Interaction of Micro- and Nanoplastics with Enzymes: The Case of Carbonic Anhydrase
Abstract
1. Introduction

2. Interaction of MPs and NPs with Enzymes
2.1. Enzymatic Degradation of MPs and NPs
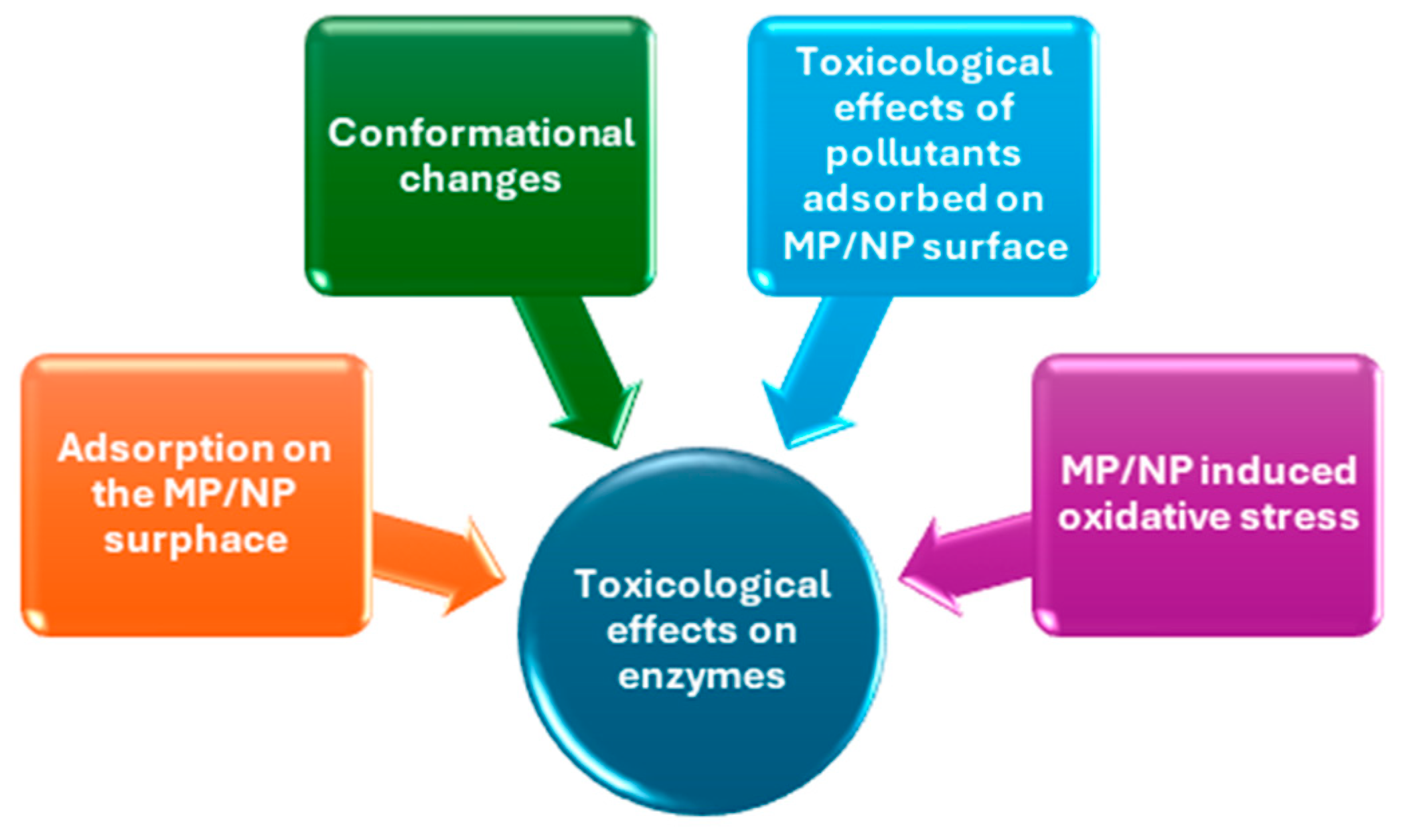
2.2. Toxicological and Ecotoxicological Effects of MPs and NPs on Enzymes
3. Carbonic Anhydrase
3.1. Sensitivity of CA Activity and Expression to Micro- and Nanoplastic Exposure
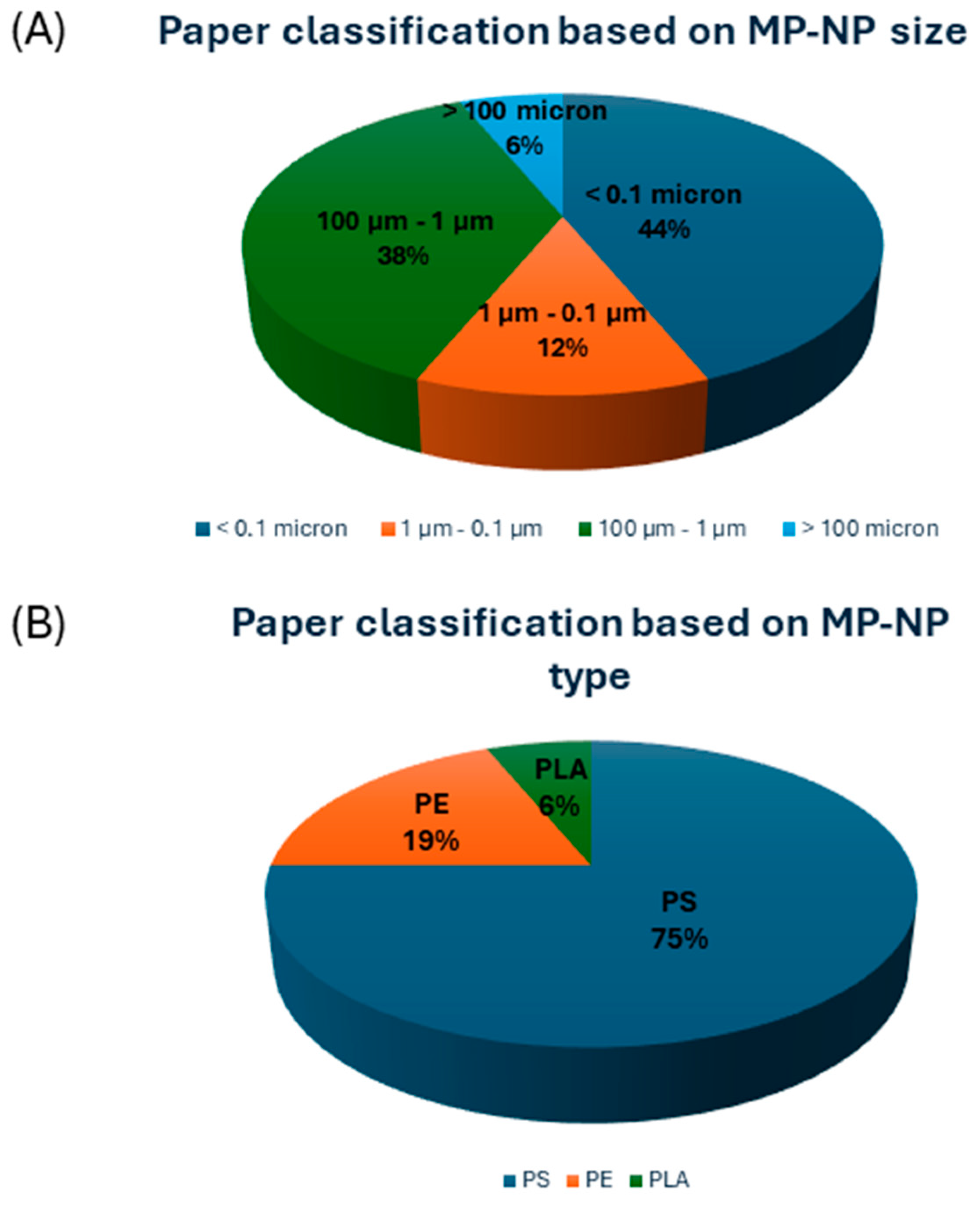
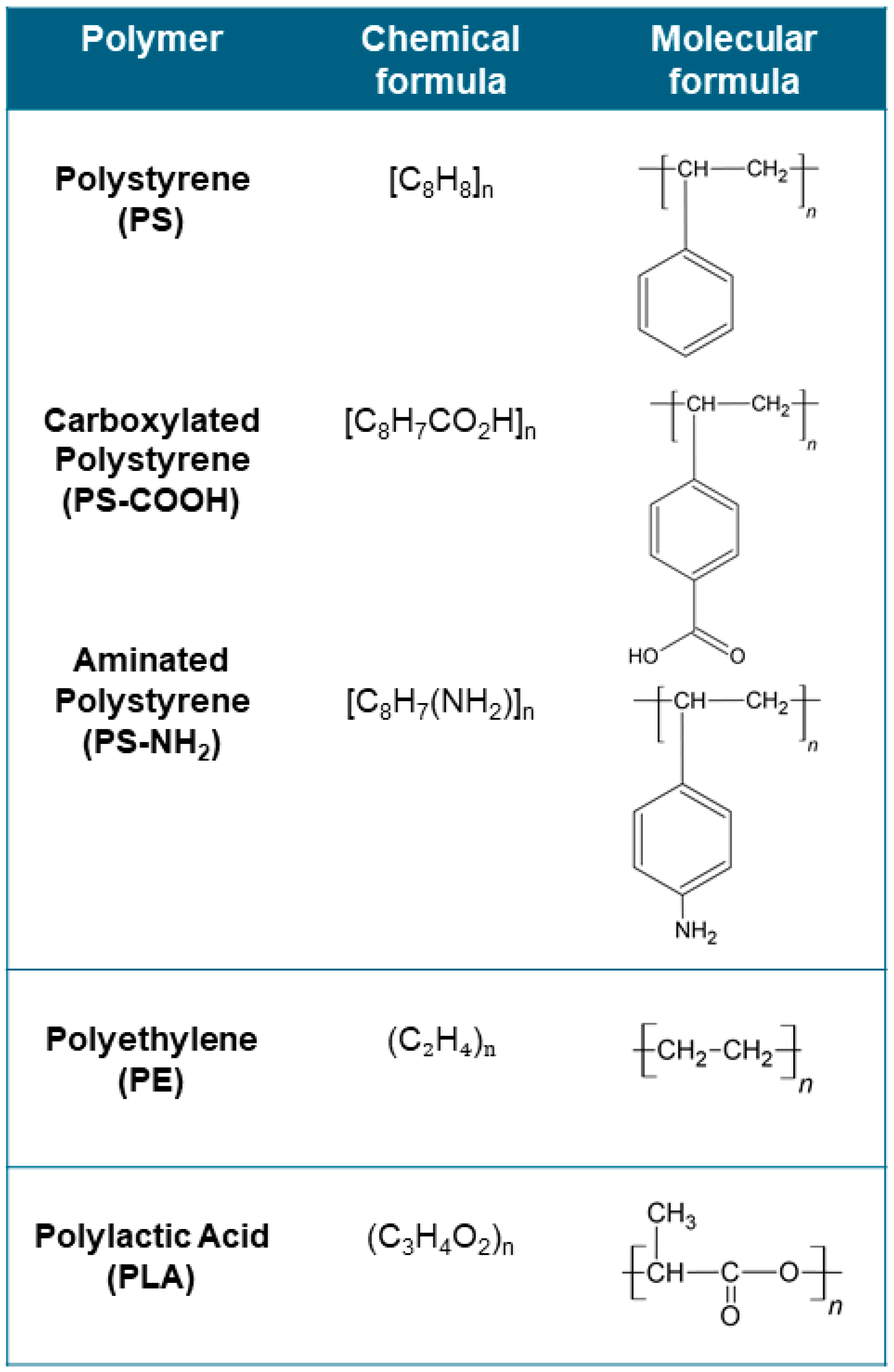
3.1.1. In Vitro and In Silico Studies
3.1.2. In Vivo Studies
4. CA Esterase Activity: Potentiality for Use in Plastic Degradation
5. Limitations and Challenges
6. Updates and Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mojiri, A.; Vishkaei, M.N.; Zhou, J.L.; Trzcinski, A.P.; Lou, Z.; Kasmuri, N.; Rezania, S.; Gholami, A.; Vakili, M.; Kazeroon, R.A. Impact of polystyrene microplastics on the growth and photosynthetic efficiency of diatom Chaetoceros neogracile. Mar. Environ. Res. 2024, 194, 106343. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, J.; Yanotti, M.; Tocock, M.; Hatton MacDonald, D. The Abundance of Microplastics in the World’s Oceans: A Systematic Review. Oceans 2024, 5, 398–428. [Google Scholar] [CrossRef]
- Singh, P.; Varshney, G.; Kaur, R. Primary Microplastics in the Ecosystem: Ecological Effects, Risks, and Comprehensive Perspectives on Toxicology and Detection Methods. J. Environ. Sci. Health Part C 2024, 1–52. [Google Scholar] [CrossRef]
- Lionetto, F.; Corcione, C.E.; Rizzo, A.; Maffezzoli, A. Production and Characterization of Polyethylene Terephthalate Nanoparticles. Polymers 2021, 13, 3745. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Lionetto, M.G.; Mele, C.; Corcione, C.E.; Bagheri, S.; Udayan, G.; Maffezzoli, A. Autofluorescence of Model Polyethylene Terephthalate Nanoplastics for Cell Interaction Studies. Nanomaterials 2022, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Acarer, S. Microplastics in wastewater treatment plants: Sources, properties, removal efficiency, removal mechanisms, and interactions with pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Padrão, J.; Khan, M.T.; Walker, T.R. Do’s and don’ts of microplastic research: A comprehensive guide. Water Emerg. Contam. Nanoplast. 2024, 3, 8. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Y.; Zuo, J.; Tian, W.; Wang, Y.; Duan, X.; Wang, S. Emerging microplastics in the environment: Properties, distributions, and impacts. Chemosphere 2022, 297, 134118. [Google Scholar] [CrossRef]
- Kothawale, S.S.; Kumar, L.; Singh, S.P. Role of organisms and their enzymes in the biodegradation of microplastics and nanoplastics: A review. Environ. Res. 2023, 232, 116281. [Google Scholar] [CrossRef]
- Mandal, M.; Roy, A.; Popek, R.; Sarkar, A. Micro- and nano- plastic degradation by bacterial enzymes: A solution to “White Pollution”. Microbe 2024, 3, 100072. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, D.P. Microplastic degradation by bacteria in aquatic ecosystem. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 431–467. [Google Scholar]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- Ahmaditabatabaei, S.; Kyazze, G.; Iqbal, H.M.N.; Keshavarz, T. Fungal Enzymes as Catalytic Tools for Polyethylene Terephthalate (PET) Degradation. J. Fungi 2021, 7, 931. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Das, S.; Roy, A.; Rakwal, R.; Jones, O.A.H.; Popek, R.; Agrawal, G.K.; Sarkar, A. Interactive relations between plants, the phyllosphere microbial community, and particulate matter pollution. Sci. Total Environ. 2023, 890, 164352. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.-K. Plastic Eating Enzymes: A Step Towards Sustainability. Indian J. Microbiol. 2022, 62, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Habib, S.; Shukor, M.Y.; Alias, S.A.; Smykla, J.; Yasid, N.A. Evaluation of the Deterioration of Untreated Commercial Polystyrene by Psychrotrophic Antarctic Bacterium. Polymers 2023, 15, 1841. [Google Scholar] [CrossRef]
