MIND Diet Impact on Multiple Sclerosis Patients: Biochemical Changes after Nutritional Intervention
Abstract
1. Introduction
2. Results
2.1. Characteristics of Participants
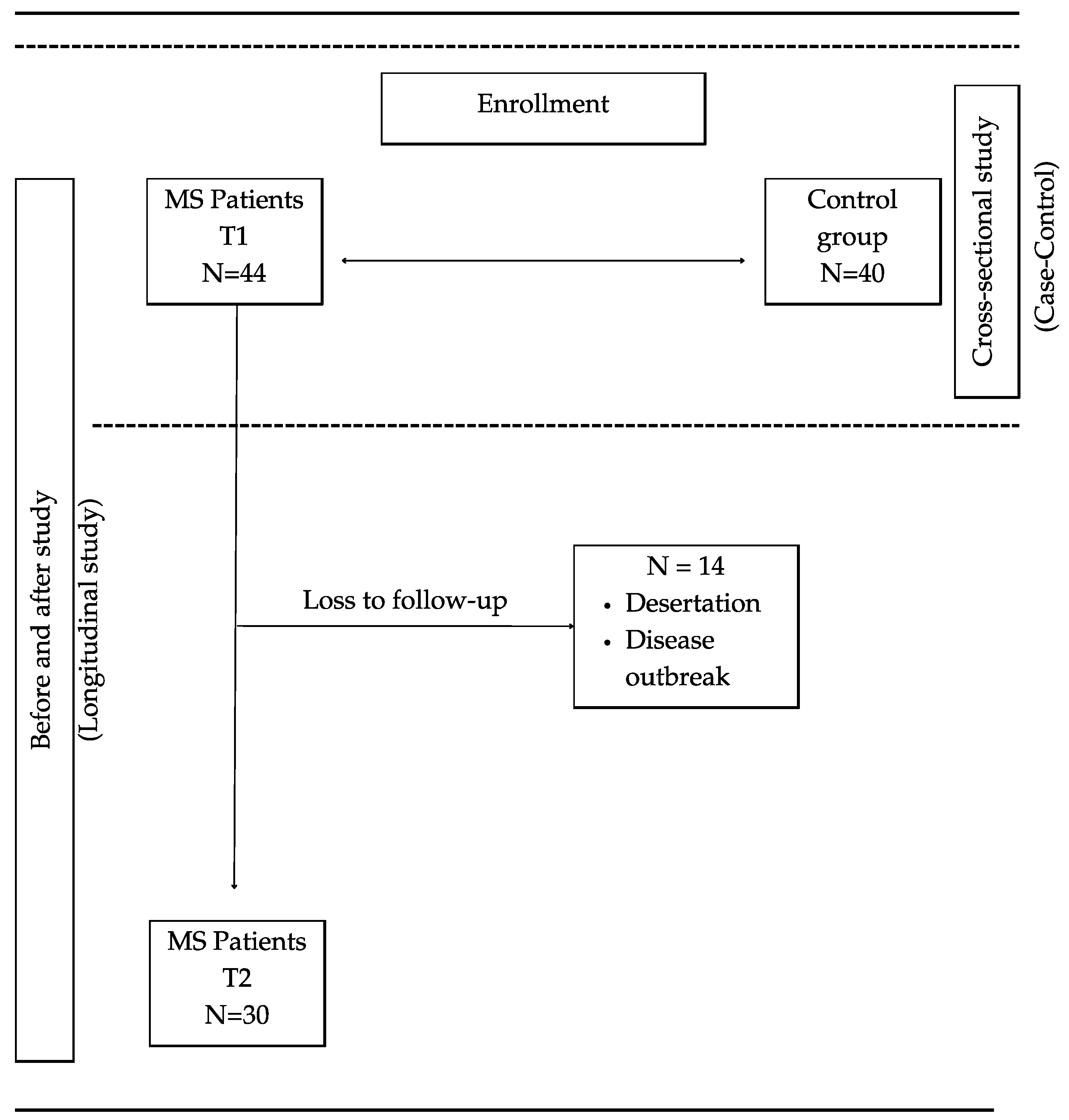
2.2. Case–Control Study
2.2.1. Lifestyles and Scale Scores
2.2.2. Measurements of Serum Biochemical Magnitudes
2.2.3. Neurofilaments and Neurotrophic Factors
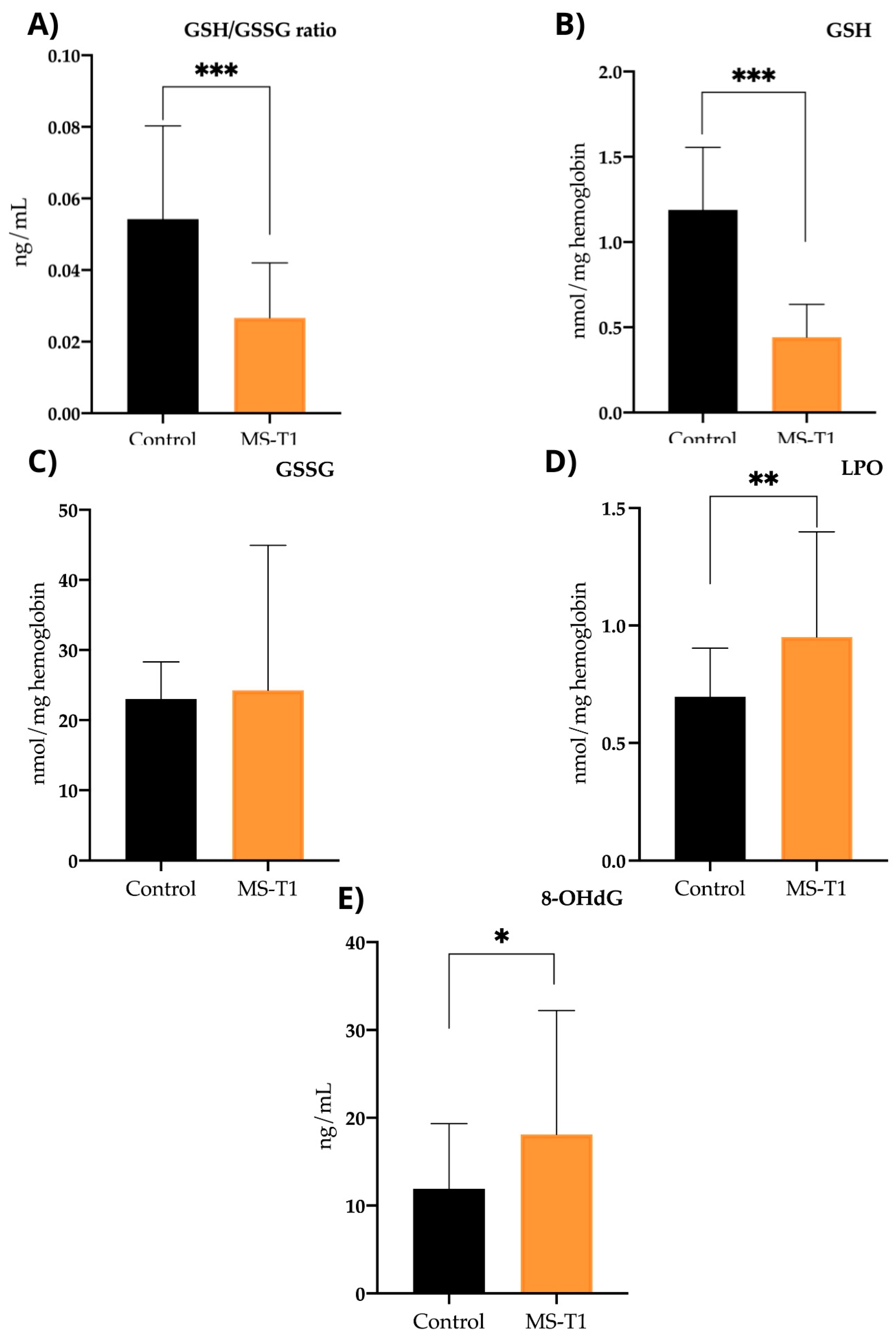
2.2.4. Oxidative Stress Parameters
2.3. Follow-Up Study: Assessment after Nutritional Intervention
2.3.1. Lifestyles and Scale Scores
2.3.2. Measurements of Serum Biochemical Magnitudes
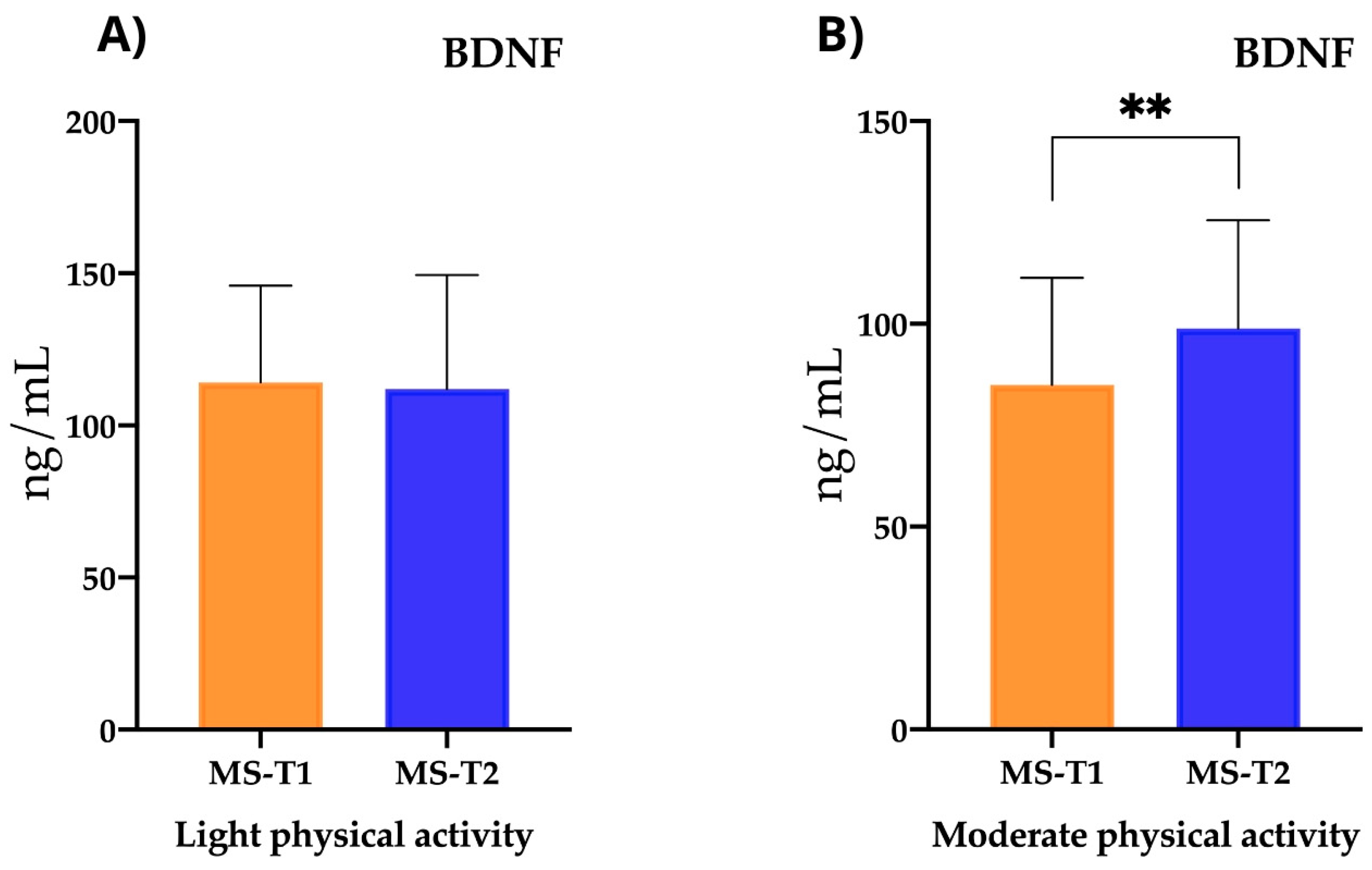
2.3.3. Neurofilaments and Neurotrophic Factors
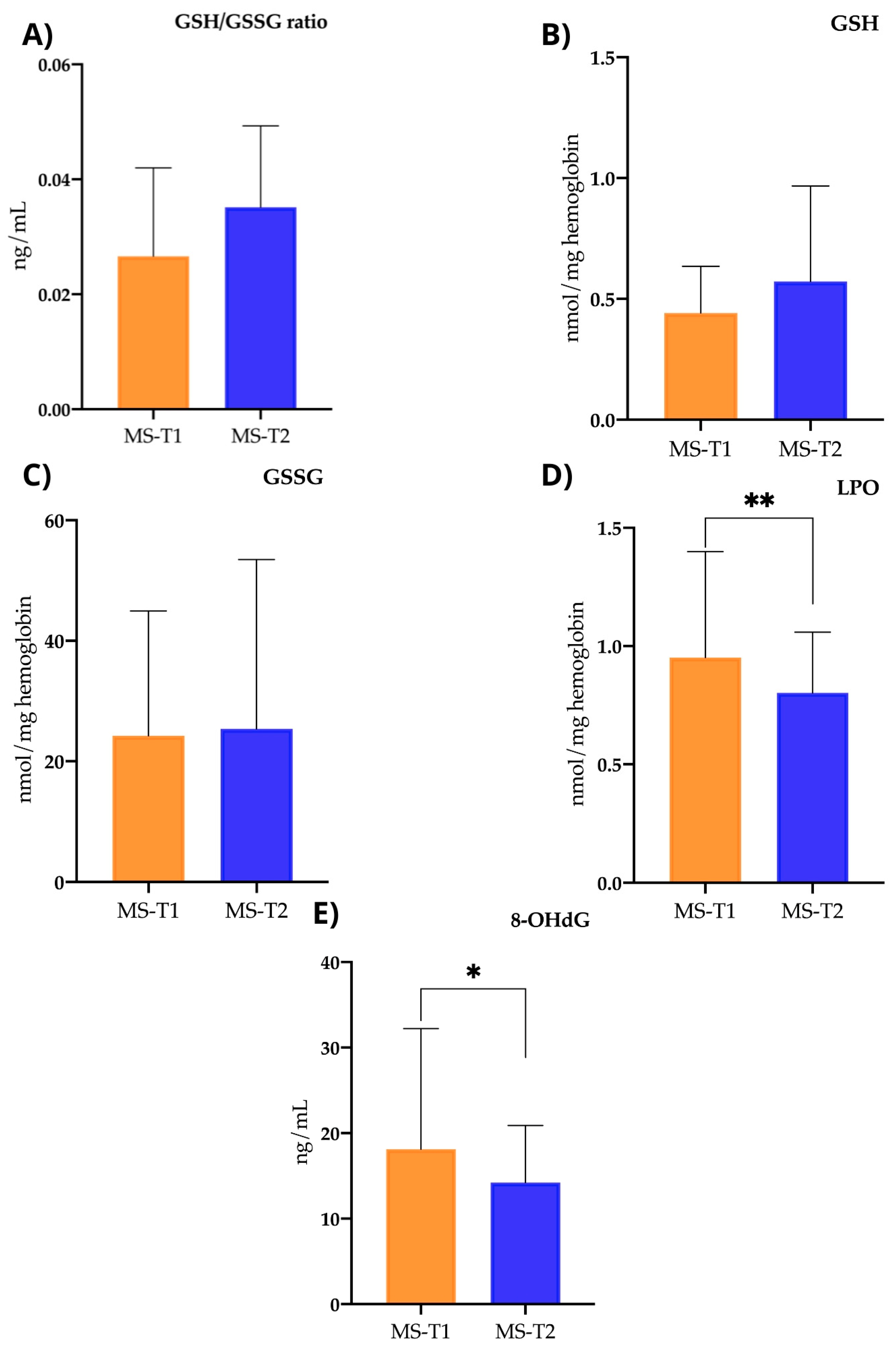
2.3.4. Oxidative Stress Parameters
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Participants and Ethical Concerns
4.3. Nutritional Intervention
4.4. Assessment of Parameters
4.4.1. Socio-Demographic Variables and Life Habits
4.4.2. Fatigue Scale
4.4.3. Quality of Life in MS
4.4.4. Symbol Digit Modalities Test (SDMT)
4.5. Serum Biomarkers
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Río, J.; Montalbán, X. Current description of multiple sclerosis. Med. Clin. 2014, 143 (Suppl. S3), 3–6. [Google Scholar] [CrossRef]
- Cuevas-García, C. Multiple sclerosis: Current immunological aspects. Rev. Alerg. México 2017, 64, 76–86. [Google Scholar] [CrossRef]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef]
- Razavi, S.; Nazem, G.; Mardani, M.; Esfandiari, E.; Salehi, H.; Esfahani, S.Z. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv. Biomed. Res. 2015, 4, 53. [Google Scholar] [CrossRef]
- Naegelin, Y.; Saeuberli, K.; Schaedelin, S.; Dingsdale, H.; Magon, S.; Baranzini, S.; Amann, M.; Parmar, K.; Tsagkas, C.; Calabrese, P.; et al. Levels of brain-derived neurotrophic factor in patients with multiple sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 2251–2261. [Google Scholar] [CrossRef]
- Nociti, V.; Romozzi, M. The Role of BDNF in Multiple Sclerosis Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 8447. [Google Scholar] [CrossRef]
- Talebi, M.; Majdi, A.; Nasiri, E.; Naseri, A.; Sadigh-Eteghad, S. The correlation between circulating inflammatory, oxidative stress, and neurotrophic factors level with the cognitive outcomes in multiple sclerosis patients. Neurol. Sci. 2020, 42, 2291–2300. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as biomarkers in neurological disorders—towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef]
