Distinct Variations in Gene Expression and Cell Composition across Lichen Planus Subtypes
Abstract
:1. Introduction
2. Results
2.1. Clinically Known LP Subtypes Show Distinct Gene Expression Patterns
2.2. LP Subtypes Exhibit Pathway-Specific Differences in Gene Expression
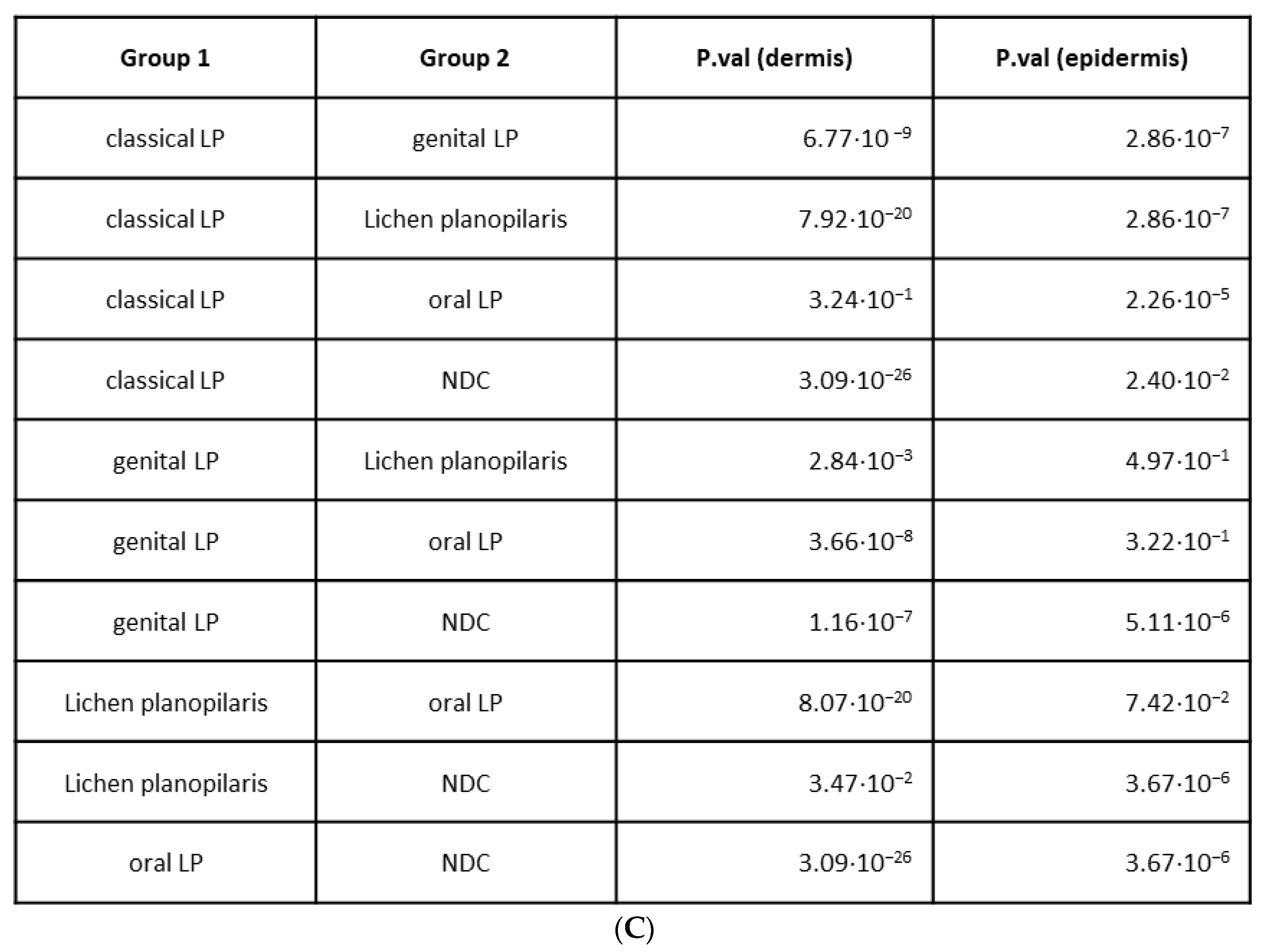
2.3. Variations in Cellular Compositions Distinguish Clinical LP Subtypes
3. Discussion
4. Materials and Methods
4.1. Human Skin Samples
4.2. NanoString Analyses
4.3. Multiplex Immunohistochemistry
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solimani, F.; Forchhammer, S.; Schloegl, A.; Ghoreschi, K.; Meier, K. Lichen planus—A clinical guide. J. Dtsch. Dermatol. Ges. 2021, 19, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Rose, C.; Sachse, M.M. Clinical variants of lichen planus. J. Dtsch. Dermatol. Ges. 2013, 11, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Le Cleach, L.; Chosidow, O. Clinical practice. Lichen planus. N. Engl. J. Med. 2012, 366, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, D.; Vakirlis, E.; Kemeny, L.; Marinovic, B.; Massone, C.; Murphy, R.; Nast, A.; Ronnevig, J.; Ruzicka, T.; Cooper, S.; et al. European S1 guidelines on the management of lichen planus: A cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1403–1414. [Google Scholar] [CrossRef]
- Zhou, X.J.; Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Seymour, G.J. Intra-epithelial CD8+ T cells and basement membrane disruption in oral lichen planus. J. Oral. Pathol. Med. 2002, 31, 23–27. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Han, Q.; Zhao, X.; Zeng, X.; Xu, Y.; Sun, Z.; Chen, Q. Role of distinct CD4+ T helper subset in pathogenesis of oral lichen planus. J. Oral. Pathol. Med. 2016, 45, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Tsoi, L.C.; Sarkar, M.K.; Xing, X.; Xue, K.; Uppala, R.; Berthier, C.C.; Zeng, C.; Patrick, M.; Billi, A.C.; et al. IFN-gamma enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci. Transl. Med. 2019, 11, eaav7561. [Google Scholar] [CrossRef] [PubMed]
- Balestri, R.; Bortolotti, R.; Rech, G.; Girardelli, C.R.; Zorzi, M.G.; Magnano, M. Treatment of Oral Erosive Lichen Planus with Upadacitinib. JAMA Dermatol. 2022, 158, 457–458. [Google Scholar] [CrossRef]
- Kooybaran, N.R.; Petzold, G.; Strobel, P.; Schon, M.P.; Mossner, R. Alleviation of erosive oral and esophageal lichen planus by the JAK1 inhibitor upadacitinib. J. Dtsch. Dermatol. Ges. 2021, 19, 1778–1780. [Google Scholar] [CrossRef]
