Pyrroloquinoline Quinone Alleviates Intestinal Inflammation and Cell Apoptosis via the MKK3/6-P38 Pathway in a Piglet Model
Abstract
:1. Introduction
2. Results
2.1. The Growth Performance
2.2. The Serum and Jejunal Mucosal Factors Contents
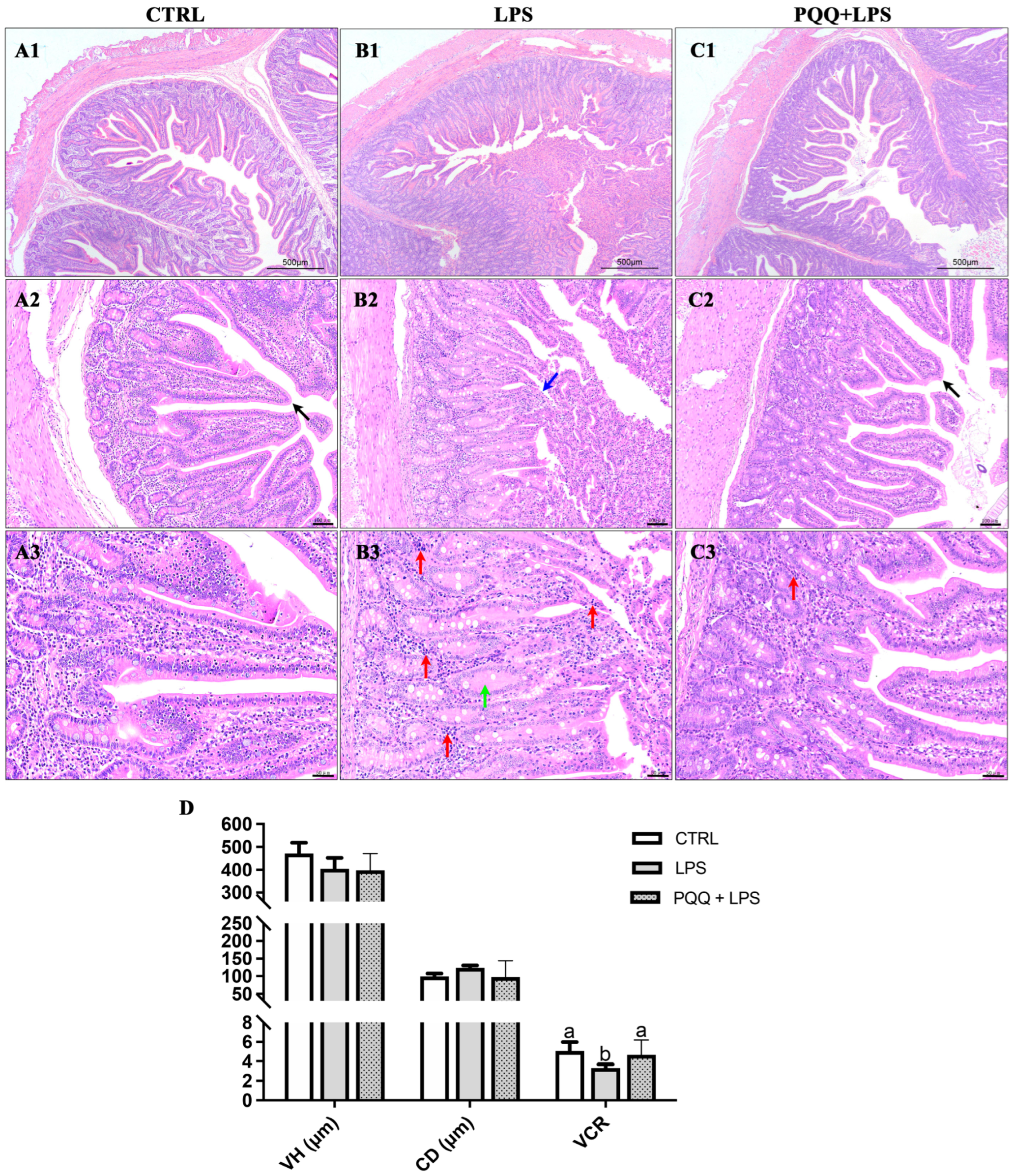
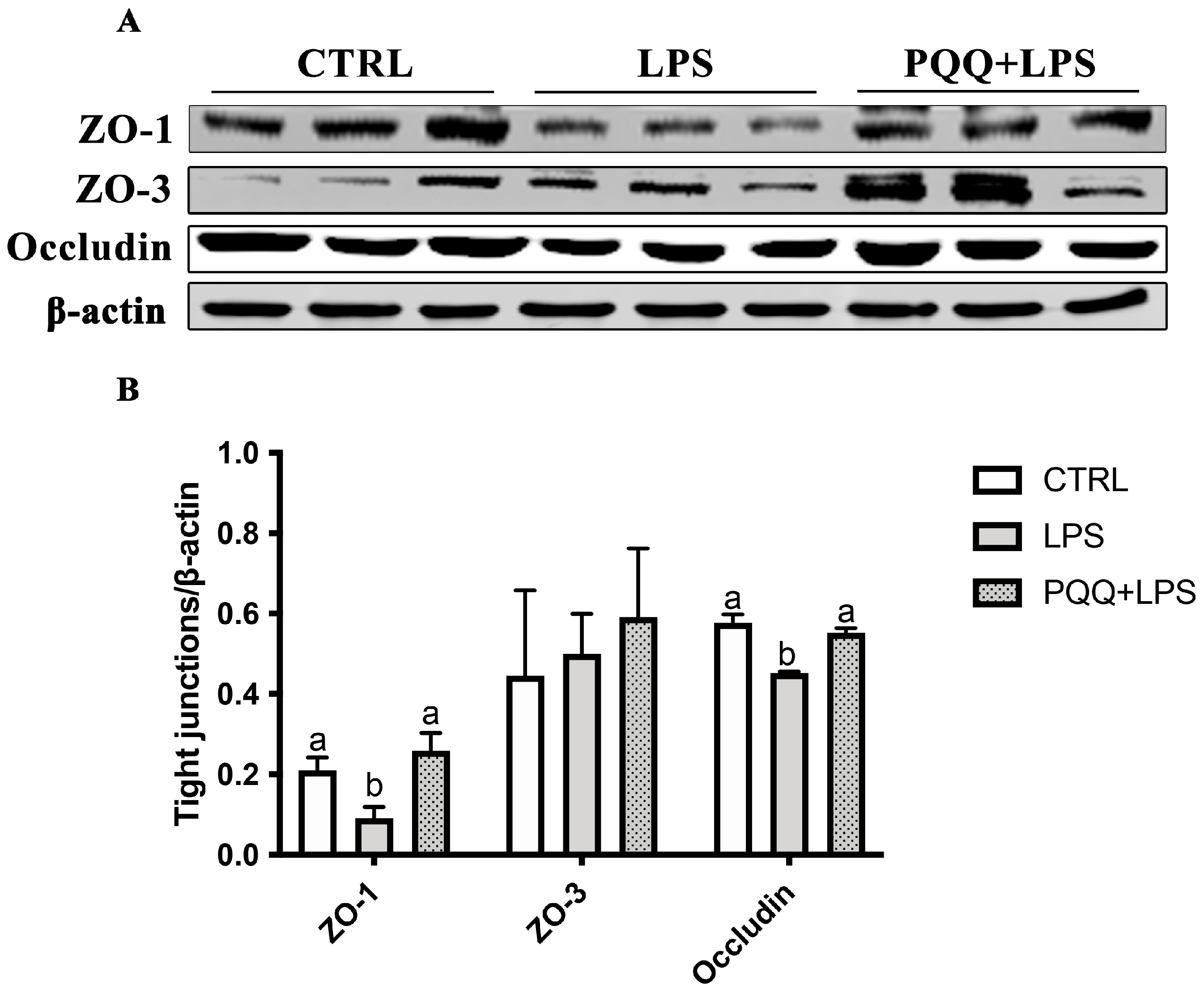
2.3. The Morphology and Tight Junction Proteins Expression in Jejunum
2.4. Analysis of Proteomic Profile in the Jejunal Mucosa
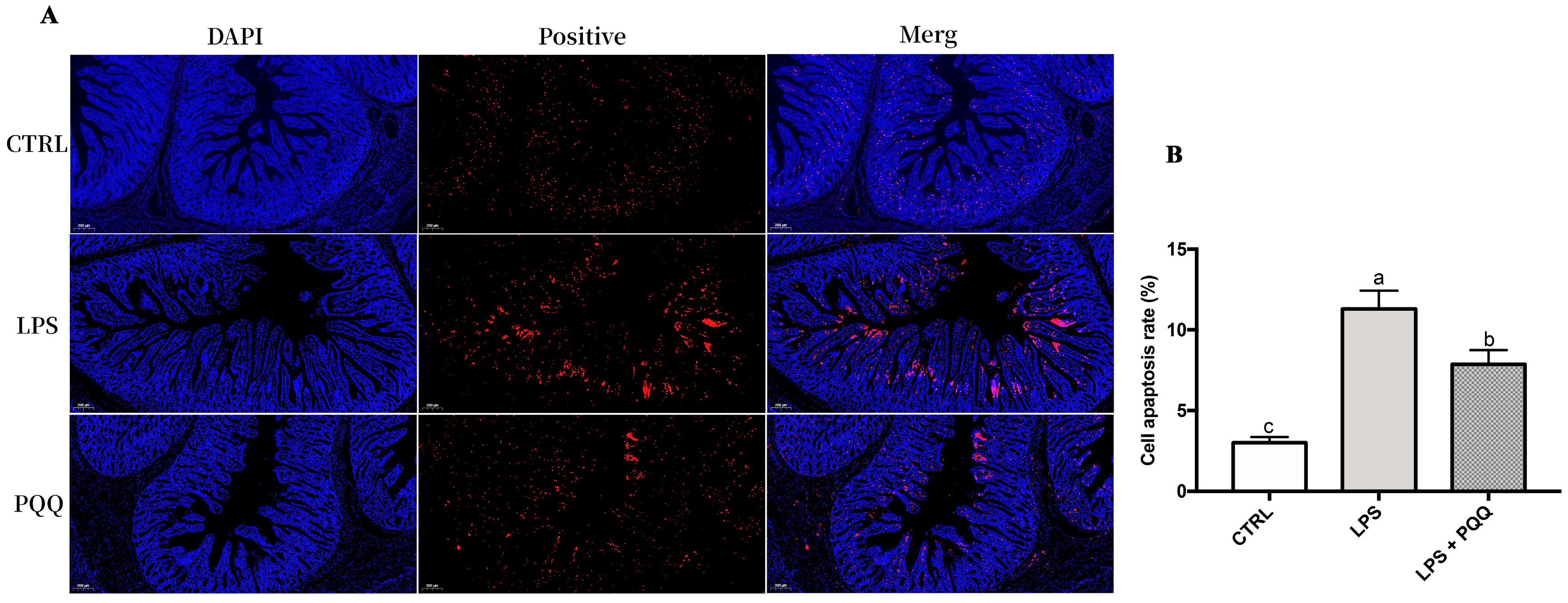
2.5. MKK3/6-p38 Signaling Pathway and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Animals and Experimental Design
4.3. Growth Performance and Sample Collection
(total number of piglets × number of days in the experiment)] × 100%.
4.4. Intestinal Morphology Analysis and TUNEL Staining
4.5. Serum and Mucosal Cytokines
4.6. Western Blot Analysis
4.7. LC-MS/MS Experiments
4.8. DDA Data Search and DIA Data Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Wu, J.M.; Wang, J.P.; Lin, Z.S.; Liu, C.C.; Zhang, Y.C.; Zhang, S.M.; Zhou, M.; Zhao, J.B.; Liu, H.; Ma, X. Clostridium butyricum alleviates weaned stress of piglets by improving intestinal immune function and gut microbiota. Food Chem. 2023, 405, 135014. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Raingeaud, J.; Whitmarsh, A.J.; Barrett, T.; Dérijard, B.; Davis, R.J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 1996, 16, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Killgore, J.; Smidt, C.; Duich, L.; Romero-Chapman, N.; Tinker, D.; Reiser, K.; Melko, M.; Hyde, D.; Rucker, R.B. Nutritional importance of pyrroloquinoline quinone. Science 1989, 245, 850–852. [Google Scholar] [CrossRef]
- Matsushita, K.; Shinagawa, E.; Adachi, O.; Ameyama, M. Quinoprotein D-glucose dehydrogenase of the Acinetobacter calcoaceticus respiratory chain: Membrane-bound and soluble forms are different molecular species. Biochemistry 1989, 28, 6276–6280. [Google Scholar] [CrossRef]