- Sanabria-Castro, A.; Alape-Girón, A.; Flores-Díaz, M.; Echeverri-McCandless, A.; Parajeles-Vindas, A. Oxidative stress involvement in the molecular pathogenesis and progression of multiple sclerosis: A literature review. Prog. Neurobiol. 2024, 35, 355–371. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R.; Larocca, M.; Trotta, V.; Mennella, I.; Vitaglione, P.; Ettorre, M.; Graverini, A.; De Santis, A.; Di Monte, E.; et al. Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: A pilot study. Exp. Biol. Med. 2016, 241, 620–635. [Google Scholar] [CrossRef]
- Mousavi-Shirazi-Fard, Z.; Mazloom, Z.; Izadi, S.; Fararouei, M. The effects of modified anti-inflammatory diet on fatigue, quality of life, and inflammatory biomarkers in relapsing-remitting multiple sclerosis patients: A randomized clinical trial. Int. J. Neurosci. 2021, 131, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Olsson, T.; Hedström, A.K. Inverse association between Mediterranean diet and risk of multiple sclerosis. Mult. Scler. J. 2023, 29, 1118–1125. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Yu, A.C.; Golz, E.; Cirstea, M.; Sundvick, K.; Kliger, D.; Foulger, L.H.; Mackenzie, M.; Finlay, B.B.; Appel-Cresswell, S. MIND and Mediterranean Diets Associated with Later Onset of Parkinson’s Disease. Mov. Disord. 2021, 36, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; A Welsh-Bohmer, K. Prospective study of Dietary Approaches to Stop Hypertension– and Mediterranean-style dietary patterns and age-related cognitive change: The Cache County Study on Memory, Health and Aging. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dhana, K.; Furtado, J.D.; Agarwal, P.; Aggarwal, N.T.; Tangney, C.; Laranjo, N.; Carey, V.; Barnes, L.L.; Sacks, F.M. Higher circulating α-carotene was associated with better cognitive function: An evaluation among the MIND trial participants. J. Nutr. Sci. 2021, 10, e64. [Google Scholar] [CrossRef] [PubMed]
- Wesselman, L.M.P.; van Lent, D.M.; Schröder, A.; van de Rest, O.; Peters, O.; Menne, F.; Fuentes, M.; Priller, J.; Spruth, E.J.; Altenstein, S.; et al. Dietary patterns are related to cognitive functioning in elderly enriched with individuals at increased risk for Alzheimer’s disease. Eur. J. Nutr. 2021, 60, 849–860. [Google Scholar] [CrossRef]
- Hosking, D.E.; Eramudugolla, R.; Cherbuin, N.; Anstey, K.J. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimer’s Dement. 2019, 15, 581–589. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Holland, T.; Agarwal, P.; Aggarwal, N.; Morris, M.C. DASH and Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diets Are Associated With Fewer Depressive Symptoms Over Time. J. Gerontol. Ser. A 2021, 76, 151–156. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Glime, G.N.E.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef] [PubMed]
