ETS Transcription Factors in Immune Cells and Immune-Related Diseases
Abstract
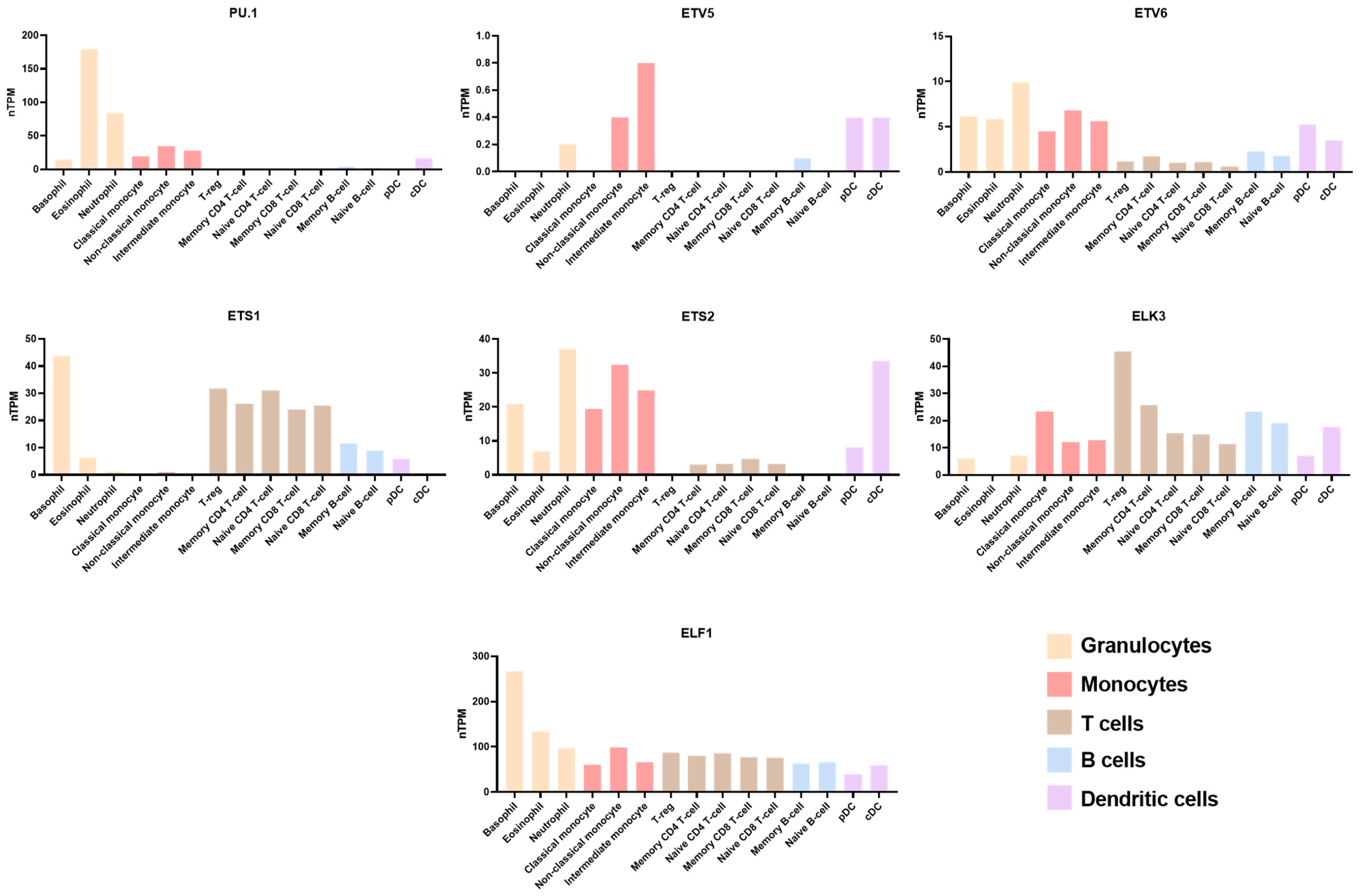
:1. Introduction
1.1. ETS Family Profile
2. ETS Family in Immune Cells
2.1. T Cell
2.2. B Cell
2.3. Macrophage
2.4. Neutrophil
2.5. Dendritic Cells
2.6. Other Immune Cells
3. ETS in Immune-Related Diseases
3.1. Cancer
3.2. Allergies and Autoimmune Diseases
3.3. Arteriosclerosis
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Leprince, D.; Gegonne, A.; Coll, J.; de Taisne, C.; Schneeberger, A.; Lagrou, C.; Stehelin, D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature 1983, 306, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Findlay, V.J.; LaRue, A.C.; Turner, D.P.; Watson, P.M.; Watson, D.K. Understanding the role of ETS-mediated gene regulation in complex biological processes. Adv. Cancer Res. 2013, 119, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Ray-Gallet, D.; Mao, C.; Tavitian, A.; Moreau-Gachelin, F. DNA binding specificities of Spi-1/PU.1 and Spi-B transcription factors and identification of a Spi-1/Spi-B binding site in the c-fes/c-fps promoter. Oncogene 1995, 11, 303–313. [Google Scholar] [PubMed]
- Oettgen, P.; Finger, E.; Sun, Z.; Akbarali, Y.; Thamrongsak, U.; Boltax, J.; Grall, F.; Dube, A.; Weiss, A.; Brown, L.; et al. PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J. Biol. Chem. 2000, 275, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst Peter, C.; Ferris Mary, W.; Hull Megan, A.; Heejoon, C.; Sun, K.; Graves Barbara, J. Oncogenic ETS proteins mimic activated RAS/MAPK signaling in prostate cells. Genes Dev. 2011, 25, 2147–2157. [Google Scholar] [CrossRef]
- Sizemore, G.M.; Pitarresi, J.R.; Balakrishnan, S.; Ostrowski, M.C. The ETS family of oncogenic transcription factors in solid tumours. Nat. Rev. Cancer 2017, 17, 337–351. [Google Scholar] [CrossRef]
- Lopez James, P.; Turner Jerrold, R.; Philipson Louis, H. Glucose-induced ERM protein activation and translocation regulates insulin secretion. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E772–E785. [Google Scholar] [CrossRef]
- Chen, S.-R.; Liu, Y.-X. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167. [Google Scholar] [CrossRef]
- Denechaud, P.D.; Lopez-Mejia, I.C.; Giralt, A.; Lai, Q.; Blanchet, E.; Delacuisine, B.; Nicolay, B.N.; Dyson, N.J.; Bonner, C.; Pattou, F.; et al. E2F1 mediates sustained lipogenesis and contributes to hepatic steatosis. J. Clin. Investig. 2016, 126, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Newton, K.; Kummerfeld, S.K.; Webster, J.; Kirkpatrick, D.S.; Phu, L.; Eastham-Anderson, J.; Liu, J.; Lee, W.P.; Wu, J.; et al. Transcription factor Etv5 is essential for the maintenance of alveolar type II cells. Proc. Natl. Acad. Sci. USA 2017, 114, 3903–3908. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Li, G.; Zhang, M.; Xing, P.; Li, Z.; Chen, B.; Yang, H.; Wu, Z. Establishment of Etv5 gene knockout mice as a recipient model for spermatogonial stem cell transplantation. Biol. Open 2021, 10, bio056804. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, T.; Ji, X.; Zhang, Q.; Zhang, X.; Wu, Z.; Li, Z.; Yang, H. Wdr17 Regulates Cell Proliferation, Cell Cycle Progression and Apoptosis in Mouse Spermatocyte Cell Line. Animals 2024, 14, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Watson, D.K. ETS transcription factors and their emerging roles in human cancer. Eur. J. Cancer 2005, 41, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; Gentile, L.N.; Donaldson, L.W.; Mackereth, C.D.; Seidel, J.J.; Graves, B.J.; McIntosh, L.P. Structure of the Ets-1 pointed domain and mitogen-activated protein kinase phosphorylation site. Proc. Natl. Acad. Sci. USA 1998, 95, 12129–12134. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, P.C.; McIntosh, L.P.; Graves, B.J. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 2011, 80, 437–471. [Google Scholar] [CrossRef] [PubMed]
- Turkistany, S.A.; DeKoter, R.P. The transcription factor PU.1 is a critical regulator of cellular communication in the immune system. Arch. Immunol. Ther. Exp. 2011, 59, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Jojic, V.; Shay, T.; Sylvia, K.; Zuk, O.; Sun, X.; Kang, J.; Regev, A.; Koller, D. Immunological Genome Project Conso. Identification of transcriptional regulators in the mouse immune system. Nat. Immunol. 2013, 14, 633–643. [Google Scholar] [CrossRef]
- Hock, H.; Shimamura, A. ETV6 in hematopoiesis and leukemia predisposition. Semin. Hematol. 2017, 54, 98–104. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper T lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Sehra, S.; Sun, X.; Kaplan, M.H. The transcription factor Etv5 controls TH17 cell development and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.; Hufford, M.M.; Sun, X.; Kaplan, M.H. Etv5 Regulates IL-10 Production in Th Cells. J. Immunol. 2017, 198, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Jacobson, N.G.; Bhattacharya, D.; Gorham, J.D.; Fenoglio, D.; Sha, W.C.; Murphy, T.L.; Murphy, K.M. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 3888–3893. [Google Scholar] [CrossRef]
- Hosokawa, H.; Ungerbäck, J.; Wang, X.; Matsumoto, M.; Nakayama, K.I.; Cohen, S.M.; Tanaka, T.; Rothenberg, E.V. Transcription Factor PU.1 Represses and Activates Gene Expression in Early T Cells by Redirecting Partner Transcription Factor Binding. Immunity 2018, 48, 1119–1134.e7. [Google Scholar] [CrossRef]
- Ungerbäck, J.; Hosokawa, H.; Wang, X.; Strid, T.; Williams, B.A.; Sigvardsson, M.; Rothenberg, E.V. Pioneering, chromatin remodeling, and epigenetic constraint in early T-cell gene regulation by SPI1 (PU.1). Genome Res. 2018, 28, 1508–1519. [Google Scholar] [CrossRef]
- Ramming, A.; Druzd, D.; Leipe, J.; Schulze-Koops, H.; Skapenko, A. Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood 2012, 119, 4665–4674. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. J. Immunol. 2012, 189, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Rivera Vargas, T.; Cai, Z.; Shen, Y.; Dosset, M.; Benoit-Lizon, I.; Martin, T.; Roussey, A.; Flavell, R.A.; Ghiringhelli, F.; Apetoh, L. Selective degradation of PU.1 during autophagy represses the differentiation and antitumour activity of TH9 cells. Nat. Commun. 2017, 8, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.; Hufford, M.M.; Pham, D.; Olson, M.R.; Wu, T.; Jabeen, R.; Sun, X.; Kaplan, M.H. The ETS Family Transcription Factors Etv5 and PU.1 Function in Parallel To Promote Th9 Cell Development. J. Immunol. 2016, 197, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Guidez, F.; Li, A.C.; Horvai, A.; Welch, J.S.; Glass, C.K. Differential utilization of Ras signaling pathways by macrophage colony-stimulating factor (CSF) and granulocyte-macrophage CSF receptors during macrophage differentiation. Mol. Cell. Biol. 1998, 18, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Ohki, M. Molecular basis of the t(8;21) translocation in acute myeloid leukaemia. Semin. Cancer Biol. 1993, 4, 369–375. [Google Scholar] [PubMed]
