The Role of the Endometrium in Implantation: A Modern View
Abstract
1. Introduction
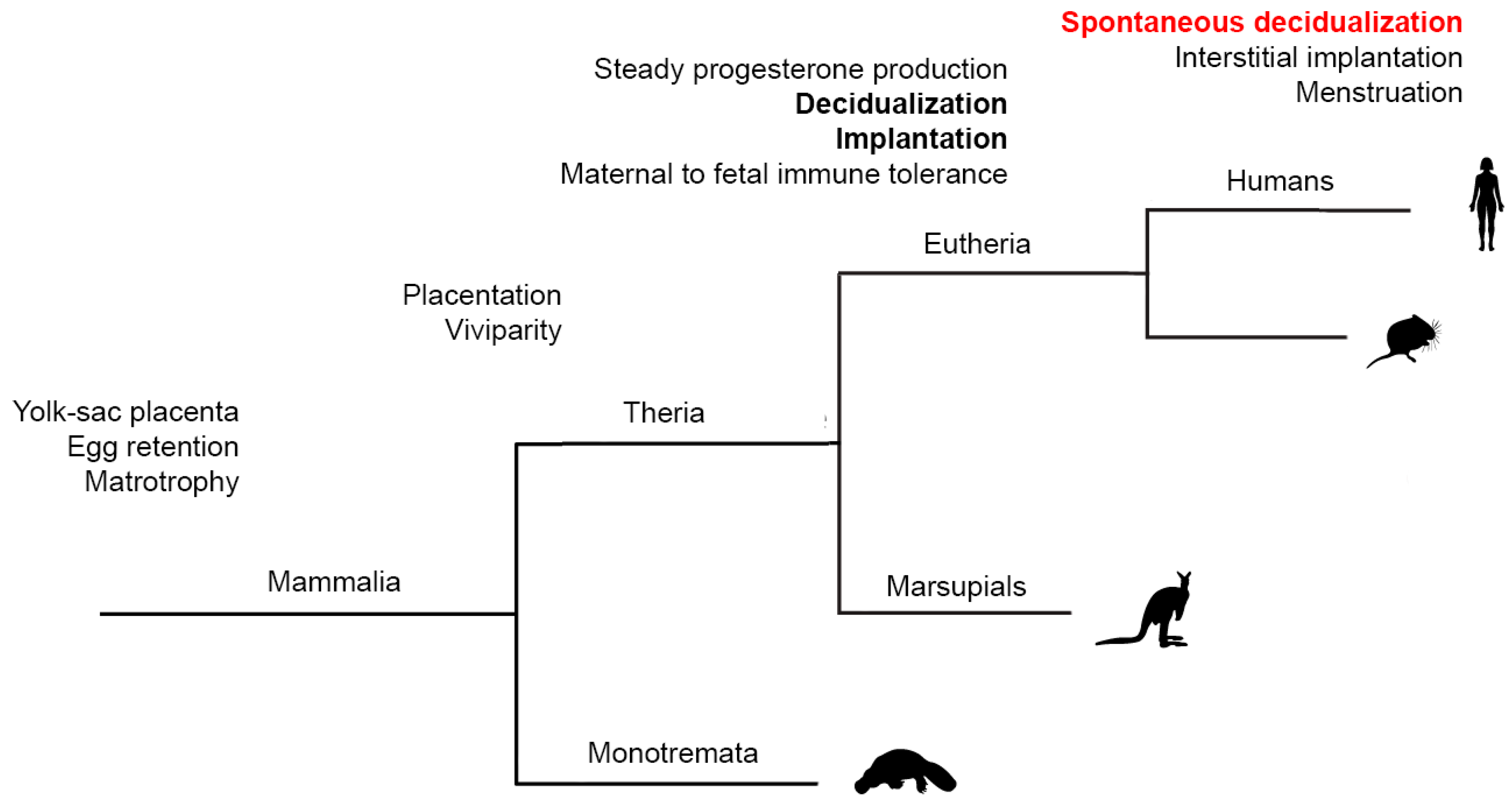
2. “Spontaneous” Decidualization of the Endometrium Is an Evolutionary Sign of the Transition of Control over Implantation from the Embryonic to Maternal One
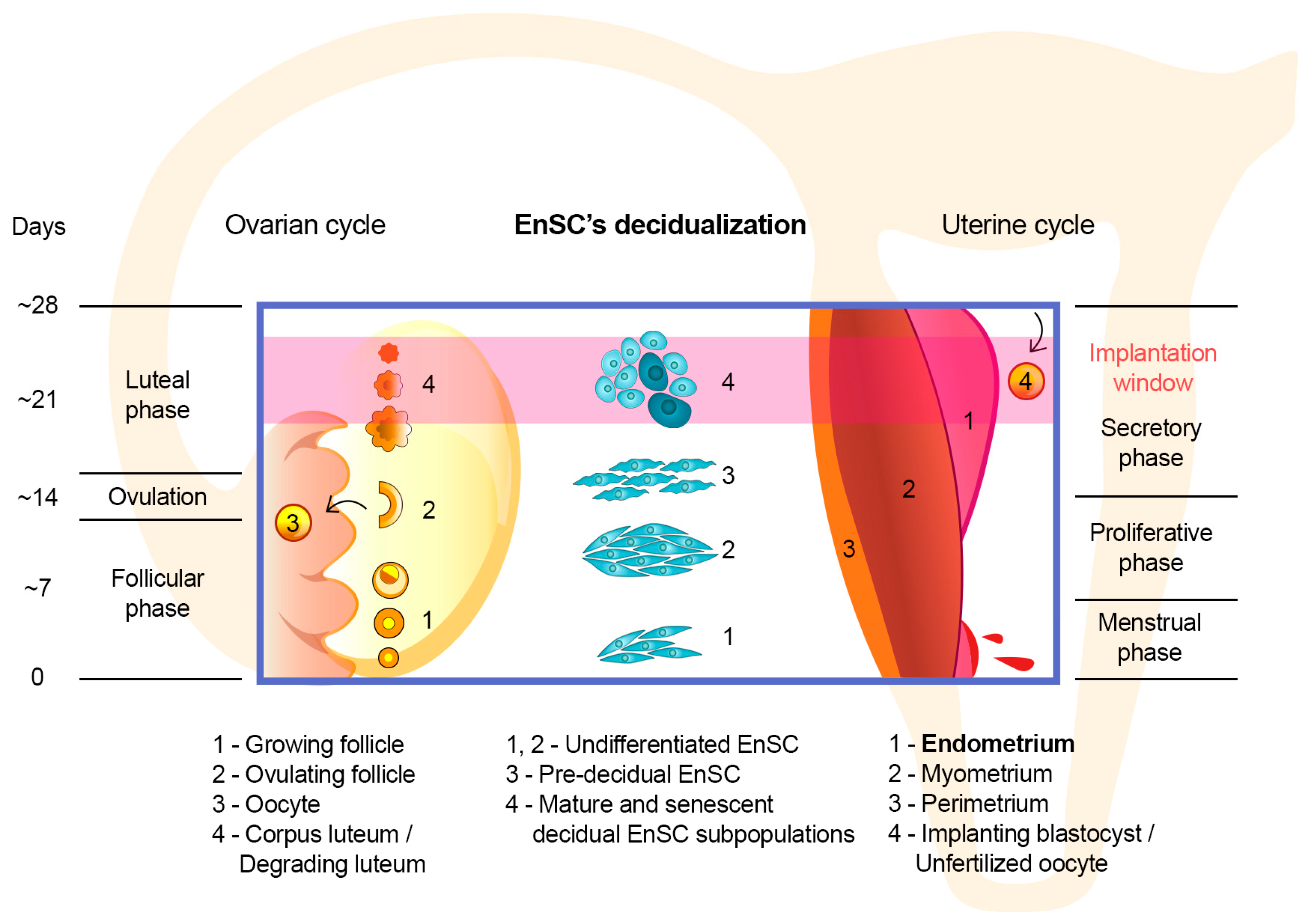
3. Implementation of the “Sensory” Function of the Endometrium at the Cellular Level
4. The Issue of Assessing the Functional Status of the Endometrium
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ART | Assisted reproductive technologies |
| EnSC | Endometrial stromal cells |
| IVF | In vitro fertilization |
| LH | Luteinizing hormone |
| P | Progesterone |
| PR | Progesterone receptor |
References
- European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Cuevas Saiz, I.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; et al. ART in Europe, 2019: Results generated from European registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar] [CrossRef] [PubMed]
- Amini, P.; Ramezanali, F.; Parchehbaf-Kashani, M.; Maroufizadeh, S.; Omani-Samani, R.; Ghaheri, A. Factors Associated with In Vitro Fertilization Live Birth Outcome: A Comparison of Different Classification Methods. Int. J. Fertil. Steril. 2021, 15, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wen, Q.; Li, J.; Zeng, D.; Huang, P. A systematic review and meta-analysis: Clinical outcomes of recurrent pregnancy failure resulting from preimplantation genetic testing for aneuploidy. Front. Endocrinol. 2023, 14, 1178294. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Koel, M.; Võsa, U.; Adler, P.; Suhorutšenko, M.; Laisk-Podar, T.; Kukushkina, V.; Saare, M.; Velthut-Meikas, A.; Krjutškov, K.; et al. Metasignature of human endometrial receptivity: A meta-analysis and validation study of transcriptomic biomarkers. Sci. Rep. 2017, 7, 10077. [Google Scholar] [CrossRef]
- Tomari, H.; Kawamura, T.; Asanoma, K.; Egashira, K.; Kawamura, K.; Honjo, K.; Nagata, Y.; Kato, K. Contribution of senescence in human endometrial stromal cells during proliferative phase to embryo receptivity. Biol. Reprod. 2020, 103, 104–113. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Borodkina, A.V. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum. Rep. 2022, 37, 1505–1524. [Google Scholar] [CrossRef]