- Moussa, A.; Colla, T.; Morrison, B.; Sinclair, R. Effective treatment of oral lichen planus with the JAK inhibitor baricitinib. Australas. J. Dermatol. 2022, 63, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Afzali, S.; Mohammadisoleimani, E.; Mansoori, Y.; Mohaghegh, P.; Bahmanyar, M.; Mansoori, B.; Pezeshki, B.; Nikfar, G.; Tavassoli, A.; Shahi, A.; et al. The potential roles of Th17 cells in the pathogenesis of oral lichen planus. Inflamm. Res. 2023, 72, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Pollmann, R.; Schmidt, T.; Schmidt, A.; Zheng, X.; Savai, R.; Mühlenbein, S.; Pickert, J.; Eubel, V.; Möbs, C.; et al. Therapeutic Targeting of Th17/Tc17 Cells Leads to Clinical Improvement of Lichen Planus. Front. Immunol. 2019, 10, 1808. [Google Scholar] [CrossRef]
- Braga, T.T.; Agudelo, J.S.; Camara, N.O. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita-Ise, M. Lichen planopilaris and frontal fibrosing alopecia: Branches from the same tree bear different fruits. Br. J. Dermatol. 2020, 183, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Pietschke, K.; Holstein, J.; Meier, K.; Schafer, I.; Muller-Hermelink, E.; Gonzalez-Menendez, I.; Quintanilla-Martinez, L.; Ghoreschi, F.C.; Solimani, F.; Ghoreschi, K. The inflammation in cutaneous lichen planus is dominated by IFN-Upsilon and IL-21-A basis for therapeutic JAK1 inhibition. Exp. Dermatol. 2021, 30, 262–270. [Google Scholar] [CrossRef]
- Wenzel, J.; Peters, B.; Zahn, S.; Birth, M.; Hofmann, K.; Küsters, D.; Tomiuk, S.; Baron, J.M.; Merk, H.F.; Mauch, C.; et al. Gene expression profiling of lichen planus reflects CXCL9+-mediated inflammation and distinguishes this disease from atopic dermatitis and psoriasis. J. Invest. Dermatol. 2008, 128, 67–78. [Google Scholar] [CrossRef]
- Kassels, A.; Edwards, L.; Kraus, C.N. Treatment of erosive vulvovaginal lichen planus with tofacitinib: A case series. JAAD Case Rep. 2023, 40, 14–18. [Google Scholar] [CrossRef]
- Miyahara, Y.; Chen, H.; Moriyama, M.; Mochizuki, K.; Kaneko, N.; Haque, A.S.M.R.; Chinju, A.; Kai, K.; Sakamoto, M.; Kakizoe-Ishiguro, N.; et al. Toll-like receptor 9-positive plasmacytoid dendritic cells promote Th17 immune responses in oral lichen planus stimulated by epithelium-derived cathepsin K. Sci. Rep. 2023, 13, 19320. [Google Scholar] [CrossRef]
- Ge, X.; Xie, H.; Nguyen, T.; Zhao, B.; Xu, J.; Du, J. Renin Promotes STAT4 Phosphorylation to Induce IL-17 Production in Keratinocytes of Oral Lichen Planus. iScience 2020, 23, 100983. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Bao, Z.; Shen, X. A potential reciprocal emergence of regulatory T cells in oral lichen planus and pemphigus vulgaris: A meta-analysis. J. Dent. Sci. 2023, 18, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Gao, X.; Ma, L.; Zhou, Z.; Shen, X.; Liu, W. Expression of Foxp3 and interleukin-17 in lichen planus lesions with emphasis on difference in oral and cutaneous variants. Arch. Dermatol. Res. 2014, 306, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.A.; Xia, J.; Chen, X.B.; Wang, H.; Dai, Y.H.; Rhodus, N.L.; Cheng, B. FOXP3 T regulatory cells in lesions of oral lichen planus correlated with disease activity. Oral Dis. 2010, 16, 76–82. [Google Scholar] [CrossRef]
