Comparison between USPIOs and SPIOs for Multimodal Imaging of Extracellular Vesicles Extracted from Adipose Tissue-Derived Adult Stem Cells
Abstract
:1. Introduction
2. Results
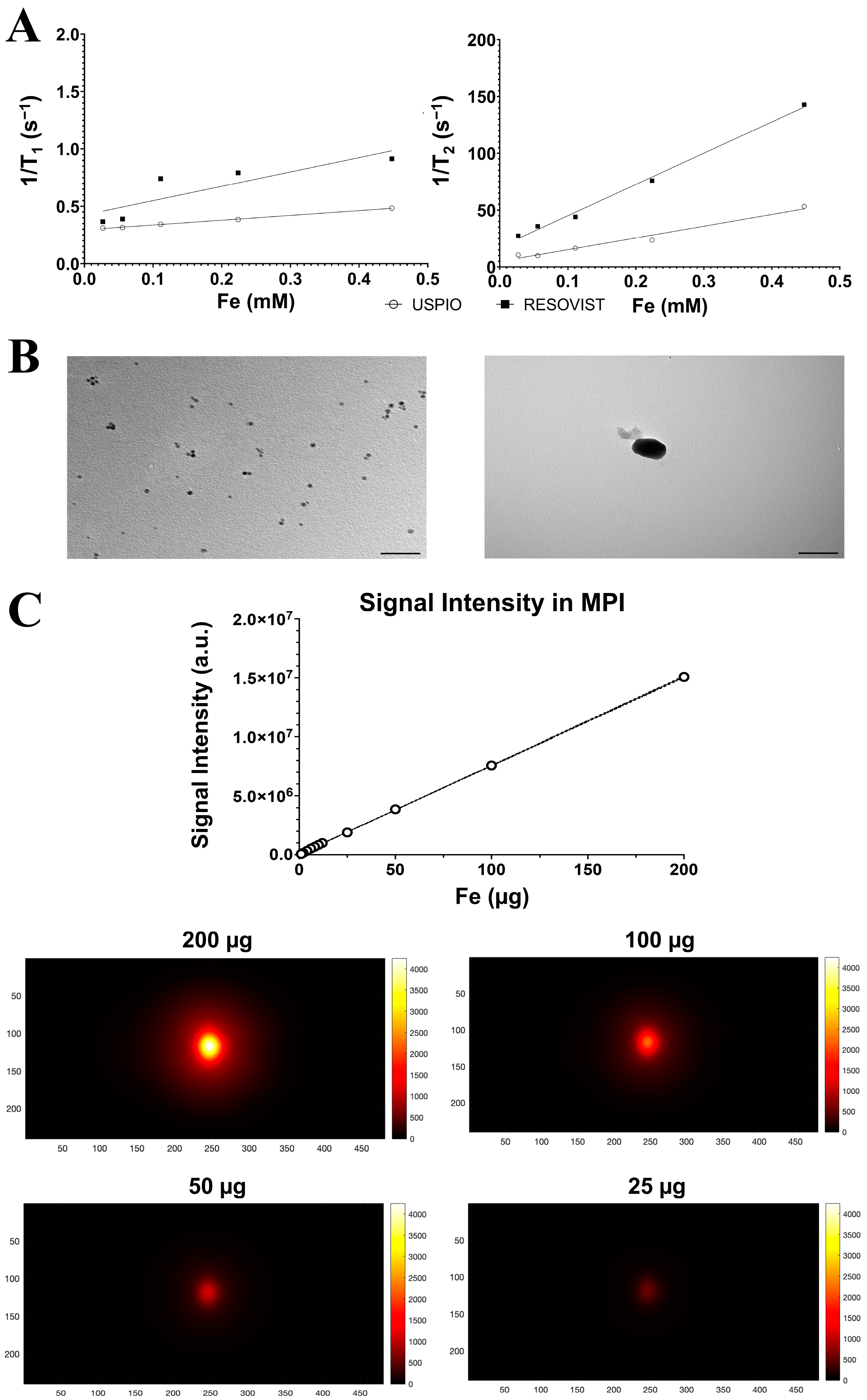
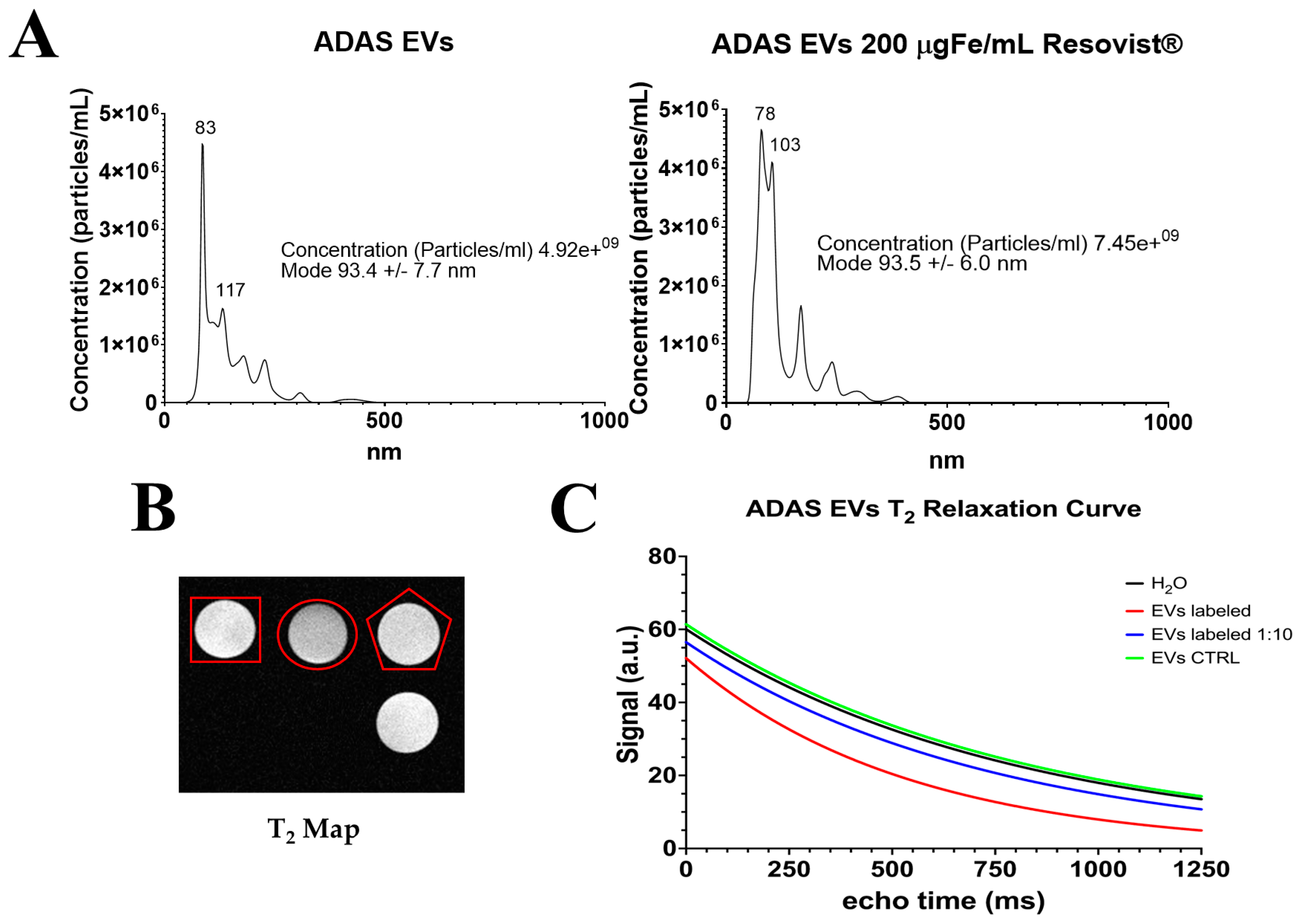
2.1. Nanoparticle Characterization
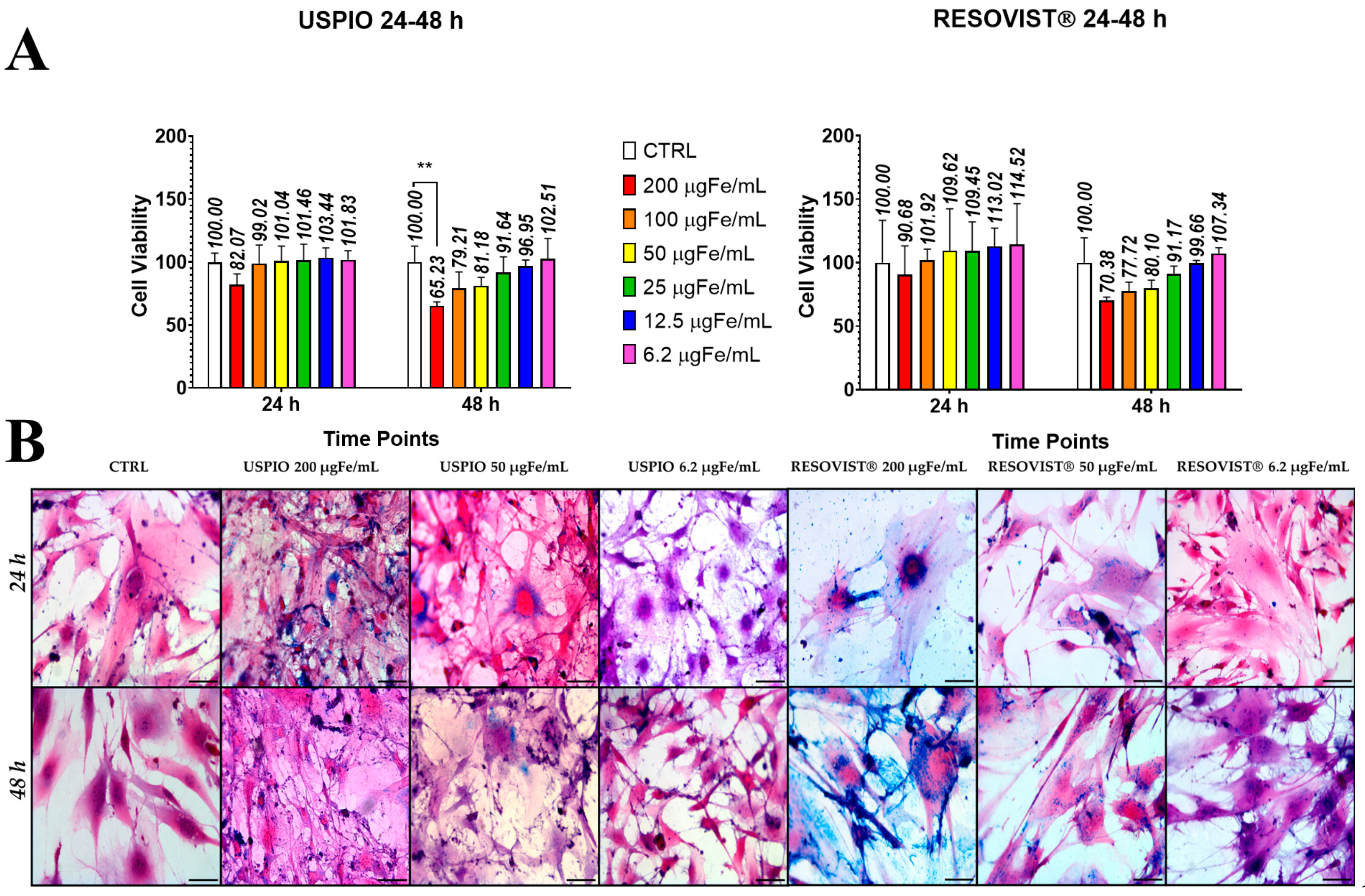
2.2. Toxicity and Internalization of NPs in ADAS
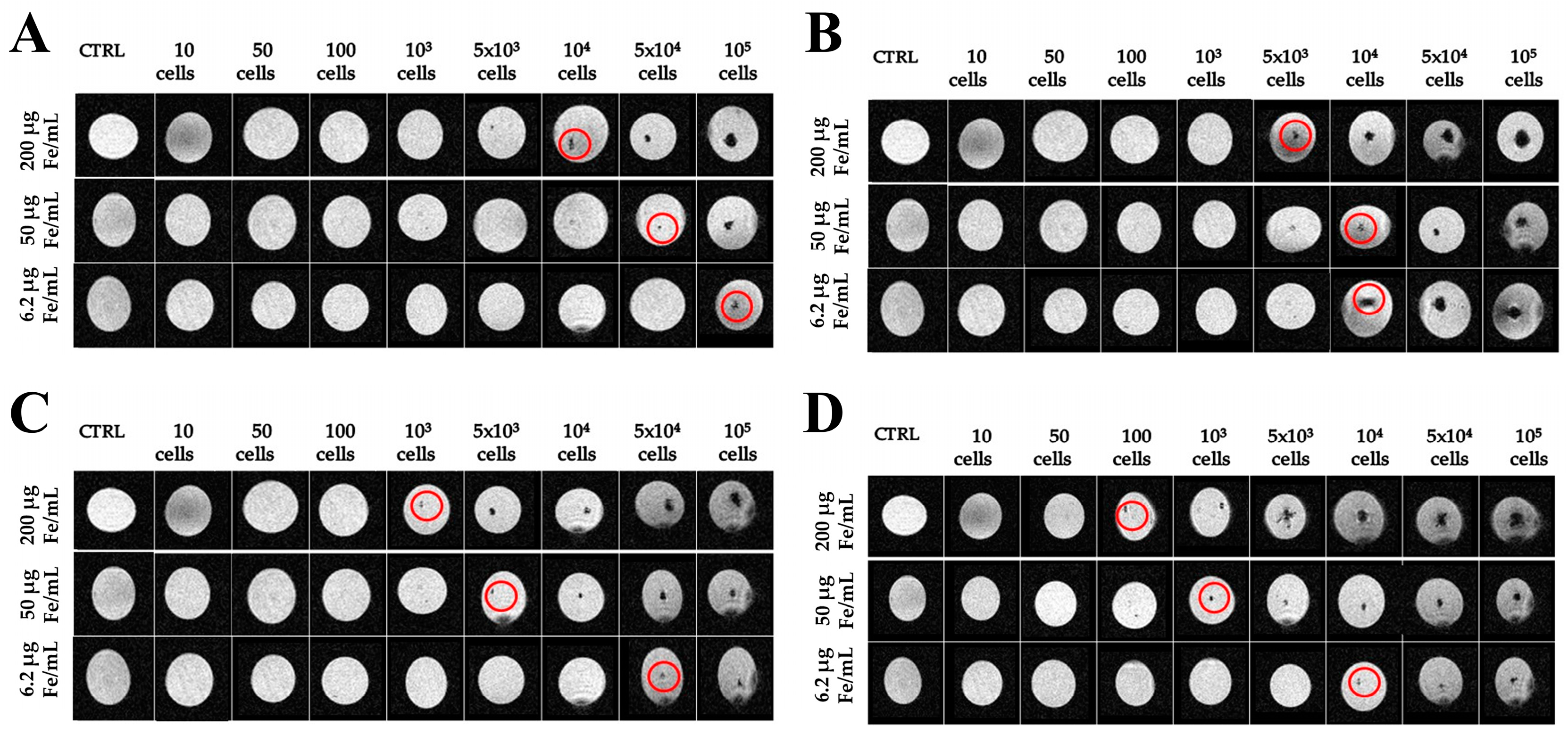
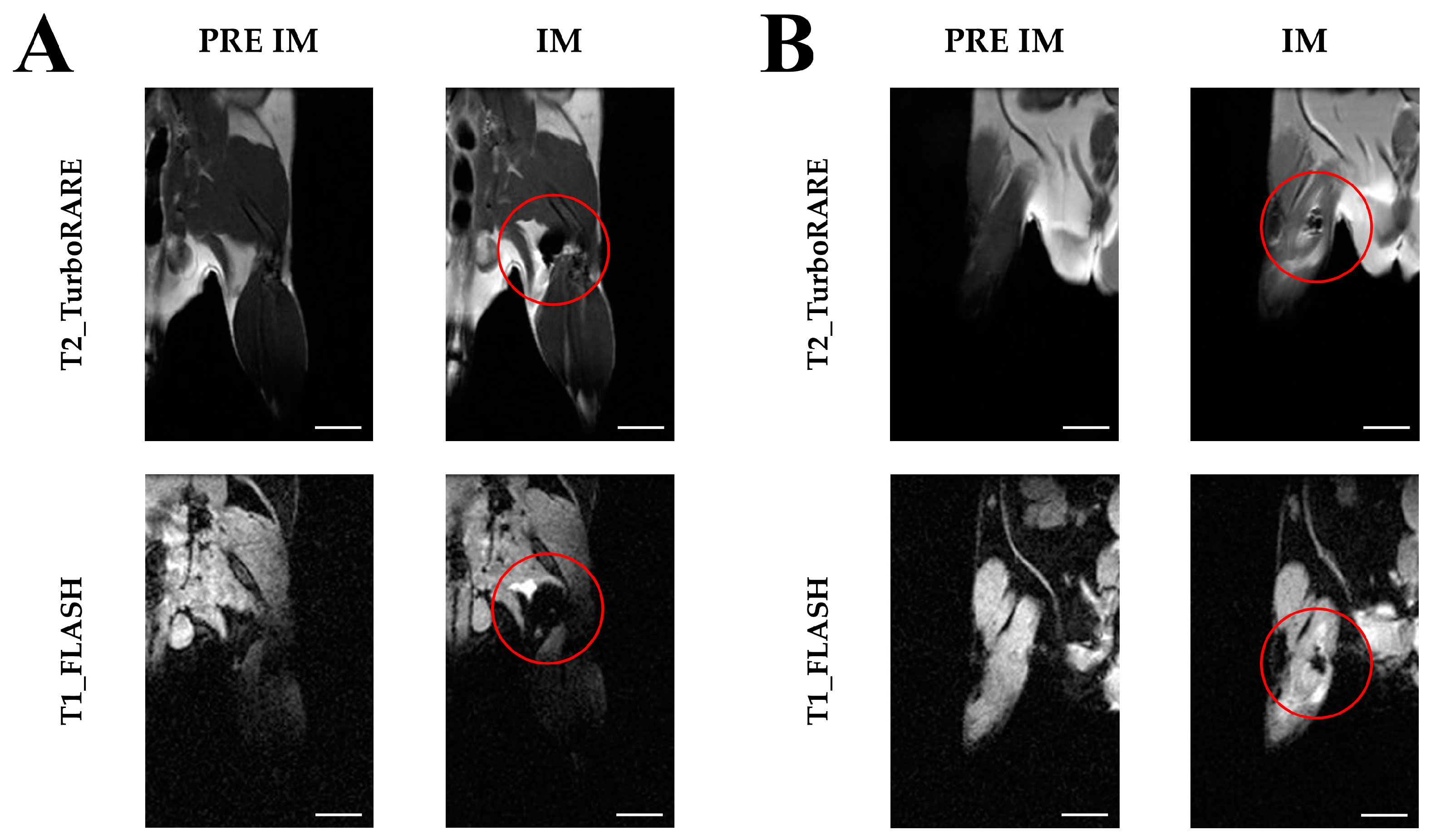
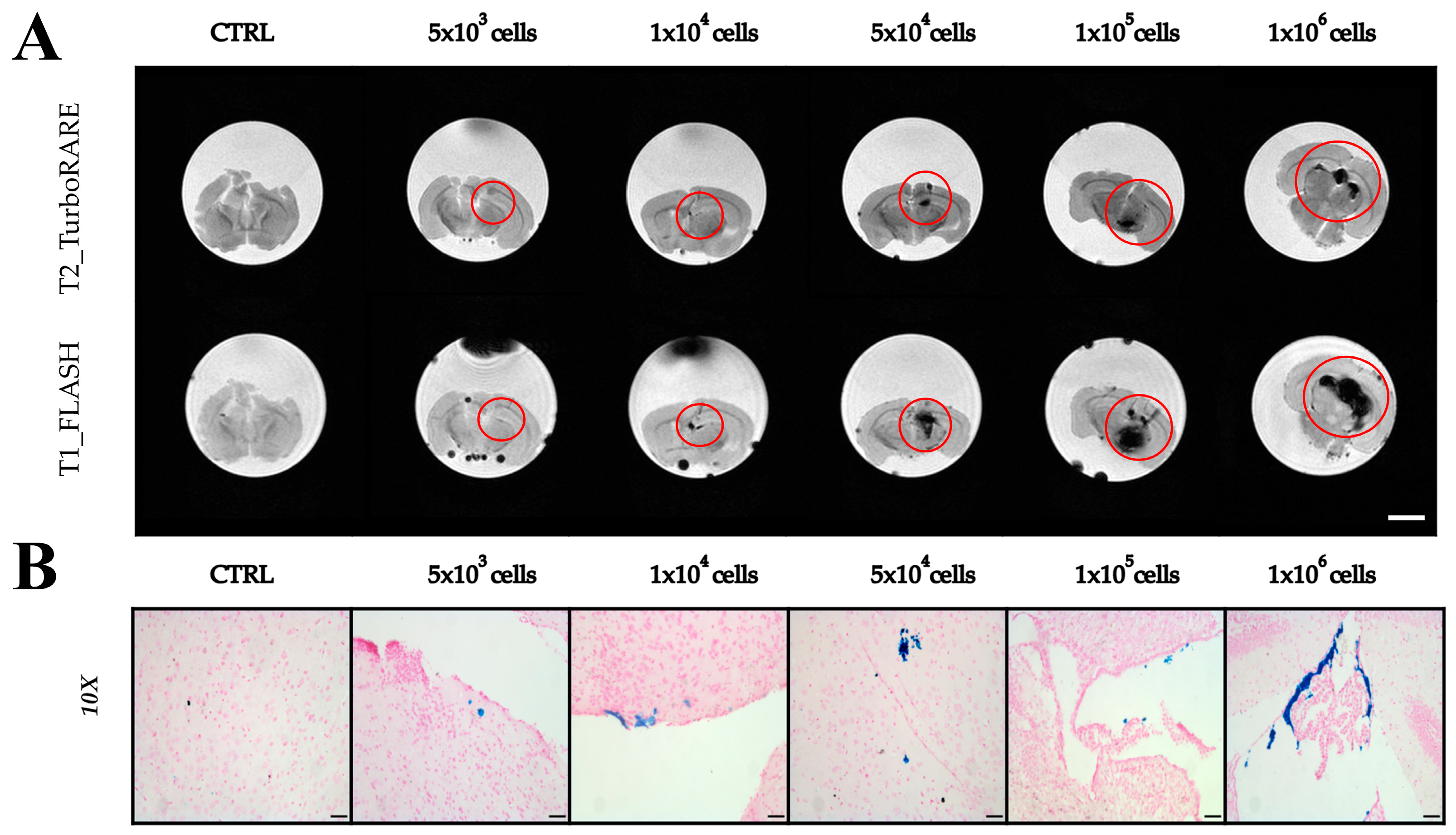
2.3. Imaging with Magnetic Resonance
2.4. Extraction of Extracellular Vesicles from ADAS-Labeled Cells
3. Discussion
4. Materials and Methods
4.1. Physical–Chemical Characterization of Nanoparticles
4.2. Internalization of Nanoparticles into Stem Cells
4.3. Magnetic Resonance Imaging
4.4. Extraction of Extracellular Vesicles from ADAS-Labeled Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Bonafede, R.; Mariotti, R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front. Cell. Neurosci. 2017, 11, 80. [Google Scholar] [CrossRef]
- Bonafede, R.; Turano, E.; Scambi, I.; Busato, A.; Bontempi, P.; Virla, F.; Schiaffino, L.; Marzola, P.; Bonetti, B.; Mariotti, R. ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS. Int. J. Mol. Sci. 2020, 21, 3651. [Google Scholar] [CrossRef]
- Turano, E.; Scambi, I.; Virla, F.; Bonetti, B.; Mariotti, R. Extracellular Vesicles from Mesenchymal Stem Cells: Towards Novel Therapeutic Strategies for Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 2917. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, R.; Bonafede, R.; Buoso, C.; Scambi, I.; Schiaffino, L. Evaluation of the Effect of Exosomes Isolated from Stem Cells in in Vivo Model of ALS. Ital. J. Anat. Embryol. 2018, 123, 139. [Google Scholar] [CrossRef]