- Huang, J.-N.; Wen, B.; Zhu, J.-G.; Zhang, Y.-S.; Gao, J.-Z.; Chen, Z.-Z. Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Chronic toxicity of polystyrene nanoparticles in the marine mussel Mytilus galloprovincialis. Chemosphere 2022, 287, 132356. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, M.; Jiang, Q.; Wu, D.; Huang, Y.; Jiao, Y.; Zhang, M.; Zhao, Y. Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J. Hazard. Mater. 2020, 398, 122990. [Google Scholar] [CrossRef]
- Gu, H.; Wang, S.; Wang, X.; Yu, X.; Hu, M.; Huang, W.; Wang, Y. Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J. Hazard. Mater. 2020, 397, 122773. [Google Scholar] [CrossRef] [PubMed]
- Covello, C.; Di Vincenzo, F.; Cammarota, G.; Pizzoferrato, M. Micro (nano) plastics and their potential impact on human gut health: A narrative review. Curr. Issues Mol. Biol. 2024, 46, 2658–2677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, L.; Xu, H.; Xu, E.G.; Li, M.; Wang, Y. Long-term exposure to polystyrene nanoplastics impairs the liver health of medaka. Water 2022, 14, 2767. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, F.; Liang, K.; Niu, W.; Duan, X.; Jia, X.; Wu, X.; Xu, P.; Zhou, L. Polystyrene nanoplastics affect digestive function and growth in juvenile groupers. Sci. Total Environ. 2022, 808, 152098. [Google Scholar] [CrossRef]
- Miranda, T.; Vieira, L.R.; Guilhermino, L. Neurotoxicity, behavior, and lethal effects of cadmium, microplastics, and their mixtures on Pomatoschistus microps juveniles from two wild populations exposed under laboratory conditions―implications to environmental and human risk assessment. Int. J. Environ. Res. Public Health 2019, 16, 2857. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Schettino, T. The complex relationship between metals and carbonic anhydrase: New insights and perspectives. Int. J. Mol. Sci. 2016, 17, 127. [Google Scholar] [CrossRef]
- DiMario, R.J.; Machingura, M.C.; Waldrop, G.L.; Moroney, J.V. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 2018, 268, 11–17. [Google Scholar] [CrossRef]
- Panda, T.; Gowrishankar, B.S. Production and applications of esterases. Appl. Microbiol. Biotechnol. 2005, 67, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Saika, A.; Shinozaki, Y.; Watanabe, T.; Suzuki, K.; Sameshima-Yamashita, Y.; Fukuoka, T.; Habe, H.; Morita, T.; Kitamoto, H. Degradation profiles of biodegradable plastic films by biodegradable plastic-degrading enzymes from the yeast Pseudozyma antarctica and the fungus Paraphoma sp. B47-9. Polym. Degrad. Stab. 2017, 141, 26–32. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef]
- Wierckx, N.; Narancic, T.; Eberlein, C.; Wei, R.; Drzyzga, O.; Magnin, A.; Ballerstedt, H.; Kenny, S.T.; Pollet, E.; Averous, L. Plastic biodegradation: Challenges and opportunities. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Biodegradation and Bioremediation; Springer: Cham, Switzerland, 2018; pp. 1–29. [Google Scholar]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro-and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef]
- Cárdenas-Alcaide, M.F.; Godínez-Alemán, J.A.; González-González, R.B.; Iqbal, H.M.N.; Parra-Saldívar, R. Environmental impact and mitigation of micro (nano) plastics pollution using green catalytic tools and green analytical methods. Green Anal. Chem. 2022, 3, 100031. [Google Scholar] [CrossRef]
- Mandal, M.; Chatterjee, S.; Majumdar, S. Outside the cell surface: Encoding the role of exopolysaccharide producing rhizobacteria to boost the drought tolerance in plants. In Plant Stress: Challenges and Management in the New Decade; Springer: Berlin/Heidelberg, Germany, 2022; pp. 295–310. [Google Scholar]
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368. [Google Scholar] [CrossRef]
- Thapa, S.; Li, H.; OHair, J.; Bhatti, S.; Chen, F.-C.; Al Nasr, K.; Johnson, T.; Zhou, S. Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol. Biotechnol. 2019, 61, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.; Müller, R.-J.; Deckwer, W.-D. Mechanism and kinetics of the enzymatic hydrolysis of polyester nanoparticles by lipases. Polym. Degrad. Stab. 2006, 91, 2486–2498. [Google Scholar] [CrossRef]