- Graham, C.H.; Lala, P.K. Mechanism of control of trophoblast invasion in situ. J. Cell. Physiol. 1991, 148, 228–234. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Dong, N.; Lou, J.; Srinivasan, D.K.; Cheng, W.; Huang, X.; Liu, M.; Fang, C.; Peng, J.; et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012, 484, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Salker, M.S.; Teklenburg, G.; Nautiyal, J.; Salter, S.; Lucas, E.S.; Steel, J.H.; Christian, M.; Chan, Y.W.; Boomsma, C.M.; et al. Uterine selection of human embryos at implantation. Sci. Rep. 2014, 4, 3894–3898. [Google Scholar] [CrossRef]
- Kong, C.S.; Ordoñez, A.A.; Turner, S.; Tremaine, T.; Muter, J.; Lucas, E.S.; Salisbury, E.; Vassena, R.; Tiscornia, G.; Fouladi-Nashta, A.A.; et al. Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation. FASEB J. 2021, 35, e21336. [Google Scholar] [CrossRef]
- Brosens, J.J.; Bennett, P.R.; Abrahams, V.M.; Ramhorst, R.; Coomarasamy, A.; Quenby, S.; Lucas, E.S.; McCoy, R.C. Maternal selection of human embryos in early gestation: Insights from recurrent miscarriage. Semin. Cell Dev. Biol. 2022, 131, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, E6566–E6575. [Google Scholar] [CrossRef]
- Mika, K.; Whittington, C.M.; McAllan, B.M.; Lynch, V.J. Gene expression phylogenies and ancestral transcriptome reconstruction resolves major transitions in the origins of pregnancy. eLife 2022, 11, e74297. [Google Scholar] [CrossRef] [PubMed]
- Chavan, A.R.; Griffith, O.W.; Stadtmauer, D.J.; Maziarz, J.; Pavlicev, M.; Fishman, R.; Koren, L.; Romero, R.; Wagner, G.P. Evolution of embryo implantation was enabled by the origin of decidual stromal cells in eutherian mammals. Mol. Biol. Evol. 2021, 38, 1060–1074. [Google Scholar] [CrossRef]
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human embryo implantation. Development 2023, 150, dev201507. [Google Scholar] [CrossRef]
- Muter, J.; Kong, C.S.; Brosens, J.J. The Role of Decidual Subpopulations in Implantation, Menstruation and Miscarriage. Front. Reprod. Health 2021, 3, 804921. [Google Scholar] [CrossRef]
- Diessler, M.E.; Hernández, R.; Gomez Castro, G.; Barbeito, C.G. Decidual cells and decidualization in the carnivoran endotheliochorial placenta. Front. Cell Dev. Biol. 2023, 11, 1134874. [Google Scholar] [CrossRef]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Wooding, P.; Burton, G. Comparative placentation. In Structures, Functions and Evolution; Springer: Heidelberg, Germany, 2008; p. 301. [Google Scholar]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Catalini, L.; Fedder, J. Characteristics of the endometrium in menstruating species: Lessons learned from the animal kingdomdagger. Biol. Reprod. 