- Sykova, E.; Jendelova, P. In Vivo Tracking of Stem Cells in Brain and Spinal Cord Injury. In Progress in Brain Research; Weber, J.T., Maas, A.I.R., Eds.; Neurotrauma: New Insights into Pathology and Treatment; Elsevier: Amsterdam, The Netherlands, 2007; Volume 161, pp. 367–383. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Zhou, L.; Mao, Y.; Yang, B.; Gao, L.; Xie, R.; Xu, F.; Zhang, D.; Liu, J.; et al. In Vivo Tracking of Superparamagnetic Iron Oxide Nanoparticle–Labeled Mesenchymal Stem Cell Tropism to Malignant Gliomas Using Magnetic Resonance Imaging: Laboratory Investigation. J. Neurosurg. 2008, 108, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M. In Vivo MRI Cell Tracking: Clinical Studies. Am. J. Roentgenol. 2009, 193, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.M.; Kwak, B.K.; Shim, H.J.; Ahn, C.; Lee, H.S.; Suh, Y.J.; Park, E.S. In Vivo Tracking of Mesenchymal Stem Cells Labeled with a Novel Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles Using 3.0T MRI. J. Korean Med. Sci. 2010, 25, 211. [Google Scholar] [CrossRef]
- Guldris, N.; Argibay, B.; Gallo, J.; Iglesias-Rey, R.; Carbó-Argibay, E.; Kolen’ko, Y.V.; Campos, F.; Sobrino, T.; Salonen, L.M.; Bañobre-López, M.; et al. Magnetite Nanoparticles for Stem Cell Labeling with High Efficiency and Long-Term in Vivo Tracking. Bioconjugate Chem. 2017, 28, 362–370. [Google Scholar] [CrossRef]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Labeling and Magnetic Resonance Imaging of Exosomes Isolated from Adipose Stem Cells. Curr. Protoc. Cell Biol. 2017, 75, 3.44.1–3.44.15. [Google Scholar] [CrossRef]
