The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss
Abstract
1. Introduction
2. Aging and Hearing Loss: Integrated Pathways
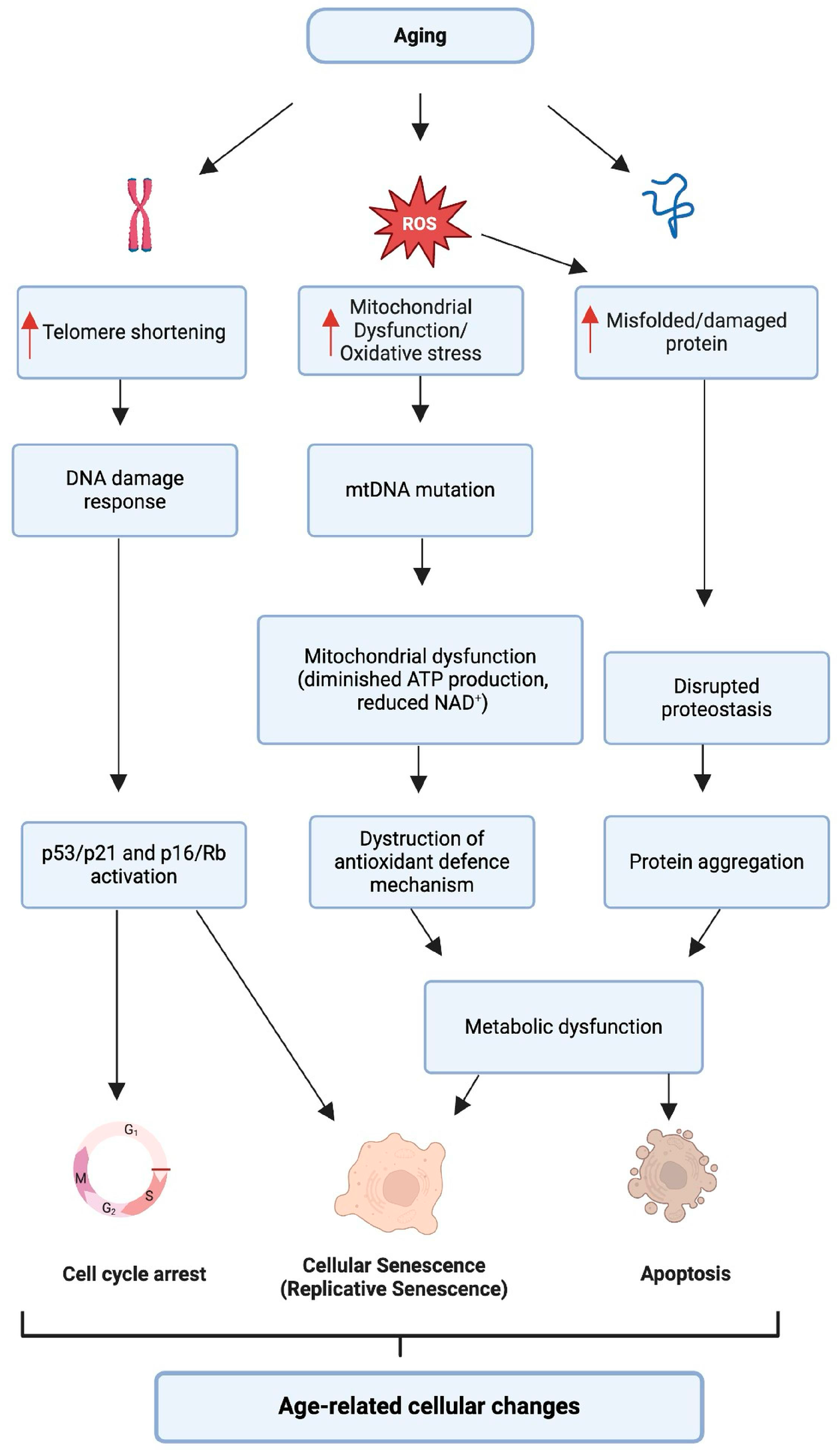
2.1. Cellular Aging and Its Impact on Hearing
2.1.1. Telomere Attrition
2.1.2. Oxidative Stress
2.1.3. Proteostasis
2.1.4. Autophagy
2.1.5. Cellular Senescence
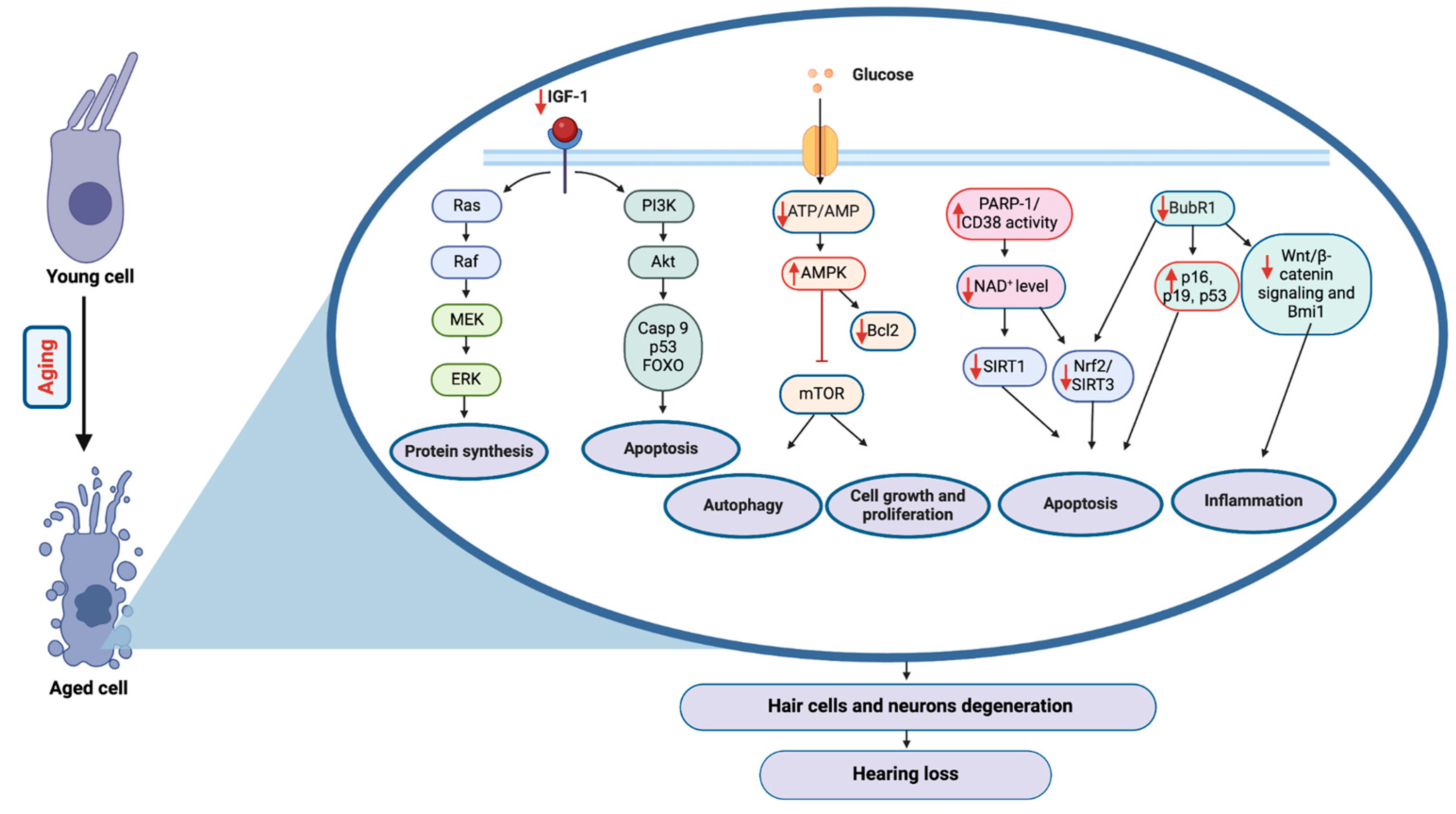
2.2. Molecular Aging Pathways and Their Role in Hearing Loss
2.2.1. Insulin/IGF-1 Pathway
2.2.2. mTOR Pathway
2.2.3. AMPK Pathway
2.2.4. Sirtuins
2.2.5. BubR1
2.2.6. Key Aging Pathways and Their Role in ARHL
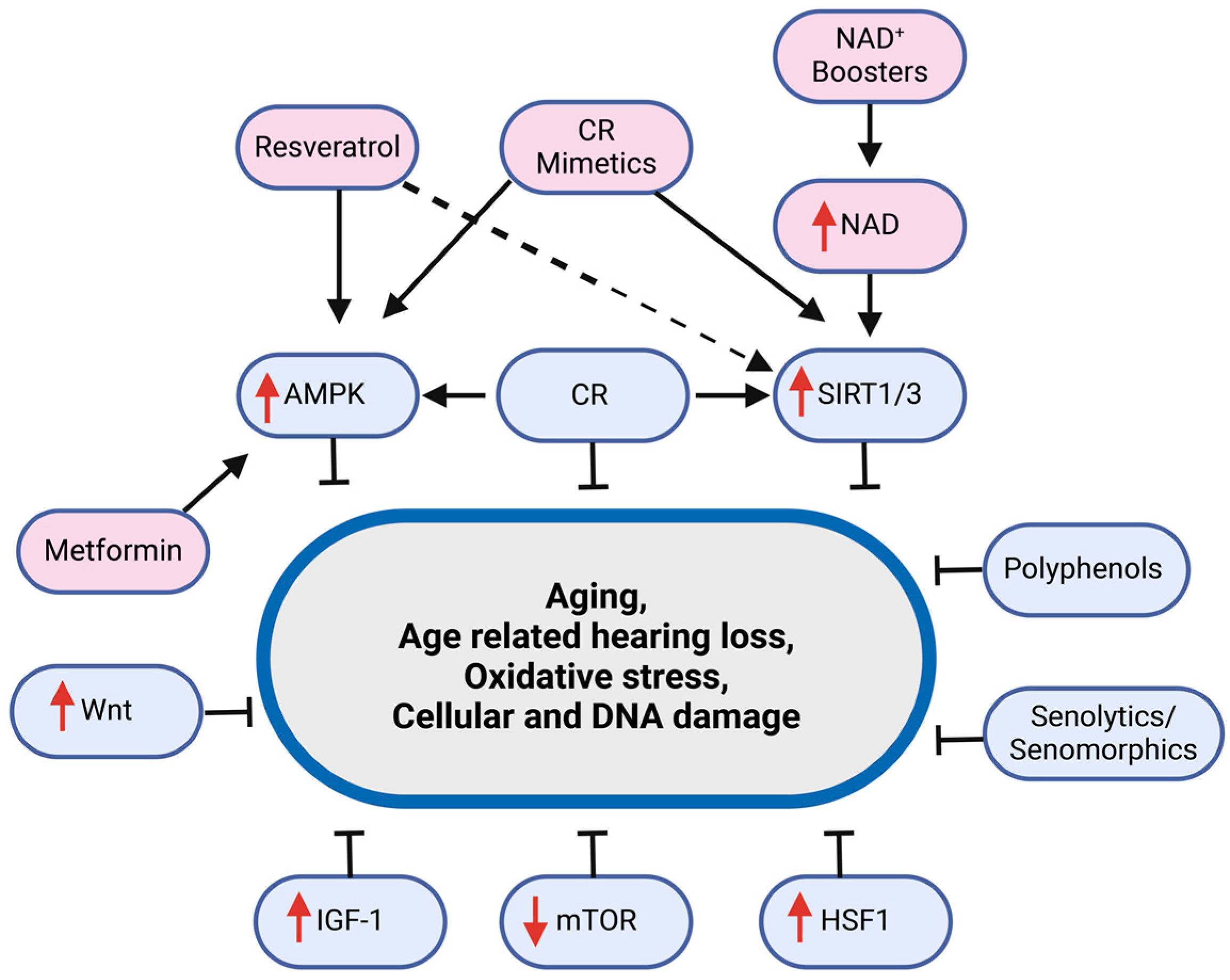
3. Aging Interventions and Hearing Loss
3.1. Rapamycin
3.2. Metformin
3.3. NAD+ Boosters
3.4. Resveratrol/Polyphenols
3.5. Senolytics/Senomorphics
3.6. Calorie Restriction and CR Mimetics
4. Conclusions
Funding
Conflicts of Interest
References
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of P16 Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular Senescence in Cancer and Aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef]
- Someya, S.; Kim, M.J. Cochlear Detoxification: Role of Alpha Class Glutathione Transferases in Protection against Oxidative Lipid Damage, Ototoxicity, and Cochlear Aging. Hear. Res. 2021, 402, 108002. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Charles Liberman, M. Age-Related Hearing Loss Is Dominated by Damage to Inner Ear Sensory Cells, Not the Cellular Battery That Powers Them. J. Neurosci. 2020, 40, 6357–6366. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.S.; Cruickshanks, K.J.; Klein, B.E.K.; Klein, R.; Wiley, T.L.; Nondahl, D.M. The Impact of Hearing Loss on Quality of Life in Older Adults. J. Gerontol. 2003, 43, 661–668. [Google Scholar] [CrossRef]
- Bowl, M.R.; Dawson, S.J. Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Walford, R.L.; Mock, D.; Verdery, R.; MacCallum, T.; Diego, S.; Reynolds, D. Calorie Restriction in Biosphere 2: Alterations in Physiologic, Hematologic, Hormonal, and Biochemical Parameters in Humans Restricted for a 2-Year Period. J. Gerontol. 2002, 57, 211–224. [Google Scholar] [CrossRef]
- Fowler, C.G.; Chiasson, K.B.; Leslie, T.H.; Thomas, D.; Beasley, T.M.; Kemnitz, J.W.; Weindruch, R. Auditory Function in Rhesus Monkeys: Effects of Aging and Caloric Restriction in the Wisconsin Monkeys Five Years Later. Hear. Res. 2010, 261, 75–81. [Google Scholar] [CrossRef]
- Seidman, M.D. Effects of Dietary Restriction and Antioxidants on Presbyacusis. Laryngoscope 2000, 110, 727–738. [Google Scholar] [CrossRef]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic Control of Longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, V.J.; Gerhard, G.S.; Pignolo, R.J. Molecular Biology of Aging. Surg. Clin. North Am. 1994, 74, 1–21. [Google Scholar] [CrossRef]
- Carney, J.M.; Starke-Reedt, P.E.; Olivert, C.N.; Landum, R.W.; Cheng, M.S.; Wu, J.F.; Floyd, R.A. Reversal of Age-Related Increase in Brain Protein Oxidation, Decrease in Enzyme Activity, and Loss in Temporal and Spatial Memory by Chronic Administration of the Spin Compound N-Tert-Butyl-a-Phenylnitrone. Proc. Natl. Acad. Sci. USA 1991, 88, 3633–3636. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.R.; Feeny, D.; Tomlinson, G.; Cushing, S.; Chen, J.M.; Krahn, M.D. Health-Related Quality of Life Changes Associated with Hearing Loss. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Yamasoba, T.; Lin, F.R.; Someya, S.; Kashio, A.; Sakamoto, T.; Kondo, K. Current Concepts in Age-Related Hearing Loss: Epidemiology and Mechanistic Pathways. Hear. Res. 2013, 303, 30–38. [Google Scholar] [CrossRef]
- LeMasurier, M.; Gillespie, P.G. Hair-Cell Mechanotransduction and Cochlear Amplification. Neuron 2005, 48, 403–415. [Google Scholar] [CrossRef]
- Raphael, Y.; Altschuler, R.A. Structure and Innervation of the Cochlea. Brain Res. Bull. 2003, 60, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Dallos, P. Cochlear Amplification, Outer Hair Cells and Prestin. Curr. Opin. Neurobiol. 2008, 18, 370–376. [Google Scholar] [CrossRef]
- Wright, A.; Davis, A.; Bredberg, G.; Ülehlová, L.; Spencer, H. Hair Cell Distributions in the Normal Human Cochlea: A Report of a European Working Group. Acta Otolaryngol. 1987, 104, 15–24. [Google Scholar] [CrossRef]
- Liu, X.Z.; Yan, D. Ageing and Hearing Loss. J. Pathol. 2007, 211, 188–197. [Google Scholar] [CrossRef]
- Ottersen, O.P.; Takumi, Y.; Matsubara6, A.; Landsend, A.S.; Laake, J.H.; Usami6, S.-I. Molecular Organization of a Type of Peripheral Glutamate Synapse: The Afferent Synapses of Hair Cells in the Inner Ear. Prog. Neurobiol. 1998, 54, 127–148. [Google Scholar] [CrossRef]
- Lalwani, A.K.; Walsh, B.J.; Reilly, P.G.; Carvalho, G.J.; Zolotukhin, S.; Muzyczka, N.; Mhatre, A.N. Long-Term in Vivo Cochlear Transgene Expression Mediated by Recombinant Adeno-Associated Virus. Gene Ther. 1998, 5, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.D. Age-Related Hearing Loss: Ear and Brain Mechanisms. Ann. N. Y. Acad. Sci. 2009, 1170, 708–717. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J.L. Presbycusis: An Update on Cochlear Mechanisms and Therapies. J. Clin. Med. 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhao, L.; Pu, L.; Wang, M.; Zhang, Q.; He, D.Z.Z. How Well Can Centenarians Hear? PLoS ONE 2013, 8, e65565. [Google Scholar] [CrossRef]
- Ozmeral, E.J.; Eddins, A.C.; Frisina, D.R.; Eddins, D.A. Large Cross-Sectional Study of Presbycusis Reveals Rapid Progressive Decline in Auditory Temporal Acuity. Neurobiol. Aging 2016, 43, 72–78. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, J. Age-Related Hearing Loss or Presbycusis. Eur. Arch. Otorhinolaryngol. 2010, 267, 1179–1191. [Google Scholar] [CrossRef]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Li, M.; Zou, S.Y.; Liao, F.L.; Ding, Y.Y.; Su, H.G.; Wei, X.F.; Wei, C.J.; Mu, Y.R.; Kong, W.J. Protection and Prevention of Age-Related Hearing Loss. Hear. Loss Prev. Cure. 2019, 1130, 59–71. [Google Scholar] [CrossRef]
- Bouzid, A.; Smeti, I.; Dhouib, L.; Roche, M.; Achour, I.; Khalfallah, A.; Gibriel, A.A.; Charfeddine, I.; Ayadi, H.; Lachuer, J.; et al. Down-Expression of P2RX2, KCNQ5, ERBB3 and SOCS3 through DNA Hypermethylation in Elderly Women with Presbycusis. Biomarkers 2018, 23, 347–356. [Google Scholar] [CrossRef]
- Nadhimi, Y.; Llano, D.A. Does Hearing Loss Lead to Dementia? A Review of the Literature. Hear. Res. 2021, 15, 108038. [Google Scholar] [CrossRef] [PubMed]
- Keithley, E.M. Pathology and Mechanisms of Cochlear Aging. J. Neurosci. Res. 2020, 98, 1674–1684. [Google Scholar] [CrossRef]
