Hypertriglyceridemia Therapy: Past, Present and Future Perspectives
Abstract
:1. Introduction
2. Past Therapies: Fibrates and Niacin
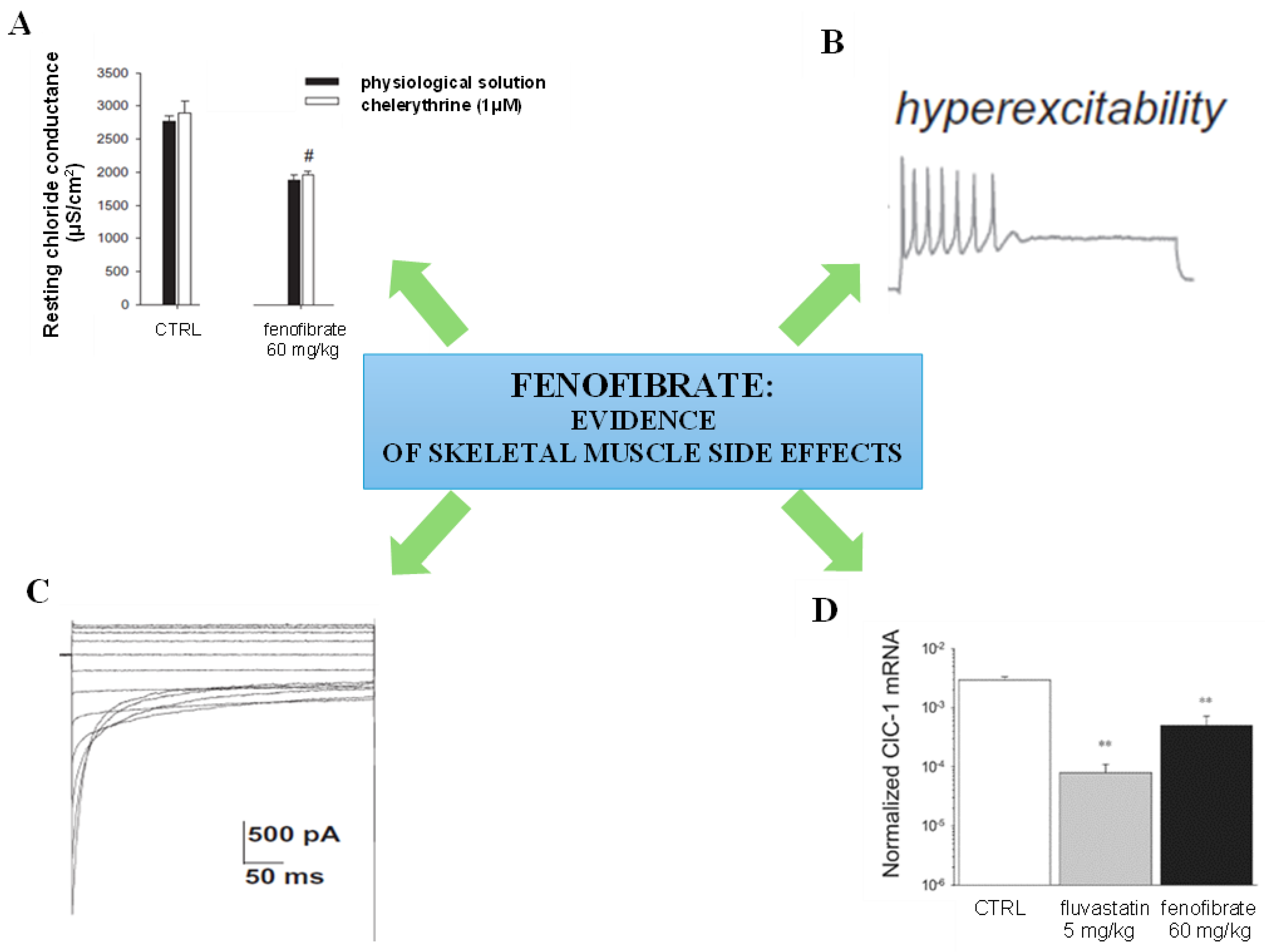
2.1. Fibrates: Mechanism of Action, Side Effects and Interaction with Other Drugs
2.2. Niacin
3. The Present: Currently Used Drugs, Nutraceuticals and Phytotherapy
3.1. Fibrates
3.2. Antisense Oligonucleotide
3.3. Human Monoclonal Antibody
3.4. Gene Therapy
3.5. Fatty Acids
3.6. Nutraceuticals and Phytotherapeutics
| Drug | Year of Approval | Mechanism of Action | Advantages | Current Status | Disadvantages | References |
|---|---|---|---|---|---|---|
| Fenofibrate | 1993 | PPAR-α agonist | increased lipoprotein lipase expression | in clinical use | side effects on skeletal muscle, elevation of serum amino- transferase | [7,11,18,33,39] |
| Volanesorsen | 2019 (EU 1/19/1360/001) authorized for the treatment of FCS, declined in US | antisense oligonucleotide, designed to inhibit apo C-III formation | lowering of TGs by over 70%, decreased pancreatitis | in clinical use | injection site reactions, careful monitoring of thrombo-cytopenia and hepatic and renal function | [44] |
| Evinacumab | 2021 (FDA, EU) | human monoclonal antibody that neutralizes angiopoietin-like 3, an inhibitor of lipoprotein lipase | reduced plasma TG and LDL-C in an LDL-receptor-independent manner, used in homozygous familial hypercholesterolemia in which statins are ineffective | in clinical use | possible flu-like illness, nausea, severe allergic reactions | [45] |
| Alipogene Tipavorvec | 2015 (FDA) | adeno-associated virus gene therapy | lipoprotein lipase synthesis and TG reduction | not clinically available | multiple intramuscular injections, lack of long-term efficacy | [34,47,48] |
| Omega-3s | 2000 (EMA) | fatty acid oxidation, decrease in hepatic lipogenesis, stimulation of lipoprotein lipase activity | reduced cholesterol andTGs, anti-inflammatory and antioxidant properties, beneficial effects on endothelial function | no longer considered effective in preventing heart disease | mild side effects: diarrhoea, nausea, dyspepsia, abdominal discomfort | [49,50,53,54,55] |
| Artichoke leaf extract; Berberine; Bergamot | - | Plasma TG reduction | contributed to the regulation of lipid levels in the blood | in use | few side effects, mainly gastrointestinal | [58,59,61,62,63,64] |
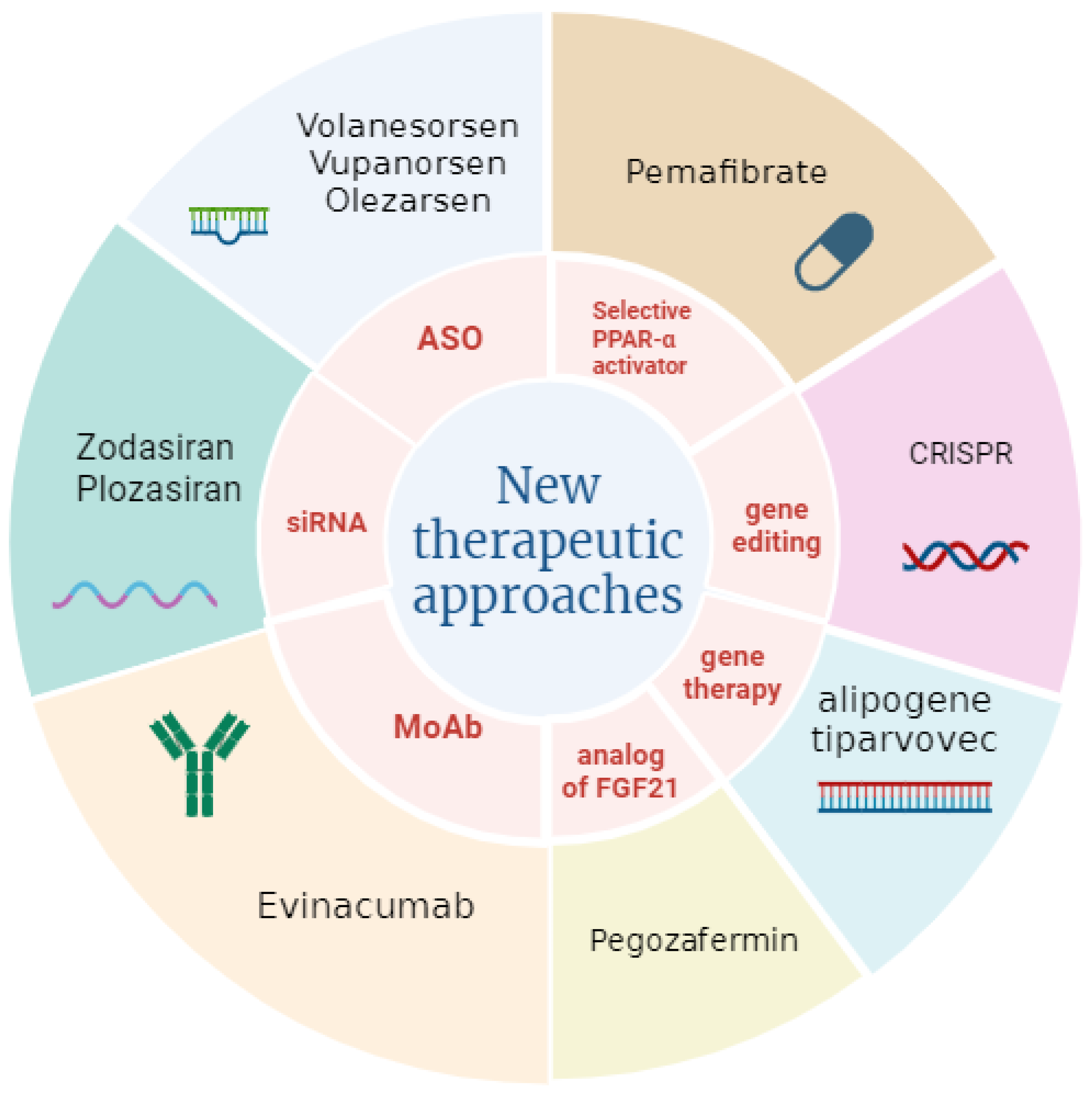
4. Future Perspectives on Hypertriglyceridemia Therapy
4.1. Selective PPARα Activator
4.2. Antisense Oligonucleotides Proposed in Therapy
4.3. RNA Interference (RNAi)
4.4. Analog of Human Fibroblast Growth Factor 21
| Drug | Mechanism of Action | Advantages | Current Status | Disadvantages | References |
|---|---|---|---|---|---|
| Pemafibrate | selective PPAR-α agonist | high selectivity of the receptor, more effective than fenofibrate | under investigation | renal adverse events and risk of venous thromboembolism | [3,57,66] |
