Mitochondria and Acute Leukemia: A Clinician’s Perspective
Abstract
1. Introduction
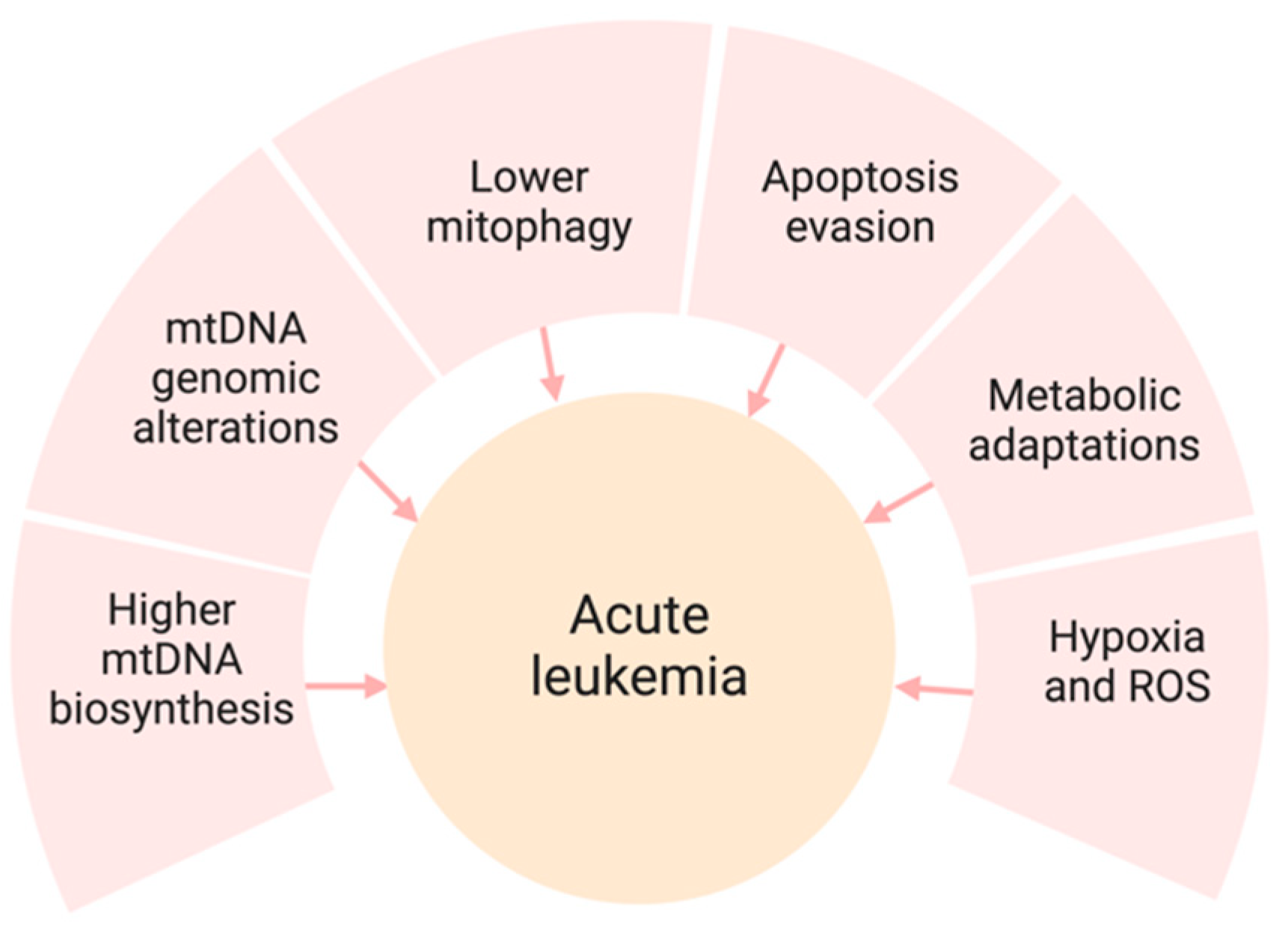
2. Mitochondria and Acute Leukemia
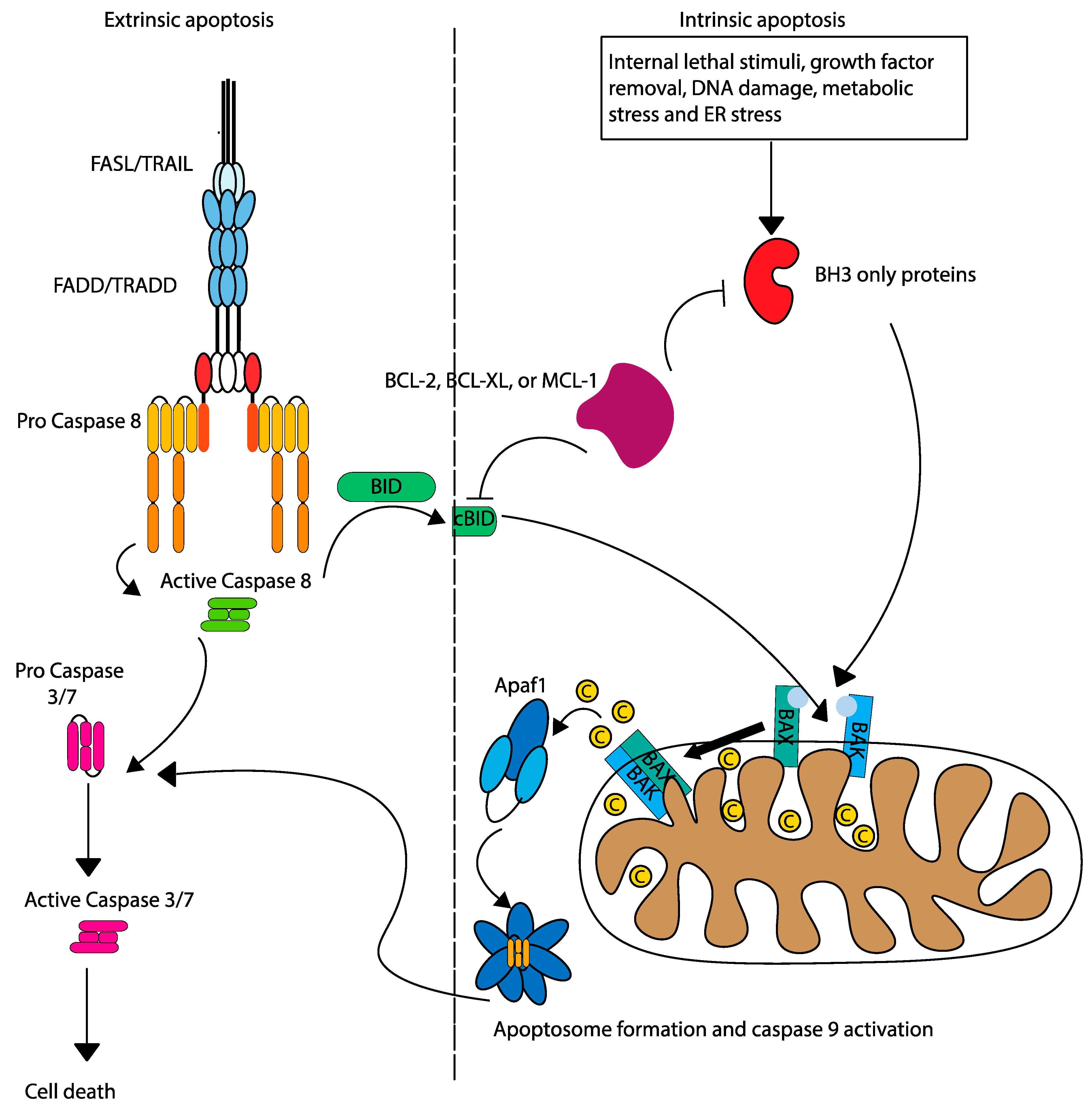
Concepts of Mitochondrial Apoptosis
3. Relevance of Mitochondrial Apoptosis in Acute Leukemia
3.1. Mitochondrial Membrane Potential
3.2. Mitochondrial Metabolism and Bioenergetics
3.3. Mitochondrial Biogenesis and Dynamics
3.4. Molecular Mechanisms and Genetic Mutations
3.4.1. Common Genetic Mutations Affecting Mitochondrial Function
3.4.2. Molecular Pathways Influenced by These Mutations