- Wu, P.Z.; Liberman, L.D.; Bennett, K.; de Gruttola, V.; O’Malley, J.T.; Liberman, M.C. Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience 2019, 407, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.K.; Chow, M.L. Hearing and Music in Dementia. In Handbook of Clinical Neurology; Elsevier, B.V.: Amsterdam, The Netherlands, 2015; Volume 129, pp. 667–687. [Google Scholar] [CrossRef]
- Liu, H.; Giffen, K.P.; Chen, L.; Henderson, H.J.; Cao, T.A.; Kozeny, G.A.; Beisel, K.W.; Li, Y.; He, D.Z. Molecular and Cytological Profiling of Biological Aging of Mouse Cochlear Inner and Outer Hair Cells. Cell Rep. 2022, 39, 110665. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres Shorten during Ageing of Human Fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Chan, K.M.; Tjia, W.M.; Deng, W.; Guan, X.; Huang, J.D.; Li, K.M.; Chau, P.Y.; Chen, D.J.; et al. Genomic Instability in Laminopathy-Based Premature Aging. Nat. Med. 2005, 11, 780–785. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative Damage and Mitochondrial Decay in Aging (BIoenergetics/Mltodcndra DNA/Arwdbipn/Acetyl-L-Cnitlne/Neurodeneration). Proc. Nail. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Apfeld, J.; O’Connor, G.; McDonagh, T.; DiStefano, P.S.; Curtis, R. The AMP-Activated Protein Kinase AAK-2 Links Energy Levels and Insulin-like Signals to Lifespan in C. elegans. Genes Dev. 2004, 18, 3004–3009. [Google Scholar] [CrossRef]
- Baker, D.J.; Jeganathan, K.B.; Cameron, J.D.; Thompson, M.; Juneja, S.; Kopecka, A.; Kumar, R.; Jenkins, R.B.; De Groen, P.C.; Roche, P.; et al. BubR1 Insufficiency Causes Early Onset of Aging-Associated Phenotypes and Infertility in Mice. Nat. Genet. 2004, 36, 744–749. [Google Scholar] [CrossRef]
- Ding, S.-L.; Shen, C.-Y. Model of Human Aging: Recent Findings on Werner’s and Hutchinson-Gilford Progeria Syndromes. Clin. Interv. Aging 2008, 3, 431–444. [Google Scholar] [CrossRef]
- Menardo, J.; Tang, Y.; Ladrech, S.; Lenoir, M.; Casas, F.; Michel, C.; Bourien, J.; Ruel, J.; Rebillard, G.; Maurice, T.; et al. Oxidative Stress, Inflammation, and Autophagic Stress as the Key Mechanisms of Premature Age-Related Hearing Loss in SAMP8 Mouse Cochlea. Antioxid. Redox Signal. 2012, 16, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and Telomerase: The Path from Maize, Tetrahymena and Yeast to Human Cancer and Aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Blackburn, E.H. Cloning Yeast Telomeres on Linear Plasmid Vectors. Cell 1982, 29, 245–255. [Google Scholar] [CrossRef]
- Greider, C.W. Commentary Telomerase Activity, Cell Proliferation, and Cancer. Proc. Natl. Acad. Sci. USA 1998, 95, 90–92. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.-P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of Life-Span by Introduction of Telomerase into Normal Human Cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Three Decades of Progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Maiti, K.; Dedman, L.; Smith, R. Is There a Role for Placental Senescence in the Genesis of Obstetric Complications and Fetal Growth Restriction? Am. J. Obstet. Gynecol. 2018, 218, S762–S773. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- Savage, S.A. Dyskeratosis Congenita and Telomere Biology Disorders. Hematology 2022, 1, 637–648. [Google Scholar] [CrossRef]
- Tanaka, H.; Abe, S.; Huda, N.; Tu, L.R.; Beam, M.J.; Grimes, B.; Gilley, D. Telomere Fusions in Early Human Breast Carcinoma. Proc. Natl. Acad. Sci. USA 2012, 109, 14098–14103. [Google Scholar] [CrossRef]
- Terry, D.F.; Nolan, V.G.; Andersen, S.L.; Perls, T.T.; Cawthon, R. Association of Longer Telomeres With Better Health in Centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Tedone, E.; Huang, E.; O’Hara, R.; Batten, K.; Ludlow, A.T.; Lai, T.P.; Arosio, B.; Mari, D.; Wright, W.E.; Shay, J.W. Telomere Length and Telomerase Activity in T Cells Are Biomarkers of High-Performing Centenarians. Aging Cell 2019, 18, e12859. [Google Scholar] [CrossRef]
- Townsley, D.M.; Dumitriu, B.; Young, N.S. Bone Marrow Failure and the Telomeropathies. Am. J. Hematol. 2014, 124, 2775–2783. [Google Scholar] [CrossRef]
- Barja, G. Towards a Unified Mechanistic Theory of Aging. Exp. Gerontol. 2019, 124, 110627. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.-L. Mitochondrial Reactive Oxygen Species in Cell Death Signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Catic, A. Cellular Metabolism and Aging. Prog. Mol. Biol. Transl. Sci. 2018, 155, 85–107. [Google Scholar] [CrossRef]
- Basisty, N.; Dai, D.F.; Gagnidze, A.; Gitari, L.; Fredrickson, J.; Maina, Y.; Beyer, R.P.; Emond, M.J.; Hsieh, E.J.; MacCoss, M.J.; et al. Mitochondrial-Targeted Catalase Is Good for the Old Mouse Proteome, but Not for the Young: ‘Reverse’ Antagonistic Pleiotropy? Aging Cell 2016, 15, 634–645. [Google Scholar] [CrossRef]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of Catalase Targeted to Mitochondria Attenuates Murine Cardiac Aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Stefanatos, R.; Sanz, A. The Role of Mitochondrial ROS in the Aging Brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef]
- Someya, S.; Prolla, T.A. Mitochondrial Oxidative Damage and Apoptosis in Age-Related Hearing Loss. Mech. Ageing Dev. 2010, 131, 480–486. [Google Scholar] [CrossRef]
- Benkafadar, N.; François, F.; Affortit, C.; Casas, F.; Ceccato, J.C.; Menardo, J.; Venail, F.; Malfroy-Camine, B.; Puel, J.L.; Wang, J. ROS-Induced Activation of DNA Damage Responses Drives Senescence-Like State in Postmitotic Cochlear Cells: Implication for Hearing Preservation. Mol. Neurobiol. 2019, 56, 5950–5969. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.; Marciano, E.; Zarrilli, F.; Mazzaccara, C.; Intrieri, M.; Calcagno, G.; Vitale, D.F.; La Manna, P.; Saulino, C.; Marcelli, V.; et al. Paraoxonase and Superoxide Dismutase Gene Polymorphisms and Noise-Induced Hearing Loss. Clin. Chem. 2004, 50, 2012–2018. [Google Scholar] [CrossRef][Green Version]
- Upreti, M.; Chu, R.; Galitovskaya, E.; Smart, S.K.; Chambers, T.C. Key Role for Bak Activation and Bak-Bax Interaction in the Apoptotic Response to Vinblastine. Mol. Cancer Ther. 2008, 7, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Xu, J.; Kondo, K.; Ding, D.; Salvi, R.J.; Yamasoba, T.; Rabinovitch, P.S.; Weindruch, R.; Leeuwenburgh, C.; Tanokura, M.; et al. Age-Related Hearing Loss in C57BL/6J Mice Is Mediated by Bak-Dependent Mitochondrial Apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 19432–19437. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Chacón, L.D.M.; Martínez-Rodríguez, S.; Madrid-García, R.; Yanes-Díaz, J.; Riestra-Ayora, J.I.; Sanz-Fernández, R.; Sánchez-Rodríguez, C. Role of Oxidative Stress in the Senescence Pattern of Auditory Cells in Age-Related Hearing Loss. Antioxidants 2021, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.K.; Jun, Y.; Jo, E.R.; Cho, S.I. Age-Related Hearing Loss in C57bl/6j Mice Is Associated with Mitophagy Impairment in the Central Auditory System. Int. J. Mol. Sci. 2020, 21, 7202. [Google Scholar] [CrossRef]