- Canese, R.; Vurro, F.; Marzola, P. Iron Oxide Nanoparticles as Theranostic Agents in Cancer Immunotherapy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34443781/ (accessed on 27 May 2024).
- Lüdtke-Buzug, K.; Haegele, J.; Biederer, S.; Sattel, T.F.; Erbe, M.; Duschka, R.L.; Barkhausen, J.; Vogt, F.M. Comparison of Commercial Iron Oxide-Based MRI Contrast Agents with Synthesized High-Performance MPI Tracers. Biomed. Tech./Biomed. Eng. 2013, 58, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, N.; Duschka, R.L.; Ahlborg, M.; Bringout, G.; Debbeler, C.; Graeser, M.; Kaethner, C.; Lüdtke-Buzug, K.; Medimagh, H.; Stelzner, J.; et al. Magnetic Particle Imaging: Current Developments and Future Directions. Int. J. Nanomed. 2015, 10, 3097–3114. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, S.; Simchi, A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic Resonance Imaging Tracking of Stem Cells in Vivo Using Iron Oxide Nanoparticles as a Tool for the Advancement of Clinical Regenerative Medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, S.; Le, W. Nanotherapeutic and Stem Cell Therapeutic Strategies in Neurodegenerative Diseases: A Promising Therapeutic Approach. Int. J. Nanomed. 2023, 18, 611–626. [Google Scholar] [CrossRef]
- Ma, M.; Shu, Y.; Tang, Y.; Chen, H. Multifaceted Application of Nanoparticle-Based Labeling Strategies for Stem Cell Therapy. Nano Today 2020, 34, 100897. [Google Scholar] [CrossRef]