4. Role of Mitochondrial Apoptosis in Leukemia Initiation and Progression
4.1. Early Apoptotic Changes
4.2. Influence on Leukemic Stem Cells (LSCs)
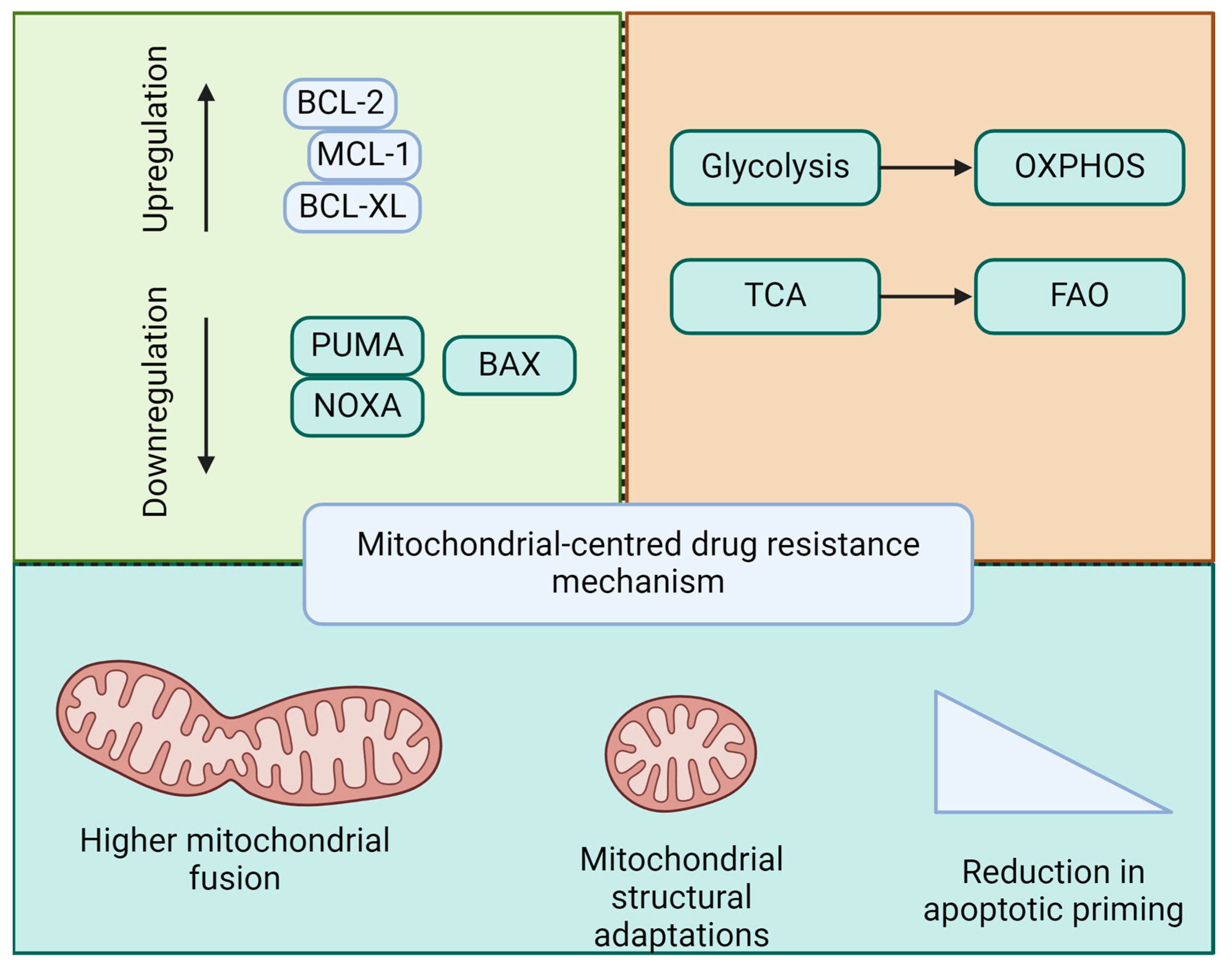
4.3. Drug Resistance Mechanisms
4.4. Evasion of Apoptosis in Resistant Leukemia
4.5. Role of Mitochondria in Drug Resistance
5. Mitochondria-Targeted Therapies in Acute Leukemia
5.1. BH3 Mimetics
5.2. Other Apoptotic Modulators
5.3. Combination Therapies
5.3.1. Synergistic Effects with Chemotherapy
5.3.2. Potential in Combination with Novel Agents
6. Clinical Trials and Outcomes
- Overview of Ongoing and Completed Clinical Trials
6.1. VIALE-A Trial
6.2. VIALE-C Trial
6.3. Combinations with Intensive Chemotherapies and Novel Agents
7. Future Directions and Conclusions
7.1. Development of Next-Generation Inhibitors
7.2. Combination Therapies
7.3. Overcoming Resistance Mechanisms
7.4. Other Optimization Strategies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Vakiti, A.; Reynolds, S.B.; Mewawalla, P. Acute Myeloid Leukemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507875/ (accessed on 25 June 2024).
