Serum Concentration of IL-5 Receptor (IL-5R) and Associations with Disease Severity in Patients with Chronic Spontaneous Urticaria (CSU) and Atopic Dermatitis (AD)
Abstract
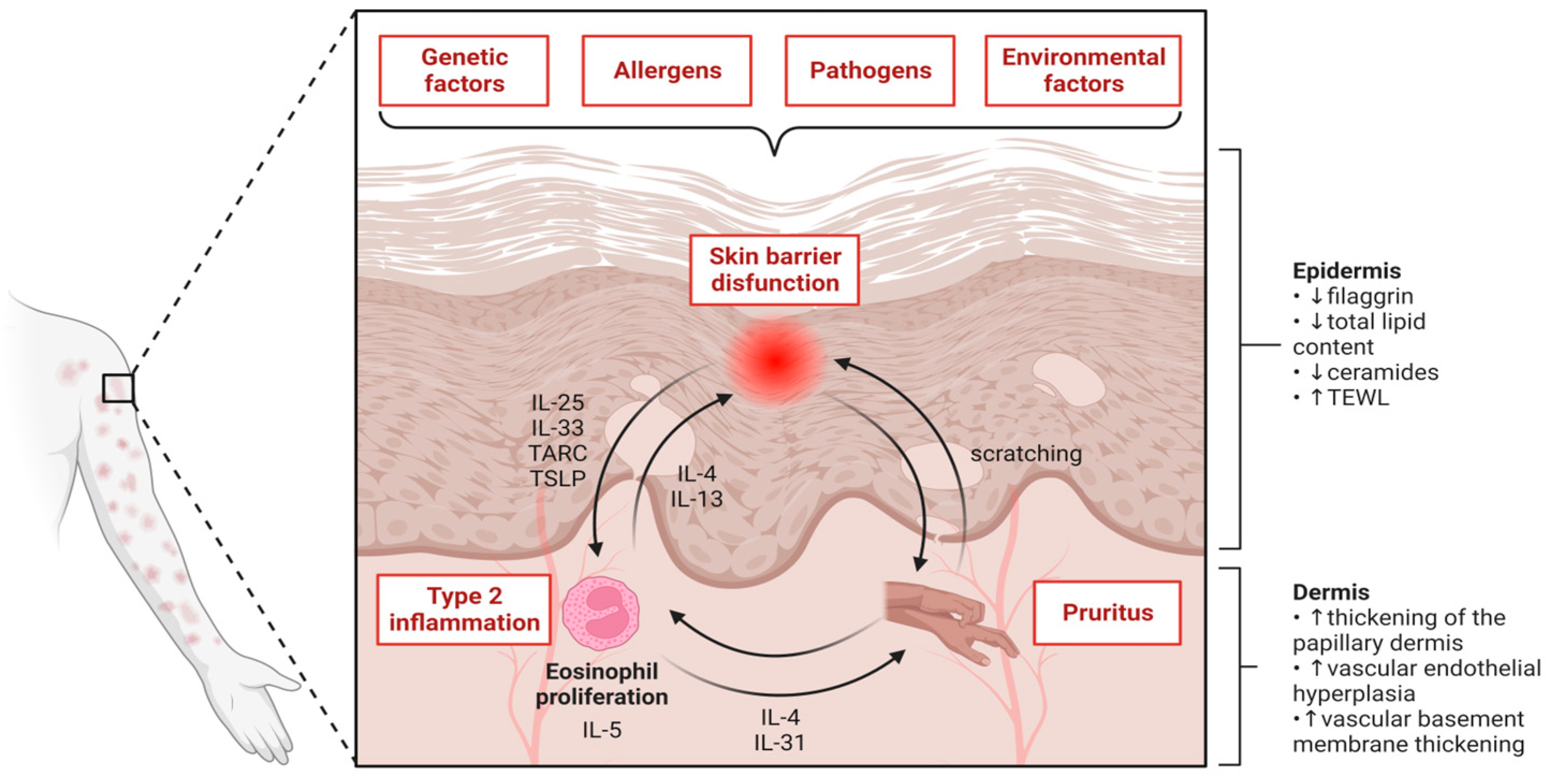
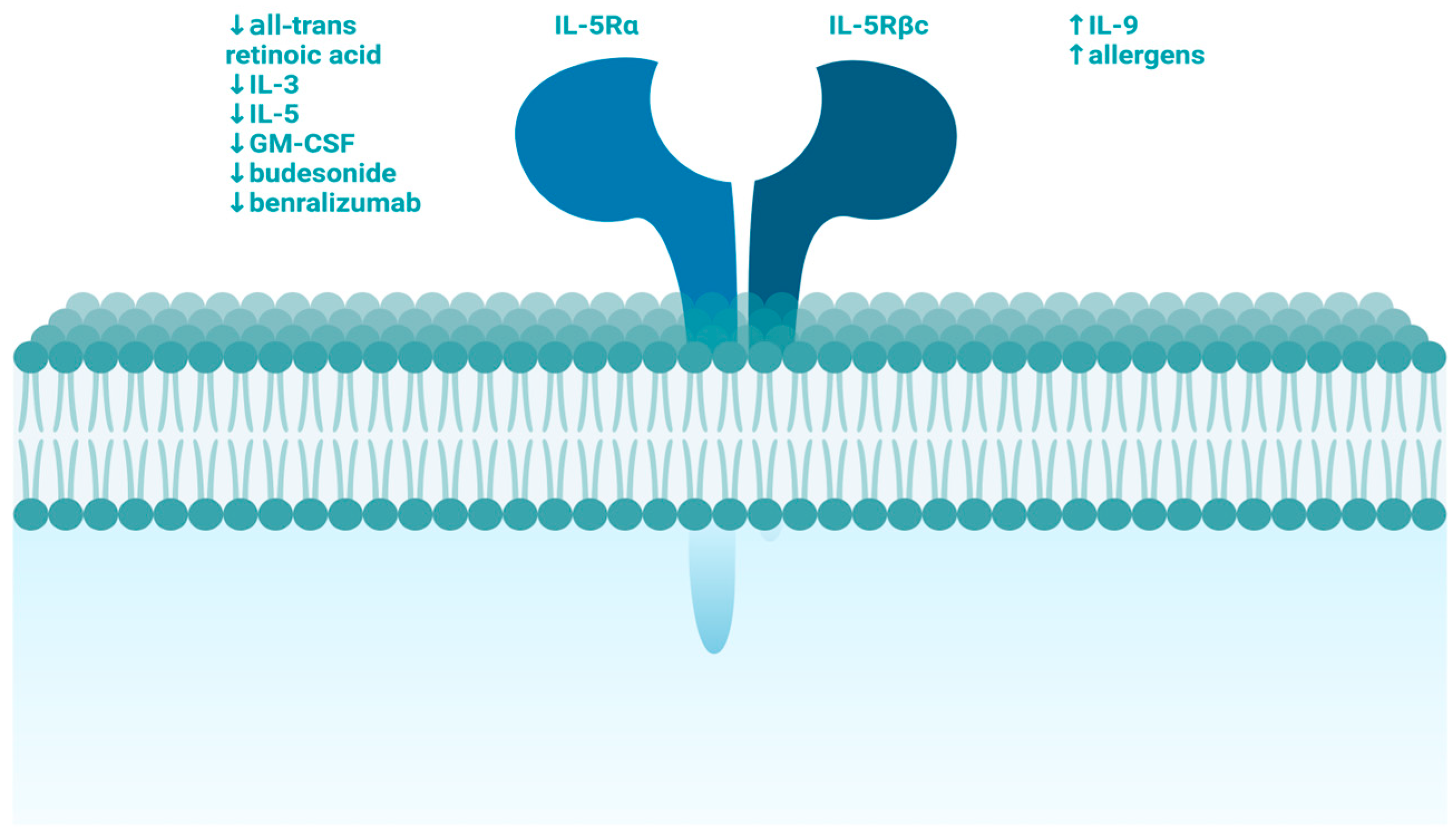
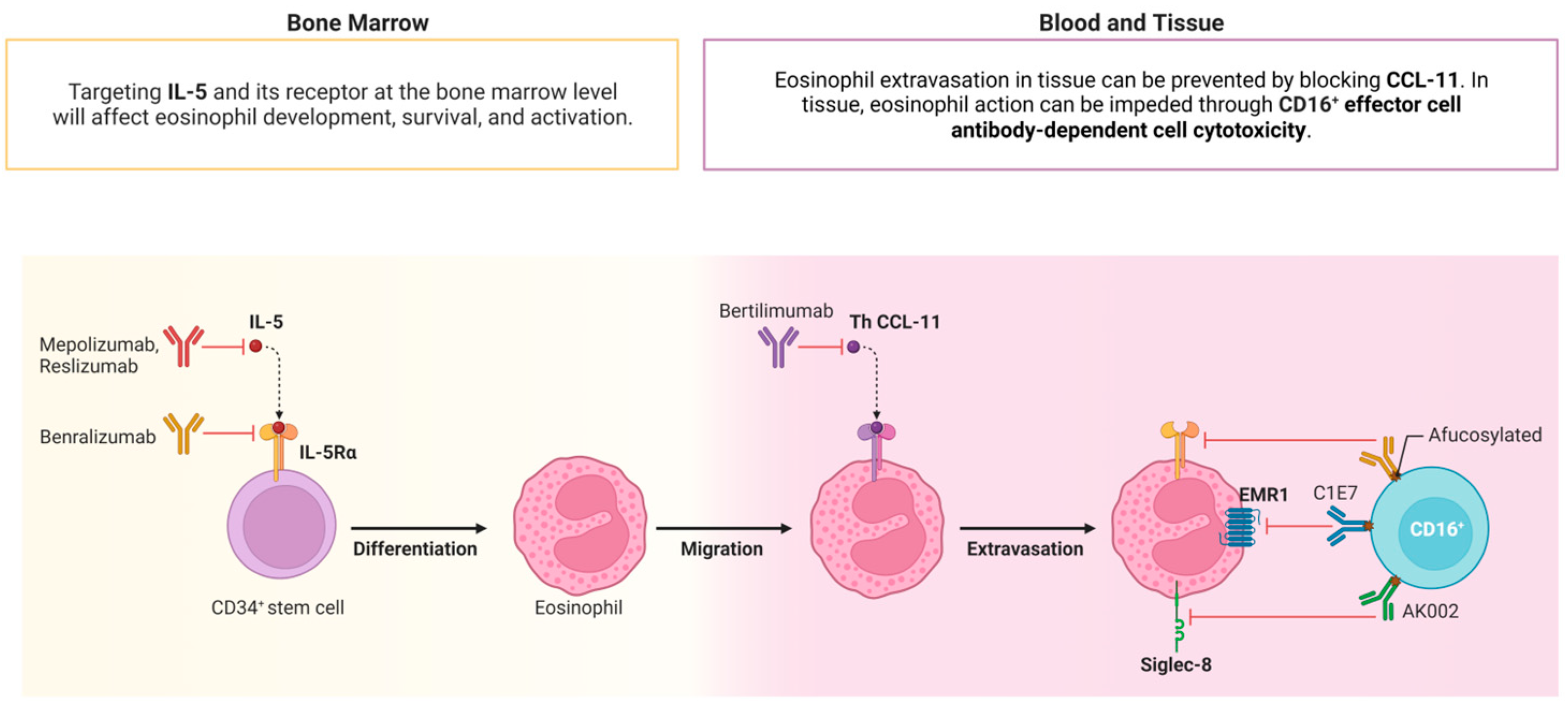
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Examined Groups
4.2. Blood Collection and Biochemical Analysis
4.3. Disease Severity
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.A.; Ensina, L.F.; Fomina, D.; Galvàn, C.A.; Godse, K.; Grattan, C.; et al. The Global Burden of Chronic Urticaria for the Patient and Society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Fasseeh, A.N.; Elezbawy, B.; Korra, N.; Tannira, M.; Dalle, H.; Aderian, S.; Abaza, S.; Kaló, Z. Burden of Atopic Dermatitis in Adults and Adolescents: A Systematic Literature Review. Dermatol. Ther. 2022, 12, 2653–2668. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Giménez-Arnau, A.M.; Kulthanan, K.; Peter, J.; Metz, M.; Maurer, M. Urticaria. Nat. Rev. Dis. Primers 2022, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Baum, A.; Ben-Shoshan, M.; Tzanani, I.; Hakroush, R.; Coster, D.; Solomon, M.; Greenberger, S. Epidemiological and Clinical Characteristics of Adult and Pediatric Patients with Chronic Spontaneous Urticaria. J. Clin. Med. 2023, 12, 7482. [Google Scholar] [CrossRef] [PubMed]
- Stepaniuk, P.; Kan, M.; Kanani, A. Natural History, Prognostic Factors and Patient Perceived Response to Treatment in Chronic Spontaneous Urticaria. Allergy Asthma Clin. Immunol. 2020, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Gomułka, K.; Panaszek, B. Contact Urticaria Syndrome Caused by Haptens. Adv. Dermatol. Allergol./Postȩpy Dermatologii i Alergologii 2014, 31, 108. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. Wytyczne EAACI/GA2LEN/EDF/WAO Dotyczące Definicji, Klasyfikacji, Diagnostyki i Leczenia Pokrzywki. Alergol. Pol. Pol. J. Allergol. 2020, 7, 1–28. [Google Scholar] [CrossRef]