- Itoh, S. Biochemistry of Vitamin B6 and PQQ. In Model Studies Directed toward the Action of Quinoprotein Methanol Dehydrogenase; Marino, G., Sannia, G., Bossa, F., Eds.; Birkhäuser: Basel, Switzerland, 1994; pp. 297–301. [Google Scholar]
- Liu, G.Q.; Sun, G.M.; Liao, X.D.; Huang, J.Z.; Guo, M.J.; Zhang, L.Y.; Guo, Y.L.; Lu, L.; Luo, X.G. Effect of dietary supplementation of pyrroloquinoline quinone disodium on growth performance, meat quality and antioxidative ability of broilers. J. Integr. Agric. 2020, 19, 1850–1856. [Google Scholar] [CrossRef]
- Shi, C.Y.; Xu, S.; Huang, C.Y.; Wang, Z.J.; Wang, W.H.; Ming, D.X.; Yin, X.D.; Liu, H.; Wang, F.L. Pyrroloquinoline Quinone Regulates Enteric Neurochemical Plasticity of Weaned Rats Challenged With Lipopolysaccharide. Front. Neurosci. 2022, 16, 878541. [Google Scholar] [CrossRef]
- Shi, C.Y.; Yu, Z.R.; Wang, Z.J.; Ning, R.; Huang, C.Y.; Gao, Y.J.; Wang, F.L. Dietary supplementation with pyrroloquinoline quinone promotes growth, relieves weaning stress, and regulates metabolism of piglets compared with adding zinc oxide. Anim. Nutr. 2023, 15, 409–419. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhao, M.L.; Lin, Z.H. Pyrroloquinoline quinone (PQQ) alleviated sepsis-induced acute liver injury, inflammation, oxidative stress and cell apoptosis by downregulating CUL3 expression. Bioengineered 2021, 12, 2459–2468. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, H.; Shi, N.Q.; Gao, S.M.; Wang, X.Y.; Zhu, S.X.; Yan, M.J. Inhibit inflammation and apoptosis of pyrroloquinoline on spinal cord injury in rat. Ann. Transl. Med. 2021, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Li, J.Z.; Cao, C.Y.; Zhang, B.R.; Yang, W.; Shi, B.M.; Shan, A.S. Pyrroloquinoline quinone inhibits the production of inflammatory cytokines via the SIRT1/NF-κB signal pathway in weaned piglet jejunum. Food Funct. 2020, 11, 2137–2153. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Zhang, J.Y.; Zhou, H.B.; Guo, Y.P.; Ma, Q.G.; Ji, C.; Zhao, L.H. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poult. Sci. 2020, 99, 5389–5398. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liang, Y.; Ran, Q.; Wu, Y.; Zhang, H.; Pan, A.; Shen, J.; Huang, T.; Fu, M.; Sun, J. Analysis on the effects of improving step cage-rearing technology for laying ducks. Hubei Acad. Agric. Sci. 2022, 103, 103938. [Google Scholar]