| Vupanorsen | antisense oligonucleotide inhibits ANGPTL3 production | reduction in cholesterol and plasma TGs | discontinuation of the clinical development programme | increase in serum transaminases | [57,72,73] |
| Olezarsen | antisense oligonucleotide targeting messenger RNA of apo C-III, responsible for the inhibition of lipoprotein lipase | significant reductions in TGs levels, does not adversely affect platelets | Phase III NCT05355402 | liver and kidney abnormalities | [74,75,76] |
| Plozasiran | siRNA silencing of apo C-III gene expression, which is responsible for the inhibition of lipoprotein lipase | decrease in TGs levels | a clinical trial is ongoing (NCT04998201) | favourable safety profile | [77,78] |
| Zodasiran | siRNA targeting ANGPTL3 | decrease in TGs levels | under investigation | transient elevation in glycated haemoglobin levels in patients with pre-existing diabetes who received the highest dose | [72,79] |
| Pegozafermin | analogue of FGF21 | reduction in TGs and LDL cholesterol | under investigation | long-term data on safety are needed | [80,81,82,83,84] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [PubMed]
- Ruscica, M.; Bertoletti, A.; Gobbi, C.; Sirtori, C.R.; Carugo, S.; Corsini, A. Lipid-lowering approaches to manage statin-intolerant patients. Eur. Heart J. Suppl. 2024, 26 (Suppl. S1), i56–i59. [Google Scholar] [CrossRef]
- Xu, J.; Ashjian, E. Treatment of Hypertriglyceridemia: A Review of Therapies in the Pipeline. J. Pharm. Pract. 2023, 36, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Nikolic, D.; Montalto, G.; Banach, M.; Mikhailidis, D.P.; Rizzo, M. The role of fibrate treatment in dyslipidemia: An overview. Curr. Pharm. Des. 2013, 19, 3124–3131. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, D.B.; Tucker, D.R.; Azarnoff, D.L. Long-term effects of clofibrate (Atromid-S) on serum lipids in man. Circulation 1969, 39, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.I.; Rifking, B.M. Lipid lowering drugs and hyperlipidaemia. Drugs 1973, 6, 12–45. [Google Scholar] [CrossRef]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Graham, D.J.; Staffa, J.A.; Shatin, D.; Andrade, S.E.; Schech, S.D.; La Grenade, L.; Gurwitz, J.H.; Chan, K.A.; Goodman, M.J.; Platt, R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004, 292, 2585–2590. [Google Scholar] [CrossRef]
- Farmer, J.A. Learning from the cerivastatin experience. Lancet 2001, 358, 1383–1385. [Google Scholar] [CrossRef]
- Furberg, C.D.; Pitt, B. Withdrawal of cerivastatin from the world market. Curr. Control. Trials Cardiovasc. Med. 2001, 2, 205–207. [Google Scholar] [CrossRef]