- Crippes, L.J.; Saxby, S.M.; Shemirani, F.; Bisht, B.; Gill, C.; Rubenstein, L.M.; Eyck, P.T.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; et al. Diet-induced changes in functional disability are mediated by fatigue in relapsing-remitting multiple sclerosis: A secondary analysis of the WAVES randomized parallel-arm trial. Mult. Scler. J. Exp. Transl. Clin. 2023, 9, 20552173231209147. [Google Scholar] [CrossRef] [PubMed]
- Noormohammadi, M.; Ghorbani, Z.; Moghadasi, A.N.; Saeedirad, Z.; Shahemi, S.; Ghanaatgar, M.; Rezaeimanesh, N.; Hekmatdoost, A.; Ghaemi, A.; Jahromi, S.R. MIND Diet Adherence Might be Associated with a Reduced Odds of Multiple Sclerosis: Results from a Case–Control Study. Neurol. Ther. 2022, 11, 397–412. [Google Scholar] [CrossRef]
- Felicetti, F.; Tommasin, S.; Petracca, M.; De Giglio, L.; Gurreri, F.; Ianniello, A.; Nistri, R.; Pozzilli, C.; Ruggieri, S. Eating Hubs in Multiple Sclerosis: Exploring the Relationship between Mediterranean Diet and Disability Status in Italy. Front. Nutr. 2022, 9, 882426. [Google Scholar] [CrossRef]
- Nazeri, M.; Bazrafshan, H.; Foroughi, A.A. Serum inflammatory markers in patients with multiple sclerosis and their association with clinical manifestations and MRI findings. Acta Neurol. Belg. 2022, 122, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; Horakova, D.; Tyblova, M.; Zeman, D.; Krasulova, E.; Mrazova, K.; Seidl, Z.; Vaneckova, M.; Krasensky, J.; Weinstock-Guttman, B.; et al. Increased albumin quotient (QAlb) in patients after first clinical event suggestive of multiple sclerosis is associated with development of brain atrophy and greater disability 48 months later. Mult. Scler. J. 2016, 22, 770–781. [Google Scholar] [CrossRef]
- Chen, K.; Li, C.; Zhao, B.; Shang, H. Albumin and multiple sclerosis: A prospective study from UK Biobank. Front. Immunol. 2024, 15, 1415160. [Google Scholar] [CrossRef]
- Fateh, H.L.; Muhammad, S.S.; Kamari, N. Associations between adherence to MIND diet and general obesity and lipid profile: A cross-sectional study. Front. Nutr. 2023, 10, 1078961. [Google Scholar] [CrossRef]