- Liu, H.B.; Holm, M.; Xie, X.Q.; Wolf-Watz, M.; Grundström, T. AML1/Runx1 recruits calcineurin to regulate granulocyte macrophage colony-stimulating factor by Ets1 activation. J. Biol. Chem. 2004, 279, 29398–29408. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.H.; Carotta, S.; Nutt, S.L. Transcriptional control of pre-B cell development and leukemia prevention. Curr. Top. Microbiol. Immunol. 2014, 381, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Carotta, S.; Willis, S.N.; Hasbold, J.; Inouye, M.; Pang, S.H.; Emslie, D.; Light, A.; Chopin, M.; Shi, W.; Wang, H.; et al. The transcription factors IRF8 and PU.1 negatively regulate plasma cell differentiation. J. Exp. Med. 2014, 211, 2169–2181. [Google Scholar] [CrossRef]
- Pang, S.H.; Minnich, M.; Gangatirkar, P.; Zheng, Z.; Ebert, A.; Song, G.; Dickins, R.A.; Corcoran, L.M.; Mullighan, C.G.; Busslinger, M.; et al. PU.1 cooperates with IRF4 and IRF8 to suppress pre-B-cell leukemia. Leukemia 2016, 30, 1375–1387. [Google Scholar] [CrossRef]
- Willis, S.N.; Tellier, J.; Liao, Y.; Trezise, S.; Light, A.; O’Donnell, K.; Garrett-Sinha, L.A.; Shi, W.; Tarlinton, D.M.; Nutt, S.L. Environmental sensing by mature B cells is controlled by the transcription factors PU.1 and SpiB. Nat. Commun. 2017, 8, 1426–1439. [Google Scholar] [CrossRef]
- DeKoter, R.P.; Lee, H.J.; Singh, H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity 2002, 16, 297–309. [Google Scholar] [CrossRef]
- Scialdone, A.; Khazaei, S.; Hasni, M.S.; Lennartsson, A.; Gullberg, U.; Drott, K. Depletion of the transcriptional coactivators CREB-binding protein or EP300 downregulates CD20 in diffuse large B-cell lymphoma cells and impairs the cytotoxic effects of anti-CD20 antibodies. Exp. Hematol. 2019, 79, 35–46.e31. [Google Scholar] [CrossRef]
- Soodgupta, D.; White, L.S.; Yang, W.; Johnston, R.; Andrews, J.M.; Kohyama, M.; Murphy, K.M.; Mosammaparast, N.; Payton, J.E.; Bednarski, J.J. RAG-Mediated DNA Breaks Attenuate PU.1 Activity in Early B Cells through Activation of a SPIC-BCLAF1 Complex. Cell Rep. 2019, 29, 829–843.e825. [Google Scholar] [CrossRef]
- Karpurapu, M.; Wang, X.; Deng, J.; Park, H.; Xiao, L.; Sadikot, R.T.; Frey, R.S.; Maus, U.A.; Park, G.Y.; Scott, E.W.; et al. Functional PU.1 in macrophages has a pivotal role in NF-κB activation and neutrophilic lung inflammation during endotoxemia. Blood 2011, 118, 5255–5266. [Google Scholar] [CrossRef] [PubMed]