- Pierno, S.; Camerino, G.M.; Cippone, V.; Rolland, J.F.; Desaphy, J.F.; De Luca, A.; Liantonio, A.; Bianco, G.; Kunic, J.D.; George, A.L., Jr.; et al. Statins and fenofibrate affect skeletal muscle chloride conductance in rats by differently impairing ClC-1 channel regulation and expression. Br. J. Pharmacol. 2009, 156, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Pellegrino, M.A.; Brocca, L.; Digennaro, C.; Camerino, D.C.; Pierno, S.; Bottinelli, R. Statin or fibrate chronic treatment modifies the proteomic profile of rat skeletal muscle. Biochem. Pharmacol. 2011, 81, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Zdebik, A.A. Statins and fibrate target ClC-1-from side effects to CLC pharmacology. Br. J. Pharmacol. 2009, 156, 1204–1205. [Google Scholar] [CrossRef]
- Pierno, S.; Musumeci, O. Pharmacotherapy of the Lipid-Lowering Drugs: Update on Efficacy and Risk. Int. J. Mol. Sci. 2023, 24, 996. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, P.D.; De Souza, A.T.; Ulrich, R.G. Profiling of hepatic gene expression in rats treated with fibric acid analogs. Mutat. Res. 2004, 549, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, P.; Mercier, J.; Vassort, G.; Lacampagne, A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 329, 1067–1075. [Google Scholar] [CrossRef]
- Liantonio, A.; Giannuzzi, V.; Cippone, V.; Camerino, G.M.; Pierno, S.; Camerino, D.C. Fluvastatin and atorvastatin affect calcium homeostasis of rat skeletal muscle fibers in vivo and in vitro by impairing the sarcoplasmic reticulum/mitochondria Ca2+-release system. J. Pharmacol. Exp. Ther. 2007, 321, 626–634. [Google Scholar] [CrossRef]
- Pierno, S.; Didonna, M.P.; Cippone, V.; De Luca, A.; Pisoni, M.; Frigeri, A.; Nicchia, G.P.; Svelto, M.; Chiesa, G.; Sirtori, C.; et al. Effects of chronic treatment with statins and fenofibrate on rat skeletal muscle: A biochemical, histological and electrophysiological study. Br. J. Pharmacol. 2006, 149, 909–919. [Google Scholar] [CrossRef]
- Camerino, G.M.; Tarantino, N.; Canfora, I.; De Bellis, M.; Musumeci, O.; Pierno, S. Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 2070. [Google Scholar] [CrossRef]
- Pierno, S.; Liantonio, A.; Camerino, G.M.; De Bellis, M.; Cannone, M.; Gramegna, G.; Scaramuzzi, A.; Simonetti, S.; Nicchia, G.P.; Basco, D.; et al. Potential benefits of taurine in the prevention of skeletal muscle impairment induced by disuse in the hindlimb-unloaded rat. Amino Acids 2012, 43, 431–445. [Google Scholar] [CrossRef]
- Pierno, S.; De Luca, A.; Beck, C.L.; George, A.L., Jr.; Conte Camerino, D. Aging-associated down-regulation of ClC-1 expression in skeletal muscle: Phenotypic-independent relation to the decrease of chloride conductance. FEBS Lett. 1999, 449, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Fonzino, A.; Conte, E.; De Bellis, M.; Mele, A.; Liantonio, A.; Tricarico, D.; Tarantino, N.; Dobrowolny, G.; Musarò, A.; et al. Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in search of new therapeutic options. Sci. Rep. 2019, 9, 3185. [Google Scholar] [CrossRef]