- Kay, A.B.; Ying, S.; Ardelean, E.; Mlynek, A.; Kita, H.; Clark, P.; Maurer, M. Elevations in Vascular Markers and Eosinophils in Chronic Spontaneous Urticarial Weals with Low-Level Persistence in Uninvolved Skin. Br. J. Dermatol. 2014, 171, 505–511. [Google Scholar] [CrossRef]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The Role and Relevance of Mast Cells in Urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef]
- Szymanski, L.; Cios, A.; Ciepielak, M.; Stankiewicz, W. Cytokines and Apoptosis in Atopic Dermatitis. Adv. Dermatol. Allergol./Postȩpy Dermatologii i Alergologii 2021, 38, 1. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Silvestre Salvador, J.F.; Romero-Pérez, D.; Encabo-Durán, B. Atopic Dermatitis in Adults: A Diagnostic Challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The Translational Revolution in Atopic Dermatitis: The Paradigm Shift from Pathogenesis to Treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic Dermatitis: The Skin Barrier and Beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef]
- Giménez-Arnau, A.M.; DeMontojoye, L.; Asero, R.; Cugno, M.; Kulthanan, K.; Yanase, Y.; Hide, M.; Kaplan, A.P. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J. Allergy Clin. Immunol. Pract. 2021, 9, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Belmesk, L.; Muntyanu, A.; Cantin, E.; AlHalees, Z.; Jack, C.S.; Le, M.; Sasseville, D.; Iannattone, L.; Ben-Shoshan, M.; Litvinov, I.V.; et al. Prominent Role of Type 2 Immunity in Skin Diseases: Beyond Atopic Dermatitis. J. Cutan. Med. Surg. 2022, 26, 33–49. [Google Scholar] [CrossRef]
- Cosmi, L.; Maggi, L.; Mazzoni, A.; Liotta, F.; Annunziato, F. Biologicals Targeting Type 2 Immunity: Lessons Learned from Asthma, Chronic Urticaria and Atopic Dermatitis. Eur. J. Immunol. 2019, 49, 1334–1343. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Maurer, M.; Metcalfe, D.D.; Pejler, G.; Sagi-Eisenberg, R.; Nilsson, G. The Ingenious Mast Cell: Contemporary Insights into Mast Cell Behavior and Function. Allergy 2022, 77, 83–99. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Liu, R.; Zhu, L.; Peng, C. The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria. Front. Immunol. 2022, 13, 879754. [Google Scholar] [CrossRef]