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Chen, S.-C.; Andersson, A.K.; Phillips, L.A.; Li, Y.; Sotzen, J.; Kundu, M.; Downing, J.R.; Melnick, A.; Mullighan, C.G. Integrated genetic and epigenetic analysis of childhood acute lymphoblastic leukemia. J. Clin. Investig. 2013, 123, 3099–3111. [Google Scholar] [CrossRef]
- Xu, H.; Wen, Y.; Jin, R.; Chen, H. Epigenetic modifications and targeted therapy in pediatric acute myeloid leukemia. Front. Pediatr. 2022, 10, 975819. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial Dysfunction in Cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012, 2012, 524308. [Google Scholar] [CrossRef]
- Vo, T.-T.; Ryan, J.; Carrasco, R.; Neuberg, D.; Rossi, D.J.; Stone, R.M.; DeAngelo, D.J.; Frattini, M.G.; Letai, A. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 2012, 151, 344–355. [Google Scholar] [CrossRef]
- Bhola, P.D.; Mar, B.G.; Lindsley, R.C.; Ryan, J.A.; Hogdal, L.J.; Vo, T.T.; DeAngelo, D.J.; Galinsky, I.; Ebert, B.L.; Letai, A. Functionally identifiable apoptosis-insensitive subpopulations determine chemoresistance in acute myeloid leukemia. J. Clin. Investig. 2016, 126, 3827–3836. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Letai, A. BCL-2 inhibition in AML: An unexpected bonus? Blood 2018, 132, 1007–1012. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Romo-González, M.; Ijurko, C.; Hernández-Hernández, Á. Reactive Oxygen Species and Metabolism in Leukemia: A Dangerous Liaison. Front Immunol. 2022, 13, 889875. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Katz, S.G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 2017, 24, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Larrue, C.; Mouche, S.; Lin, S.; Simonetta, F.; Scheidegger, N.K.; Poulain, L.; Birsen, R.; Sarry, J.-E.; Stegmaier, K.; Tamburini, J. Mitochondrial fusion is a therapeutic vulnerability of acute myeloid leukemia. Leukemia 2023, 37, 765–775. [Google Scholar] [CrossRef]
- Czegle, I.; Gray, A.L.; Wang, M.; Liu, Y.; Wang, J.; Wappler-Guzzetta, E.A. Mitochondria and Their Relationship with Common Genetic Abnormalities in Hematologic Malignancies. Life 2021, 11, 1351. [Google Scholar] [CrossRef]
- Ju, H.-Q.; Zhan, G.; Huang, A.; Sun, Y.; Wen, S.; Yang, J.; Lu, W.-H.; Xu, R.-H.; Li, J.; Li, Y.; et al. ITD mutation in FLT3 tyrosine kinase promotes Warburg effect and renders therapeutic sensitivity to glycolytic inhibition. Leukemia 2017, 31, 2143–2150. [Google Scholar] [CrossRef]
- Baran, N.; Lodi, A.; Dhungana, Y.; Herbrich, S.; Collins, M.; Sweeney, S.; Pandey, R.; Skwarska, A.; Patel, S.; Tremblay, M.; et al. Inhibition of mitochondrial complex I reverses NOTCH1-driven metabolic reprogramming in T-cell acute lymphoblastic leukemia. Nat. Commun. 2022, 13, 2801. [Google Scholar] [CrossRef]
- Nechiporuk, T.; Kurtz, S.E.; Nikolova, O.; Liu, T.; Jones, C.L.; D’Alessandro, A.; Culp-Hill, R.; d’Almeida, A.; Joshi, S.K.; Rosenberg, M.; et al. The TP53 Apoptotic Network is a Primary Mediator of Resistance to BCL2 inhibition in AML Cells. Cancer Discov. 2019, 9, 910–925. [Google Scholar] [CrossRef]
- Shin, D.-Y. TP53 Mutation in Acute Myeloid Leukemia: An Old Foe Revisited. Cancers 2023, 15, 4816. [Google Scholar] [CrossRef]
- Darici, S.; Alkhaldi, H.; Horne, G.; Jørgensen, H.G.; Marmiroli, S.; Huang, X. Targeting PI3K/Akt/mTOR in AML: Rationale and Clinical Evidence. J. Clin. Med. 2020, 9, 2934. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Challen, G.A.; Sun, D.; Jeong, M.; Luo, M.; Jelinek, J.; Berg, J.S.; Bock, C.; Vasanthakumar, A.; Gu, H.; Xi, Y.; et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 2011, 44, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Bcl-2–family proteins and hematologic malignancies: History and future prospects. Blood 2008, 111, 3322–3330. [Google Scholar] [CrossRef]