- De Luca, A.; Pierno, S.; Camerino, D.C. Electrical properties of diaphragm and EDL muscles during the life of dystrophic mice. Am. J. Physiol. 1997, 272 Pt 1, C333–C340. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Pierno, S.; De Luca, A.; Didonna, P.; Camerino, D.C. Different ability of clenbuterol and salbutamol to block sodium channels predicts their therapeutic use in muscle excitability disorders. Mol. Pharmacol. 2003, 63, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Pierno, S.; Liantonio, A.; De Bellis, M.; Cannone, M.; Sblendorio, V.; Conte, E.; Mele, A.; Tricarico, D.; Tavella, S.; et al. Effects of pleiotrophin overexpression on mouse skeletal muscles in normal loading and in actual and simulated microgravity. PLoS ONE 2013, 8, e72028. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Desaphy, J.F.; De Bellis, M.; Capogrosso, R.F.; Cozzoli, A.; Dinardo, M.M.; Caloiero, R.; Musaraj, K.; Fonzino, A.; Conte, E.; et al. Effects of Nandrolone in the Counteraction of Skeletal Muscle Atrophy in a Mouse Model of Muscle Disuse: Molecular Biology and Functional Evaluation. PLoS ONE 2015, 10, e0129686. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Farinato, A.; Altamura, C.; De Bellis, M.; Imbrici, P.; Tarantino, N.; Caccia, C.; Melloni, E.; Padoani, G.; Vailati, S.; et al. Safinamide’s potential in treating nondystrophic myotonias: Inhibition of skeletal muscle voltage-gated sodium channels and skeletal muscle hyperexcitability in vitro and in vivo. Exp. Neurol. 2020, 328, 113287. [Google Scholar] [CrossRef]
- Camerino, G.M.; Musumeci, O.; Conte, E.; Musaraj, K.; Fonzino, A.; Barca, E.; Marino, M.; Rodolico, C.; Tricarico, D.; Camerino, C.; et al. Risk of Myopathy in Patients in Therapy with Statins: Identification of Biological Markers in a Pilot Study. Front. Pharmacol. 2017, 8, 500. [Google Scholar] [CrossRef]
- Costa, J.; Graça, P.; Evangelista, T.; De Carvalho, M. Pain and calf hypertrophy associated with spontaneous repetitive discharges treated with botulinum toxin. Clin. Neurophysiol. 2005, 116, 2847–2852. [Google Scholar] [CrossRef]
- Serrao, M.; Arendt-Nielsen, L.; Ge, H.-Y.; Pierelli, F.; Sandrini, G.; Farina, D. Experimental muscle pain decreases the frequency threshold of electrically elicited muscle cramps. Exp. Brain Res. 2007, 182, 301–308. [Google Scholar] [CrossRef]
- Loiodice, F.; Ferorelli, S.; Tangari, N.; Bettoni, G.; Tortorella, V.; Pierno, S.; De Luca, A.; Tricarico, D.; Conte-Camerino, D. Carboxylic acids and chloride conductance in skeletal muscle: Influence on the pharmacological activity induced by the chain substituents and the distance between the phenolic group and the carboxylic function in 4-chloro-phenoxy alkanoic acids. Farmaco 1993, 48, 45–63. [Google Scholar]