- Huang, C.; Ming, D.; Wang, W.; Wang, Z.; Hu, Y.; Ma, X.; Wang, F. Pyrroloquinoline Quinone Alleviates Jejunal Mucosal Barrier Function Damage and Regulates Colonic Microbiota in Piglets Challenged With Enterotoxigenic Escherichia coli. Front. Microbiol. 2020, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fan, Z.; Han, D.; Johnston, L.J.; Ma, X.; Wang, F. Pyrroloquinoline quinone regulates the redox status in vitro and in vivo of weaned pigs via the Nrf2/HO-1 pathway. J. Anim. Sci. Biotechnol. 2021, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Zhang, W.; Yang, Z.; Ding, B.; Zhu, H.; Liu, Y.; Qiu, Y.; Yin, Y.; Wu, G. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar] [CrossRef]
- Kimura, K.; Takada, M.; Ishii, T.; Tsuji-Naito, K.; Akagawa, M. Pyrroloquinoline quinone stimulates epithelial cell proliferation by activating epidermal growth factor receptor through redox cycling. Free Radic. Biol. Med. 2012, 53, 1239–1251. [Google Scholar] [CrossRef]
- Shao, D.; Liu, L.; Tong, H.; Shi, S. Dietary pyrroloquinoline quinone improvement of the antioxidant capacity of laying hens and eggs are linked to the alteration of Nrf2/HO-1 pathway and gut microbiota. Food Chem. X 2023, 20, 101021. [Google Scholar] [CrossRef]
- Wang, Z.; Han, N.; Zhao, K.; Li, Y.; Chi, Y.; Wang, B. Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int. Immunopharmacol. 2019, 72, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Zhao, Q.; Wang, J.; Qi, G.H.; Wu, S.G.; Zhang, H.J. Effects of Pyrroloquinoline Quinone on Lipid Metabolism and Anti-Oxidative Capacity in a High-Fat-Diet Metabolic Dysfunction-Associated Fatty Liver Disease Chick Model. Int. J. Mol. Sci. 2021, 22, 1458. [Google Scholar] [CrossRef]