- Seicol, B.J.; Lin, S.; Xie, R. Age-Related Hearing Loss Is Accompanied by Chronic Inflammation in the Cochlea and the Cochlear Nucleus. Front. Aging Neurosci. 2022, 14, 846804. [Google Scholar] [CrossRef]
- Koga, H.; Kaushik, S.; Cuervo, A.M. Protein Homeostasis and Aging: The Importance of Exquisite Quality Control. Ageing Res. Rev. 2011, 10, 205–215. [Google Scholar] [CrossRef]
- Bennett, E.J.; Bence, N.F.; Jayakumar, R.; Kopito, R.R. Global Impairment of the Ubiquitin-Proteasome System by Nuclear or Cytoplasmic Protein Aggregates Precedes Inclusion Body Formation. Mol. Cell 2005, 17, 351–365. [Google Scholar] [CrossRef]
- Bence, N.F.; Sampat, R.M.; Kopito, R.R. Impairment of the Ubiquitin-Proteasome System by Protein Aggregation. Science 2001, 292, 1552–1555. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Molecular Chaperones in the Cytosol: From Nascent Chain to Folded Protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, J.; Kunisada, T.; Fornace, A.J.; Schneider, E.L.; Holbrook, N.J. Decreased Expression of Heat Shock Protein 70 MRNA and Protein after Heat Treatment in Cells of Aged Rats (Stress/Aging). Proc. Natl. Acad. Sci. USA 1990, 87, 846–850. [Google Scholar] [CrossRef]
- Trivedi, R.; Jurivich, D.A. A Molecular Perspective on Age-Dependent Changes to the Heat Shock Axis. Exp. Gerontol. 2020, 137, 110969. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Gil, E.S.; Jeong, I.H.; Kim, H.; Jang, J.H.; Choung, Y.H. Heat Shock Factor 1 Prevents Age-Related Hearing Loss by Decreasing Endoplasmic Reticulum Stress. Cells 2021, 10, 2054. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The Coming of Age of Chaperone-Mediated Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Heat Shock Proteins Multiple Neuroprotective Functions and Implications for Neurologic Disease. Neurology 2011, 76, 660–667. [Google Scholar] [CrossRef]
- Koyuncu, S.; Loureiro, R.; Lee, H.J.; Wagle, P.; Krueger, M.; Vilchez, D. Rewiring of the Ubiquitinated Proteome Determines Ageing in C. elegans. Nature 2021, 596, 285–290. [Google Scholar] [CrossRef]
- Stefani, M.; Dobson, C.M. Protein Aggregation and Aggregate Toxicity: New Insights into Protein Folding, Misfolding Diseases and Biological Evolution. J. Mol. Med. 2003, 81, 678–699. [Google Scholar] [CrossRef]
- Mikuriya, T.; Sugahara, K.; Sugimoto, K.; Fujimoto, M.; Takemoto, T.; Hashimoto, M.; Hirose, Y.; Shimogori, H.; Hayashida, N.; Inouye, S.; et al. Attenuation of Progressive Hearing Loss in a Model of Age-Related Hearing Loss by a Heat Shock Protein Inducer, Geranylgeranylacetone. Brain Res. 2008, 1212, 9–17. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Chen, S.; Zhou, X.; Wu, X.; Kong, W.; Kong, W. Impaired Unfolded Protein Response in the Degeneration of Cochlea Cells in a Mouse Model of Age-Related Hearing Loss. Exp. Gerontol. 2015, 70, 61–70. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Mathiassen, S.G.; De Zio, D.; Cecconi, F. Autophagy and the Cell Cycle: A Complex Landscape. Front. Oncol. 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in Immunity and Inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in Mammalian Cells: Revisiting a 40-Year-Old Conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Bourdenx, M.; Luengo, E.; Diaz, A.; Sohn, P.D.; Chen, X.; Wang, C.; Juste, Y.R.; Wegmann, S.; Patel, B.; et al. Acetylated Tau Inhibits Chaperone-Mediated Autophagy and Promotes Tau Pathology Propagation in Mice. Nat. Commun. 2021, 12, 2238. [Google Scholar] [CrossRef]
- Pyo, J.O.; Yoo, S.M.; Ahn, H.H.; Nah, J.; Hong, S.H.; Kam, T.I.; Jung, S.; Jung, Y.K. Overexpression of Atg5 in Mice Activates Autophagy and Extends Lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a Promoter of Longevity: Insights from Model Organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Escobar, K.A.; Cole, N.H.; Mermier, C.M.; VanDusseldorp, T.A. Autophagy and Aging: Maintaining the Proteome through Exercise and Caloric Restriction. Aging Cell 2019, 18, e12876. [Google Scholar] [CrossRef]
- Fujimoto, C.; Iwasaki, S.; Urata, S.; Morishita, H.; Sakamaki, Y.; Fujioka, M.; Kondo, K.; Mizushima, N.; Yamasoba, T. Autophagy Is Essential for Hearing in Mice. Cell Death Dis. 2017, 8, e2780. [Google Scholar] [CrossRef]
- Tsuchihashi, N.A.; Hayashi, K.; Dan, K.; Goto, F.; Nomura, Y.; Fujioka, M.; Kanzaki, S.; Komune, S.; Ogawa, K. Autophagy through 4EBP1 and AMPK Regulates Oxidative Stress-Induced Premature Senescence in Auditory Cells. Oncotarget 2015, 6, 3644. [Google Scholar] [CrossRef]
- He, Z.; Guo, L.; Shu, Y.; Fang, Q.; Zhou, H.; Liu, Y.; Liu, D.; Lu, L.; Zhang, X.; Ding, X.; et al. Autophagy Protects Auditory Hair Cells against Neomycin-Induced Damage. Autophagy 2017, 13, 1884–1904. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, F.; Li, X.; Wu, Q.; Dai, C. Rapamycin Ameliorates Age-Related Hearing Loss in C57BL/6J Mice by Enhancing Autophagy in the SGNs. Neurosci. Lett. 2022, 772, 136493. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Hill, K.; Chen, J.; Lemasters, J.; Yang, S.M.; Sha, S.H. Autophagy Attenuates Noise-Induced Hearing Loss by Reducing Oxidative Stress. Antioxid. Redox Signal. 2015, 22, 1308–1324. [Google Scholar] [CrossRef]
- Xiao, F.H.; Chen, X.Q.; Yu, Q.; Ye, Y.; Liu, Y.W.; Yan, D.; Yang, L.Q.; Chen, G.; Lin, R.; Yang, L.; et al. Transcriptome Evidence Reveals Enhanced Autophagy-Lysosomal Function in Centenarians. Genome Res. 2018, 28, 1601–1610. [Google Scholar] [CrossRef]
- Emanuele, E.; Minoretti, P.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Yilmaz, Y.; Garatachea, N.; Lucia, A. Can Enhanced Autophagy Be Associated with Human Longevity? Serum Levels of the Autophagy Biomarker Beclin-1 Are Increased in Healthy Centenarians. Rejuvenation Res. 2014, 17, 518–524. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tyler, J.K. Epigenetics and Aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Avelar, R.A.; Ortega, J.G.; Tacutu, R.; Tyler, E.J.; Bennett, D.; Binetti, P.; Budovsky, A.; Chatsirisupachai, K.; Johnson, E.; Murray, A.; et al. A Multidimensional Systems Biology Analysis of Cellular Senescence in Aging and Disease. Genome Biol. 2020, 21, 91. [Google Scholar] [CrossRef]