- Lloberas, J.; Soler, C.; Celada, A. The key role of PU.1/SPI-1 in B cells, myeloid cells and macrophages. Immunol. Today 1999, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Celada, A.; Borras, F.E.; Soler, C.; Lloberas, J.; Klemsz, M.; vanBeveren, C.; McKercher, S.; Maki, R.A. The transcription factor PU.1 is involved in macrophage proliferation. J. Exp. Med. 1996, 184, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tagore, M.; McAndrew, M.J.; Gjidoda, A.; Floer, M. The Lineage-Specific Transcription Factor PU.1 Prevents Polycomb-Mediated Heterochromatin Formation at Macrophage-Specific Genes. Mol. Cell. Biol. 2015, 35, 2610–2625. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Kwon, M.; Cho, Y.J.; Hu, N.; Pedchenko, T.V.; Sadikot, R.T.; Blackwell, T.S.; Christman, J.W. Lipopolysaccharide-dependent interaction between PU.1 and cJun determines production of lipocalin-type prostaglandin D synthase and prostaglandin D2 in macrophages. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L771–L779. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.C.; Wang, X.; Park, G.Y.; Sadikot, R.T.; Cao, H.; Mathew, B.; Atchison, M.; Van Breemen, R.B.; Joo, M.; Christman, J.W. NF-κB-inducing kinase regulates cyclooxygenase 2 gene expression in macrophages by phosphorylation of PU.1. J. Immunol. 2007, 179, 7868–7875. [Google Scholar] [CrossRef]
- Karpurapu, M.; Kakarala, K.K.; Chung, S.; Nie, Y.; Koley, A.; Dougherty, P.; Christman, J.W. Epigallocatechin gallate regulates the myeloid-specific transcription factor PU.1 in macrophages. PLoS ONE 2024, 19, e0301904. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, L.; Ghorbani, S.; Talebi, F.; Noorbakhsh, F. MicroRNA-150 targets PU.1 and regulates macrophage differentiation and function in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018, 323, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Yoon, J.H.; Kim, K.S.; Ahn, D.W. c-Ets1 inhibits the interaction of NF-κB and CREB, and downregulates IL-1β-induced MUC5AC overproduction during airway inflammation. Mucosal Immunol. 2012, 5, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, Z.; Li, N.; Jiang, W.; Gao, P.; Yang, M.; Yu, X.; Wang, G.; Zhang, Y. Ets2 suppresses inflammatory cytokines through MAPK/NF-κB signaling and directly binds to the IL-6 promoter in macrophages. Aging 2019, 11, 10610–10625. [Google Scholar] [CrossRef]
- Chen, Y.H.; Layne, M.D.; Chung, S.W.; Ejima, K.; Baron, R.M.; Yet, S.F.; Perrella, M.A. Elk-3 is a transcriptional repressor of nitric-oxide synthase 2. J. Biol. Chem. 2003, 278, 39572–39577. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Chen, Y.H.; Yet, S.F.; Layne, M.D.; Perrella, M.A. Endotoxin-induced down-regulation of Elk-3 facilitates heme oxygenase-1 induction in macrophages. J. Immunol. 2006, 176, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.; Schlee, M.; Roth, S.; Mraheil, M.A.; Barchet, W.; Böttcher, J.; Hain, T.; Geiger, S.; Hayakawa, Y.; Fritz, J.H.; et al. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 2012, 31, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Kiene, N.J.; Tatarian, A.; Eix, E.F.; Schorey, J.S. Host cytosolic RNA sensing pathway promotes T Lymphocyte-mediated mycobacterial killing in macrophages. PLoS Pathog. 2020, 16, e1008569. [Google Scholar] [CrossRef]
- McIvor, Z.; Hein, S.; Fiegler, H.; Schroeder, T.; Stocking, C.; Just, U.; Cross, M. Transient expression of PU.1 commits multipotent progenitors to a myeloid fate whereas continued expression favors macrophage over granulocyte differentiation. Exp. Hematol. 2003, 31, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Geldart, A.M.; Christou, H.; Liu, X.; Chung, S.W.; Perrella, M.A. Elk-3 is a KLF4-regulated gene that modulates the phagocytosis of bacteria by macrophages. J. Leukoc. Biol. 2015, 97, 171–180. [Google Scholar] [CrossRef]
