, H.; Larsson, N.-G. Increased Mitochondrial Mass in Mitochondrial Myopathy Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 15066–15071. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.; Youle, R.J. Mitochondrial Fission and Fusion. Essays Biochem. 2010, 47, 85–98. [Google Scholar] [CrossRef]
- Jenkins, B.C.; Neikirk, K.; Katti, P.; Claypool, S.M.; Kirabo, A.; McReynolds, M.R.; Hinton, A. Mitochondria in Disease: Changes in Shapes and Dynamics. Trends Biochem. Sci. 2024, 49, 346–360. [Google Scholar] [CrossRef]
- Ahuja, P.; Wanagat, J.; Wang, Z.; Wang, Y.; Liem, D.A.; Ping, P.; Antoshechkin, I.A.; Margulies, K.B.; Maclellan, W.R. Divergent mitochondrial biogenesis responses in human cardiomyopathy. Circulation 2013, 127, 1957–1967. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Totoń-Żurańska, J.; Mikolajczyk, T.P.; Saju, B.; Guzik, T.J. Vascular remodelling in cardiovascular disease: Hypertension, oxidation, and inflammation. Clin. Sci. 2024, 138, 817–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, P.; Nie, B.; Zhang, Z.; Shi, R.; Liu, Q.; Wang, S.; Xu, T.; Tian, J. Identification and verification of oxidative stress-related signature markers for intracranial aneurysm: Applied bioinformatics. Front. Biosci. 2024, 29, 294. [Google Scholar] [CrossRef]
- Lam, J.; Katti, P.; Biete, M.; Mungai, M.; AshShareef, S.; Neikirk, K.; Garza Lopez, E.; Vue, Z.; Christensen, T.A.; Beasley, H.K.; et al. A Universal Approach to Analyzing Transmission Electron Microscopy with ImageJ. Cells 2021, 10, 2177. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic Type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Morelli, A.M.; Ravera, S.; Panfoli, I. The Aerobic Mitochondrial ATP Synthesis from a Comprehensive Point of View. Open Biol. 2020, 10, 200224. [Google Scholar] [CrossRef]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal Structure of Mitochondrial Respiratory Membrane Protein Complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoschikova, I.V.; Gon-calves, R.L.; Hey-Mogensen, M.; Brand, M.D. The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. Enzymol. Methods 2013, 526, 189–217. [Google Scholar] [CrossRef]
- Guan, H.; Sun, J.; Liang, X.; Yao, W. Protective Role of Cytochrome C Oxidase 5A (COX5A) against Mitochondrial Disorder and Oxidative Stress in VSMC Phenotypic Modulation and Neointima Formation. Curr. Vasc. Pharmacol. 2023, 21, 128–142. [Google Scholar] [CrossRef]
- Kamarauskaite, J.; Baniene, R.; Trumbeckas, D.; Strazdauskas, A.; Trumbeckaite, S. Increased succinate accumulation induces ROS generation in in vivo ischemia/reperfusion-affected rat kidney mitochondria. Biomed Res. Int. 2020, 2020, 8855585. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; d’Amati, G. Evaluation of Gastrointestinal mtDNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE). Methods Mol. Biol. 2011, 755, 223–232. [Google Scholar]
- Goetzman, E.; Gong, Z.; Zhang, B.; Muzumdar, R. Complex II Biology in Aging, Health, and Disease. Antioxidants 2023, 12, 1477. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol Induces Mitochondrial Biogenesis in Endothelial Cells. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Millare, B.; O’Rourke, B.; Trayanova, N. Hydrogen Peroxide Diffusion and Scavenging Shapes Mitochondrial Network Instability and Failure by Sensitizing ROS-Induced ROS Release. Sci. Rep. 2020, 10, 15758. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-Induced ROS Release: An Update and Review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Raj Rai, S.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Bălăeţ, C.; Coculescu, B.I.; Manole, G.; Bălăeţ, M.; Dincă, G.V. Gamma-Glutamyltransferase, Possible Novel Biomarker in Colon Diverticulosis: A Case-Control Study. J. Enzym. Inhib. Med. Chem. 2018, 33, 428–432. [Google Scholar] [CrossRef]
- Chalmers, S.; Saunter, C.; Wilson, C.; Coats, P.; Girkin, J.M.; McCarron, J.G. Mitochondrial Motility and Vascular Smooth Muscle Proliferation. Arter. Thromb. Vasc. Biol. 2012, 32, 3000–3011. [Google Scholar] [CrossRef]
- Marsboom, G.; Toth, P.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.-H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-Related Protein 1–Mediated Mitochondrial Mitotic Fission Permits Hyperproliferation of Vascular Smooth Muscle Cells and Offers a Novel Therapeutic Target in Pulmonary Hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; An, P.; Luo, J.; Luo, Y. Mitochondrial Homeostasis in VSMCs as a Central Hub in Vascular Remodeling. Int. J. Mol. Sci. 2023, 24, 3483. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 75–89. [Google Scholar] [CrossRef]