2020, 102, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Emera, D.; Romero, R.; Wagner, G. The evolution of menstruation: A new model for genetic assimilation: Explaining molecular origins of maternal responses to fetal invasiveness. BioEssays 2012, 34, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Teklenburg, G.; Salker, M.; Molokhia, M.; Lavery, S.; Trew, G.; Aojanepong, T.; Mardon, H.J.; Lokugamage, A.U.; Rai, R.; Landles, C.; et al. Natural selection of human embryos: Decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE 2010, 5, e10258. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.J.; Leclerc, R.D.; May, G.; Wagner, G.P. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 2011, 43, 1154–1159. [Google Scholar] [CrossRef]
- Mika, K.; Lynch, V.J. Transposable elements continuously remodel the regulatory landscape, transcriptome, and function of decidual stromal cells. Genome Biol. Evol. 2022, 14, evac164. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical endometrial repair and regeneration. Development 2021, 148, dev199577. [Google Scholar] [CrossRef]
- Evans, J.; Salamonsen, L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef]
- Henriet, P.; Chevronnay, H.P.G.; Marbaix, E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012, 358, 197–207. [Google Scholar] [CrossRef]
- Weimar, C.H.; Kavelaars, A.; Brosens, J.J.; Gellersen, B.; de Vreeden-Elbertse, J.M.; Heijnen, C.J.; Macklon, N.S. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS ONE 2012, 7, e41424. [Google Scholar] [CrossRef]
- Lucas, E.S.; Dyer, N.P.; Murakami, K.; Hou Lee, Y.; Chan, Y.W.; Grimaldi, G.; Muter, J.; Brighton, P.J.; Moore, J.D.; Patel, G.; et al. Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cells 2016, 34, 346–356. [Google Scholar] [CrossRef]
- Jarrell, J. The significance and evolution of menstruation. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Imir, A.; Fenkci, V.; Yilmaz, M.B.; Bulun, S.E. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: A mechanism for defective estradiol metabolism. Am. J. Obstet. Gynecol. 2007, 196, 391-e1. [Google Scholar] [CrossRef] [PubMed]
- Rac, M.W.; Wells, C.E.; Twickler, D.M.; Moschos, E.; McIntire, D.D.; Dashe, J.S. Placenta accreta and vaginal bleeding according to gestational age at delivery. Obstet. Gynecol. 2015, 125, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, A.; Bergeron, C. Histology of the human endometrium: From birth to senescence. Ann. N. Y. Acad. Sci. 1991, 622, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef]
- Paria, B.C.; Huet-Hudson, Y.M.; Dey, S.K. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc. Natl. Acad. Sci. USA 1993, 90, 10159–10162. [Google Scholar] [CrossRef]
- Psychoyos, A. Uterine receptivity for nidation. Ann. N. Y. Acad. Sci. 1986, 476, 36–42. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef]