- Dutta, K.; Sen, S.; Veeranki, V.D. Production, characterization and applications of microbial cutinases. Process Biochem. 2009, 44, 127–134. [Google Scholar] [CrossRef]
- Baker, P.J.; Poultney, C.; Liu, Z.; Gross, R.; Montclare, J.K. Identification and comparison of cutinases for synthetic polyester degradation. Appl. Microbiol. Biotechnol. 2012, 93, 229–240. [Google Scholar] [CrossRef]
- Wei, R.; Oeser, T.; Then, J.; Kühn, N.; Barth, M.; Schmidt, J.; Zimmermann, W. Functional characterization and structural modeling of synthetic polyester-degrading hydrolases from Thermomonospora curvata. AMB Express 2014, 4, 44. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal enzymes involved in plastics biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
- Zimmermann, W.; Billig, S. Enzymes for the biofunctionalization of poly (ethylene terephthalate). Biofunction. Polym. Their Appl. 2011, 125, 97–120. [Google Scholar]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.A.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Murata, N.; Tanabe, E.; Kubota, K.; Kubo, M. Isolation and characterization of an ether-type polyurethane-degrading micro-organism and analysis of degradation mechanism by Alternaria sp. J. Appl. Microbiol. 2010, 108, 1946–1953. [Google Scholar] [PubMed]
- Hollóczki, O.; Gehrke, S. Nanoplastics can change the secondary structure of proteins. Sci. Rep. 2019, 9, 16013. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Esposito Corcione, C. An Overview of the Sorption Studies of Contaminants on Poly (Ethylene Terephthalate) Microplastics in the Marine Environment. J. Mar. Sci. Eng. 2021, 9, 445. [Google Scholar] [CrossRef]
- Luo, H.; Tu, C.; He, D.; Zhang, A.; Sun, J.; Li, J.; Xu, J.; Pan, X. Interactions between microplastics and contaminants: A review focusing on the effect of aging process. Sci. Total Environ. 2023, 899, 165615. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.; Narayan Vaidya, A.; Kumar, A.R. Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environ. Sci. Pollut. Res. 2022, 29, 49490–49512. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C.; Messa, F.; Perrone, S.; Salomone, A.; Maffezzoli, A. The Sorption of Amoxicillin on Engineered Polyethylene Terephthalate Microplastics. J. Polym. Environ. 2023, 31, 1383–1397. [Google Scholar] [CrossRef]
- Patra, I.; Huy, D.T.N.; Alsaikhan, F.; Opulencia, M.J.C.; Van Tuan, P.; Nurmatova, K.C.; Majdi, A.; Shoukat, S.; Yasin, G.; Margiana, R. Toxic effects on enzymatic activity, gene expression and histopathological biomarkers in organisms exposed to microplastics and nanoplastics: A review. Environ. Sci. Eur. 2022, 34, 80. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584, 1022–1031. [Google Scholar] [CrossRef]
- Tan, H.; Yue, T.; Xu, Y.; Zhao, J.; Xing, B. Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ. Sci. Technol. 2020, 54, 12285–12294. [Google Scholar] [CrossRef]
- Rajendran, D.; Chandrasekaran, N. Unveiling the Modification of Esterase-like Activity of Serum Albumin by Nanoplastics and Their Cocontaminants. ACS Omega 2023, 8, 43719–43731. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Hou, J.; You, G.; Liu, Z.; Liu, S.; Li, T.; Mo, Y.; Guo, S.; Qu, H. Acute effects of nanoplastics and microplastics on periphytic biofilms depending on particle size, concentration and surface modification. Environ. Pollut. 2019, 255, 113300. [Google Scholar] [CrossRef]
- Gu, H.; Chang, X.; Huang, W.; Sokolova, I.M.; Wei, S.; Sun, L.; Li, S.; Wang, X.; Hu, M.; Zeng, J. Oxidative stress induced by nanoplastics in the liver of juvenile large yellow croaker Larimichthys crocea. Mar. Pollut. Bull. 2021, 170, 112661. [Google Scholar]