- Jones, C.L.; Inguva, A.; Jordan, C.T. Targeting Energy Metabolism in Cancer Stem Cells: Progress and Challenges in Leukemia and Solid Tumors. Cell Stem Cell 2021, 28, 378–393. [Google Scholar] [CrossRef]
- Khaldoyanidi, S.K.; Hindoyan, A.; Stein, A.; Subklewe, M. Leukemic stem cells as a target for eliminating acute myeloid leukemia: Gaps in translational research. Crit. Rev. Oncol. Hematol. 2022, 175, 103710. [Google Scholar] [CrossRef] [PubMed]
- de Beauchamp, L.; Himonas, E.; Helgason, G.V. Mitochondrial metabolism as a potential therapeutic target in myeloid leukaemia. Leukemia 2022, 36, 1–12. [Google Scholar] [CrossRef]
- Barbato, A.; Scandura, G.; Puglisi, F.; Cambria, D.; La Spina, E.; Palumbo, G.A.; Lazzarino, G.; Tibullo, D.; Di Raimondo, F.; Giallongo, C.; et al. Mitochondrial Bioenergetics at the Onset of Drug Resistance in Hematological Malignancies: An Overview. Front. Oncol. 2020, 10, 604143. [Google Scholar] [CrossRef]
- Griffioen, M.S.; de Leeuw, D.C.; Janssen, J.J.W.M.; Smit, L. Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies. Cancers 2022, 14, 3456. [Google Scholar] [CrossRef]
- Aumann, S.; Shaulov, A.; Haran, A.; Gross Even-Zohar, N.; Vainstein, V.; Nachmias, B. The Emerging Role of Venetoclax-Based Treatments in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2022, 23, 10957. [Google Scholar] [CrossRef]
- Jain, N.; Stevenson, K.E.; Winer, E.S.; Garcia, J.S.; Stone, R.M.; Jabbour, E.; Ravandi, F.; Stewart, J.M.; Legg, D.R.; Kantarjian, H.M.; et al. A Multicenter Phase I Study Combining Venetoclax with Mini-Hyper-CVD in Older Adults with Untreated and Relapsed/Refractory Acute Lymphoblastic Leukemia. Blood 2019, 134, 3867. [Google Scholar] [CrossRef]
- Richard-Carpentier, G.; Jabbour, E.; Short, N.J.; Rausch, C.R.; Savoy, J.M.; Bose, P.; Yilmaz, M.; Jain, N.; Borthakur, G.; Ohanian, M.; et al. Clinical Experience With Venetoclax Combined With Chemotherapy for Relapsed or Refractory T-Cell Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2020, 20, 212–218. [Google Scholar] [CrossRef]
- Gibson, B.E.; Webb, D.K.; Howman, A.J.; De Graaf, S.S.; Harrison, C.J.; Wheatley, K. Results of a randomized trial in children with Acute Myeloid Leukaemia: Medical research council AML12 trial. Br. J. Haematol. 2011, 155, 366–376. [Google Scholar] [CrossRef]
- Palmisiano, N.; Lee, J.-W.; Claxton, D.F.; Paietta, E.; Alkhateeb, H.B.; Park, J.H.; Podoltsev, N.; Atallah, E.L.; Schaar, D.G.; Dinner, S.; et al. Maximal Tolerated Dose Determined for Venetoclax in Combination with Liposomal Vincristine in Patients with Relapsed or Refractory Ph-Negative T-Cell or B-Cell Acute Lymphoblastic Leukemia: Results of Phase 1 Portion of ECOG-ACRIN EA9152. Blood 2021, 138, 3407. [Google Scholar] [CrossRef]
- Pullarkat, V.A.; Lacayo, N.J.; Jabbour, E.; Rubnitz, J.E.; Bajel, A.; Laetsch, T.W.; Leonard, J.; Colace, S.I.; Khaw, S.L.; Fleming, S.A.; et al. Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov. 2021, 11, 1440–1453. [Google Scholar] [CrossRef]
- Short, N.J.; Konopleva, M.; Kadia, T.; Kebriaei, P.; Daver, N.; Huang, X.; Masarova, L.; Cook, R.; Jain, N.; Jabbour, E.; et al. An Effective Chemotherapy-Free Regimen of Ponatinib plus Venetoclax for Relapsed/Refractory Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Am. J. Hematol. 2021, 96, E229–E232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, C.; Shi, T.; Zhang, Y.; Qian, J.; Wang, Y.; Hu, Y.; Mao, L.; Ye, X.; Liu, F.; et al. Venetoclax-ponatinib for T315I/compound-mutated Ph+ acute lymphoblastic leukemia. Blood Cancer J. 2022, 12, 20. [Google Scholar] [CrossRef]
- Chonghaile, T.N.; Letai, A. Mimicking the BH3 domain to kill cancer cells. Oncogene 2008, 27, S149–S157. [Google Scholar] [CrossRef]