- Yokoyama, H.; Peralta, R.C.; Diaz, R.; Sendo, S.; Ikemori, Y.; Kodama, Y. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect. Immun. 1992, 60, 998–1007. [Google Scholar] [CrossRef]
- Martín-Venegas, R.; Roig-Pérez, S.; Ferrer, R.; Moreno, J.J. Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. J. Lipid Res. 2006, 47, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yu, X.; Shi, C.; Wang, M.; Li, A.; Wang, F. Pyrroloquinoline quinone supplementation attenuates inflammatory liver injury by STAT3/TGF-β1 pathway in weaned piglets challenged with lipopolysaccharide. Br. J. Nutr. 2024, 131, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Stähelin, B.J.; Marti, U.; Solioz, M.; Zimmermann, H.; Reichen, J. False positive staining in the TUNEL assay to detect apoptosis in liver and intestine is caused by endogenous nucleases and inhibited by diethyl pyrocarbonate. Mol. Pathol. 1998, 51, 204–208. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, L.; Jia, W.; Xiao, J.; Huang, G.; Tian, G.; Li, J.; Xiao, Y. Serum diamine oxidase as a hemorrhagic shock biomarker in a rabbit model. PLoS ONE 2014, 9, e102285. [Google Scholar] [CrossRef]
- Funaoka, H.; Kanda, T.; Fujii, H. Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases. Rinsho Byori. Jpn. J. Clin. Pathol. 2010, 58, 162–168. [Google Scholar]
- Wang, X.; Wang, W.; Wang, L.; Yu, C.; Zhang, G.; Zhu, H.; Wang, C.; Zhao, S.; Hu, C.A.; Liu, Y. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Xie, Y.; Chen, Y.; Lu, C.; Yang, Z.; Zhu, Y.; Ouyang, Y.; Zhang, Y.; Wang, X. Mo3Se4 nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice. Redox Biol. 2022, 56, 102441. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef]
- Pedersen, J.; LaCasse, E.C.; Seidelin, J.B.; Coskun, M.; Nielsen, O.H. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol. Med. 2014, 20, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A.; Ichijo, H. Molecular mechanisms of the decision between life and death: Regulation of apoptosis by apoptosis signal-regulating kinase 1. J. Biochem. 