- Pereira, J.S.; Monteiro, B.V.; Nonaka, C.F.; Silveira, E.J.; Miguel, M.C. FoxP3+ T regulatory cells in oral lichen planus and its correlation with the distinct clinical appearance of the lesions. Int. J. Exp. Pathol. 2012, 93, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cao, T.; Wang, Y.; Yao, H.; Du, G.; Chen, G.; Niu, X.; Tang, G. Frequently Increased but Functionally Impaired CD4+CD25+ Regulatory T Cells in Patients with Oral Lichen Planus. Inflammation 2016, 39, 1205–1215. [Google Scholar] [CrossRef]
- Domingues, R.; de Carvalho, G.C.; da Silva Oliveira, L.M.; Futata Taniguchi, E.; Zimbres, J.M.; Aoki, V.; da Silva Duarte, A.J.; Sato, M.N. The dysfunctional innate immune response triggered by Toll-like receptor activation is restored by TLR7/TLR8 and TLR9 ligands in cutaneous lichen planus. Br. J. Dermatol. 2015, 172, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell. 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Laeijendecker, R.; Van Joost, T.H.; Tank, B.; Neumann, H.A. Oral lichen planus and hepatitis C virus infection. Arch. Dermatol. 2005, 141, 906–907. [Google Scholar] [CrossRef]
- Wang, J.H.; Hung, S.J. Lichen planus associated with hepatitis B, hepatitis C, and liver cirrhosis in a nationwide cohort study. J. Am. Acad. Dermatol. 2021, 84, 1085–1086. [Google Scholar] [CrossRef]
- Lucchese, A.; Di Stasio, D.; Romano, A.; Fiori, F.; De Felice, G.P.; Lajolo, C.; Serpico, R.; Cecchetti, F.; Petruzzi, M. Correlation between Oral Lichen Planus and Viral Infections Other Than HCV: A Systematic Review. J. Clin. Med. 2022, 11, 5487. [Google Scholar] [CrossRef]
- Meier-Schiesser, B.; Zeccha, C.; Zierold, S.; Kolm, I.; Röckel, M.; Fröhlich, W.; Mittag, N.; Schmitt, C.; Kumbrink, J.; Hassel, J.C.; et al. Checkpoint inhibitore induced lichen planus differs from spontaneous lichen planus on the clinical, histological, and gene expression level. JAAD Int. 2024, 15, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. mixR: An R package for Finite Mixture Modeling for Both Raw and Binned Data. J. Open Source Softw. 2022, 7, 4031, R Package Version 0.2.0. Available online: https://CRAN.R-project.org/package=mixR (accessed on 3 September 2024). [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.B.; Simpson, G.L. vegan: Community Ecology Package. R Package Version 2.6-4. 2012. Available online: https://CRAN.R-project.org/package=vegan (accessed on 3 September 2024).