- Hu, R.D.; Zhang, W.; Li, L.; Zuo, Z.Q.; Ma, M.; Ma, J.F.; Yin, T.T.; Gao, C.Y.; Yang, S.H.; Zhao, Z.B.; et al. Chromatin accessibility analysis identifies the transcription factor ETV5 as a suppressor of adipose tissue macrophage activation in obesity. Cell Death Dis. 2021, 12, 1023–1033. [Google Scholar] [CrossRef]
- DiSpirito, J.R.; Fang, B.; Wang, F.; Lazar, M.A. Pruning of the Adipocyte Peroxisome Proliferator-Activated Receptor γ Cistrome by Hematopoietic Master Regulator PU.1. Mol. Cell. Biol. 2013, 33, 3354–3364. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Haimovici, A.; Brigger, D.; Torbett, B.E.; Fey, M.F.; Tschan, M.P. Induction of the autophagy-associated gene MAP1S via PU.1 supports APL differentiation. Leuk. Res. 2014, 38, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Federzoni, E.A.; Valk, P.J.; Torbett, B.E.; Haferlach, T.; Löwenberg, B.; Fey, M.F.; Tschan, M.P. PU.1 is linking the glycolytic enzyme HK3 in neutrophil differentiation and survival of APL cells. Blood 2012, 119, 4963–4970. [Google Scholar] [CrossRef] [PubMed]
- Malu, K.; Garhwal, R.; Pelletier, M.G.; Gotur, D.; Halene, S.; Zwerger, M.; Yang, Z.F.; Rosmarin, A.G.; Gaines, P. Cooperative Activity of GABP with PU.1 or C/EBPε Regulates Lamin B Receptor Gene Expression, Implicating Their Roles in Granulocyte Nuclear Maturation. J. Immunol. 2016, 197, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Walter, C.; Tönges, A.; Aleth, H.; Jordão, M.J.C.; Leddin, M.; Gröning, V.; Erdmann, T.; Lenz, G.; Roth, J.; et al. Safeguard function of PU.1 shapes the inflammatory epigenome of neutrophils. Nat. Immunol. 2019, 20, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Keightley, M.C.; Carradice, D.P.; Layton, J.E.; Pase, L.; Bertrand, J.Y.; Wittig, J.G.; Dakic, A.; Badrock, A.P.; Cole, N.J.; Traver, D.; et al. The Pu.1 target gene Zbtb11 regulates neutrophil development through its integrase-like HHCC zinc finger. Nat. Commun. 2017, 8, 14911. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Steinman, R.M.; Kaplan, G.; Witmer, M.D.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J. Exp. Med. 1979, 149, 1–16. [Google Scholar] [CrossRef]
- Metlay, J.P.; Witmer-Pack, M.D.; Agger, R.; Crowley, M.T.; Lawless, D.; Steinman, R.M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990, 171, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Muhlethaler-Mottet, A.; Otten, L.A.; Steimle, V.; Mach, B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997, 16, 2851–2860. [Google Scholar] [CrossRef]
- Smith, M.A.; Wright, G.; Wu, J.; Tailor, P.; Ozato, K.; Chen, X.; Wei, S.; Piskurich, J.F.; Ting, J.P.; Wright, K.L. Positive regulatory domain I (PRDM1) and IRF8/PU.1 counter-regulate MHC class II transactivator (CIITA) expression during dendritic cell maturation. J. Biol. Chem. 2011, 286, 7893–7904. [Google Scholar] [CrossRef]