- Scirocco, A.; Pallotta, L.; Rengo, M.; Ignazzi, A.; Carabotti, M.; Cicenia, A.; Vona, R.; Chirletti, P.; Maselli, M.A.; Donghia, R.; et al. Myogenic Oxidative Imbalance Interferes with Antral Motility in Obese Subjects. Dig. Liver Dis. 2018, 50, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Petitta, C.; Scirocco, A.; Ascione, B.; Ammoscato, F.; Di Natale, G.; Anastasi, E.; Marconi, M.; Chirletti, P.; Malorni, W.; et al. Antioxidants Counteract Lipopolysaccharide-Triggered Alterations of Human Colonic Smooth Muscle Cells. Free. Radic. Biol. Med. 2012, 53, 2102–2111. [Google Scholar] [CrossRef]
- Romagnoli, C.; Marcucci, T.; Picariello, L.; Tonelli, F.; Vincenzini, M.T.; Iantomasi, T. Role of N-Acetylcysteine and GSH Redox System on Total and Active MMP-2 in Intestinal Myofibroblasts of Crohn’s Disease Patients. Int. J. Color. Dis. 2013, 28, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Cremon, C.; Fuschi, D.; Marasco, G.; Palombo, M.; Stanghellini, V.; Barbara, G. Pathophysiology of Diverticular Disease: From Diverticula Formation to Symptom Generation. Int. J. Mol. Sci. 2022, 23, 6698. [Google Scholar] [CrossRef]
- Zhou, J.; Terluk, M.R.; Orchard, P.J.; Cloyd, J.C.; Kartha, R.V. N-Acetylcysteine Reverses the Mitochondrial Dysfunction Induced by Very Long-Chain Fatty Acids in Murine Oligodendrocyte Model of Adrenoleukodystrophy. Biomedicines 2021, 9, 1826. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramírez-Camacho, I.; Correa, F.; El Hafidi, M.; Silva-Palacios, A.; Ostolga-Chavarría, M.; Esparza-Perusquía, M.; Olvera-Sánchez, S.; Flores-Herrera, O.; Zazueta, C. Cardioprotective strategies preserve the stability of respiratory chain supercomplexes and reduce oxidative stress in reperfused ischemic hearts. Free Radic. Biol. Med. 2018, 129, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Corleto, V.D.; Taffuri, M.; Campanini, N.; Rindi, G.; Caprilli, R.; Delle Fave, G.; Severi, C. Optimisation of Isolation of Richly Pure and Homogeneous Primary Human Colonic Smooth Muscle Cells. Dig. Liver Dis. 2004, 36, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Scirocco, A.; Matarrese, P.; Petitta, C.; Cicenia, A.; Ascione, B.; Mannironi, C.; Ammoscato, F.; Cardi, M.; Fanello, G.; Guarino, M.P.; et al. Exposure of Toll-like Receptors 4 to Bacterial Lipopolysaccharide (LPS) Impairs Human Colonic Smooth Muscle Cell Function. J. Cell. Physiol. 2010, 223, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial Dynamics, Mitophagy and Cardiovascular Disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]

| Controls | Diverticulosis | Complicated DD | |
|---|---|---|---|
| Gender | 7 women/6 men | 8 women/9 men | 8 women/7 men |
| Age (years) median (95% CI) | 68 (53–92) | 76 (46–89) | 67 (42–85) |
| BMI (kg/m2) median (95% CI) | 23 (21–30) | 24.0 (21–29) | 26.0 (20–34) |
| Contraction | Relaxation | |
|---|---|---|
| LONGITUDINAL layer | ||
| DIV | 45.7% ± 7.1 | 56.9% * ± 5.3 |
| cDD | 61.7% * ± 9.6 | 56.9% * ± 5.3 |
| CIRCULAR layer | ||
| DIV | 46.7% ± 5.8 | 21.9% ± 10.2 |
| cDD | 56.4% ± 14.2 | 54.6% ± 13.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappelletti, M.; Pallotta, L.; Vona, R.; Tinari, A.; Pisano, A.; Casella, G.; Crocetti, D.; Carlomagno, D.; Tattoli, I.; Giordano, C.; et al. The Unexplored Role of Mitochondria-Related Oxidative Stress in Diverticular Disease. Int. J. Mol. Sci. 2024, 25, 9680. https://doi.org/10.3390/ijms25179680
Cappelletti M, Pallotta L, Vona R, Tinari A, Pisano A, Casella G, Crocetti D, Carlomagno D, Tattoli I, Giordano C, et al. The Unexplored Role of Mitochondria-Related Oxidative Stress in Diverticular Disease. International Journal of Molecular Sciences. 2024; 25(17):9680. https://doi.org/10.3390/ijms25179680
Chicago/Turabian StyleCappelletti, Martina, Lucia Pallotta, Rosa Vona, Antonella Tinari, Annalinda Pisano, Giovanni Casella, Daniele Crocetti, Dominga Carlomagno, Ivan Tattoli, Carla Giordano, and et al. 2024. "The Unexplored Role of Mitochondria-Related Oxidative Stress in Diverticular Disease" International Journal of Molecular Sciences 25, no. 17: 9680. https://doi.org/10.3390/ijms25179680
APA StyleCappelletti, M., Pallotta, L., Vona, R., Tinari, A., Pisano, A., Casella, G., Crocetti, D., Carlomagno, D., Tattoli, I., Giordano, C., Matarrese, P., & Severi, C. (2024). The Unexplored Role of Mitochondria-Related Oxidative Stress in Diverticular Disease. International Journal of Molecular Sciences, 25(17), 9680. https://doi.org/10.3390/ijms25179680