- Noori, H.; Gheini, M.R.; Rezaeimanesh, N.; Saeedi, R.; Aliabadi, H.R.; Sahraian, M.A.; Moghadasi, A.N. The correlation between dyslipidemia and cognitive impairment in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2019, 36, 101415. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Kalantari, N.; Gorgani-Firouzjaee, T.; Rostami-Mansoor, S.; Shirafkan, H. Association between insulin resistance and multiple sclerosis: A systematic review and meta-analysis. Metab. Brain Dis. 2024, 39, 1015–1026. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Koskinen, J.O.; Laaksonen, M.; Hänninen, A.; Lilius, E.M.; Waris, M. High sensitivity measurement of CRP and disease progression in multiple sclerosis. Neurology 2005, 65, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Miller, D.; Losseff, N.; Sailer, M.; Lewellyn-Smith, N.; Thompson, A.; Thompson, E. Serum inflammatory markers and clinical/MRI markers of disease progression in multiple sclerosis. J. Neurol. 2001, 248, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, D.; Jockers-Wretou, E. Serum creatine kinase B levels in diseases of the central nervous system. Eur. Neurol. 1987, 27, 78–81. [Google Scholar] [CrossRef]
- Steen, C.; Wilczak, N.; Hoogduin, J.M.; Koch, M.; De Keyser, J. Reduced Creatine Kinase B Activity in Multiple Sclerosis Normal Appearing White Matter. PLoS ONE 2010, 5, e10811. [Google Scholar] [CrossRef][Green Version]
- Karimi, N.; Ashourizadeh, H.; Pasha, B.A.; Haghshomar, M.; Jouzdani, T.; Shobeiri, P.; Teixeira, A.L.; Rezaei, N. Blood levels of brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis (MS): A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022, 65, 103984. [Google Scholar] [CrossRef]
- Liguori, M.; Fera, F.; Gioia, M.C.; Valentino, P.; Manna, I.; Condino, F.; Cerasa, A.; La Russa, A.; Clodomiro, A.; Paolillo, A.; et al. Investigating the role of brain-derived neurotrophic factor in relapsing-remitting multiple sclerosis. Genes, Brain Behav. 2007, 6, 177–183. [Google Scholar] [CrossRef]
- Văcăraş, V.; Major, Z.Z.; Buzoianu, A.D. Brain-derived neurotrophic factor levels under chronic natalizumab treatment in multiple sclerosis. A preliminary report. Neurol. i Neurochir. Polska 2017, 51, 221–226. [Google Scholar] [CrossRef]
- Diechmann, M.D.; Campbell, E.; Coulter, E.; Paul, L.; Dalgas, U.; Hvid, L.G. Effects of Exercise Training on Neurotrophic Factors and Subsequent Neuroprotection in Persons with Multiple Sclerosis—A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 1499. [Google Scholar] [CrossRef]
- Oraby, M.I.; El Masry, H.A.; El Shafy, S.S.A.; Galil, E.M.A. Serum Level of Brain-Derived Neurotrophic Factor in Patients with Relapsing Remitting Multiple Sclerosis: A Potential Biomarker for Disease Activity. Mult. Scler. Relat. Disord. 2023, 71, 104277. [Google Scholar] [CrossRef]