2001, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, L.; Tao, W.; Pei, X.; Wang, G.; Wang, M. Clostridium Tyrobutyricum Protect Intestinal Barrier Function from LPS-Induced Apoptosis via P38/JNK Signaling Pathway in IPEC-J2 Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1779–1792. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, Y.; Hou, X.Y. MLK3-MKK3/6-P38MAPK cascades following N-methyl-D-aspartate receptor activation contributes to amyloid-β peptide-induced apoptosis in SH-SY5Y cells. J. Neurosci. Res. 2014, 92, 808–817. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; He, X.; Jiao, N.; Zhang, X.; Qiu, K.; Piao, X.; Yin, J. The involvement of NF-κB/P38 pathways in Scutellaria baicalensis extracts attenuating of Escherichia coli K88-induced acute intestinal injury in weaned piglets. Br. J. Nutr. 2019, 122, 152–161. [Google Scholar] [CrossRef]
- Luise, D.; Lauridsen, C.; Bosi, P.; Trevisi, P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]



| Item | CTRL | LPS | PQQ + LPS | SEM | p Value |
|---|---|---|---|---|---|
| Initial BW (kg) | 7.16 | 7.16 | 7.16 | 0.85 | 0.96 |
| Final BW (kg) | 11.79 a | 10.05 b | 10.48 ab | 1.14 | 0.03 |
| ADG (g) | 330.00 a | 237.00 b | 206.00 b | 0.05 | <0.01 |
| ADFI (g) | 575.00 | 463.00 | 445.00 | 0.10 | 0.08 |
| FCR | 1.76 | 2.35 | 1.92 | 0.62 | 0.33 |
| Diarrhea index (%) | 1.98 b | 7.54 a | 4.37 ab | 0.33 | 0.02 |
| Items | CTRL | LPS | PQQ + LPS | SEM | p Value |
|---|---|---|---|---|---|
| sDAO (U/mL) | 4.95 c | 6.02 a | 5.73 b | 0.06 | <0.01 |
| sI-FABP (pg/mL) | 200.06 b | 234.82 a | 210.76 b | 5.58 | <0.01 |
| sD-LA (nmol/mL) | 13.27 b | 14.40 ab | 14.95 a | 0.37 | 0.02 |
| sALP (U/L) | 336.61 a | 224.97 b | 209.52 b | 18.06 | <0.01 |
| mI-FABP (pg/mg) | 8.03 | 9.37 | 8.41 | 0.42 | 0.12 |
| mD-LA (nmol/mg) | 0.86 a | 1.01 b | 1.02 b | 0.04 | 0.03 |
| mTNF-α (pg/mg) | 18.69 b | 23.98 a | 20.60 b | 0.77 | <0.01 |
| mTGF-β1 (pg/mg) | 15.59 | 15.20 | 13.79 | 1.46 | 0.67 |
| mIFN-γ (pg/mg) | 9.36 b | 12.23 a | 10.85 ab | 0.47 | <0.01 |
| mIL-1β (pg/mg) | 5.77 | 6.39 | 6.53 | 0.31 | 0.23 |