- Van de Walle, A.; Perez, J.E.; Wilhelm, C. Magnetic Bioprinting of Stem Cell-Based Tissues. Bioprinting 2023, 30, e00265. [Google Scholar] [CrossRef]
- Bontempi, P.; Busato, A.; Bonafede, R.; Schiaffino, L.; Scambi, I.; Sbarbati, A.; Mariotti, R.; Marzola, P. MRI Reveals Therapeutical Efficacy of Stem Cells: An Experimental Study on the SOD1(G93A) Animal Model. Magn. Reson. Med. 2018, 79, 459–469. [Google Scholar] [CrossRef]
- Marzola, P.; Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Mariotti, R. Magnetic Resonance Imaging of Ultrasmall Superparamagnetic Iron Oxide-Labeled Exosomes from Stem Cells: A New Method to Obtain Labeled Exosomes. Int. J. Nanomed. 2016, 11, 2481–2490. [Google Scholar] [CrossRef]
- Sun, R.; Dittrich, J.; Le-Huu, M.; Mueller, M.M.; Bedke, J.; Kartenbeck, J.; Lehmann, W.D.; Krueger, R.; Bock, M.; Huss, R.; et al. Physical and Biological Characterization of Superparamagnetic Iron Oxide- and Ultrasmall Superparamagnetic Iron Oxide-Labeled Cells: A Comparison. Investig. Radiol. 2005, 40, 504–513. [Google Scholar] [CrossRef]
- Oude Engberink, R.D.; van der Pol, S.M.A.; Döpp, E.A.; de Vries, H.E.; Blezer, E.L.A. Comparison of SPIO and USPIO for in Vitro Labeling of Human Monocytes: MR Detection and Cell Function. Radiology 2007, 243, 467–474. [Google Scholar] [CrossRef]
- Saito, S.; Tsugeno, M.; Koto, D.; Mori, Y.; Yoshioka, Y.; Nohara, S.; Murase, K. Impact of Surface Coating and Particle Size on the Uptake of Small and Ultrasmall Superparamagnetic Iron Oxide Nanoparticles by Macrophages. Int. J. Nanomed. 2012, 7, 5415–5421. [Google Scholar] [CrossRef]