- Liu, Z.; Cai, M.; Yu, P.; Chen, M.; Wu, D.; Zhang, M.; Zhao, Y. Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposure to a polystyrene nanoplastic. Aquat. Toxicol. 2018, 204, 1–8. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Trestrail, C.; Nugegoda, D.; Shimeta, J. Invertebrate responses to microplastic ingestion: Reviewing the role of the antioxidant system. Sci. Total Environ. 2020, 734, 138559. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ. 2020, 723, 138180. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Nocentini, A.; Supuran, C.T.; Capasso, C. Bacterial ι-carbonic anhydrase: A new active class of carbonic anhydrase identified in the genome of the Gram-negative bacterium Burkholderia territorii. J. Enzym. Inhib. Med. Chem. 2020, 35, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem. 2007, 15, 4336–4350. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Giordano, M.E.; Vilella, S.; Schettino, T. Inhibition of eel enzymatic activities by cadmium. Aquat. Toxicol. 2000, 48, 561–571. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Erroi, E.; Giordano, M.E.; Schettino, T. Carbonic anhydrase-based environmental bioassay. Int. J. Environ. Anal. Chem. 2005, 85, 895–903. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Erroi, E.; Giordano, M.E.; Schettino, T. Potential application of carbonic anhydrase activity in bioassay and biomarker studies. Chem. Ecol. 2006, 22, S119–S125. [Google Scholar] [CrossRef]
- Roberto, C.; Giulia, L.M.; Francesco, D.; Aldo, V.; Trifone, S. Carbonic anhydrase activity in Mytilus galloprovincialis digestive gland: Sensitivity to heavy metal exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 241–247. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Erroi, E.; Schettino, T. Carbonic anhydrase as pollution biomarker: An ancient enzyme with a new use. Int. J. Environ. Res. Public Health 2012, 9, 3965–3977. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Carbonic Anhydrase Sensitivity to Pesticides: Perspectives for Biomarker Development. Int. J. Mol. Sci. 2020, 21, 3562. [Google Scholar] [CrossRef] [PubMed]
- Caricato, R.; Giordano, M.E.; Schettino, T.; Maisano, M.; Mauceri, A.; Giannetto, A.; Cappello, T.; Parrino, V.; Ancora, S.; Caliani, I.; et al. Carbonic anhydrase integrated into a multimarker approach for the detection of the stress status induced by pollution exposure in Mytilus galloprovincialis: A field case study. Sci. Total Environ. 2019, 690, 140–150. [Google Scholar] [CrossRef]
- Caricato, R.; Giordano, M.E.; Schettino, T.; Lionetto, M.G. Functional involvement of carbonic anhydrase in the lysosomal response to cadmium exposure in Mytilus galloprovincialis digestive gland. Front. Physiol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Zebral, Y.D.; da Silva Fonseca, J.; Marques, J.A.; Bianchini, A. Carbonic anhydrase as a biomarker of global and local impacts: Insights from calcifying animals. Int. J. Mol. Sci. 2019, 20, 3092. [Google Scholar] [CrossRef]
- Güleç, Ö.; Türkeş, C.; Arslan, M.; Demir, Y.; Yeni, Y.; Hacımüftüoğlu, A.; Ereminsoy, E.; Küfrevioğlu, Ö.İ.; Beydemir, Ş. Cytotoxic effect, enzyme inhibition, and in silico studies of some novel N-substituted sulfonyl amides incorporating 1, 3, 4-oxadiazol structural motif. Mol. Divers. 2022, 26, 2825–2845. [Google Scholar] [CrossRef]
- Perussolo, M.C.; Guiloski, I.C.; Lirola, J.R.; Fockink, D.H.; Corso, C.R.; Bozza, D.C.; Prodocimo, V.; Mela, M.; Ramos, L.P.; Cestari, M.M. Integrated biomarker response index to assess toxic effects of environmentally relevant concentrations of paracetamol in a neotropical catfish (Rhamdia quelen). Ecotoxicol. Environ. Saf. 2019, 182, 109438. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Lundqvist, M. The effect of nanoparticles on the structure and enzymatic activity of human carbonic anhydrase I and II. Molecules 2020, 25, 4405. [Google Scholar] [CrossRef]
- Anna, A.; Isabel, P.-S.; Celia, C.-L. Inactivation and Adsorption of Human Carbonic Anhydrase II by Nanoparticles. Langmuir 2014, 30, 9448–9456. [Google Scholar]