- Montero, J.; Haq, R. Adapted to Survive: Targeting Cancer Cells with BH3 Mimetics. Cancer Discov. 2022, 12, 1217–1232. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.V.; Luger, S.; Mangan, J.; Zebrowski, A.; Loren, A.W.; Minderman, H.; Baird, J.; Porter, D.L.; Hexner, E.O.; Kumar, A.J.; et al. A Phase I Study Using Single Agent Birinapant in Patients with Relapsed Myelodysplastic Syndrome and Acute Myelogenous Leukemia. Blood 2014, 124, 3758. [Google Scholar] [CrossRef]
- Klener, P.; Sovilj, D.; Renesova, N.; Andera, L. BH3 Mimetics in Hematologic Malignancies. Int. J. Mol. Sci. 2021, 22, 10157. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Kadia, T.; Daver, N.; Xiao, L.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed acute myeloid leukemia. Am. J. Hematol. 2022, 97, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Karol, S.E.; Alexander, T.B.; Budhraja, A.; Pounds, S.B.; Canavera, K.; Wang, L.; Wolf, J.; Klco, J.M.; Mead, P.E.; Gupta, S.D.; et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: A phase 1, dose-escalation study. Lancet Oncol. 2020, 21, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Panayiotidis, P.; Montesinos, P.; Laribi, K.; Ivanov, V.; Kim, I.; Novak, J.; Champion, R.; Fiedler, W.; Pagoni, M.; et al. Long-term follow-up of VIALE-C in patients with untreated AML ineligible for intensive chemotherapy. Blood 2022, 140, 2754–2756. [Google Scholar] [CrossRef]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 4048–4059. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Ge, S.-S.; Qiu, Q.-C.; Du, J.-H.; Shan, S.-S.; Shen, X.-D.; Wan, C.-L.; Wang, B.-R.; Wu, D.-P.; et al. Combination of Venetoclax and Midostaurin Efficiently Suppressed Relapsed t(8;21)Acute Myeloid Leukemia With Mutant KIT After Failure of Venetoclax Plus Azacitidine Treatment. Front. Oncol. 2022, 12, 841276. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Loghavi, S.; Zeng, Z.; Tanaka, T.; Kim, Y.J.; Uryu, H.; Turkalj, S.; Jakobsen, N.A.; Luskin, M.R.; Duose, D.Y.; et al. A Phase Ib/II Study of Ivosidenib with Venetoclax ± Azacitidine in IDH1-Mutated Myeloid Malignancies. Blood Cancer Discov. 2023, 4, 276–293. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Zheng, F.; Wang, D.; Zhao, L.; Liu, J.; Li, L.; Zhang, Z.; Zhang, C.; Li, Y.; Yang, S.; et al. Venetoclax combined with daunorubicin and cytarabine (2 + 6) as induction treatment in adults with newly diagnosed acute myeloid leukemia: A phase 2, multicenter, single-arm trial. Exp. Hematol. Oncol. 2023, 12, 45. [Google Scholar] [CrossRef]
- Wang, H.; Mao, L.; Yang, M.; Qian, P.; Lu, H.; Tong, H.; Xie, W.; Zhou, D.; Huang, X.; Wang, Y.; et al. Venetoclax plus 3 + 7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2022, 9, e415–e424. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.C.; Roberts, A.W.; Reynolds, J.; Fong, C.Y.; Ting, S.B.; Salmon, J.M.; MacRaild, S.; Ivey, A.; Tiong, I.S.; Fleming, S.; et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial (CAVEAT): A Phase Ib Dose-Escalation Study of Venetoclax Combined With Modified Intensive Chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3506–3517. [Google Scholar] [CrossRef]
- Kadia, T.M.; Reville, P.K.; Borthakur, G.; Yilmaz, M.; Kornblau, S.; Alvarado, Y.; Dinardo, C.D.; Daver, N.; Jain, N.; Pemmaraju, N.; et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e552–e561. [Google Scholar] [CrossRef] [PubMed]
- Astellas Pharma Global Development, Inc. A Phase 1/2, Multicenter, Open-Label, Randomized Dose Ranging and Expansion Study of the Combination of Gilteritinib, Venetoclax and Azacitidine in Patients With Newly Diagnosed FLT3 Mutated Acute Myeloid Leukemia (AML) Not Eligible for Intensive Induction Chemotherapy. clinicaltrials.gov; 2024 May. Report No.: NCT05520567. Available online: https://clinicaltrials.gov/study/NCT05520567 (accessed on 1 January 2024).