- Takatsu, K. Interleukin-5 and IL-5 Receptor in Health and Diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463. [Google Scholar] [CrossRef]
- Di Filippo, P.; Russo, D.; Attanasi, M.; Di Pillo, S.; Chiarelli, F. Immunological Targets of Biologic Drugs in Allergic Skin Diseases in Children. Biomedicines 2021, 9, 1615. [Google Scholar] [CrossRef]
- Pitlick, M.M.; Pongdee, T. Combining Biologics Targeting Eosinophils (IL-5/IL-5R), IgE, and IL-4/IL-13 in Allergic and Inflammatory Diseases. World Allergy Organ. J. 2022, 15, 100707. [Google Scholar] [CrossRef]
- Maurer, M.; Khan, D.A.; Elieh Ali Komi, D.; Kaplan, A.P. Biologics for the Use in Chronic Spontaneous Urticaria: When and Which. J. Allergy Clin. Immunol. Pract. 2021, 9, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Braathen, L.R.; Simon, H.U. Eosinophils and Atopic Dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Gomułka, K.; Wójcik, E.; Szepietowski, J.C. Serum Levels of Eosinophil-Derived Neurotoxin, Platelet-Activating Factor and Vascular Endothelial Growth Factor in Adult Patients with Atopic Dermatitis—A Pilot Study. Biomedicines 2022, 10, 3109. [Google Scholar] [CrossRef]
- Haddad, E.B.; Cyr, S.L.; Arima, K.; McDonald, R.A.; Levit, N.A.; Nestle, F.O. Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis. Dermatol. Ther. 2022, 12, 1501–1533. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Weinstein, S.; Janka, L.; Zangrilli, J.; Garin, M. Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. Chest 2016, 150, 799–810. [Google Scholar] [CrossRef]
- Oldhoff, J.M.; Darsow, U.; Werfel, T.; Katzer, K.; Wulf, A.; Laifaoui, J.; Hijnen, D.J.; Plötz, S.; Knol, E.F.; Kapp, A.; et al. Anti-IL-5 Recombinant Humanized Monoclonal Antibody (Mepolizumab) for the Treatment of Atopic Dermatitis. Allergy 2005, 60, 693–696. [Google Scholar] [CrossRef]