| KEGG Term (p Value)/Protein Name | Gene Name | Protein ID in UniProt | Log2FC |
|---|---|---|---|
| Antigen processing and presentation (p = 0.002) | |||
| MHC I6 | SLA-6 | A1YH88 | 1.53 |
| MHC I2 | SLA-2 | A0A2S1PUH7 | −4.65 |
| Cathepsin L1 | CTSL | Q28944 | −1.61 |
| Apoptosis (p = 0.01) | |||
| IgG heavy chain | IGHG | L8B139 | 1.64 |
| Poly-Ig receptor | unknow | Q9N2H7 | 5.79 |
| Fc epsilon RI signaling pathway (p = 0.02) | |||
| IgG heavy chain | IGHG | L8B139 | 1.64 |
| MKK3/6 | MAP2K6 | F1RV28 | −2 |
| Inflammatory mediator regulation of TRP channels (p = 0.008) | |||
| MKK3/6 | MAP2K6 | F1RV28 | −2 |
| Cytochrome P450 2C42 | CYP2C42 | I3LCZ8 | −2.89 |
| Calcium/calmodulin-dependent protein kinase | CAMK2G | A0A286ZKT1 | −2.26 |
| Intestinal immune network for IgA production (p = 0.01) | |||
| IgG heavy chain | IGHG | L8B139 | 1.64 |
| Poly-Ig receptor | unknow | Q9N2H7 | 5.79 |
| Tight junction (p = 0.01) | |||
| MAGUK p55 subfamily member 5 | MPP5 | F1SA41 | 3.15 |
| Claudin | CLDN7 | C3VPJ4 | −1.59 |
| PATJ crumbs cell polarity complex component | PATJ | A0A287AG82 | −2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Yu, X.; Du, Z.; Zhu, Z.; Shi, C.; Li, A.; Wang, F. Pyrroloquinoline Quinone Alleviates Intestinal Inflammation and Cell Apoptosis via the MKK3/6-P38 Pathway in a Piglet Model. Int. J. Mol. Sci. 2024, 25, 9723. https://doi.org/10.3390/ijms25179723
Huang C, Yu X, Du Z, Zhu Z, Shi C, Li A, Wang F. Pyrroloquinoline Quinone Alleviates Intestinal Inflammation and Cell Apoptosis via the MKK3/6-P38 Pathway in a Piglet Model. International Journal of Molecular Sciences. 2024; 25(17):9723. https://doi.org/10.3390/ijms25179723
Chicago/Turabian StyleHuang, Caiyun, Xuanci Yu, Ziyuan Du, Zhihao Zhu, Chenyu Shi, Ang Li, and Fenglai Wang. 2024. "Pyrroloquinoline Quinone Alleviates Intestinal Inflammation and Cell Apoptosis via the MKK3/6-P38 Pathway in a Piglet Model" International Journal of Molecular Sciences 25, no. 17: 9723. https://doi.org/10.3390/ijms25179723