- Carotta, S.; Dakic, A.; D’Amico, A.; Pang, S.H.; Greig, K.T.; Nutt, S.L.; Wu, L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity 2010, 32, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Laiosa, C.V.; Stadtfeld, M.; Xie, H.; de Andres-Aguayo, L.; Graf, T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBPα and PU.1 transcription factors. Immunity 2006, 25, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L.; Perkin, H.; Surh, C.D.; Venturini, S.; Maki, R.A.; Torbett, B.E. Transcription factor PU.1 is necessary for development of thymic and myeloid progenitor-derived dendritic cells. J. Immunol. 2000, 164, 1855–1861. [Google Scholar] [CrossRef]
- Yashiro, T.; Kasakura, K.; Oda, Y.; Kitamura, N.; Inoue, A.; Nakamura, S.; Yokoyama, H.; Fukuyama, K.; Hara, M.; Ogawa, H.; et al. The hematopoietic cell-specific transcription factor PU.1 is critical for expression of CD11c. Int. Immunol. 2017, 29, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Yokoyama, H.; Yashiro, T.; Nakano, N.; Nishiyama, M.; Kanada, S.; Fukai, T.; Hara, M.; Ikeda, S.; Ogawa, H.; et al. Role of PU.1 in MHC class II expression through transcriptional regulation of class II transactivator pI in dendritic cells. J. Allergy Clin. Immunol. 2012, 129, 814–824.e816. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Kasakura, K.; Nakano, N.; Hara, M.; Maeda, K.; Okumura, K.; Ogawa, H.; Yashiro, T.; Nishiyama, C. Role of PU.1 in MHC Class II Expression via CIITA Transcription in Plasmacytoid Dendritic Cells. PLoS ONE 2016, 11, e0154094. [Google Scholar] [CrossRef] [PubMed]
- Kanada, S.; Nishiyama, C.; Nakano, N.; Suzuki, R.; Maeda, K.; Hara, M.; Kitamura, N.; Ogawa, H.; Okumura, K. Critical role of transcription factor PU.1 in the expression of CD80 and CD86 on dendritic cells. Blood 2011, 117, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, T.; Hara, M.; Ogawa, H.; Okumura, K.; Nishiyama, C. Critical Role of Transcription Factor PU.1 in the Function of the OX40L/TNFSF4 Promoter in Dendritic Cells. Sci. Rep. 2016, 6, 34825. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, T.; Nakano, S.; Nomura, K.; Uchida, Y.; Kasakura, K.; Nishiyama, C. A transcription factor PU.1 is critical for Ccl22 gene expression in dendritic cells and macrophages. Sci. Rep. 2019, 9, 1161–1169. [Google Scholar] [CrossRef]
- Yashiro, T.; Takeuchi, H.; Nakamura, S.; Tanabe, A.; Hara, M.; Uchida, K.; Okumura, K.; Kasakura, K.; Nishiyama, C. PU.1 plays a pivotal role in dendritic cell migration from the periphery to secondary lymphoid organs via regulating CCR7 expression. FASEB J. 2019, 33, 11481–11491. [Google Scholar] [CrossRef]
- Domínguez-Soto, A.; Puig-Kröger, A.; Vega, M.A.; Corbí, A.L. PU.1 regulates the tissue-specific expression of dendritic cell-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin. J. Biol. Chem. 2005, 280, 33123–33131. [Google Scholar] [CrossRef] [PubMed]
- Chopin, M.; Lun, A.T.; Zhan, Y.; Schreuder, J.; Coughlan, H.; D’Amico, A.; Mielke, L.A.; Almeida, F.F.; Kueh, A.J.; Dickins, R.A.; et al. Transcription Factor PU.1 Promotes Conventional Dendritic Cell Identity and Function via Induction of Transcriptional Regulator DC-SCRIPT. Immunity 2019, 50, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Vissers, J.L.; Hartgers, F.C.; Lindhout, E.; Teunissen, M.B.; Figdor, C.G.; Adema, G.J. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J. Leukoc. Biol. 2001, 69, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yang, B.; He, Q.; Wang, J.; Weng, Q. New Insights of CCR7 Signaling in Dendritic Cell Migration and Inflammatory Diseases. Front. Pharmacol. 2022, 13, 841687. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, Y.; Hao, L.; Zhang, Y. DC-SIGN and immunoregulation. Cell Mol. Immunol. 2006, 3, 279–283. [Google Scholar] [CrossRef]