- Reimann, M.; Lee, S.; Schmitt, C.A. Cellular Senescence: Neither Irreversible nor Reversible. J. Exp. Med. 2024, 221, e20232136. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The Role of Cellular Senescence in Ageing and Endocrine Disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Özcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased Analysis of Senescence Associated Secretory Phenotype (SASP) to Identify Common Components Following Different Genotoxic Stresses. Aging 2016, 8, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR Regulates the Pro-Tumorigenic Senescence-Associated Secretory Phenotype by Promoting IL1A Translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, Z.; Sunchu, B.; Shoaf, J.; Dang, I.; Zhao, S.; Caples, K.; Bradley, L.; Beaver, L.M.; Ho, E.; et al. Rapamycin Inhibits the Secretory Phenotype of Senescent Cells by a Nrf2-Independent Mechanism. Aging Cell 2017, 16, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Yamasoba, T. Oxidative Stresses and Mitochondrial Dysfunction in Age-Related Hearing Loss. Oxid. Med. Cell. Longev. 2014, 1, 582849. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses 2017, 9, 289. [Google Scholar] [CrossRef]
- Escames, G.; López, L.C.; García, J.A.; García-Corzo, L.; Ortiz, F.; Acuña-Castroviejo, D. Mitochondrial DNA and Inflammatory Diseases. Hum. Genet. 2012, 131, 161–173. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf Expression Is a Biomarker of Aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef]
- Baker, D.J.; Perez-Terzic, C.; Jin, F.; Pitel, K.; Niederländer, N.J.; Jeganathan, K.; Yamada, S.; Reyes, S.; Rowe, L.; Hiddinga, H.J.; et al. Opposing Roles for P16Ink4a and P19Arf in Senescence and Ageing Caused by BubR1 Insufficiency. Nat. Cell Biol. 2008, 10, 825–836. [Google Scholar] [CrossRef]
- Hao, C.; Wu, X.; Zhou, R.; Zhang, H.; Zhou, Y.; Wang, X.; Feng, Y.; Mei, L.; He, C.; Cai, X.; et al. Downregulation of P66Shc Can Reduce Oxidative Stress and Apoptosis in Oxidative Stress Model of Marginal Cells of Stria Vascularis in Sprague Dawley Rats. Drug Des. Devel. Ther. 2019, 13, 3199–3206. [Google Scholar] [CrossRef]
- Lebiedzinska, M.; Karkucinska-Wieckowska, A.; Giorgi, C.; Karczmarewicz, E.; Pronicka, E.; Pinton, P.; Duszynski, J.; Pronicki, M.; Wieckowski, M.R. Oxidative Stress-Dependent P66Shc Phosphorylation in Skin Fibroblasts of Children with Mitochondrial Disorders. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choo, O.S.; Lee, J.S.; Jang, J.H.; Woo, H.G.; Choung, Y.H. BCL2 Interacting Protein 3-like/NIX-Mediated Mitophagy Plays an Important Role in the Process of Age-Related Hearing Loss. Neuroscience 2021, 455, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xiong, H.; Su, Z.; Pang, J.; Lai, L.; Zhang, H.; Jian, B.; Zhang, W.; Zheng, Y. Inhibition of DRP-1-Dependent Mitophagy Promotes Cochlea Hair Cell Senescence and Exacerbates Age-Related Hearing Loss. Front Cell Neurosci 2019, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Mazucanti, C.; Cabral-Costa, J.; Vasconcelos, A.; Andreotti, D.; Scavone, C.; Kawamoto, E. Longevity Pathways (MTOR, SIRT, Insulin/IGF-1) as Key Modulatory Targets on Aging and Neurodegeneration. Curr. Top. Med. Chem. 2015, 15, 2116–2138. [Google Scholar] [CrossRef]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans Mutant That Lives Twice as Long as Wild Type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Deford, J.H.; Flurkey, K.; Harrison, D.E.; Papaconstantinou, J. Implications for the Insulin Signaling Pathway in Snell Dwarf Mouse Longevity: A Similarity with the C. elegans Longevity Paradigm. Mech. Ageing Dev. 2002, 123, 1229–1244. [Google Scholar] [CrossRef]
- Brown-Borg, H.M.; Borg, K.E.; Meliska, C.J.; Bartke, A. Dwarf Mice and the Ageing Process. Nature 1996, 384, 33. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, B.C.; Maheshwari, H.G.; He, L.I.; Reed, M.; Lozykowski, M.; Okada, S.; Cataldo, L.; Coschigamo, K.; Wagner, T.E.; et al. A Mammalian Model for Laron Syndrome Produced by Targeted Disruption of the Mouse Growth Hormone Receptorbinding Protein Gene (the Laron Mouse). Proc. Natl. Acad. Sci. USA 1997, 94, 13215–13220. [Google Scholar] [CrossRef]
- Riquelme, R.; Cediel, R.; Contreras, J.; Rodriguez-de la Rosa, L.; Murillo-Cuesta, S.; Hernandez-Sanchez, C.; Zubeldia, J.M.; Cerdan, S.; Varela-Nieto, I. A Comparative Study of Age-Related Hearing Loss in Wild Type and Insulin-like Growth Factor I Deficient Mice. Front. Neuroanat. 2010, 4, 1707. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. AMP-Activated Protein Kinase (AMPK) Controls the Aging Process via an Integrated Signaling Network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef]
- Denduluri, S.K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like Growth Factor (IGF) Signaling Intumorigenesis and the Development Ofcancer Drug Resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef]
- Hou, X.; Li, Z.; Higashi, Y.; Delafontaine, P.; Sukhanov, S. Insulin-Like Growth Factor Prevents Cellular Aging via Activation of Mitophagy. J. Aging Res. 2020, 2020, 4939310. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. Insulin/IGF-1 Paradox of Aging: Regulation via AKT/IKK/NF-ΚB Signaling. Cell. Signal. 2010, 22, 573–577. [Google Scholar] [CrossRef]
- Paulsen, A.J.; Cruickshanks, K.J.; Pinto, A.; Schubert, C.R.; Dalton, D.S.; Fischer, M.E.; Klein, B.E.K.; Klein, R.; Tsai, M.Y.; Tweed, T.S. Neuroprotective Factors and Incident Hearing Impairment in the Epidemiology of Hearing Loss Study. Laryngoscope 2019, 129, 2178–2183. [Google Scholar] [CrossRef]
- Gao, L.; Nakagawa, T. Insulin-like Growth Factor 1: Role in the Auditory System and Therapeutic Potential in Otology. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 286–290. [Google Scholar] [CrossRef]
- RodriGuez-De La Rosa, L.; Lassaletta, L.; Calvino, M.; Murillo-Cuesta, S.; Varela-Nieto, I. The Role of Insulin-like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front. Aging Neurosci. 2017, 12, 411. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Rodríguez-De la Rosa, L.; Cediel, R.; Lassaletta, L.; Varela-Nieto, I. The Role of Insulin-like Growth Factor-i in the Physiopathology of Hearing. Front. Mol. Neurosci. 2011, 4, 12336. [Google Scholar] [CrossRef] [PubMed]
- Williamson, T.T.; Ding, B.; Zhu, X.; Frisina, R.D. Hormone Replacement Therapy Attenuates Hearing Loss: Mechanisms Involving Estrogen and the IGF-1 Pathway. Aging Cell 2019, 18, e12939. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like Growth Factor 1 Inhibits Hair Cell Apoptosis and Promotes the Cell Cycle of Supporting Cells by Activating Different Downstream Cascades after Pharmacological Hair Cell Injury in Neonatal Mice. Mol. Cell. Neurosci. 2013, 56, 29–38. [Google Scholar] [CrossRef]
- Yamahara, K.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like Growth Factor 1: A Novel Treatment for the Protection or Regeneration of Cochlear Hair Cells. Hear. Res. 2015, 330, 2–9. [Google Scholar] [CrossRef]