- van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. R Package Version 2.0-8. 2024. Available online: https://CRAN.R-project.org/package=compositions (accessed on 3 September 2024).



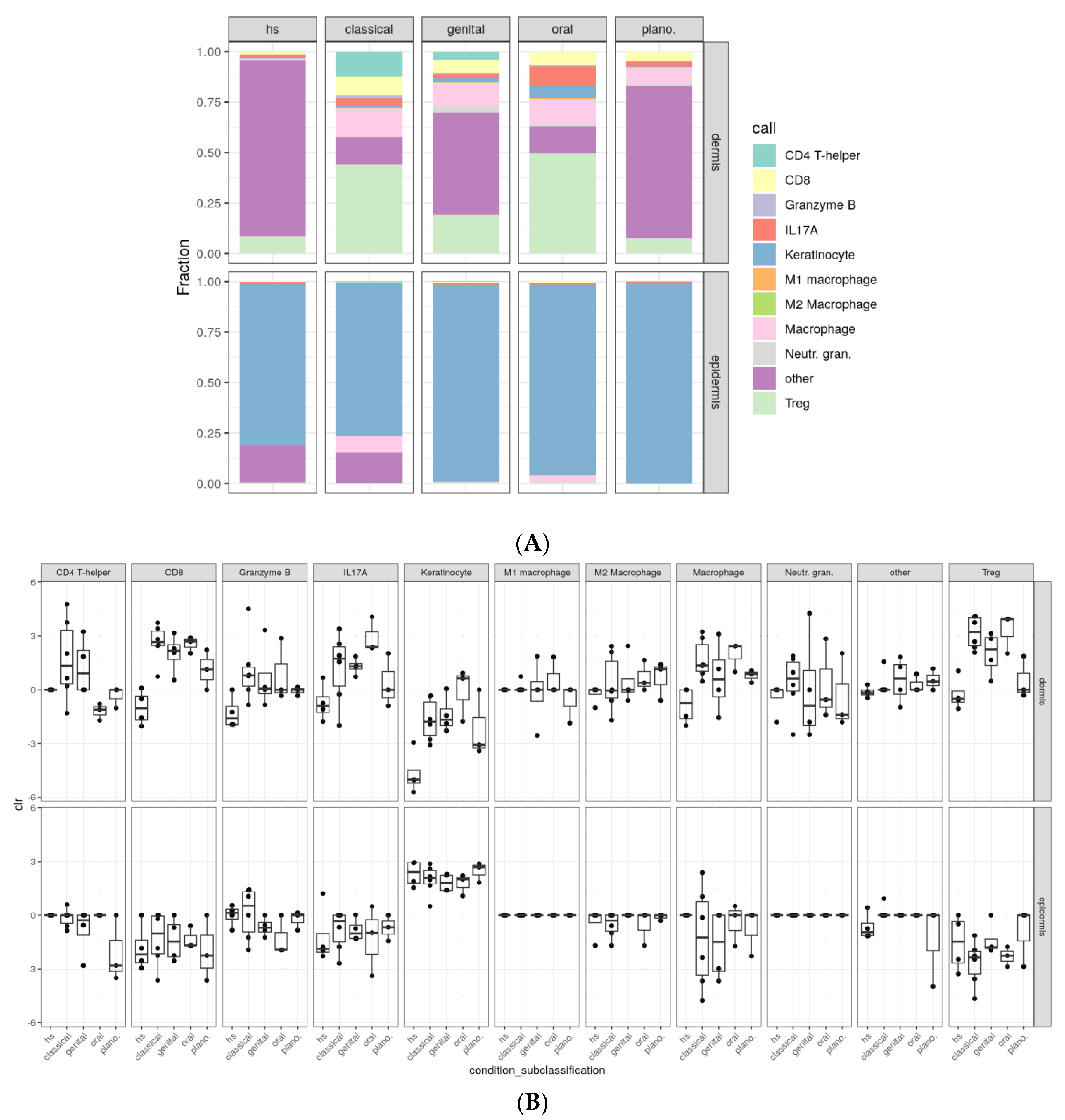
| Cell Types | Classical LP | Genital LP | Oral LP | Lichen Planopilaris | NDC |
|---|---|---|---|---|---|
| Total T cells | 48.8% | 24.7% | 53.0% | 7.04% | 3.61% |
| CD4 + T cells | 8.5% | 3.14% | 0.108% | 0.069% | 0% |
| CD8 + T cells | 6.44% | 4.87% | 5.27% | 2.17% | 0.373% |
| Tregs | 30.3% | 14.6% | 39.2% | 3.51% | 2.29% |
| Granzyme B+ cells | 1.15% | 0.619% | 0.434% | 0.110% | 0.117% |
| IL17A+ cells | 2.39% | 1.54% | 8.02% | 1.18% | 0.831% |
| Keratinocytes | 24.9% | 25.4% | 24.6% | 54.0% | 62.1% |
| Total macrophages | 12.3% | 9.26% | 11.2% | 3.96% | 0.193% |
| M1 macrophages | 0.0397% | 0.243% | 0.266% | 0.0092% | 0% |
| M2 macrophages | 0.176% | 0.191% | 0.124% | 0.212% | 0.0126% |
| Neutrophilic granulocytes | 0.301% | 2.51% | 0.730% | 0.446% | 0.0084% |
| Other | 13.8% | 38.1% | 10.5% | 34.6% | 34.1% |
| Characteristics of Patients | Number of Patients, No. | Age y, Mean (SD) | Female/Male Sex, Ratio | Other Autoimmune Disease, No. | HBV or HCV Infection, No. | Topical Therapy at Time of Biopsy *, No. | Disease Onset Until Biopsy Months, Mean (SD) ** | |
|---|---|---|---|---|---|---|---|---|
| Classical LP | NanoString | 5 | 64.0 (13.2) | 2/3 | 0 | 0 | 0 | 4 (1.8) |
| IHC | 6 | 65.0 (15.3) | 3/3 | 0 | 0 | 0 | 10 (14.8) | |
| Oral LP | NanoString | 3 | 49.0 (8.9) | 1/2 | 0 | 0 | 0 | 2 (0.6) |
| IHC | 3 | 52.0 (6.6) | 1/2 | 0 | 0 | 0 | 11 (5.7) | |
| Genital LP | NanoString | 4 | 59.8 (17.4) | 0/4 | 0 | 1 | 1 | 14 (14.1) |