- Oda, Y.; Kasakura, K.; Fujigaki, I.; Kageyama, A.; Okumura, K.; Ogawa, H.; Yashiro, T.; Nishiyama, C. The effect of PU.1 knockdown on gene expression and function of mast cells. Sci. Rep. 2018, 8, 2005. [Google Scholar] [CrossRef]
- Ito, T.; Nishiyama, C.; Nishiyama, M.; Matsuda, H.; Maeda, K.; Akizawa, Y.; Tsuboi, R.; Okumura, K.; Ogawa, H. Mast cells acquire monocyte-specific gene expression and monocyte-like morphology by overproduction of PU.1. J. Immunol. 2005, 174, 376–383. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Maeda, K.; Yashiro, T.; Inage, E.; Niyonsaba, F.; Hara, M.; Suzuki, R.; Ohtsuka, Y.; Shimizu, T.; Ogawa, H.; et al. Involvement of PU.1 in Mast Cell/Basophil-Specific Function of the Human IL1RL1 ST2 Promoter. Allergol. Int. 2012, 61, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nishiyama, C.; Nakano, N.; Nishiyama, M.; Usui, Y.; Takeda, K.; Kanada, S.; Fukuyama, K.; Akiba, H.; Tokura, T.; et al. Roles of PU.1 in monocyte- and mast cell-specific gene regulation: PU.1 transactivates CIITA pIV in cooperation with IFN-γ. Int. Immunol. 2009, 21, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C.; DeKoter, R.P.; Lee, H.J.; Smith, E.D.; Lancki, D.W.; Gurish, M.F.; Friend, D.S.; Stevens, R.L.; Anastasi, J.; Singh, H. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity 2002, 17, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, C.M.; Brandal, S.; Jegga, A.G.; Lee, Y.N.; Shahlaee, A.; Ying, Y.; DeKoter, R.; McDevitt, M.A. PU.1 Positively Regulates GATA-1 Expression in Mast Cells. J. Immunol. 2010, 184, 4349–4361. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, Z.; Chen, Y.; Hou, W.; Zhong, W.; Zhou, Y.; Zhang, A.; Xu, Y. PU.1 induces tumor-associated macrophages promoting glioma progression through BTK-mediated Akt/mTOR pathway activation. Am. J. Cancer Res. 2024, 14, 1139–1156. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhou, C.; Feng, H.; Li, J.; Xia, T.; Cheng, X.; Zhao, R.; Zou, D. ETV1 Positively Correlated With Immune Infiltration and Poor Clinical Prognosis in Colorectal Cancer. Front. Immunol. 2022, 13, 939806. [Google Scholar] [CrossRef]
- Zhou, C.; Uluisik, R.; Rowley, J.W.; David, C.; Jones, C.L.; Scharer, C.D.; Noetzli, L.; Fisher, M.H.; Kirkpatrick, G.D.; Bark, K.; et al. Germline ETV6 mutation promotes inflammation and disrupts lymphoid development of early hematopoietic progenitors. Exp. Hematol. 2022, 112, 24–34. [Google Scholar] [CrossRef]
- Kodgule, R.; Goldman, J.W.; Monovich, A.C.; Saari, T.; Aguilar, A.R.; Hall, C.N.; Rajesh, N.; Gupta, J.; Chu, S.C.A.; Ye, L.; et al. ETV6 Deficiency Unlocks ERG-Dependent Microsatellite Enhancers to Drive Aberrant Gene Activation in B-Lymphoblastic Leukemia. Blood Cancer Discov. 2023, 4, 34–53. [Google Scholar] [CrossRef]