- Kapahi, P.; Zid, B.M.; Harper, T.; Koslover, D.; Sapin, V.; Benzer, S. Regulation of Lifespan in Drosophila by Modulation of Genes in the TOR Signaling Pathway. Curr. Biol. 2004, 14, 885–890. [Google Scholar] [CrossRef]
- Kamada, Y.; Funakoshi, T.; Shintani, T.; Nagano, K.; Ohsumi, M.; Ohsumi, Y. Tor-Mediated Induction of Autophagy via an Apg1 Protein Kinase Complex. J. Cell Biol. 2000, 150, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Mittnacht, S.; Paterson, H.; Olson, M.F.; Marshall, C.J. Ras Signalling Is Required for Inactivation of the Tumour Suppressor PRb Cell-Cycle Control Protein. Curr. Biol. 1997, 7, 219–221. [Google Scholar] [CrossRef]
- Kenyon, C. The First Long-Lived Mutants: Discovery of the Insulin/IGF-1 Pathway for Ageing. Philos. Trans. R. Soc. B Biol. 2011, 366, 9–16. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C. PI3-Kinase/Akt/MTOR Signaling: Impaired on/off Switches in Aging, Cognitive Decline and Alzheimer’s Disease. Exp. Gerontol. 2013, 48, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Melick, C.H.; Jewell, J.L. Regulation of Mtorc1 by Upstream Stimuli. Genes 2020, 11, 989. [Google Scholar] [CrossRef]
- Fu, X.; Sun, X.; Zhang, L.; Jin, Y.; Chai, R.; Yang, L.; Zhang, A.; Liu, X.; Bai, X.; Li, J.; et al. Tuberous Sclerosis Complex-Mediated MTORC1 Overactivation Promotes Age-Related Hearing Loss. J. Clin. Investig. 2018, 128, 4938–4955. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Rana, A.; Rera, M.; Graniel, J.; Walker, D.W. AMPK Modulates Tissue and Organismal Aging in a Non-Cell-Autonomous Manner. Cell Rep. 2014, 8, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD + Metabolism and SIRT1 Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Burkewitz, K.; Zhang, Y.; Mair, W.B. AMPK at the Nexus of Energetics and Aging. Cell Metab. 2014, 20, 10–25. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Selman, C.; Tullet, J.M.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef]
- Redpath, N.T.; Foulstone, E.J.; Proud, C.G. Regulation of Translation Elongation Factor-2 by Insulin via a Rapamycin-Sensitive Signalling Pathway. EMBO J. 1996, 15, 2291–2297. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and MTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, X.; Hu, Y.; Sun, H.; Gong, G.; Huang, X.; Chen, X.; Xia, M.; Sun, C.; Huang, Q.; et al. Autophagy Regulates the Degeneration of the Auditory Cortex through the AMPK-MTOR-ULK1 Signaling Pathway. Int. J. Mol. Med. 2018, 41, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Zhao, X.; Lin, X.; Gao, Y.; Raimundo, N.; Li, G.L.; Shang, W.; Wu, H.; Song, L. Down-Regulation of AMPK Signaling Pathway Rescues Hearing Loss in TFB1 Transgenic Mice and Delays Age-Related Hearing Loss. Aging 2020, 12, 5590. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Austriaco, N.R.; Zhang, J.; Guarente, L. Mutation in the Silencing Gene SIR4 Can Delay Aging in S. Cerevisiae. Cell 1995, 80, 485–496. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Gotta, M.; Sinclair, D.A.; Mills, K.; McNabb, D.S.; Murthy, M.; Pak, S.M.; Laroche, T.; Gasser, S.M.; Guarente, L. Redistribution of Silencing Proteins from Telomeres to the Nucleolus Is Associated with Extension of Life Span in S. Cerevisiae. Cell 1997, 89, 381–391. [Google Scholar] [CrossRef]
- Longo, V.D.; Kennedy, B.K. Sirtuins in Aging and Age-Related Disease. Cell 2006, 126, 257–268. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of FOXO Transcription Factors by the SIRT1 Deacetylase. Science 2024, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, X. Nucleus or Cytoplasm? The Mysterious Case of SIRT1’s Subcellular Localization. Cell Cycle 2016, 15, 3337–3338. [Google Scholar] [CrossRef]
- Verdin, E. NAD + in Aging, Metabolism, and Neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Pagans, S.; Pedal, A.; North, B.J.; Kaehlcke, K.; Marshall, B.L.; Dorr, A.; Hetzer-Egger, C.; Henklein, P.; Frye, R.; McBurney, M.W.; et al. SIRT1 Regulates HIV Transcription via Tat Deacetylation. PLoS Biol. 2005, 3, e41. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small Molecule Activators of SIRT1 as Therapeutics for the Treatment of Type 2 Diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Lemos, V.; de Oliveira, R.M.; Naia, L.; Szegö, É.; Ramos, E.; Pinho, S.; Magro, F.; Cavadas, C.; Cristina Rego, A.; Costa, V.; et al. The NAD+-Dependent Deacetylase SIRT2 Attenuates Oxidative Stress and Mitochondrial Dysfunction and Improves Insulin Sensitivity in Hepatocytes. Hum. Mol. Genet. 2017, 26, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; A Rosenberg, M.; Jeganathan, K.B.; Hafner, A.V.; Michan, S.; Dai, J.; Baker, D.J.; Cen, Y.; E Wu, L.; A Sauve, A.; et al. SIRT 2 Induces the Checkpoint Kinase BubR1 to Increase Lifespan. EMBO J. 2014, 33, 1438–1453. [Google Scholar] [CrossRef]
- Liu, M.; Liang, K.; Zhen, J.; Zhou, M.; Wang, X.; Wang, Z.; Wei, X.; Zhang, Y.; Sun, Y.; Zhou, Z.; et al. Sirt6 Deficiency Exacerbates Podocyte Injury and Proteinuria through Targeting Notch Signaling. Nat. Commun. 2017, 8, 413. [Google Scholar] [CrossRef]
- Takumida, M.; Takumida, H.; Katagiri, Y.; Anniko, M. Localization of Sirtuins (SIRT1-7) in the Aged Mouse Inner Ear. Acta Oto-Laryngol. 2016, 136, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, R.; Munoz, F.M.; Lau, S.S.; Monks, T.J. PARP-1 Hyperactivation and Reciprocal Elevations in Intracellular Ca2+ during ROS-Induced Nonapoptotic Cell Death. Toxicol. Sci. 2014, 140, 118–134. [Google Scholar] [CrossRef]
- Aksoy, P.; Escande, C.; White, T.A.; Thompson, M.; Soares, S.; Benech, J.C.; Chini, E.N. Regulation of SIRT 1 Mediated NAD Dependent Deacetylation: A Novel Role for the Multifunctional Enzyme CD38. Biochem. Biophys. Res. Commun. 2006, 349, 353–359. [Google Scholar] [CrossRef]
- Lee, J.; Padhye, A.; Sharma, A.; Song, G.; Miao, J.; Mo, Y.Y.; Wang, L.; Kemper, J.K. A Pathway Involving Farnesoid X Receptor and Small Heterodimer Partner Positively Regulates Hepatic Sirtuin 1 Levels via MicroRNA-34a Inhibition. J. Biol. Chem. 2010, 285, 12604–12611. [Google Scholar] [CrossRef]
- Kim, H.; Cao, W.; Oh, G.; Lee, S.; Shen, A.; Khadka, D.; Lee, S.; Sharma, S.; Kim, S.Y.; Choe, S.; et al. Augmentation of Cellular NAD+ by NQO1 Enzymatic Action Improves Age-Related Hearing Impairment. Aging Cell 2019, 18, e13016. [Google Scholar] [CrossRef]