- Assarsson, A.; Nasir, I.; Lundqvist, M.; Cabaleiro-Lago, C. Kinetic and thermodynamic study of the interactions between human carbonic anhydrase variants and polystyrene nanoparticles of different size. Rsc Adv. 2016, 6, 35868–35874. [Google Scholar] [CrossRef]
- Nasir, I.; Lundqvist, M.; Cabaleiro-Lago, C. Size and surface chemistry of nanoparticles lead to a variant behavior in the unfolding dynamics of human carbonic anhydrase. Nanoscale 2015, 7, 17504–17515. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yu, R.; Fan, M.; Yang, Z.; Liao, Z.; Yang, Z.; Xie, C.; Xuan, Y.; Wang, J.; Zhang, X.; et al. Physiological and transcriptome analysis of Mytilus coruscus in response to Prorocentrum lima and microplastics. Front. Mar. Sci. 2022, 9, 1087667. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, D.; Han, Y.; Huang, L.; Tang, Y.; Zhang, W.; Zhou, W.; Shi, W.; Yu, Y.; Liu, G. Impacts of microplastics and carbamazepine on the shell formation of thick-shell mussels and the underlying mechanisms of action. Sci. Total Environ. 2022, 838, 156442. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, M.; Franzellitti, S.; Valbonesi, P.; Lanzas, C.S.; Fabbri, E. Uptake and transcriptional effects of polystyrene microplastics in larval stages of the Mediterranean mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 1038–1047. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Jiang, Q.; Ye, Y.; Zhao, Y. Effects of nanoplastic on cell apoptosis and ion regulation in the gills of Macrobrachium nipponense. Environ. Pollut. 2022, 300, 118989. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, X.; Zhang, B.; Li, L.; Zhao, Y.; Lv, M.; Li, J.; Kan, C.; Zhao, Y. The effects of two sized polystyrene nanoplastics on the growth, physiological functions, and toxin production of Alexandrium tamarense. Chemosphere 2022, 291, 132943. [Google Scholar] [CrossRef] [PubMed]
- Rondon, R.; Valdés, C.; Cosseau, C.; Bergami, E.; Cárdenas, C.A.; Balbi, T.; Pérez-Toledo, C.; Garrido, I.; Perrois, G.; Chaparro, C.; et al. Transcriptomic responses of Antarctic clam Laternula elliptica to nanoparticles, at single and combined exposures reveal ecologically relevant biomarkers. Ecotoxicol. Environ. Saf. 2024, 280, 116523. [Google Scholar] [CrossRef]
- Bojic, S.; Falco, M.M.; Stojkovic, P.; Ljujic, B.; Gazdic Jankovic, M.; Armstrong, L.; Markovic, N.; Dopazo, J.; Lako, M.; Bauer, R.; et al. Platform to study intracellular polystyrene nanoplastic pollution and clinical outcomes. Stem Cells 2020, 38, 1321–1325. [Google Scholar] [CrossRef]
- Balbi, T.; Camisassi, G.; Montagna, M.; Fabbri, R.; Franzellitti, S.; Carbone, C.; Dawson, K.; Canesi, L. Impact of cationic polystyrene nanoparticles (PS-NH2) on early embryo development of Mytilus galloprovincialis: Effects on shell formation. Chemosphere 2017, 186, 1–9. [Google Scholar] [CrossRef]
- Lopes, L.G.A.; Lopes, F.C.; Quintana, K.G.; Costa, P.G.; de Martinez Gaspar Martins, C.; Souza, M.M. Biomineralization biomarkers to assess microplastics toxic effects in the freshwater snail Pomacea canaliculata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 268, 109585. [Google Scholar] [CrossRef]
- Roda, J.F.B.; Lauer, M.M.; Risso, W.E.; Bueno dos Reis Martinez, C. Microplastics and copper effects on the neotropical teleost Prochilodus lineatus: Is there any interaction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110659. [Google Scholar] [CrossRef] [PubMed]
- de Campos, J.M.; Wintruff, L.T.T.; de Souza-Bastos, L.R.; Dal Pont, G.; Dolatto, R.G.; Westphal, G.G.C.; Grassi, M.T.; Ostrensky, A.; Sadauskas-Henrique, H. Osmoregulatory responses in the neotropical fish species Astyanax lacustris, exposed to single and combined microplastics, polycyclic aromatic hydrocarbons, and their mixture. Aquat. Toxicol. 2023, 263, 106693. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, L.; Salam, M.; Yang, B.; He, Q.; Yang, Y.; Li, H. Revealing the environmental hazard posed by biodegradable microplastics in aquatic ecosystems: An investigation of polylactic acid’s effects on Microcystis aeruginosa. Environ. Pollut. 2024, 344, 123347. [Google Scholar] [CrossRef]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Andersson, D.; Freskgård, P.-O.; Jonsson, B.-H.; Carlsson, U. Formation of Local Native-like Tertiary Structures in the Slow Refolding Reaction of Human Carbonic Anhydrase II as Monitored by Circular Dichroism on Tryptophan Mutants. Biochemistry 1997, 36, 4623–4630. [Google Scholar] [CrossRef]