- Fracchiolla, G.; Laghezza, A.; Piemontese, L.; Tortorella, P.; Mazza, F.; Montanari, R.; Pochetti, G.; Lavecchia, A.; Novellino, E.; Pierno, S.; et al. New 2-aryloxy-3-phenyl-propanoic acids as peroxisome proliferator-activated receptors alpha/gamma dual agonists with improved potency and reduced adverse effects on skeletal muscle function. J. Med. Chem. 2009, 52, 6382–6393. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188, Erratum in Eur. Heart J. 2020, 41, 4255. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Triglyceride Lowering Drugs. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2024. [Google Scholar]
- Yadav, M.K.; Sarma, P.; Maharana, J.; Ganguly, M.; Mishra, S.; Zaidi, N.; Dalal, A.; Singh, V.; Saha, S.; Mahajan, G.; et al. Structure-guided engineering of biased-agonism in the human niacin receptor via single amino acid substitution. Nat. Commun. 2024, 15, 1939. [Google Scholar] [CrossRef]
- Packard, C.J.; Boren, J.; Taskinen, M.R. Causes and consequences of hypertriglyceridemia. Front. Endocrinol. 2020, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.E.J.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: A study of more than 115000 individuals from the general population. Clin. Chem. 2019, 65, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, F.; Khalaj, L.; Ahmadiani, A.; Rahmani, B. Gemfibrozil pretreatment affecting antioxidant defense system and inflammatory, but not Nrf-2 signaling pathways resulted in female neuroprotection and male neurotoxicity in the rat models of global cerebral ischemia-reperfusion. Neurotox. Res. 2013, 23, 225–237. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Elisaf, M.S. Safety considerations with fenofibrate/simvastatin combination. Expert Opin. Drug Saf. 2015, 14, 1481–1493. [Google Scholar] [CrossRef]
- Spagnuolo, C.M.; Hegele, R.A. Recent advances in treating hypertriglyceridemia in patients at high risk of cardiovascular disease with apolipoprotein C-III inhibitors. Expert Opin. Pharmacother. 2023, 24, 1013–1020. [Google Scholar] [CrossRef]
- Gesner, M.; Frishman, W.H. Drug Therapy for Hypertriglyceridemia and Familial Chylomicronemia Syndrome: Focus on Volnesorsen. Cardiol. Rev. 2023, 31, 325–329. [Google Scholar] [CrossRef]
- Tomlinson, B.; Wu, Q.Y.; Zhong, Y.M.; Li, Y.H. Advances in Dyslipidaemia Treatments: Focusing on ApoC3 and ANGPTL3 Inhibitors. J. Lipid Atheroscler. 2024, 13, 2–20. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Santos, R.D.; Sirtori, C.R.; Corsini, A. Lipid Lowering Drugs: Present Status and Future Developments. Curr. Atheroscler. Rep. 2021, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Duggan, S. Volanesorsen: First Global Approval. Drugs 2019, 79, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Adach, W.; Surma, S.; Rosenson, R.S.; Banach, M. Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia. J. Clin. Med. 2022, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Watts, G.F.; Tamehri Zadeh, S.S.; Chan, D.C. ANGPTL3 as a therapeutic target for treating homozygous familial hypercholesterolaemia: A shot in the arm for evinacumab. Eur. Heart J. 2024, 45, 2435–2438. [Google Scholar] [CrossRef]