- Brown, K.D.; Maqsood, S.; Huang, J.Y.; Pan, Y.; Harkcom, W.; Li, W.; Sauve, A.; Verdin, E.; Jaffrey, S.R. Activation of SIRT3 by the NAD+ Precursor Nicotinamide Riboside Protects from Noise-Induced Hearing Loss. Cell Metab. 2014, 20, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Puel, J.L.; Ruel, J.; d’Aldin, C.G.; Pujol, R. Excitotoxicity and Repair of Cochlear Synapses after Noise-Trauma Induced Hearing Loss. Neuroreport 1998, 9, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shentu, T.P.; Wen, L.; Johnson, D.A.; Shyy, J.Y.J. Regulation of SIRT1 by Oxidative Stress-Responsive MiRNAs and a Systematic Approach to Identify Its Role in the Endothelium. Antioxid. Redox Signal. 2013, 19, 1522–1538. [Google Scholar] [CrossRef]
- Pang, J.; Xiong, H.; Ou, Y.; Yang, H.; Xu, Y.; Chen, S.; Lai, L.; Ye, Y.; Su, Z.; Lin, H.; et al. SIRT1 Protects Cochlear Hair Cell and Delays Age-Related Hearing Loss via Autophagy. Neurobiol. Aging 2019, 80, 127–137. [Google Scholar] [CrossRef]
- Bolanos-Garcia, V.M.; Blundell, T.L. BUB1 and BUBR1: Multifaceted Kinases of the Cell Cycle. Trends Biochem. Sci. 2011, 36, 141–150. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Liu, X.; Ye, S.; Yao, P.Y.; Wang, W.; Yang, F.; Gao, X.; Li, J.; Zhang, Y.; et al. BubR1 Phosphorylates CENP-E as a Switch Enabling the Transition from Lateral Association to End-on Capture of Spindle Microtubules. Cell Res. 2019, 29, 562–578. [Google Scholar] [CrossRef]
- Cho, C.H.; Yang, Z.; Yoo, K.H.; Oliveros, A.; Jang, M.H. Bubr1 Insufficiency Impairs Affective Behavior and Memory Function in Mice. Int. Neurourol. J. 2018, 22, S122–S130. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Ito, E.; Tauchi, H.; Komatsu, K.; Ikeuchi, T.; Kajii, T. Chromosomal Instability Syndrome of Total Premature Chromatid Separation with Mosaic Variegated Aneuploidy Is Defective in Mitotic-Spindle Checkpoint. Am. J. Hum. Genet. 2000, 67, 483–486. [Google Scholar] [CrossRef][Green Version]
- Tang, R.; Jiang, Z.; Chen, F.; Yu, W.; Fan, K.; Tan, J.; Zhang, Z.; Liu, X.; Li, P.; Yuan, K. The Kinase Activity of Drosophila BubR1 Is Required for Insulin Signaling-Dependent Stem Cell Maintenance. Cell Rep. 2020, 31, 107794. [Google Scholar] [CrossRef]
- Miyamoto, T.; Porazinski, S.; Wang, H.; Borovina, A.; Ciruna, B.; Shimizu, A.; Kajii, T.; Kikuchi, A.; Furutani-Seiki, M.; Matsuura, S. Insufficiency of BUBR1, a Mitotic Spindle Checkpoint Regulator, Causes Impaired Ciliogenesis in Vertebrates. Hum. Mol. Genet. 2011, 20, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Y.; Zhao, X.Y.; Huang, Q.L.; Sun, H.Y.; Sun, C.; Yuan, J.; He, C.; Sun, Y.; Huang, X.; Kong, W.; et al. Activation of Wnt/β-Catenin Signaling by Lithium Chloride Attenuates d-Galactose-Induced Neurodegeneration in the Auditory Cortex of a Rat Model of Aging. FEBS Open Bio 2017, 7, 759–776. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Hu, Y.; Sun, H.; He, Z.; Yuan, J.; Cai, H.; Sun, Y.; Huang, X.; Kong, W.; et al. Age-Associated Decline in Nrf2 Signaling and Associated MtDNA Damage May Be Involved in the Degeneration of the Auditory Cortex: Implications for Central Presbycusis. Int. J. Mol. Med. 2018, 42, 3371–3385. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Qi, J.; Zhang, Y.; He, Y.; Ni, W.; Li, W.; Zhang, S.; Sun, S.; Taketo, M.M.; et al. Wnt Activation Protects against Neomycin-Induced Hair Cell Damage in the Mouse Cochlea. Cell Death Dis. 2016, 7, e2136. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; De Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 2015, 14, 497–510. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin Fed Late in Life Extends Lifespan in Genetically Heterogeneous Mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR Is a Key Modulator of Ageing and Age-Related Disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Schinaman, J.M.; Rana, A.; Ja, W.W.; Clark, R.I.; Walker, D.W. Rapamycin Modulates Tissue Aging and Lifespan Independently of the Gut Microbiota in Drosophila. Sci. Rep. 2019, 9, 7824. [Google Scholar] [CrossRef]
- Altschuler, R.A.; Kanicki, A.; Martin, C.; Kohrman, D.C.; Miller, R.A. Rapamycin but Not Acarbose Decreases Age-Related Loss of Outer Hair Cells in the Mouse Cochlea. Hear. Res. 2018, 370, 11–15. [Google Scholar] [CrossRef]
- Zuber, J.; Anglicheau, D.; Elie, C.; Bererhi, L.; Timsit, M.O.; Mamzer-Bruneel, M.F.; Ciroldi, M.; Martinez, F.; Snanoudj, R.; Hiesse, C.; et al. Sirolimus May Reduce Fertility in Male Renal Transplant Recipients. Am. J. Transplant. 2008, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Mahé, E.; Morelon, E.; Lechaton, S.; Sang, K.H.L.Q.; Mansouri, R.; Ducasse, M.F.; Mamzer-Bruneel, M.F.; De Prost, Y.; Kreis, H.; Bodemer, C. Cutaneous Adverse Events in Renal in Renal Transplant Recipients Receiving Sirolimus-Based Therapy. Transplantation 2005, 79, 476–482. [Google Scholar] [CrossRef]
- Glossmann, H.H.; Lutz, O.M.D. Metformin and Aging: A Review. Gerontology 2019, 65, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Slack, C.; Foley, A.; Partridge, L. Activation of AMPK by the Putative Dietary Restriction Mimetic Metformin Is Insufficient to Extend Lifespan in Drosophila. PLoS ONE 2012, 7, e47699. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Berstein, L.M.; Egormin, P.A.; Piskunova, T.S.; Popovich, I.G.; Zabezhinski, M.A.; Tyndyk, M.L.; Yurova, M.V.; Kovalenko, I.G.; Poroshina, T.E.; et al. Metformin Slows down Aging and Extends Life Span of Female SHR Mice. Cell Cycle 2008, 7, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Elam, C.F.; Mattison, J.A.; Lane, M.A.; Roth, G.S.; Ingram, D.K.; Allison, D.B. Metformin Supplementation and Life Span in Fischer-344 Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Berstein, L.M.; Egormin, P.A.; Piskunova, T.S.; Popovich, I.G.; Zabezhinski, M.A.; Kovalenko, I.G.; Poroshina, T.E.; Semenchenko, A.V.; Provinciali, M.; et al. Effect of Metformin on Life Span and on the Development of Spontaneous Mammary Tumors in HER-2/Neu Transgenic Mice. Exp. Gerontol. 2005, 40, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kanigur Sultuybek, G.; Soydas, T.; Yenmis, G. NF-ΚB as the Mediator of Metformin’s Effect on Ageing and Ageing-Related Diseases. Clin. Exp. Pharmacol. Physiol. 2019, 46, 413–422. [Google Scholar] [CrossRef]
- Chester, J.; Johnston, E.; Walker, D.; Jones, M.; Ionescu, C.M.; Wagle, S.R.; Kovacevic, B.; Brown, D.; Mikov, M.; Mooranian, A.; et al. A Review on Recent Advancement on Age-Related Hearing Loss: The Applications of Nanotechnology, Drug Pharmacology, and Biotechnology. Pharmaceutics 2021, 13, 1041. [Google Scholar] [CrossRef]
- Kesici, G.G.; Öcal, F.C.A.; Gürgen, S.G.; Erdem, Ş.R.; Öğüş, E.; Erbek, H.S.; Özlüoğlu, L.N. The Protective Effect of Metformin against the Noise-Induced Hearing Loss. Eur. Arch. Otorhinolaryngol. 2018, 275, 2957–2966. [Google Scholar] [CrossRef]
- Cai, H.; Han, B.; Hu, Y.; Zhao, X.; He, Z.; Chen, X.; Sun, H.; Yuan, J.; Li, Y.; Yang, X.; et al. Metformin Attenuates the D-Galactose-Induced Aging Process via the UPR through the AMPK/ERK1/2 Signaling Pathways. Int. J. Mol. Med. 2020, 45, 715–730. [Google Scholar] [CrossRef]