- Bulte, J.W.M. Superparamagnetic Iron Oxides as MPI Tracers: A Primer and Review of Early Applications. Adv. Drug Deliv. Rev. 2019, 138, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Harvell-Smith, S.; Tung, L.D.; Thanh, N.T.K. Magnetic Particle Imaging: Tracer Development and the Biomedical Applications of a Radiation-Free, Sensitive, and Quantitative Imaging Modality. Nanoscale 2022, 14, 3658–3697. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Tracer Design for Magnetic Particle Imaging (Invited). J. Appl. Phys. 2012, 111, 07B318. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, S.; Hu, L.; Feng, S.; Wu, Z.; Liu, S.; Duan, S.; Chen, Z.; Zhou, C.; Zhao, X. Imaging Characteristics of USPIO Nanoparticles (<5 Nm) as MR Contrast Agent In Vitro and in the Liver of Rats. Contrast Media Mol. Imaging 2019, 2019, e3687537. [Google Scholar] [CrossRef]
- Azzoz, M.; Zaghloul, M.; Sayed, M.; Soliman, H.; Mounir, N.; Abdel Shakour, N.; El-Dabae, W.; Aly, S. Efficiency of MTT and Trypan Blue Assays for Detection of Viability and Recovery of Different Frozen Cell Lines. Egypt. J. Vet. Sci. 2024, 55, 1649–1657. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Daldrup-Link, H.E. Clinical Tracking of Cell Transfer and Cell Transplantation: Trials and Tribulations. Radiology 2018, 289, 604–615. [Google Scholar] [CrossRef]
- Canese, R.; Vurro, F.; Marzola, P. Iron Oxide Nanoparticles as Theranostic Agents in Cancer Immunotherapy. Nanomaterials 2021, 11, 1950. [Google Scholar] [CrossRef]
- Turano, E.; Scambi, I.; Bonafede, R.; Dusi, S.; Angelini, G.; Lopez, N.; Marostica, G.; Rossi, B.; Furlan, R.; Constantin, G.; et al. Extracellular Vesicles from Adipose Mesenchymal Stem Cells Target Inflamed Lymph Nodes in Experimental Autoimmune Encephalomyelitis. Cytotherapy 2024, 26, 276–285. [Google Scholar] [CrossRef]