- Brück, W.; Stadelmann, C. The spectrum of multiple sclerosis: New lessons from pathology. Curr. Opin. Neurol. 2005, 18, 221–224. [Google Scholar] [CrossRef]
- Cacalano, G.; Fariñas, I.; Wang, L.-C.; Hagler, K.; Forgie, A.; Moore, M.; Armanini, M.; Phillips, H.; Ryan, A.M.; Reichardt, L.F.; et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 1998, 21, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Bochi, G.V.; Trevisan, G. Advanced Oxidative Protein Products Role in Multiple Sclerosis: A Systematic Review and Meta-analysis. Mol. Neurobiol. 2021, 58, 5724–5742. [Google Scholar] [CrossRef] [PubMed]
- Tavassolifar, M.J.; Moghadasi, A.N.; Esmaeili, B.; Sadatpour, O.; Vodjgani, M.; Izad, M. Redox Imbalance in CD4+ T Cells of Relapsing-Remitting Multiple Sclerosis Patients. Oxidative Med. Cell. Longev. 2020, 2020, 8860813. [Google Scholar] [CrossRef]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134 Pt 7, 1914–1924. [Google Scholar] [CrossRef]
- Bizzozero, O.A.; Reyes, S.; Ziegler, J.; Smerjac, S. Lipid peroxidation scavengers prevent the carbonylation of cytoskeletal brain proteins induced by glutathione depletion. Neurochem. Res. 2007, 32, 2114–2122. [Google Scholar] [CrossRef]
- Bizoń, A.; Chojdak-Łukasiewicz, J.; Budrewicz, S.; Pokryszko-Dragan, A.; Piwowar, A. Exploring the Relationship between Antioxidant Enzymes, Oxidative Stress Markers, and Clinical Profile in Relapsing–Remitting Multiple Sclerosis. Antioxidants 2023, 12, 1638. [Google Scholar] [CrossRef] [PubMed]
- Khajenobar, N.B.; Mahboob, S.; Nourazarian, A.; Shademan, B.; Laghousi, D.; Moayed, Z.B.; Hassanpour, M.; Nikanfar, M. Comparison between cerebrospinal fluid and serum levels of myelin-associated glycoprotein, total antioxidant capacity, and 8-hydroxy-2′-deoxyguanosine in patients with multiple sclerosis. Clin. Neurol. Neurosurg. 2021, 200, 106377. [Google Scholar] [CrossRef]
- Burgetova, A.; Dusek, P.; Uher, T.; Vaneckova, M.; Vejrazka, M.; Burgetova, R.; Horakova, D.; Srpova, B.; Krasensky, J.; Lambert, L. Oxidative Stress Markers in Cerebrospinal Fluid of Newly Diagnosed Multiple Sclerosis Patients and Their Link to Iron Deposition and Atrophy. Diagnostics 2022, 12, 1365. [Google Scholar] [CrossRef]
- Jaworski, M.G.; Gillies, J.K.; Youngs, M.; Wojcik, C.; Santivasci, C.; Jakimovski, D.; Bergsland, N.; Weinstock-Guttman, B.; Benedict, R.H. Predicting employment deterioration with the Processing Speed Test (PST) and SDMT in multiple sclerosis. Mult. Scler. J. 2023, 29, 1327–1336. [Google Scholar] [CrossRef]