- Muri, L.; Le, N.D.; Zemp, J.; Grandgirard, D.; Leib, S.L. Metformin Mediates Neuroprotection and Attenuates Hearing Loss in Experimental Pneumococcal Meningitis. J. Neuroinflamm. 2019, 16, 156. [Google Scholar] [CrossRef]
- Okur, M.N.; Mao, B.; Kimura, R.; Haraczy, S.; Fitzgerald, T.; Edwards-Hollingsworth, K.; Tian, J.; Osmani, W.; Croteau, D.L.; Kelley, M.W.; et al. Short-Term NAD+ Supplementation Prevents Hearing Loss in Mouse Models of Cockayne Syndrome. NPJ Aging Mech. Dis. 2020, 6, 1. [Google Scholar] [CrossRef]
- Han, S.; Du, Z.; Liu, K.; Gong, S. Nicotinamide Riboside Protects Noise-Induced Hearing Loss by Recovering the Hair Cell Ribbon Synapses. Neurosci. Lett. 2020, 725, 134910. [Google Scholar] [CrossRef]
- Okur, M.N.; Sahbaz, B.D.; Kimura, R.; Manor, U.; Patel, J.; Park, J.H.; Andrade, L.; Puligilla, C.; Croteau, D.L.; Bohr, V.A. Long-Term NAD+ Supplementation Prevents the Progression of Age-Related Hearing Loss in Mice. Aging Cell 2023, 22, e13909. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Fernández, R.; Sánchez-Rodriguez, C.; Granizo, J.J.; Durio-Calero, E.; Martín-Sanz, E. Accuracy of Auditory Steady State and Auditory Brainstem Responses to Detect the Preventive Effect of Polyphenols on Age-Related Hearing Loss in Sprague–Dawley Rats. Eur. Arch. Otorhinolaryngol. 2016, 273, 341–347. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Xiong, H.; Pang, J.; Yang, H.; Dai, M.; Liu, Y.; Ou, Y.; Huang, Q.; Chen, S.; Zhang, Z.; Xu, Y.; et al. Activation of MiR-34a/SIRT1/P53 Signaling Contributes to Cochlear Hair Cell Apoptosis: Implications for Age-Related Hearing Loss. Neurobiol. Aging 2015, 36, 1692–1701. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.; Babu, S.; Tang, W.; Naem, E.; Quirk, W.S. Effects of Resveratrol on Acoustic Trauma. Otolaryngol. Head Neck Surg. 2003, 129, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Tang, W.; Bai, V.U.; Ahmad, N.; Jiang, H.; Media, J.; Patel, N.; Rubin, C.J.; Standring, R.T. Resveratrol Decreases Noise-Induced Cyclooxygenase-2 Expression in the Rat Cochlea. Otolaryngol. Head Neck Surg. 2013, 148, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.Q.; Shu, F.Q.; Zhang, M.; Kai, Y.Z.; Tang, Z.Q. Resveratrol Prevents Hearing Loss and a Subregion Specific- Reduction of Serotonin Reuptake Transporter Induced by Noise Exposure in the Central Auditory System. Front. Neurosci. 2023, 17, 1134153. [Google Scholar] [CrossRef] [PubMed]
- Muderris, T.; Sağlam, A.; Unsal, D.; Mülazimoğlu, S.; Sevil, E.; Kayhan, H. Efficiency of Resveratrol in the Prevention and Treatment of Age-related Hearing Loss. Exp. Ther. Med. 2022, 23, 1–7. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Yang, S.; Ding, Y.; Qu, Y. Low-Dose Resveratrol Inhibits RIPK3-Mediated Necroptosis and Delays the Onset of Age-Related Hearing Loss. Front. Pharmacol. 2022, 13, 40. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, S.; Cazevieille, C.; Caumes, B. Resveratrol Treatment Reduces Neuromotor Impairment and Hearing Loss in a Mouse Model of Diabetic Neuropathy and Nerve Injury. J. Diabetes Res. 2020, 2, 59–67. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, C.; Cuadrado, E.; Riestra-Ayora, J.; Sanz-Fernández, R. Polyphenols Protect against Age-Associated Apoptosis in Female Rat Cochleae. Biogerontology 2018, 19, 159–169. [Google Scholar] [CrossRef] [PubMed]
- García-Alcántara, F.; Murillo-Cuesta, S.; Pulido, S.; Bermúdez-Muñoz, J.M.; Martínez-Vega, R.; Milo, M.; Varela-Nieto, I.; Rivera, T. The Expression of Oxidative Stress Response Genes Is Modulated by a Combination of Resveratrol and N-Acetylcysteine to Ameliorate Ototoxicity in the Rat Cochlea. Hear. Res. 2018, 358, 10–21. [Google Scholar] [CrossRef]
- Nevado, J.; Sanz, R.; Sánchez-Rodríguez, C.; García-Berrocal, J.R.; Martín-Sanz, E.; González-García, J.Á.; Esteban-Sánchez, J.; Ramírez-Camacho, R. Ginkgo Biloba Extract (EGb761) Protects against Aging-Related Caspase-Mediated Apoptosis in Rat Cochlea. Acta Otolaryngol. 2010, 130, 1101–1112. [Google Scholar] [CrossRef]
- Li, N.; Yan, X.; Huang, W.; Chu, M.; Dong, Y.; Song, H.; Peng, Y.; Shi, J.; Liu, Q. Curcumin Protects against the Age-Related Hearing Loss by Attenuating Apoptosis and Senescence via Activating Nrf2 Signaling in Cochlear Hair Cells. Biochem. Pharmacol. 2023, 212, 115575. [Google Scholar] [CrossRef]
- van Deursen, J.M. Senolytic Therapies for Healthy Longevity. Science 2019, 364, 636–637. [Google Scholar] [CrossRef]
- Kim, E.C.; Kim, J.R. Senotherapeutics: Emerging Strategy for Healthy Aging and Age-Related Disease. BMB Rep. 2019, 52, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, H.; Riabowol, K. Senolytics: A Translational Bridge Between Cellular Senescence and Organismal Aging. Front. Cell Dev. Biol. 2020, 7, 367. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 Inhibitors as a Novel Class of Senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, D.Y.; Kim, H.S.; Park, S.C.; Park, J.T.; Kim, H.S.; Oh, W.K.; Cho, K.A. Identification of a Novel Senomorphic Agent, Avenanthramide C, via the Suppression of the Senescence-Associated Secretory Phenotype. Mech. Ageing Dev. 2020, 192, 111355. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Birch, J.; Fielder, E.; Rahmatika, D.; Taylor, J.; Chapman, J.; Lagnado, A.; Carroll, B.M.; Miwa, S.; Richardson, G.; et al. Rapamycin Improves Healthspan but Not Inflammaging in Nfκb1−/− Mice. Aging Cell 2019, 18, e12882. [Google Scholar] [CrossRef] [PubMed]
- Erbaba, B.; Arslan-Ergul, A.; Adams, M.M. Effects of Caloric Restriction on the Antagonistic and Integrative Hallmarks of Aging. Ageing Res. Rev. 2021, 66, 101228. [Google Scholar] [CrossRef]
- Fontana, L.; Nehme, J.; Demaria, M. Caloric Restriction and Cellular Senescence. Mech. Ageing Dev. 2018, 176, 19–23. [Google Scholar] [CrossRef]
- Vaquero, A.; Reinberg, D. Calorie Restriction and the Exercise of Chromatin. Genes Dev. 2009, 23, 1849–1869. [Google Scholar] [CrossRef]
- Anderson, R.M.; Bitterman, K.J.; Wood, J.G.; Medvedik, O.; Sinclair, D.A. Nicotinamide and PNC1 Govern Lifespan Extension by Calorie Restriction in Saccharomyces Cerevisiae. Nature 2003, 423, 181–185. [Google Scholar] [CrossRef]
- Lakowski, B.; Hekimi, S. The Genetics of Caloric Restriction in Caenorhabditis Elegans. Proc. Natl. Acad. Sci. USA 1998, 95, 13091–13096. [Google Scholar] [CrossRef]