- Capuzzo, A.; Scambi, I.; Mariotti, R.; Marzola, P. From Isolation of Adult Adipose Tissue Derived Stem Cells ADAS to Labelling with Superparamagnetic Iron Oxide Nanoparticles: First Approaches to Unleash the Potential. In Proceedings of the XXVII School of Pure and Applied Biophysics Extracellular Vesicles: From Biophysical to Translational Challenges, Venice, Italy, 6–10 February 2023. [Google Scholar]
- Capuzzo, A.; Ossanna, R.; Quintero Sierra, L.; Virla, F.; Negri, A.; Conti, A.; Veronese, S.; Sbarbati, A. From the Classification of Stem Cells to the Release of Potential in Cell Therapies: Limits, Considerations and Future Aspects in Regenerative Medicine. In Possibilities and Limitations in Current Translational Stem Cell Research; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Arifin, D.R.; Witwer, K.W.; Bulte, J.W.M. Non-Invasive Imaging of Extracellular Vesicles: Quo Vaditis in Vivo? J. Extracell. Vesicles 2022, 11, 12241. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic Iron Oxide Contrast Agents: Physicochemical Characteristics and Applications in MR Imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Namestnikova, D.; Gubskiy, I.; Kholodenko, I.; Melnikov, P.; Sukhinich, K.; Gabashvili, A.; Vishnevskiy, D.; Soloveva, A.; Abakumov, M.; Vakhrushev, I.; et al. Methodological Aspects of MRI of Transplanted Superparamagnetic Iron Oxide-Labeled Mesenchymal Stem Cells in Live Rat Brain. PLoS ONE 2017, 12, e0186717. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. The Sensitivity Performance of the Human Eye on an Absolute Scale. J. Opt. Soc. Am. 1948, 38, 196–208. [Google Scholar] [CrossRef]