- Stadtmauer, D.J.; Wagner, G.P. Single-cell analysis of prostaglandin E2-induced human decidual cell in vitro differentiation: A minimal ancestral deciduogenic signal. Biol. Reprod. 2022, 106, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021, 10, e69603. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef]
- Kabacik, S.; Lowe, D.; Fransen, L.; Leonard, M.; Ang, S.L.; Whiteman, C.; Corsi, S.; Cohen, H.; Felton, S.; Bali, R.; et al. The relationship between epigenetic age and the hallmarks of aging in human cells. Nat. Aging 2022, 2, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, A.V.; Deryabin, P.I.; Giukova, A.A.; Nikolsky, N.N. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Nat. 2018, 10, 4–14. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Keijser, R.; Repping, S.; Lambalk, C.B.; Afink, G.B.; Mastenbroek, S.; Hamer, G. High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a. Hum. Reprod. 2020, 35, 1797–1807. [Google Scholar] [CrossRef]
- Grewal, S.; Carver, J.G.; Ridley, A.J.; Mardon, H.J. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc. Natl. Acad. Sci. USA 2008, 105, 16189–16194. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Lambalk, C.B.; Huirne, J.; Mijatovic, V.; Repping, S.; Hamer, G.; Mastenbroek, S. High-quality human preimplantation embryos actively influence endometrial stromal cell migration. J. Assist. Reprod. Genet. 2018, 35, 659–667. [Google Scholar] [CrossRef]
- Zeng, S.; Liang, Y.; Lai, S.; Bi, S.; Huang, L.; Li, Y.; Deng, W.; Xu, P.; Liu, M.; Xiong, Z.; et al. TNFα/TNFR1 signal induces excessive senescence of decidua stromal cells in recurrent pregnancy loss. J. Reprod. Immunol. 2023, 155, 103776. [Google Scholar] [CrossRef]
- Bergh, P.A.; Navot, D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil. Steril. 1992, 58, 537–542. [Google Scholar] [CrossRef]
- Ruiz-Alonso, M.; Valbuena, D.; Gomez, C.; Cuzzi, J.; Simon, C. Endometrial Receptivity Analysis (ERA): Data versus opinions. Hum. Reprod. Open 2021, 2021, hoab011. [Google Scholar] [CrossRef] [PubMed]
- Creus, M.; Ordi, J.; Fábregues, F.; Casamitjana, R.; Ferrer, B.; Coll, E.; Vanrell, J.A.; Balasch, J. Alphavbeta3 integrin expression and pinopod formation in normal and out-of-phase endometria of fertile and infertile women. Hum. Reprod. 2002, 17, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Coutifaris, C.; Myers, E.R.; Guzick, D.S.; Diamond, M.P.; Carson, S.A.; Legro, R.S.; McGovern, P.G.; Schlaff, W.D.; Carr, B.R.; Steinkampf, M.P.; et al. NICHD National Cooperative Reproductive Medicine Network. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil. Steril. 2004, 82, 1264–1272. [Google Scholar] [CrossRef]