- Barger, J.L.; Walford, R.L.; Weindruch, R. The Retardation of Aging by Caloric Restriction: Its Significance in the Transgenic Era. Exp. Gerontol. 2003, 38, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Bernardes de Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. Telomerase Reverse Transcriptase Synergizes with Calorie Restriction to Increase Health Span and Extend Mouse Longevity. PLoS ONE 2013, 8, e53760. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural Mechanisms of Ageing and Cognitive Decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, S.H.; Colman, R.J.; Lopez, M.; Beasley, T.M.; Aiken, J.M.; Anderson, R.M.; Weindruch, R. Caloric Restriction Delays Aging-Induced Cellular Phenotypes in Rhesus Monkey Skeletal Muscle. Exp. Gerontol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Duszka, K.; Gregor, A.; Guillou, H.; König, J.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Caloric Restriction-Common Pathways Affecting Metabolism, Health, and Longevity. Cells 2020, 9, 1708. [Google Scholar] [CrossRef]
- Yu, W.; Dittenhafer-Reed, K.E.; Denu, J.M. SIRT3 Protein Deacetylates Isocitrate Dehydrogenase 2 (IDH2) and Regulates Mitochondrial Redox Status. J. Biol. Chem 2012, 287, 14078–14086. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yamasoba, T.; Weindruch, R.; Prolla, T.A.; Tanokura, M. Caloric Restriction Suppresses Apoptotic Cell Death in the Mammalian Cochlea and Leads to Prevention of Presbycusis. Neurobiol. Aging 2007, 28, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Willott, J.F.; Erway, L.C.; Archer, J.R.; Harrison, D.E. Genetics of Age-Related Hearing Loss in Mice. II. Strain Differences and Effects of Caloric Restriction on Cochlear Pathology and Evoked Response Thresholds. Hear. Res. 1995, 88, 143–155. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Pietrocola, F.; Eisenberg, T.; Kroemer, G. Caloric Restriction Mimetics: Towards a Molecular Definition. Nat. Rev. Drug Discov. 2014, 13, 727–740. [Google Scholar] [CrossRef]
- Kim, D.H.; Bang, E.; Jung, H.J.; Noh, S.G.; Yu, B.P.; Choi, Y.J.; Chung, H.Y. Anti-aging Effects of Calorie Restriction (CR) and CR Mimetics Based on the Senoinflammation Concept. Nutrients 2020, 12, 422. [Google Scholar] [CrossRef]
- Gabandé-Rodríguez, E.; Mittelbrunn, M.; de las Heras, M.M.G. Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells 2019, 9, 82. [Google Scholar] [CrossRef]
| Pathway | Role in Aging | Effects on Hearing | References |
|---|---|---|---|
| IGF-1 Signaling | Decreases with age |
| [128,129,130,131,132,133] |
| mTORC1 Signaling | Hyperactivated in aged cochlear cells |
| [139,140] |
| AMPK Pathway | Activated by ROS; inhibited in AMPKα1 depleted mice |
| [148,149] |
| Sirtuin Pathway | SIRT1, SIRT3, SIRT5 decrease with age in cochlea; NAD+ levels decline |
| [35,164,168,169,170,171,172] |
| BubR1/Wnt/β-Catenin Signalling | Decreased BubR1, Wnt/β-catenin signalling in the aged auditory system, involves CDC20, p16, p19, p53 |
| [179,180,181] |
| ROS and Mitochondrial Dysfunction | Increased ROS and decreased antioxidant enzymes with age |
| [61,62,63,64,65,66,67,68] |
| Mitophagy and Autophagy | Impaired mitophagy and autophagy with age |
| [90,91,92,93,94] |
| Cellular Senescence | Accumulation of senescent cells with age; regulated by mTOR and AMPK pathways |
| [42,62,98,102,103,104,105,111,113,114] |
| Intervention | Mechanism of Action | Effect on ARHL | Benefits | Limitations/Considerations | Research Stage | References |
|---|---|---|---|---|---|---|
| Rapamycin | mTOR inhibition, enhances autophagy | Delays ARHL by reducing mTORC1 activity in cochlear cells | Protects cochlear hair cells; Enhances autophagy | Potential immunosuppressive effects: Alternative mTOR inhibitors may be needed | In vivo (mouse models); Clinical trials for other conditions | [140,186] |
| Metformin | AMPK activation, mimics calorie restriction | Reduces inflammation, delays ARHL, reduces neuronal apoptosis | Well-established safety profile; Multiple beneficial effects | Dose-dependent effects; Not consistently effective across all species | In vivo (mouse models); Clinically approved (for diabetes) | [197,198,199] |
| NAD+ Boosters (e.g., NR, NMN) | Restores NAD+ levels, activates sirtuins | Protects cochlea, enhances survival of hair cells, reduces oxidative stress | Can protect against hearing loss even in advanced age; Enhances mitochondrial function | Optimal dosage and long-term effects still under investigation | In vivo (mouse models); Early-stage clinical trials | [200,201,202] |
| Resveratrol/ Polyphenols | SIRT1 activation, reduces oxidative stress, enhances autophagy | Delays ARHL, protects against cochlear damage and neuronal loss | Natural compounds with multiple health benefits; Acts as a SIRT1 agonist | Bioavailability issues; Optimal dosage unclear | In vivo (mouse models); Clinical trials for other conditions | [172,207,208,209,210,211,212,213,214,215,216] |
| Senolytics (e.g., Dasatinib + Quercetin) | Induces apoptosis in senescent cells, reduces SASP | Potential to mitigate tissue degeneration and extend healthspan | Targets fundamental aging processes; Potential for periodic treatment | More research needed for ARHL-specific effects; Optimal timing and dosing unclear | Preclinical research; Early clinical trials for other conditions | [217,218,219,220,221,222,223] |
| Calorie Restriction and CR Mimetics | Activates autophagy, modulates mitochondrial energy metabolism, promotes protein deacetylation | Slows ARHL progression, reduces oxidative damage in mitochondria, potential to delay ARHL by targeting aging-related pathways | Comprehensive health benefits beyond hearing; No drug side effects; May provide CR benefits without dietary restriction; Multiple targets | Challenging to implement long-term; Effectiveness may vary with genetics; Specific effects on ARHL need more research; Optimal compounds still under investigation | In vivo (mouse models); Limited human studies; Preclinical research; Some compounds in early clinical trials | [235,236,237,238,239,240,241] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ege, T.; Tao, L.; North, B.J. The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss. Int. J. Mol. Sci. 2024, 25, 9705. https://doi.org/10.3390/ijms25179705
Ege T, Tao L, North BJ. The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss. International Journal of Molecular Sciences. 2024; 25(17):9705. https://doi.org/10.3390/ijms25179705
Chicago/Turabian StyleEge, Tuba, Litao Tao, and Brian J. North. 2024. "The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss" International Journal of Molecular Sciences 25, no. 17: 9705. https://doi.org/10.3390/ijms25179705
APA StyleEge, T., Tao, L., & North, B. J. (2024). The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss. International Journal of Molecular Sciences, 25(17), 9705. https://doi.org/10.3390/ijms25179705