- Maartensson, L.G.; Jonsson, B.H.; Freskgaard, P.O.; Kihlgren, A.; Svensson, M.; Carlsson, U. Characterization of folding intermediates of human carbonic anhydrase II: Probing substructure by chemical labeling of sulfhydryl groups introduced by site-directed mutagenesis. Biochemistry 1993, 32, 224–231. [Google Scholar] [CrossRef]
- Rosner, A.; Ballarin, L.; Barnay-Verdier, S.; Borisenko, I.; Drago, L.; Drobne, D.; Concetta Eliso, M.; Harbuzov, Z.; Grimaldi, A.; Guy-Haim, T.; et al. A broad-taxa approach as an important concept in ecotoxicological studies and pollution monitoring. Biol. Rev. 2024, 99, 131–176. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; De la Vieja, A.; de Alba Gonzalez, M.; Esteban Lopez, M.; Castaño Calvo, A.; Cañas Portilla, A.I. Toxicity of nanoplastics for zebrafish embryos, what we know and where to go next. Sci. Total Environ. 2021, 797, 149125. [Google Scholar] [CrossRef]
- Babaei, A.A.; Rafiee, M.; Khodagholi, F.; Ahmadpour, E.; Amereh, F. Nanoplastics-induced oxidative stress, antioxidant defense, and physiological response in exposed Wistar albino rats. Environ. Sci. Pollut. Res. 2022, 29, 11332–11344. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Pérez, E.; Jiang, Q.; Chen, Q.; Jiao, Y.; Huang, Y.; Yang, Y.; Zhao, Y. Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: Application of transcriptome profiling in risk assessment of nanoplastics. J. Hazard. Mater. 2021, 402, 123778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, G.; Jiang, H.; Pan, K.; Liu, W. Nanoplastics induces oxidative stress and triggers lysosome-associated immune-defensive cell death in the earthworm Eisenia fetida. Environ. Int. 2023, 174, 107899. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, A.; Donà, G.; Brunati, A.M.; Clari, G.; Armanini, D.; Ragazzi, E.; Ambrosini, G.; Bordin, L. Increased oxidation-related glutathionylation and carbonic anhydrase activity in endometriosis. Reprod. Biomed. Online 2014, 28, 773–779. [Google Scholar] [CrossRef][Green Version]
- Dal Pizzol, J.L.; Marques, J.A.; da Silva Fonseca, J.; Costa, P.G.; Bianchini, A. Metal accumulation induces oxidative stress and alters carbonic anhydrase activity in corals and symbionts from the largest reef complex in the South Atlantic ocean. Chemosphere 2022, 290, 133216. [Google Scholar] [CrossRef] [PubMed]
- Senturk, A.; Alver, A.; Karkucak, M.; Küçük, M.; Ahmadi Rendi, T. Oxidative modification of carbonic anhydrase by peroxynitrite trigger immune response in mice and rheumatic disease patients. Am. J. Med. Sci. 2023, 366, 438–448. [Google Scholar] [CrossRef]
- Winum, J.-Y.; Colinas, P. Chapter 21—Carbonic Anhydrases as Esterases and Their Biotechnological Applications. In Carbonic Anhydrases as Biocatalysts; Supuran, C.T., De Simone, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 361–371. ISBN 978-0-444-63258-6. [Google Scholar]
- Angeli, A.; Carta, F.; Supuran, C.T. Carbonic Anhydrases: Versatile and Useful Biocatalysts in Chemistry and Biochemistry. Catalysts 2020, 10, 1008. [Google Scholar] [CrossRef]
- Tashian, R.E.; Douglas, D.P.; Ya-Shiou, L.Y. Esterase and hydrase activity of carbonic anhydrase-I from primate erythrocytes. Biochem. Biophys. Res. Commun. 1964, 14, 256–261. [Google Scholar] [CrossRef]
- Gould, S.M.; Tawfik, D.S. Directed evolution of the promiscuous esterase activity of carbonic anhydrase II. Biochemistry 2005, 44, 5444–5452. [Google Scholar] [CrossRef]
- Lopez, M.; Vu, H.; Wang, C.K.; Wolf, M.G.; Groenhof, G.; Innocenti, A.; Supuran, C.T.; Poulsen, S.-A. Promiscuity of carbonic anhydrase II. Unexpected ester hydrolysis of carbohydrate-based sulfamate inhibitors. J. Am. Chem. Soc. 2011, 133, 18452–18462. [Google Scholar] [CrossRef]