- M.D. Anderson Cancer Center. Phase 1b/2 Study of Oral Decitabine/Cedazuridine (ASTX727) and Venetoclax in Combination With the Targeted Mutant IDH1 Inhibitor Ivosidenib or the Targeted Mutant IDH2 Inhibitor Enasidenib. clinicaltrials.gov; 2024 May. Report No.: NCT04774393. Available online: https://clinicaltrials.gov/study/NCT04774393 (accessed on 1 January 2024).
- Garciaz, S.; Hospital, M.-A.; Collette, Y.; Vey, N. Venetoclax Resistance in Acute Myeloid Leukemia. Cancers 2024, 16, 1091. [Google Scholar] [CrossRef] [PubMed]
- Amgen. A Phase 1 First in Human Study Evaluating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AMG 176 in Subjects With Relapsed or Refractory Multiple Myeloma and Subjects With Relapsed or Refractory Acute Myeloid Leukemia. clinicaltrials.gov; 2024 Feb. Report No.: NCT02675452. Available online: https://clinicaltrials.gov/study/NCT02675452 (accessed on 1 January 2024).
- Institut de Recherches Internationales Servier. An International Phase Ib Multicentre Study to Characterize the Safety and Tolerability of Intravenously Administered S64315, a Selective Mcl-1 Inhibitor, in Combination with Orally Administered Venetoclax, a Selective Bcl-2 Inhibitor in Patients With Acute Myeloid Leukaemia (AML). clinicaltrials.gov; 2024 Feb. Report No.: NCT03672695. Available online: https://clinicaltrials.gov/study/NCT03672695 (accessed on 1 January 2024).
- Institut de Recherches Internationales Servier. Phase I, International, Multicentre, Open-Label, Non-Randomised, Non-Comparative Study of Intravenously Administered S64315, a Mcl-1 Inhibitor, in Patients with Acute Myeloid Leukaemia (AML) or Myelodysplastic Syndrome (MDS). clinicaltrials.gov; 2022 May. Report No.: NCT02979366. Available online: https://clinicaltrials.gov/study/NCT02979366 (accessed on 1 January 2024).
- Kargbo, R.B. Redefining Cancer Therapy: Toward BCL-XL/BCL-2 Dual Inhibitors with Diminished Platelet Toxicity. ACS Med. Chem. Lett. 2023, 14, 1156–1158. [Google Scholar] [CrossRef]
- Hird, A.W.; Tron, A.E. Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol. Ther. 2019, 198, 59–67. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. A Phase II Multi-center, Single Arm, Safety and Efficacy Study of MBG453 in Combination with Azacitidine and Venetoclax for the Treatment of Acute Myeloid Leukemia (AML) in Adult Patients Unfit for Chemotherapy. clinicaltrials.gov; 2024 May. Report No.: NCT04150029. Available online: https://clinicaltrials.gov/study/NCT04150029 (accessed on 1 January 2024).
- Carter, B.Z.; Mak, P.Y.; Tao, W.; Ayoub, E.; Ostermann, L.B.; Huang, X.; Loghavi, S.; Boettcher, S.; Nishida, Y.; Ruvolo, V.; et al. Combined inhibition of BCL-2 and MCL-1 overcomes BAX deficiency-mediated resistance of TP53-mutant acute myeloid leukemia to individual BH3 mimetics. Blood Cancer J. 2023, 13, 57. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef]
- Bhatia, K.; Sandhu, V.; Wong, M.H.; Iyer, P.; Bhatt, S. Therapeutic biomarkers in acute myeloid leukemia: Functional and genomic approaches. Front. Oncol. 2024, 14, 1275251. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; Tierney, C.; Dunphy, K.; Miettinen, J.J.; Heckman, C.A.; Bazou, D.; O’Gorman, P. Identification of Protein Biomarker Signatures for Acute Myeloid Leukemia (AML) Using Both Nontargeted and Targeted Approaches. Proteomes 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Zúñiga, C.D.; Garza-Veloz, I.; Martínez-Rendón, J.; Ureño-Segura, M.; Delgado-Enciso, I.; Martinez-Fierro, M.L. Circulating Biomarkers Associated with the Diagnosis and Prognosis of B-Cell Progenitor Acute Lymphoblastic Leukemia. Cancers 2023, 15, 4186. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial ID | Phase | Patient’s Age Group | Drug Tested | Participation Criteria |
|---|---|---|---|---|
| NCT04500587 | I | ≥18 years | BCL-2 inhibitor ZN-d5 | Relapsed or refractory (R/R) AML (primary, secondary or treatment related) |