- Oldhoff, J.M.; Darsow, U.; Werfel, T.; Bihari, I.C.; Katzer, K.; Laifaoui, J.; Plötz, S.; Kapp, A.; Knol, E.F.; Bruijnzeel-Koomen, C.A.F.M.; et al. No Effect of Anti-Interleukin-5 Therapy (Mepolizumab) on the Atopy Patch Test in Atopic Dermatitis Patients. Int. Arch. Allergy Immunol. 2006, 141, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Corren, J. Inhibition of Interleukin-5 for the Treatment of Eosinophilic Diseases. Discov. Med. 2012, 13, 305–312. [Google Scholar] [PubMed]
- Semic-Jusufagic, A.; Gevaert, P.; Bachert, C.; Murray, C.; Simpson, A.; Custovic, A. Increased Serum-Soluble Interleukin-5 Receptor Alpha Level Precedes the Development of Eczema in Children. Pediatr. Allergy Immunol. 2010, 21, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.A.; Leung, D.Y.M.; Ghaffar, O.; Boguniewicz, M.; Hamid, Q. In Vivo Expression of Cytokine Receptor MRNA in Atopic Dermatitis. J. Allergy Clin. Immunol. 1998, 102, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Namkung, J.H.; Lee, J.E.; Kim, E.; Cho, H.J.; Kim, S.; Shin, E.S.; Cho, E.Y.; Yang, J.M. IL-5 and IL-5 Receptor Alpha Polymorphisms Are Associated with Atopic Dermatitis in Koreans. Allergy 2007, 62, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Mansueto, P.; Melluso, M.; Pellitteri, M.E.; Di Salvo, A.; Caruso, C.; Candore, G.; Cigna, D. Blood Eosinophils and Serum Eosinophil Cationic Protein in Patients with Acute and Chronic Urticaria. Mediat. Inflamm. 1996, 5, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Gomułka, K.; Mędrala, W. Serum Levels of Vascular Endothelial Growth Factor, Platelet Activating Factor and Eosinophil-Derived Neurotoxin in Chronic Spontaneous Urticaria—A Pilot Study in Adult Patients. Int. J. Mol. Sci. 2022, 23, 9631. [Google Scholar] [CrossRef]