- Sharma, G.; Tran, T.M.; Bansal, I.; Beg, M.S.; Bhardwaj, R.; Bassi, J.; Tan, Y.; Jaiswal, A.K.; Tso, C.; Jain, A.; et al. RNA binding protein IGF2BP1 synergizes with ETV6-RUNX1 to drive oncogenic signaling in B-cell Acute Lymphoblastic Leukemia. J. Exp. Clin. Cancer Res. 2023, 42, 231–250. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Sun, M.; Huang, W.; Xia, L. ETS transcription factors: Multifaceted players from cancer progression to tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188872. [Google Scholar] [CrossRef]
- Qian, F.; Deng, J.; Lee, Y.G.; Zhu, J.; Karpurapu, M.; Chung, S.; Zheng, J.N.; Xiao, L.; Park, G.Y.; Christman, J.W. The transcription factor PU.1 promotes alternative macrophage polarization and asthmatic airway inflammation. J. Mol. Cell Biol. 2015, 7, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dai, S.; Qiu, C.; Wang, T.; Zhou, Y.; Xue, C.; Yao, J.; Xu, Y. MicroRNA-219a-5p suppresses intestinal inflammation through inhibiting Th1/Th17-mediated immune responses in inflammatory bowel disease. Mucosal Immunol. 2020, 13, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mistri, S.K.; Hilton, B.M.; Horrigan, K.J.; Andretta, E.S.; Savard, R.; Dienz, O.; Hampel, K.J.; Gerrard, D.L.; Rose, J.T.; Sidiropoulos, N.; et al. SLAM/SAP signaling regulates discrete gammadelta T cell developmental checkpoints and shapes the innate-like gammadelta TCR repertoire. bioRxiv Prepr. Serv. Biol. 2024. [Google Scholar]
- Wei, X.; Niu, X. T follicular helper cells in autoimmune diseases. J. Autoimmun. 2023, 134, 102976. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Regulation and function of capicua in mammals. Exp. Mol. Med. 2020, 52, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.; Kim, E.; Lee, Y. The Capicua/ETS Translocation Variant 5 Axis Regulates Liver-Resident Memory CD8+ T-Cell Development and the Pathogenesis of Liver Injury. Hepatology 2019, 70, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Hur, Y.; Kim, C.J.; Kim, H.B.; Um, D.; Kim, D.S.; Lee, J.Y.; Park, S.; Park, Y.; et al. ETV5 promotes lupus pathogenesis and follicular helper T cell differentiation by inducing osteopontin expression. Proc. Natl. Acad. Sci. USA 2024, 121, e2322009121. [Google Scholar] [CrossRef]
- Xiong, X.; Yan, Z.; Jiang, W.; Jiang, X. ETS variant transcription factor 6 enhances oxidized low-density lipoprotein-induced inflammatory response in atherosclerotic macrophages via activating NF-κB signaling. Int. J. Immunopathol. Pharmacol. 2022, 36, 1–12. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Han, X.; Sun, L.; Shao, F.; Yin, Y.; Zhang, W. ETS Transcription Factors in Immune Cells and Immune-Related Diseases. Int. J. Mol. Sci. 2024, 25, 10004. https://doi.org/10.3390/ijms251810004
Yang Y, Han X, Sun L, Shao F, Yin Y, Zhang W. ETS Transcription Factors in Immune Cells and Immune-Related Diseases. International Journal of Molecular Sciences. 2024; 25(18):10004. https://doi.org/10.3390/ijms251810004
Chicago/Turabian StyleYang, Yaxu, Xue Han, Lijun Sun, Fangyu Shao, Yue Yin, and Weizhen Zhang. 2024. "ETS Transcription Factors in Immune Cells and Immune-Related Diseases" International Journal of Molecular Sciences 25, no. 18: 10004. https://doi.org/10.3390/ijms251810004
APA StyleYang, Y., Han, X., Sun, L., Shao, F., Yin, Y., & Zhang, W. (2024). ETS Transcription Factors in Immune Cells and Immune-Related Diseases. International Journal of Molecular Sciences, 25(18), 10004. https://doi.org/10.3390/ijms251810004