- González-Márquez, A.; Loera-Corral, O.; Santacruz-Juárez, E.; Tlécuitl-Beristain, S.; García-Dávila, J.; Viniegra-González, G.; Sánchez, C. Biodegradation patterns of the endocrine disrupting pollutant di(2-ethyl hexyl) phthalate by Fusarium culmorum. Ecotoxicol. Environ. Saf. 2019, 170, 293–299. [Google Scholar] [CrossRef]
- Ahmad, A.; Tsutsui, A.; Iijima, S.; Suzuki, T.; Shah, A.A.; Nakajima-Kambe, T. Gene structure and comparative study of two different plastic-degrading esterases from Roseateles depolymerans strain TB-87. Polym. Degrad. Stab. 2019, 164, 109–117. [Google Scholar] [CrossRef]
- Leitão, A.L.; Enguita, F.J. Structural Insights into Carboxylic Polyester-Degrading Enzymes and Their Functional Depolymerizing Neighbors. Int. J. Mol. Sci. 2021, 22, 2332. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.C.; Figueiredo, P.; Carvalho, A.T.P. Polycaprolactone Enzymatic Hydrolysis: A Mechanistic Study. ACS Omega 2019, 4, 6769–6774. [Google Scholar] [CrossRef]
- Hentschel, C.; Wagner, H.; Smiatek, J.; Heuer, A.; Fuchs, H.; Zhang, X.; Studer, A.; Chi, L. AFM-based Force Spectroscopy on Polystyrene Brushes: Effect of Brush Thickness on Protein Adsorption. Langmuir 2013, 29, 1850–1856. [Google Scholar] [CrossRef]
- Choong, C.; Foord, J.S.; Griffiths, J.-P.; Parker, E.M.; Baiwen, L.; Bora, M.; Moloney, M.G. Post-polymerisation modification of surface chemical functionality and its effect on protein binding. New J. Chem. 2012, 36, 1187–1200. [Google Scholar] [CrossRef]
- Vismara, A.; Gautieri, A. Molecular insights into nanoplastics-peptides binding and their interactions with the lipid membrane. Biophys. Chem. 2024, 308, 107213. [Google Scholar] [CrossRef]
- Cox, J.D.; Hunt, J.A.; Compher, K.M.; Fierke, C.A.; Christianson, D.W. Structural Influence of Hydrophobic Core Residues on Metal Binding and Specificity in Carbonic Anhydrase II. Biochemistry 2000, 39, 13687–13694. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhang, W.; Zhu, X.; Zhang, S. An updated overview of some factors that influence the biological effects of nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1254861. [Google Scholar] [CrossRef]
- Abarghouei, S.; Hedayati, A.; Raeisi, M.; Hadavand, B.S.; Rezaei, H.; Abed-Elmdoust, A. Size-dependent effects of microplastic on uptake, immune system, related gene expression and histopathology of goldfish (Carassius auratus). Chemosphere 2021, 276, 129977. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, P.; Kazemi, S.; Zivary, S.; Karbalaei, S.; Abolghasem Ghadami, S. The effect of polyethylene terephthalate and abamectin on oxidative damages and expression of vtg and cyp1a genes in juvenile zebrafish. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100565. [Google Scholar] [CrossRef]
- Martin-Folgar, R.; González-Caballero, M.C.; Torres-Ruiz, M.; Cañas-Portilla, A.I.; de Alba González, M.; Liste, I.; Morales, M. Molecular effects of polystyrene nanoplastics on human neural stem cells. PLoS ONE 2024, 19, e0295816. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Di Paolo, C.; Ottermanns, R.; Keiter, S.; Ait-Aissa, S.; Bluhm, K.; Brack, W.; Breitholtz, M.; Buchinger, S.; Carere, M.; Chalon, C.; et al. Bioassay battery interlaboratory investigation of emerging contaminants in spiked water extracts—Towards the implementation of bioanalytical monitoring tools in water quality assessment and monitoring. Water Res. 2016, 104, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Aissa, S.A.; Backhaus, T.; Dulio, V.; Escher, B.I.; Faust, M.; Hilscherova, K.; Hollender, J.; Hollert, H.; Müller, C.; et al. Effect-based methods are key. The European Collaborative Project SOLUTIONS recommends integrating effect-based methods for diagnosis and monitoring of water quality. Environ. Sci. Eur. 2019, 31, 10. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in the Framework of Marine Biodiversity Conservation: State of Art and Perspectives. Water 2021, 13, 1847. [Google Scholar] [CrossRef]