| NCT03113643 | I | ≥18 years | SL-401, azacytidine (AZA) and venetoclax (VEN) | Treatment-naïve AML not eligible for induction therapy, or R/R AML |
| NCT05190471 | I/Ib | ≥18 years | BP1002 (a Liposomal Bcl-2 Antisense Oligodeoxynucleotide) monotherapy or combination with decitabine | R/R AML |
| NCT05287568 | I | ≥18 years | CC-486 (oral azacitidine) with VEN | R/R AML |
| NCT05829226 | I | ≥18 years | LYT-200 (a monoclonal antibody targeting galectin-9) alone or in combination with VEN and AZA | R/R AML |
| NCT05682170 | I/II | ≥18 years | Wee1 inhibitor ZN-c3 monotherapy followed by combination with BCL-2 inhibitor ZN-d5 | R/R AML |
| NCT04017546 | I | ≥18 years | CYC065 in combination with venetoclax | R/R AML |
| NCT04501120 | I | ≥18 years | APG-2575 (BCL-2 inhibitor) single agent and in combination with Homoharringtonine or AZA | R/R AML |
| NCT05986240 | I | ≥18 years | Danvatirsen alone followed by combination with VEN | R/R AML |
| NCT05909293 | - | 60–85 years | VEN maintenance therapy for 12 cycles | AML patients who received induction therapy and achieved complete remission or incomplete complete remission |
| NCT06191263 | I | ≥18 years | RVU120 (CDK8 inhibitor) in combination with VEN | R/R AML on venetoclax and azacytidine |
| NCT03113643 | I | ≥18 years | AZA with SL-401 or AZA with SL-401 and VEN | R/R AML |
| NCT03709758 | I | 18–60 years | VEN in combination with cytarabine and daunorubicin | Treatment-naïve AML |
| NCT04771130 | II | ≥18 years | Bcl-2 inhibitor BGB-11417 alone or in combination with AZA | AML/MDS/MPN |
| NCT06030089 | - | ≥18 years | VEN and AZA | AML patients ineligible for induction therapy |
| NCT04937166 | I | ≥18 years | DSP107 with AZA or DSP107 with AZA and VEN | R/R AML |
| NCT05918198 | II | 18–75 years | VEN with CAG (cytarabine, Acla and G-CSF) | R/R AML |
| NCT03471260 | Ib/II | ≥18 years | VEN, AZA and ivosidenib | R/R AML or treatment-naïve not eligible for induction therapy |
| NCT06046313 | II | ≥60 years | VEN with decitabine | Treatment-naïve AML |
| NCT05262465 | - | 60–85 years | Micro transplantation with AZA and VEN | Treatment-naïve elderly AML |
| NCT05287568 | I | 18–100 years | CC-486 (oral azacitidine) with VEN | R/R AML |
| NCT05576532 | II | 14–45 years | VEN plus IM2 (Ifosfamide plus Mitoxantrone or Idarubicin plus methotrexate) | R/R T-ALL |
| NCT03319901 | I | ≥18 years | VEN with standard of care | Treatment-naïve ALL (B/T) or R/R ALL |
| NCT05594784 | II | ≥14 years | Olverembatinib with VEN, prednisone and vincristine | De novo Ph+ ALL |
| NCT05660473 | II | 14–60 years | VEN with pediatric-inspired regimen (vincristine, daunorubicin, cyclophosphamide, pegaspargase, prednisone, cytarabine, 6-mercaptopurine, dexamethasone and methotrexate) | De novo Ph- ALL |
| NCT04872790 | Ib | ≥18 years | Prednisone, dasatinib, venetoclax, methotrexate, rituximab and Blinatumomab | Newly diagnosed or relapsed Ph+ ALL or Mixed Phenotype Acute Leukemia (MPAL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, P.; Jasdanwala, S.S.; Bhatia, K.; Bhatt, S. Mitochondria and Acute Leukemia: A Clinician’s Perspective. Int. J. Mol. Sci. 2024, 25, 9704. https://doi.org/10.3390/ijms25179704
Iyer P, Jasdanwala SS, Bhatia K, Bhatt S. Mitochondria and Acute Leukemia: A Clinician’s Perspective. International Journal of Molecular Sciences. 2024; 25(17):9704. https://doi.org/10.3390/ijms25179704
Chicago/Turabian StyleIyer, Prasad, Shaista Shabbir Jasdanwala, Karanpreet Bhatia, and Shruti Bhatt. 2024. "Mitochondria and Acute Leukemia: A Clinician’s Perspective" International Journal of Molecular Sciences 25, no. 17: 9704. https://doi.org/10.3390/ijms25179704
APA StyleIyer, P., Jasdanwala, S. S., Bhatia, K., & Bhatt, S. (2024). Mitochondria and Acute Leukemia: A Clinician’s Perspective. International Journal of Molecular Sciences, 25(17), 9704. https://doi.org/10.3390/ijms25179704