- Mehta, N.; Gilbert, R.; Chahal, P.S.; Moreno, M.J.; Nassoury, N.; Coulombe, N.; Lytvyn, V.; Mercier, M.; Fatehi, D.; Lin, W.; et al. Preclinical Development and Characterization of Novel Adeno-Associated Viral Vectors for the Treatment of Lipoprotein Lipase Deficiency. Hum. Gene Ther. 2023, 34, 927–946. [Google Scholar] [CrossRef]
- Florentin, M.; Kostapanos, M.S.; Anagnostis, P.; Liamis, G. Recent developments in pharmacotherapy for hypertriglyceridemia: What’s the current state of the art? Expert Opin. Pharmacother. 2020, 21, 107–120. [Google Scholar] [CrossRef]
- Wolska, A.; Yang, Z.H.; Remaley, A.T. Hypertriglyceridemia: New approaches in management and treatment. Curr. Opin. Lipidol. 2020, 31, 331–339. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Ferrari, R.; Censi, S.; Cimaglia, P. The journey of omega-3 fatty acids in cardiovascular medicine. Eur. Heart J. Suppl. 2020, 22 (Suppl. J), J49–J53. [Google Scholar] [CrossRef]
- Santos-Baez, L.S.; Ginsberg, H.N. Hypertriglyceridemia-Causes, Significance, and Approaches to Therapy. Front. Endocrinol. 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098, Erratum in Lancet 2007, 370, 220. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendía, L.E.; Mikhailidis, D.P.; Pirro, M.; Banach, M.; Sirtori, C.R.; Reiner, Ž. Effect of omega-3 supplements on plasma apolipoprotein C-III concentrations: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2018, 50, 565–575. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. How to Handle Elevated Triglycerides: Life after PROMINENT. Curr. Atheroscler. Rep. 2023, 25, 921–929. [Google Scholar] [CrossRef]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-optimal Lipid Levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.R.; Oh, G.T.; Park, H.S.; Lee, K.U.; Lane, M.D.; et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef]
- Meng, S.; Wang, L.S.; Huang, Z.Q.; Zhou, Q.; Sun, Y.G.; Cao, J.T.; Li, Y.G.; Wang, C.Q. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin. Exp. Pharmacol. Physiol. 2012, 39, 406–411. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Nikolic, D.; Li Volti, G.; Al-Rasadi, K.; Katsiki, N.; Mikhailidis, D.P.; Montalto, G.; Ivanova, E.; Orekhov, A.N.; et al. The effect of bergamot on dyslipidemia. Phytomedicine 2016, 23, 1175–1181. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother. Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc. Diabetol. 2017, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Abe, K.; Toma, T.; Nishikawa, M.; Ozawa, H.; Okuda, A.; Araki, T.; Oda, S.; Inoue, K.; Shibuya, K.; et al. Design and synthesis of highly potent and selective human peroxisome proliferator-activated receptor alpha agonists. Bioorg. Med. Chem. Lett. 2007, 17, 4689–4693. [Google Scholar] [CrossRef]