- Tota, M.; Łacwik, J.; Laska, J.; Sędek, Ł.; Gomułka, K. The Role of Eosinophil-Derived Neurotoxin and Vascular Endothelial Growth Factor in the Pathogenesis of Eosinophilic Asthma. Cells 2023, 12, 1326. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Frischbutter, S.; Fok, J.S.; Kolkhir, P.; Jiao, Q.; Skov, P.S.; Metz, M.; Church, M.K.; Maurer, M. The Role of Eosinophils in Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. 2020, 145, 1510–1516. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Singh, U.; Rao, M.B.; Berendts, K.; Zhang, X.; Mutasim, D. Treatment of Chronic Spontaneous Urticaria with Benralizumab: Report of Primary Endpoint per-Protocol Analysis and Exploratory Endpoints. Allergy 2021, 76, 1277–1280. [Google Scholar] [CrossRef]
- Kaplan, A.P.; Joseph, K.; Maykut, R.J.; Geba, G.P.; Zeldin, R.K. Treatment of Chronic Autoimmune Urticaria with Omalizumab. J. Allergy Clin. Immunol. 2008, 122, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic Dermatitis. Acta Derm. Venereol. 1980, 60, 44–47. [Google Scholar] [CrossRef]
- Stalder, J.F.; Taïeb, A.; Atherton, D.J.; Bieber, P.; Bonifazi, E.; Broberg, A.; Calza, A.; Coleman, R.; De Prost, Y.; Stalder, J.F.; et al. Severity Scoring of Atopic Dermatitis: The SCORAD Index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Storck, M.; Sandmann, S.; Bruland, P.; Pereira, M.P.; Steinke, S.; Riepe, C.; Soto-Rey, I.; Garcovich, S.; Augustin, M.; Blome, C.; et al. Pruritus Intensity Scales across Europe: A Prospective Validation Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1176–1185. [Google Scholar] [CrossRef]

| IL-5R | CSU Group (n= 15) | AD Group (n = 15) | Control Group (n = 15) |
|---|---|---|---|
| Mean ± SD (pg/mL) | 37.92 ± 4.68 | 35.83 ± 3.79 | 27.12 ± 3.23 |
| Minimal–Maximal Value (pg/mL) | 31.55–42.03 | 23.21–74.57 | 24.82–51.25 |
| p-value | |||
| CSU Group vs. Control Group | 0.038 | ||
| AD Group vs. Control Group | 0.151 | ||
| CSU Group vs. AD Group | 0.348 | ||
| Parameters Mean ± SD (Points) Minimal–Maximal Value (Points) | CSU Group | AD Group | Correlation with IL-5R |
|---|---|---|---|
| UAS7 scale | 31.0 ± 4.95 22–37 | - | r = −0.3; p = 0.847 |