| IHC | 4 | 56.5 (19.6) | 3/1 | 0 | 0 | 0 | 11 (2.7) | |
| Lichen planopilaris | NanoString | 5 | 56.2 (17.6) | 5/0 | 2 | 0 | 2 | 24 (14.4) |
| IHC | 3 | 45.7 (17.5) | 2/1 | 1 | 0 | 0 | 24 (20.2) | |
| Healthy skin (NDC) | NanoString | 3 | 81.0 (7.9) | 1/2 | 0 | 0 | 0 | 0 |
| IHC | 4 | 57.0 (20.4) | 0/4 | 0 | 0 | 0 | 0 | |
| Opal | T-Cell Panel | Macrophage Panel | ||
|---|---|---|---|---|
| Antibody | Dilution | Antibody | Dilution | |
| 480 | CD8 | 1:200 | pSTAT1 | 1:50 |
| 520 | FoxP3 | 1:100 | ||
| 570 | IL17A | 1:200 | MPO | 1:150 |
| 620 | GranzymeB | 1:100 | c-Maf | 1:50 |
| 690 | PanCK | 1:100 | PanCK | 1:100 |
| 780 | CD4 | 1:100 | CD68 | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoch, C.; Baghin, V.; Turko, P.; Winkelbeiner, N.; Staeger, R.; Wei, K.; Banzola, I.; Mellett, M.; Levesque, M.P.; Kuendig, T.; et al. Distinct Variations in Gene Expression and Cell Composition across Lichen Planus Subtypes. Int. J. Mol. Sci. 2024, 25, 9720. https://doi.org/10.3390/ijms25179720
Knoch C, Baghin V, Turko P, Winkelbeiner N, Staeger R, Wei K, Banzola I, Mellett M, Levesque MP, Kuendig T, et al. Distinct Variations in Gene Expression and Cell Composition across Lichen Planus Subtypes. International Journal of Molecular Sciences. 2024; 25(17):9720. https://doi.org/10.3390/ijms25179720
Chicago/Turabian StyleKnoch, Cadri, Veronika Baghin, Patrick Turko, Nicola Winkelbeiner, Ramon Staeger, Kongchang Wei, Irina Banzola, Mark Mellett, Mitchell P. Levesque, Thomas Kuendig, and et al. 2024. "Distinct Variations in Gene Expression and Cell Composition across Lichen Planus Subtypes" International Journal of Molecular Sciences 25, no. 17: 9720. https://doi.org/10.3390/ijms25179720