- Galiè, M.; Pignatti, M.; Scambi, I.; Sbarbati, A.; Rigotti, G. Comparison of Different Centrifugation Protocols for the Best Yield of Adipose-Derived Stromal Cells from Lipoaspirates. Plast. Reconstr. Surg. 2008, 122, 233e. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Bonaconsa, M.; Scambi, I.; Squintani, G.M.; Rui, W.; Turano, E.; Ungaro, D.; D’Agostino, S.; Barbieri, F.; Angiari, S.; et al. Systemic Treatment with Adipose-Derived Mesenchymal Stem Cells Ameliorates Clinical and Pathological Features in the Amyotrophic Lateral Sclerosis Murine Model. Neuroscience 2013, 248, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.; Scambi, I.; Peroni, D.; Potrich, V.; Boschi, F.; Benati, D.; Bonetti, B.; Mariotti, R. Exosome Derived from Murine Adipose-Derived Stromal Cells: Neuroprotective Effect on in Vitro Model of Amyotrophic Lateral Sclerosis. Exp. Cell Res. 2016, 340, 150–158. [Google Scholar] [CrossRef]
- Scambi, I.; Peroni, D.; Nodari, A.; Merigo, F.; Benati, D.; Boschi, F.; Mannucci, S.; Frontini, A.; Visonà, S.; Sbarbati, A.; et al. The Transcriptional Profile of Adipose-Derived Stromal Cells (ASC) Mirrors the Whitening of Adipose Tissue with Age. Eur. J. Cell Biol. 2022, 101, 151206. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| USPIO in Distilled Water | USPIO in Agar Gel 2% | Resovist® in Distilled Water | Resovist® in Agar Gel 2% | |
|---|---|---|---|---|
| Size on Light Scattering (Size Peak nm ± SDev) | 9.5 ± 2.01 | 69.18 ± 1.56 | ||