- Hanassab, S.; Abbara, A.; Yeung, A.C.; Voliotis, M.; Tsaneva-Atanasova, K.; Kelsey, T.W.; Trew, G.H.; Nelson, S.M.; Heinis, T.; Dhillo, W.S. The prospect of artificial intelligence to personalize assisted reproductive technology. npj Digit. Med. 2024, 7, 55. [Google Scholar] [CrossRef]
- Schuff, R.H.A.; Suarez, J.; Laugas, N.; Ramirez, M.L.Z.; Alkon, T. Artificial intelligence model utilizing endometrial analysis to contribute as a predictor of assisted reproductive technology success. J. IVF-Worldw. 2024, 2, 1–8. [Google Scholar] [CrossRef]
- Fjeldstad, J.; Qi, W.; Siddique, N.; Mercuri, N.; Krivoi, A.; Nayot, D. An artificial intelligence (AI) model non-invasively evaluates endometrial receptivity from ultrasound images, surpassing endometrial thickness (EMT) in predicting implantation. Hum. Reprod. 2024, 39, deae108.025. [Google Scholar] [CrossRef]
- Lee, S.; Arffman, R.K.; Komsi, E.K.; Lindgren, O.; Kemppainen, J.A.; Metsola, H.; Rossi, H.R.; Ahtikoski, A.; Kask, K.; Saare, M.; et al. AI-algorithm training and validation for identification of endometrial CD138+ cells in infertility-associated conditions; polycystic ovary syndrome (PCOS) and recurrent implantation failure (RIF). J. Pathol. Inform. 2024, 29, 100380. [Google Scholar] [CrossRef]
- Lee, S.; Arffman, R.K.; Komsi, E.K.; Lindgren, O.; Kemppainen, J.A.; Kask, K.; Saare, M.; Samulets, A.; Piltonen, T.T. Dynamic changes in AI-based analysis of endometrial cellular composition: Analysis of PCOS and RIF endometrium. J. Pathol. Inform. 2024, 15, 100364. [Google Scholar] [CrossRef]
- Cohen, A.M.; Ye, X.Y.; Colgan, T.J.; Greenblatt, E.M.; Chan, C. Comparing endometrial receptivity array to histologic dating of the endometrium in women with a history of implantation failure. Syst. Biol. Reprod. Med. 2020, 66, 347–354. [Google Scholar] [CrossRef]
- Maziotis, E.; Kalampokas, T.; Giannelou, P.; Grigoriadis, S.; Rapani, A.; Anifantakis, M.; Kotsifaki, A.; Pantou, A.; Triantafyllidou, O.; Tzanakaki, D.; et al. Commercially Available Molecular Approaches to Evaluate Endometrial Receptivity: A Systematic Review and Critical Analysis of the Literature. Diagnostics 2022, 12, 2611. [Google Scholar] [CrossRef]