| Parameter | MS Group (n = 44) ± SD | Control Group (n = 40) ± SD | p-Value |
|---|---|---|---|
| Age (years) | 40.50 ± 10.03 | 36.82 ± 14.14 | 0.141 |
| Gender (%) | 0.822 | ||
| Male | 32 | 38 | |
| Female | 68 | 62 | |
| Educational level (%) | 0.455 | ||
| Primary school | 13.63 | 4.54 | |
| High school | 45.45 | 13.63 | |
| University | 40.90 | 72.72 | |
| Place of residence (%) | 0.095 | ||
| Countryside | 15.90 | 5 | |
| City | 84.09 | 95 | |
| Work status (%) | <0.001 * | ||
| Unemployed/retired | 63.36 | 20 | |
| Working | 36.36 | 80 | |
| Nighttime sleep hours (%) | 0.984 | ||
| <7 h | 52.26 | 52.50 | |
| ≥7 h | 47.74 | 47.50 | |
| Physical activity (%) | 0.625 | ||
| Light | 72.72 | 65 | |
| Moderate–intense | 27.27 | 35 | |
| Smoking (%) | 0.044 * | ||
| Yes | 34.09 | 15 | |
| No | 65.90 | 85 | |
| Co-habitants (%) | 0.729 | ||
| Alone | 6.82 | 5 | |
| Accompanied | 93.18 | 95 |
| Parameter | MS Group (n = 44) ± SD | Control Group (n = 40) ± SD | p-Value |
|---|---|---|---|
| Glucose (mg/dL) | 85.89 ± 8.45 | 85.1 ± 9.07 | 0.685 |
| Albumin (g/dL) | 4.72 ± 0.24 | 4.52 ± 0.27 | <0.001 * |
| Total protein (g/dL) | 7.09 ± 0.38 | 7.17 ± 0.34 | 0.34 |
| Urea (mg/dL) | 32.27 ± 6.76 | 33.1 ± 8.31 | 0.618 |
| Creatinine (mg/dL) | 0.78 ± 0.13 | 0.81 ± 0.15 | 0.309 |
| Uric acid (mg/dL) | 4.49 ± 1.07 | 4.77 ± 1.24 | 0.277 |
| Total cholesterol (mg/dL) | 190.3 ± 28.62 | 182.2 ± 29.28 | 0.205 |
| HDL (mg/dL) | 56.56 ± 14.91 | 63.56 ± 14.33 | 0.033 * |
| LDL (mg/dL) | 117 ± 22.7 | 101.2 ± 23.18 | 0.003 * |
| Triglycerides (mg/dL) | 85.14 ± 33.7 | 88.29 ± 38.26 | 0.693 |
| Bilirubin (mg/dL) | 0.69 ± 0.25 | 0.75 ± 0.39 | 0.403 |
| LDH (U/L) | 188.9 ± 27.39 | 181.6 ± 34.14 | 0.288 |
| GGT (U/L) | 17.93 ± 11.58 | 16.33 ± 8.60 | 0.482 |
| ALT (U/L) | 18.91 ± 9.59 | 23.26 ± 12.45 | 0.078 |
| CK (mg/dL) | 79.5 ± 47.64 | 151.9 ± 133.7 | 0.002 * |
| ALP (U/L) | 70.91 ± 19.44 | 64.16 ± 17.86 | 0.107 |
| Na (mEq/L) | 141.3 ± 2.48 | 140.7 ± 1.74 | 0.224 |
| K (mEq/L) | 4.29 ± 0.35 | 4.28 ± 0.33 | 0.936 |
| Cl (mEq/L) | 107.4 ± 3.08 | 105.1 ± 2.17 | <0.001 * |
| Ca (mg/L) | 9.87 ± 0.44 | 9.65 ± 0.35 | 0.021 * |
| P (mg/dL) | 3.56 ± 0.51 | 3.55 ± 0.56 | 0.953 |
| Iron (mg/dL) | 93.42 ± 34.29 | 92.08 ± 33.44 | 0.858 |
| Apo AI (mg/dL) | 130.2 ± 20.69 | 145.1 ± 20.14 | 0.002 * |
| Apo B-100 (mg/dL) | 76.86 ± 17.29 | 77.5 ± 17.08 | 0.868 |
| Ratio APO (ApoB/ApoA1) | 0.60 ± 0.15 | 0.54 ± 0.13 | 0.078 |
| Lp(a) (mg/dL) | 31.24 ± 30.82 | 40.63 ± 36.78 | 0.215 |