| CA Isoforms | MP or NP Type | IC50 | MP or NP Size | In Vitro/In Silico | Toxicological Effects on CA | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| HCAII | PS NPs: (PSCOOH, PSNH2 PS) | 0.3–0.6 nM | PSCOOH (43 nm) PS (46 nm) PSNH2 (59 nm) | in vitro | inhibition | adsorption | [82] |
| HCAI, HCAII, trHCAII | PSCOOH | - | 25, 41, 92, 114 nm | in vitro | inhibition | adsorption | [83] |
| HCAI, trCAII | PSCOOH | - | 52 nm 52 nm | in vitro | - | adsorption | [84] |
| CA estrinsic protein in Chaetoceros neogracile | PS MPs | - | 0.1 μm | in silico | - | potential interaction between the aromatic ring of polystyrene and some CA amino acids | [1] |
| Species | MP or NP Type | MP/NP Concentrations | MP or NP Size | Other Contaminant Present | Exposure Time | Tissues | Effect on CA | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mytilus coruscus (bivalve mollusc) | PS MPs | 2, 200 μg/L | 90 μm | - | - | whole organism | CA gene expression upregulation (transcriptomic analysis) | [85] |
| Mytilus coruscus (bivalve mollusc) | PS MPs | 0.26 mg/L | 5 μm | carbamazepine (CBZ) 10 μg/L | 4 weeks | mantle | CA content reduction (ELISA) | [86] |
| Mytilus galloprovincialis (bivalve mollusc) | PS MPs | 50–500 particles mL−1 | 3 μm | - | 48 hpf | whole embrio | significantly up-regulation of CA transcripts | [87] |
| Macrobrachium nipponense (river prawn) | PS NPs | 0, 5, 10, 20, 40 mg/L | 75 nm | - | 7, 14, 21, 28 days | gills | CA gene upregulation at 5, 10 and 20 mg/L; no effects at 40 mg/L | [88] |
| Alexandrium tamarense (dinoflagellate) | PS MPs/NPs | 5, 50 mg/L | Two sized: 0.1, 1 μm | - | 0, 6, 24, 48, 72, 96 h | microalgal suspension | CAext activity inhibition | [89] |
| Laternula elliptica (bivalve mollusc) | PSCOOH NPs | 5 µg/L | 62 nm | TiO2 (25 nm) 5 µg/L | 96 h | gills | CA9 gene expression upregulation when exposed to both PSCOOH NPs and TiO2 | [90] |
| Homo sapiens | PSCOOH NPs | 109 particles mL−1 | 40, 200 nm | - | 24 h | embryo Induced pluripotent stem cells (in vitro) | CA4 gene expression upregulation | [91] |
| Mytilus galloprovincialis (bivalve mollusc) | PS-NH2 | 0.150 mg/L | 50 nm | - | 24, 48 | embryos | CA gene expression downregulation after 48 h | [92] |
| Pomacea canaliculate (gastropod mollusc) | PE MPs | 20 μg/L | 10–90 μm | - | 24, 72, 120 h | digestive gland, mantle | CA activity inhibition in digestive gland after 24 h; CA activity stimulation in mantle after 24 and 72 h | [93] |
| Prochilodus lineatus (fish) | PE MPs | 20 μg/L | 10–90 μm | Copper (Cu) 10 μg/L | 24 and 96 h | gills | no significative effect on CA activity | [94] |
| Astyanax lacustris (fish) | LDPE MPs | 10 mg/L | 100, 200 µm | PAH 2.28 μg/L | 96 h | gills | no significative effect on CA activity | [95] |
| Microcystis aeruginosa (cyanobacteria) | PLA MPs | 10, 50, 200 mg/L | 2.564 μm | - | day 27, 39, 51, 63 | cells in suspension | CA content reduction (ELISA) | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo, G.; Lionetto, F.; Giordano, M.E.; Lionetto, M.G. Interaction of Micro- and Nanoplastics with Enzymes: The Case of Carbonic Anhydrase. Int. J. Mol. Sci. 2024, 25, 9716. https://doi.org/10.3390/ijms25179716
Polo G, Lionetto F, Giordano ME, Lionetto MG. Interaction of Micro- and Nanoplastics with Enzymes: The Case of Carbonic Anhydrase. International Journal of Molecular Sciences. 2024; 25(17):9716. https://doi.org/10.3390/ijms25179716
Chicago/Turabian StylePolo, Gregorio, Francesca Lionetto, Maria Elena Giordano, and Maria Giulia Lionetto. 2024. "Interaction of Micro- and Nanoplastics with Enzymes: The Case of Carbonic Anhydrase" International Journal of Molecular Sciences 25, no. 17: 9716. https://doi.org/10.3390/ijms25179716
APA StylePolo, G., Lionetto, F., Giordano, M. E., & Lionetto, M. G. (2024). Interaction of Micro- and Nanoplastics with Enzymes: The Case of Carbonic Anhydrase. International Journal of Molecular Sciences, 25(17), 9716. https://doi.org/10.3390/ijms25179716