- Haouzi, D.; Mahmoud, K.; Fourar, M.; Bendhaou, K.; Dechaud, H.; De Vos, J.; Rème, T.; Dewailly, D.; Hamamah, S. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum. Reprod. 2009, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gimeno, P.; Horcajadas, J.A.; Martinez-Conejero, J.A.; Esteban, F.J.; Alama, P.; Pellicer, A.; Simon, C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Enciso, M.; Carrascosa, J.P.; Sarasa, J.; Martínez-Ortiz, P.A.; Munné, S.; Horcajadas, J.A.; Aizpurua, J. Development of a new comprehensive and reliable endometrial receptivity map (ER Map/ER Grade) based on RT-qPCR gene expression analysis. Hum. Reprod. 2018, 33, 220–228. [Google Scholar] [CrossRef]
- Meltsov, A.; Saare, M.; Teder, H.; Paluoja, P.; Arffman, R.K.; Piltonen, T.; Laudanski, P.; Wielgoś, M.; Gianaroli, L.; Koel, M.; et al. Targeted gene expression profiling for accurate endometrial receptivity testing. Sci. Rep. 2023, 13, 13959. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, L.; Choi, J.; LeSaint, C.; Bissonnette, F.; Kadoch, I. Three Different Endometrial Receptivity Profiles Can Be Defined in Patients with Previous Failed Embryo Transfer; Oxford University Press: Oxford, UK, 2019; Volume 34, pp. 308–309. [Google Scholar]
- Drissennek, L.; Baron, C.; Brouillet, S.; Entezami, F.; Hamamah, S.; Haouzi, D. Endometrial miRNome profile according to the receptivity status and implantation failure. Hum. Fertil. 2020, 25, 356–368. [Google Scholar] [CrossRef]
- Haouzi, D.; Entezami, F.; Torre, A.; Innocenti, C.; Antoine, Y.; Mauries, C.; Vincens, C.; Bringer-Deutsch, S.; Gala, A.; Ferrieres-Hoa, A.; et al. Customized Frozen Embryo Transfer after Identification of the Receptivity Window with a Transcriptomic Approach Improves the Implantation and Live Birth Rates in Patients with Repeated Implantation Failure. Reprod. Sci. 2020, 28, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Enciso, M.; Aizpurua, J.; Rodríguez-Estrada, B.; Jurado, I.; Ferrández-Rives, M.; Rodríguez, E.; Pérez-Larrea, E.; Climent, A.B.; Marron, K.; Sarasa, J. The precise determination of the window of implantation significantly improves ART outcomes. Sci. Rep. 2021, 11, 13420. [Google Scholar] [CrossRef]
- Ben Rafael, Z. Endometrial Receptivity Analysis (ERA) test: An unproven technology. Hum. Reprod. Open 2021, 2021, hoab010. [Google Scholar] [CrossRef]
- Teh, W.T.; Chung, J.; Holdsworth-Carson, S.J.; Donoghue, J.F.; Healey, M.; Rees, H.C.; Bittinger, S.; Obers, V.; Sloggett, C.; Kendarsari, R.; et al. A molecular staging model for accurately dating the endometrial biopsy. Nat. Commun. 2023, 14, 6222. [Google Scholar] [CrossRef]
- Lipecki, J.; Mitchell, A.E.; Muter, J.; Lucas, E.S.; Makwana, K.; Fishwick, K.; Odendaal, J.; Hawkes, A.; Vrljicak, P.; Brosens, J.J.; et al. EndoTime: Non-categorical timing estimates for luteal endometrium. Hum. Reprod. 2022, 37, 747–761. [Google Scholar] [CrossRef]