| SCORAD index | - | 40.6 ± 17.20 26–70 | r = −0.9; p = 0.047 |
| Pruritus (VAS) | 6.4 ± 1.67 4–8 | 6.0 ± 2.12 4–9 | CSU group: r = 0.2; p = 0.746 AD group: r = 0.1; p = 0.785 |
| Parameters | CSU Group | AD Group | Control Group |
|---|---|---|---|
| Participants, n | 15 | 15 | 15 |
| Age (years), mean ± SD | 43.2 ± 10.62 | 30 ± 11.31 | 31.67 ± 6.77 |
| Sex female, n (%) | 12 (80%) | 12 (80%) | 11 (73.33%) |
| BMI (kg/m2), mean ± SD | 29.2 ± 7.9 | 27.8 ± 6.3 | 26.7 ± 6.8 |
| Smoker—current or former, n (%) | 7 (46.67%) | 8 (53.33%) | 6 (40%) |
| Other concomitant atopic disease: (asthma, rhinitis), n (%) | 3 (20%) | 5 (33.33%) | 3 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomułka, K.; Tota, M.; Laska, J.; Gojny, K.; Sędek, Ł. Serum Concentration of IL-5 Receptor (IL-5R) and Associations with Disease Severity in Patients with Chronic Spontaneous Urticaria (CSU) and Atopic Dermatitis (AD). Int. J. Mol. Sci. 2024, 25, 7598. https://doi.org/10.3390/ijms25147598
Gomułka K, Tota M, Laska J, Gojny K, Sędek Ł. Serum Concentration of IL-5 Receptor (IL-5R) and Associations with Disease Severity in Patients with Chronic Spontaneous Urticaria (CSU) and Atopic Dermatitis (AD). International Journal of Molecular Sciences. 2024; 25(14):7598. https://doi.org/10.3390/ijms25147598
Chicago/Turabian StyleGomułka, Krzysztof, Maciej Tota, Julia Laska, Karina Gojny, and Łukasz Sędek. 2024. "Serum Concentration of IL-5 Receptor (IL-5R) and Associations with Disease Severity in Patients with Chronic Spontaneous Urticaria (CSU) and Atopic Dermatitis (AD)" International Journal of Molecular Sciences 25, no. 14: 7598. https://doi.org/10.3390/ijms25147598
APA StyleGomułka, K., Tota, M., Laska, J., Gojny, K., & Sędek, Ł. (2024). Serum Concentration of IL-5 Receptor (IL-5R) and Associations with Disease Severity in Patients with Chronic Spontaneous Urticaria (CSU) and Atopic Dermatitis (AD). International Journal of Molecular Sciences, 25(14), 7598. https://doi.org/10.3390/ijms25147598