| Zeta Potential (mV ± ZDev) | −23.6 ± 1.7 | −31.8 ± 7.12 | ||
| Size on Transmission Electron Microscopy (nm ± SDev) | 5.0± 0.01 | 70 ± 0.2 | ||
| Transverse relaxivity (mM−1 s−1) | Not measured * | 103.3 ± 2.122 | 176.6 ± 1.868 | 274.9 ± 4.108 |
| Longitudinal relaxivity (mM−1 s−1) | Not measured * | 0.4174 ± 0.018 | 1.736 ± 0.042 | 1.072 ± 0.07 |
| Ratio r2/r1 | 247.484 | 101.73 | 256.436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuzzo, A.M.; Piccolantonio, G.; Negri, A.; Bontempi, P.; Lacavalla, M.A.; Malatesta, M.; Scambi, I.; Mariotti, R.; Lüdtke-Buzug, K.; Corsi, M.; et al. Comparison between USPIOs and SPIOs for Multimodal Imaging of Extracellular Vesicles Extracted from Adipose Tissue-Derived Adult Stem Cells. Int. J. Mol. Sci. 2024, 25, 9701. https://doi.org/10.3390/ijms25179701
Capuzzo AM, Piccolantonio G, Negri A, Bontempi P, Lacavalla MA, Malatesta M, Scambi I, Mariotti R, Lüdtke-Buzug K, Corsi M, et al. Comparison between USPIOs and SPIOs for Multimodal Imaging of Extracellular Vesicles Extracted from Adipose Tissue-Derived Adult Stem Cells. International Journal of Molecular Sciences. 2024; 25(17):9701. https://doi.org/10.3390/ijms25179701
Chicago/Turabian StyleCapuzzo, Arnaud M., Giusi Piccolantonio, Alessandro Negri, Pietro Bontempi, Maria A. Lacavalla, Manuela Malatesta, Ilaria Scambi, Raffaella Mariotti, Kerstin Lüdtke-Buzug, Mauro Corsi, and et al. 2024. "Comparison between USPIOs and SPIOs for Multimodal Imaging of Extracellular Vesicles Extracted from Adipose Tissue-Derived Adult Stem Cells" International Journal of Molecular Sciences 25, no. 17: 9701. https://doi.org/10.3390/ijms25179701