- Cao, D.D.; Wang, J.; Yao, Y.Q.; Yeung, W.S.B. Single-cell analysis in endometrial research. Reprod. Dev. Med. 2022, 6, 197–207. [Google Scholar] [CrossRef]
| Name, Year | Number of Genes, Method | Result | Evidence of the Efficiency |
|---|---|---|---|
| WIN-test, 2009 [62] | 11 genes, qPCR | 3 categories: receptive, partially receptive, non-receptive tissue | Two retrospective and one prospective trial [66,67,68]. Key findings: an increase in clinical pregnancy, ongoing pregnancy, and live birth rates when applied to IVF patients with recurrent implantation failures. |
| ERA, 2011 [63] | 238 genes, microarray | 6 categories: proliferative, early-receptive, partially receptive, receptive, late-receptive, or post-receptive tissue | Thirteen retrospective, two prospective, and one randomised controlled trial (summarized in [61]). Key findings: an increase in clinical pregnancy and live birth rates when applied to IVF patients with recurrent implantation failures; no significant shifts in the general population. |
| ER Map/ER Grade, 2018 [64] | 40 genes, qPCR | 5 categories: proliferative, pre-receptive, receptive, late-receptive, or post-receptive tissue | One retrospective trial [69]. Key findings: the probability of clinical pregnancy during embryo transfer at the predicted receptive state of the endometrium is higher than at the moment that has been estimated as non-receptive state in the general population. |
| beReady, 2019 [65] | 67 genes, targeted allele counting by sequencing | 4 categories: pre-receptive, early-receptive, late-receptive, or post-receptive tissue | No clinical trials. According to the original study, the test is effective in predicting the shift of the “implantation window” when applied for IVF patients with recurrent implantation failures [65]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabin, P.I.; Borodkina, A.V. The Role of the Endometrium in Implantation: A Modern View. Int. J. Mol. Sci. 2024, 25, 9746. https://doi.org/10.3390/ijms25179746
Deryabin PI, Borodkina AV. The Role of the Endometrium in Implantation: A Modern View. International Journal of Molecular Sciences. 2024; 25(17):9746. https://doi.org/10.3390/ijms25179746
Chicago/Turabian StyleDeryabin, Pavel I., and Aleksandra V. Borodkina. 2024. "The Role of the Endometrium in Implantation: A Modern View" International Journal of Molecular Sciences 25, no. 17: 9746. https://doi.org/10.3390/ijms25179746
APA StyleDeryabin, P. I., & Borodkina, A. V. (2024). The Role of the Endometrium in Implantation: A Modern View. International Journal of Molecular Sciences, 25(17), 9746. https://doi.org/10.3390/ijms25179746