- Raza-Iqbal, S.; Tanaka, T.; Anai, M.; Inagaki, T.; Matsumura, Y.; Ikeda, K.; Taguchi, A.; Gonzalez, F.J.; Sakai, J.; Kodama, T. Transcriptome analysis of K-877 (a novel selective PPARalpha modulator (SPPARMalpha))-regulated genes in primary human hepatocytes and the mouse liver. J. Atheroscler. Thromb. 2015, 22, 754–772. [Google Scholar] [CrossRef]
- Iwata, H.; Osborn, E.A.; Ughi, G.J.; Murakami, K.; Goettsch, C.; Hutcheson, J.D.; Mauskapf, A.; Mattson, P.C.; Libby, P.; Singh, S.A.; et al. Highly Selective PPARα (Peroxisome Proliferator-Activated Receptor α) Agonist Pemafibrate Inhibits Stent Inflammation and Restenosis Assessed by Multimodality Molecular-Microstructural Imaging. J. Am. Heart Assoc. 2021, 10, e020834. [Google Scholar] [CrossRef] [PubMed]
- Sairyo, M.; Kobayashi, T.; Masuda, D.; Kanno, K.; Zhu, Y.; Okada, T.; Koseki, M.; Ohama, T.; Nishida, M.; Sakata, Y.; et al. A Novel Selective PPARα Modulator (SPPARMα), K-877 (Pemafibrate), Attenuates Postprandial Hypertriglyceridemia in Mice. J. Atheroscler. Thromb. 2018, 25, 142–152. [Google Scholar] [CrossRef]
- Miller, M. Pemafibrate and other triglyceride-lowering therapies to reduce risk of cardiovascular and metabolic disease. Curr. Opin. Cardiol. 2024, 39, 286–291. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Schwarz, J.; Berthold, H.K. Updates in Drug Treatment of Severe Hypertriglyceridemia. Curr. Atheroscler. Rep. 2023, 25, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen Study Investigators. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Marston, N.A.; Prohaska, T.A.; Alexander, V.J.; Zimerman, A.; Moura, F.A.; Murphy, S.A.; Goodrich, E.L.; Zhang, S.; Gaudet, D.; et al. Olezarsen for Hypertriglyceridemia in Patients at High Cardiovascular Risk. N. Engl. J. Med. 2024, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Karwatowska-Prokopczuk, E.; Amour, E.S.; Ballantyne, C.M.; Shapiro, M.D.; Moriarty, P.M.; Baum, S.J.; Hurh, E.; Bartlett, V.J.; Kingsbury, J.; et al. Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk. Eur. Heart J. 2022, 43, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J.; Pirillo, A.; Tsimikas, S.; Ference, B.A.; Catapano, A.L. Exploring apolipoprotein C-III: Pathophysiological and pharmacological relevance. Cardiovasc. Res. 2024, 119, 2843–2857. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Vasas, S.; Azizad, M.; Clifton, P.; Rosenson, R.S.; Chang, T.; Melquist, S.; Zhou, R.; Mushin, M.; Leeper, N.J.; et al. Plozasiran, an RNA Interference Agent Targeting APOC3, for Mixed Hyperlipidemia. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Gaudet, D.; Pall, D.; Watts, G.F.; Nicholls, S.J.; Rosenson, R.S.; Modesto, K.; San Martin, J.; Hellawell, J.; Ballantyne, C.M. Plozasiran (ARO-APOC3) for Severe Hypertriglyceridemia: The SHASTA-2 Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 620–630. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Gaudet, D.; Hegele, R.A.; Ballantyne, C.M.; Nicholls, S.J.; Lucas, K.J.; San Martin, J.; Zhou, R.; Muhsin, M.; Chang, T.; et al. Zodasiran, an RNAi Therapeutic Targeting ANGPTL3, for Mixed Hyperlipidemia. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Jeong, C.; Han, N.; Jeon, N.; Rhee, S.J.; Staatz, C.E.; Kim, M.S.; Baek, I.H. Efficacy and Safety of Fibroblast Growth Factor-21 Analogs for the Treatment of Metabolic Dysfunction-Associated Steatohepatitis: A Systematic Review and Meta-Analysis. Clin. Pharmacol. Ther. 2024, 116, 72–81. [Google Scholar] [CrossRef]
- Alkhouri, N.; Lazas, D.; Loomba, R.; Frias, J.P.; Feng, S.; Tseng, L.; Balic, K.; Agollah, G.D.; Kwan, T.; Lyer, J.S.; et al. Clinical trial: Effects of pegozafermin on the liver and on metabolic comorbidities in subjects with biopsy-confirmed nonalcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2023, 58, 1005–1015. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Bays, H.E.; Miller, M.; Cain, J.E., 3rd; Wasilewska, K.; Andrawis, N.S.; Parli, T.; Feng, S.; Sterling, L.; Tseng, L.; et al. ENTRIGUE Principal Investigators. The FGF21 analog pegozafermin in severe hypertriglyceridemia: A randomized phase 2 trial. Nat. Med. 2023, 29, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Tokgözoğlu, L.; Libby, P. The dawn of a new era of targeted lipid-lowering therapies. Eur. Heart J. 2022, 43, 3198–3208. [Google Scholar] [CrossRef]
- Wang, X.; Raghavan, A.; Chen, T.; Qiao, L.; Zhang, Y.; Ding, Q.; Musunuru, K. CRISPR-Cas9 Targeting of PCSK9 in Human Hepatocytes In Vivo-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 783–786. [Google Scholar] [CrossRef] [PubMed]
| Drug | Year of Approval | Mechanism of Action | Advantages | Current Status | Disadvantages | References |
|---|---|---|---|---|---|---|
| Clofibrate | 1967 | PPAR-α agonist | reduced risk of heart disease | not in use | side effects on skeletal muscle, liver, gastrointestinal tissue, gallstones | [5,7] |
| Gemfibrozil | 1981 | PPAR-α agonist | reduced risk of heart disease | in clinical use | increased risk of pancreatitis | [9] |
| Niacin | 1955 | inhibition of triglyceride synthesis | reduced atherogenic lipoprotein particles | not in use in the EU | serious liver toxicity | [33,34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canfora, I.; Pierno, S. Hypertriglyceridemia Therapy: Past, Present and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 9727. https://doi.org/10.3390/ijms25179727
Canfora I, Pierno S. Hypertriglyceridemia Therapy: Past, Present and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(17):9727. https://doi.org/10.3390/ijms25179727
Chicago/Turabian StyleCanfora, Ileana, and Sabata Pierno. 2024. "Hypertriglyceridemia Therapy: Past, Present and Future Perspectives" International Journal of Molecular Sciences 25, no. 17: 9727. https://doi.org/10.3390/ijms25179727