| CRP (mg/dL) | 1.43 ± 1.86 | 1.15 ± 1.03 | <0.001 * |
| HbA1c (%) | 5.24 ± 0.25 | 5.32 ± 0.32 | 0.247 |
| Insulin (U/L) | 7.42 ± 3.04 | 6.00 ± 2.83 | 0.035 * |
| HOMA-IR index | 1.64 ± 0.73 | 1.68 ± 2.96 | 0.915 |
| Leukocyte count/µL | 6.41 ± 2.38 | 5.72 ± 1.34 | 0.115 |
| Haemoglobin (g/dL) | 14.3 ± 1.27 | 14.05 ± 1.19 | 0.364 |
| Parameter | MS Group (n = 44) ± SD | Control Group (n = 40) ± SD | p-Value |
|---|---|---|---|
| Nfl (pg/mL) | 12.09 ± 17.84 | 8.59 ± 5.05 | 0.235 |
| GDNF (pg/mL) | 254.2 ± 436.8 | 254.2 ± 341.4 | >0.999 |
| NGF (pg/mL) | 1165 ± 2505 | 1210 ± 2297 | 0.933 |
| GFAP (pg/mL) | 686.7 ± 235.6 | 689.5 ± 199.7 | 0.954 |
| BDNF (ng/mL) | 109.3 ± 31.28 | 59.19 ± 18.10 | <0.001 * |
| Parameter | Group MS-T1 (n = 44) ± SD | Group MS-T2 (n = 30) ± SD | p-Value |
|---|---|---|---|
| EDSS score | 3.2 ± 1.82 | 3.3 ± 2.09 | 0.686 |
| Work status (%) | 0.043 * | ||
| Unemployed/retired | 63.64 | 72.42 | |
| Working | 36.36 | 27.58 | |
| Nighttime sleep hours (%) | 0.745 | ||
| <7 h | 52.26 | 50 | |
| ≥7 h | 47.74 | 50 | |
| Physical activity (%) | 0.801 | ||
| Light | 72.72 | 60 | |
| Moderate–intense | 27.27 | 40 |
| Parameter | Group MS-T1 (n = 44) ± SD | Group MS-T2 (n = 30) ± SD | p-Value |
|---|---|---|---|
| Glucose (mg/dL) | 84.27 ± 8.12 | 85.17 ± 8.00 | 0.625 |
| Albumin (g/dL) | 4.66 ± 0.21 | 4.57 ± 0.26 | 0.03 * |
| Total protein (g/dL) | 7.09 ± 0.31 | 6.98 ± 0.34 | 0.058 |
| Urea (mg/dL) | 32.5 ± 7.03 | 32.07 ± 6.61 | 0.712 |
| Creatinine (mg/dL) | 0.78 ± 0.12 | 0.78 ± 0.14 | 0.766 |
| Uric acid (mg/dL) | 4.37 ± 1.21 | 4.53 ± 1.13 | 0.155 |
| Total cholesterol (mg/dL) | 192.1 ± 27.48 | 186.9 ± 27.3 | 0.121 |
| HDL (mg/dL) | 58.86 ± 14.93 | 57.1 ± 15.76 | 0.350 |
| LDL (mg/dL) | 117.8 ± 24.91 | 112.1 ± 25.49 | 0.038 * |
| Triglycerides (mg/dL) | 80.85 ± 33.1 | 72.11 ± 24.16 | 0.011 * |
| Bilirubin (mg/dL) | 0.68 ± 0.25 | 0.68 ± 0.31 | 0.864 |
| LDH (U/L) | 190.8 ± 24.07 | 196.8 ± 41.52 | 0.381 |
| GGT (U/L) | 18.97 ± 13.16 | 17.53 ± 9.67 | 0.297 |
| ALT (U/L) | 18.77 ± 10.67 | 17.47 ± 5.96 | 0.268 |
| CK (mg/dL) | 77.13 ± 54.41 | 80.17 ± 58.10 | 0.758 |
| ALP (U/L) | 70.52 ± 20.94 | 71.38 ± 22.50 | 0.640 |
| Na (mEq/L) | 140.9 ± 2.56 | 140 ± 2.32 | 0.119 |
| K (mEq/L) | 4.29 ± 0.39 | 4.26 ± 0.40 | 0.630 |
| Cl (mEq/L) | 107.4 ± 3.04 | 106.4 ± 2.88 | 0.083 |
| Ca (mg/L) | 9.83 ± 0.42 | 9.70 ± 0.48 | 0.177 |
| P (mg/dL) | 3.50 ± 0.48 | 3.73 ± 0.62 | 0.064 |
| Iron (mg/dL) | 94.83 ± 36.25 | 79.9 ± 33.14 | 0.060 |
| Apo AI (mg/dL) | 134.1 ± 20.54 | 133.6 ± 22.16 | 0.859 |
| Apo B-100 (mg/dL) | 78.45 ± 19.39 | 76.38 ± 13.65 | 0.485 |
| Ratio APO (ApoB/ApoA1) | 0.58 ± 0.15 | 0.57 ± 0.13 | 0.702 |
| Lp(a) (mg/dL) | 33.04 ± 30.65 | 36.83 ± 35.54 | 0.211 |
| CRP (mg/dL) | 1.04 ± 1.14 | 0.66 ± 0.43 | 0.117 |
| HbA1c (%) | 5.29 ± 0.21 | 5.35 ± 0.21 | 0.134 |
| Insulin (U/L) | 6.79 ± 2.91 | 6.71 ± 2.99 | 0.892 |
| HOMA-IR index | 1.52 ± 0.75 | 1.39 ± 0.59 | 0.128 |
| Leucocyte count/µL | 6.57 ± 2.38 | 6.61 ± 2.22 | 0.886 |
| Haemoglobin (g/dL) | 14.04 ± 1.32 | 13.88 ± 1.40 | 0.405 |
| Parameters | Group MS-T1 (n = 44) ± SD | Group MS-T2 (n = 30) ± SD | p-Value |
|---|---|---|---|
| Nfl (pg/mL) | 14.11 ± 21.26 | 19.53 ± 26.78 | 0.282 |
| GDNF (pg/mL) | 240.5 ± 441.9 | 198.8 ± 274.9 | 0.041 * |
| NGF (pg/mL) | 1012 ± 2269 | 855.7 ± 1943 | 0.122 |
| GFAP (pg/mL) | 697.2 ± 257.6 | 676.9 ± 241 | 0.495 |
| BDNF (ng/mL) | 106.8 ± 34.54 | 109.9 ± 32.14 | 0.603 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete-Pérez, A.; Gómez-Melero, S.; Escribano, B.M.; Galvao-Carmona, A.; Conde-Gavilán, C.; Peña-Toledo, M.Á.; Villarrubia, N.; Villar, L.M.; Túnez, I.; Agüera-Morales, E.; et al. MIND Diet Impact on Multiple Sclerosis Patients: Biochemical Changes after Nutritional Intervention. Int. J. Mol. Sci. 2024, 25, 10009. https://doi.org/10.3390/ijms251810009
Navarrete-Pérez A, Gómez-Melero S, Escribano BM, Galvao-Carmona A, Conde-Gavilán C, Peña-Toledo MÁ, Villarrubia N, Villar LM, Túnez I, Agüera-Morales E, et al. MIND Diet Impact on Multiple Sclerosis Patients: Biochemical Changes after Nutritional Intervention. International Journal of Molecular Sciences. 2024; 25(18):10009. https://doi.org/10.3390/ijms251810009
Chicago/Turabian StyleNavarrete-Pérez, Ainoa, Sara Gómez-Melero, Begoña Mª Escribano, Alejandro Galvao-Carmona, Cristina Conde-Gavilán, Mª Ángeles Peña-Toledo, Noelia Villarrubia, Luisa Mª Villar, Isaac Túnez, Eduardo Agüera-Morales, and et al. 2024. "MIND Diet Impact on Multiple Sclerosis Patients: Biochemical Changes after Nutritional Intervention" International Journal of Molecular Sciences 25, no. 18: 10009. https://doi.org/10.3390/ijms251810009
APA StyleNavarrete-Pérez, A., Gómez-Melero, S., Escribano, B. M., Galvao-Carmona, A., Conde-Gavilán, C., Peña-Toledo, M. Á., Villarrubia, N., Villar, L. M., Túnez, I., Agüera-Morales, E., & Caballero-Villarraso, J. (2024). MIND Diet Impact on Multiple Sclerosis Patients: Biochemical Changes after Nutritional Intervention. International Journal of Molecular Sciences, 25(18), 10009. https://doi.org/10.3390/ijms251810009







