Melatonin as an Efficient and Eco-Friendly Tool to Increase Yield and to Maintain Quality Attributes during Lemon Storage
Abstract
1. Introduction
2. Results
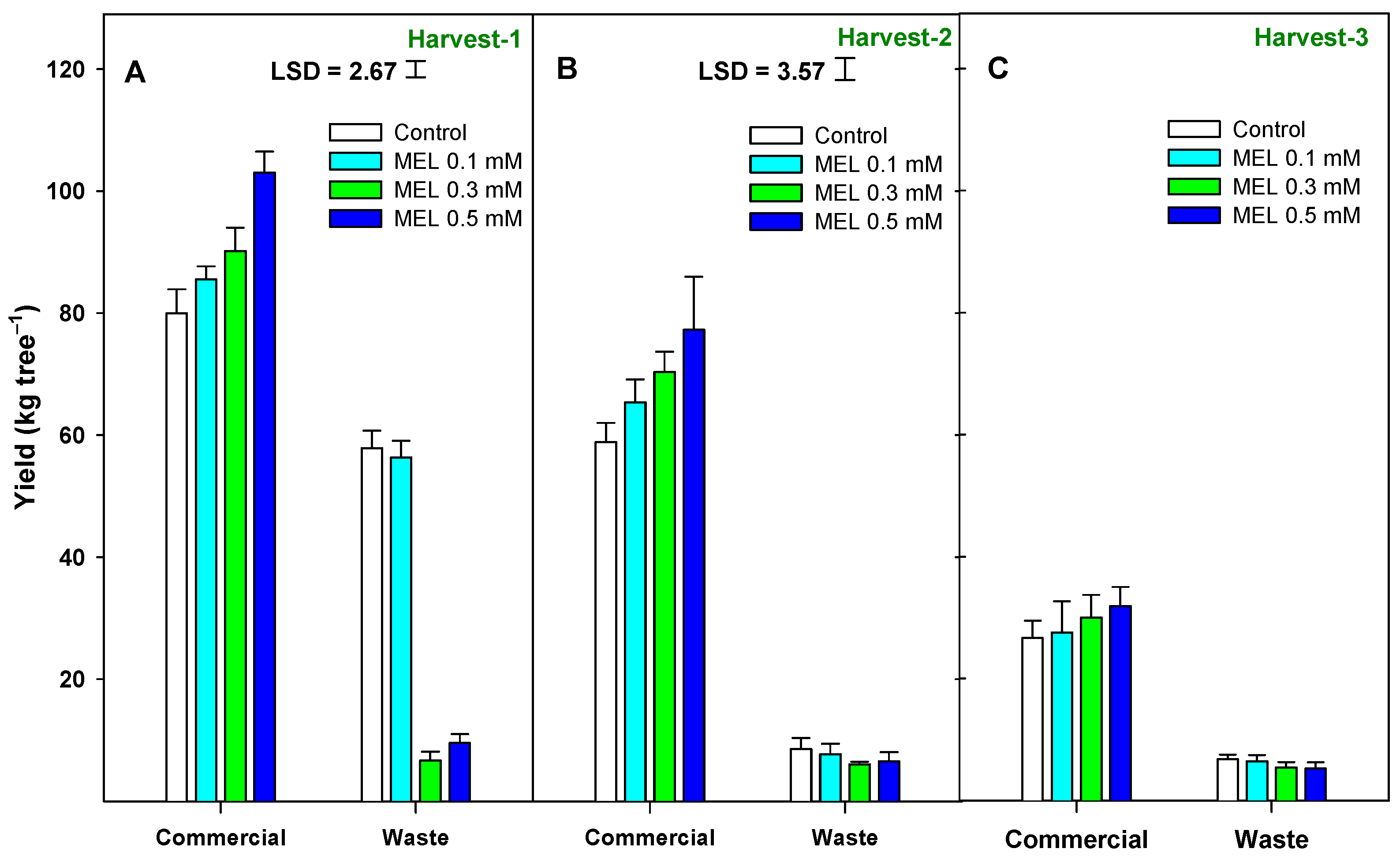
2.1. Crop Yield
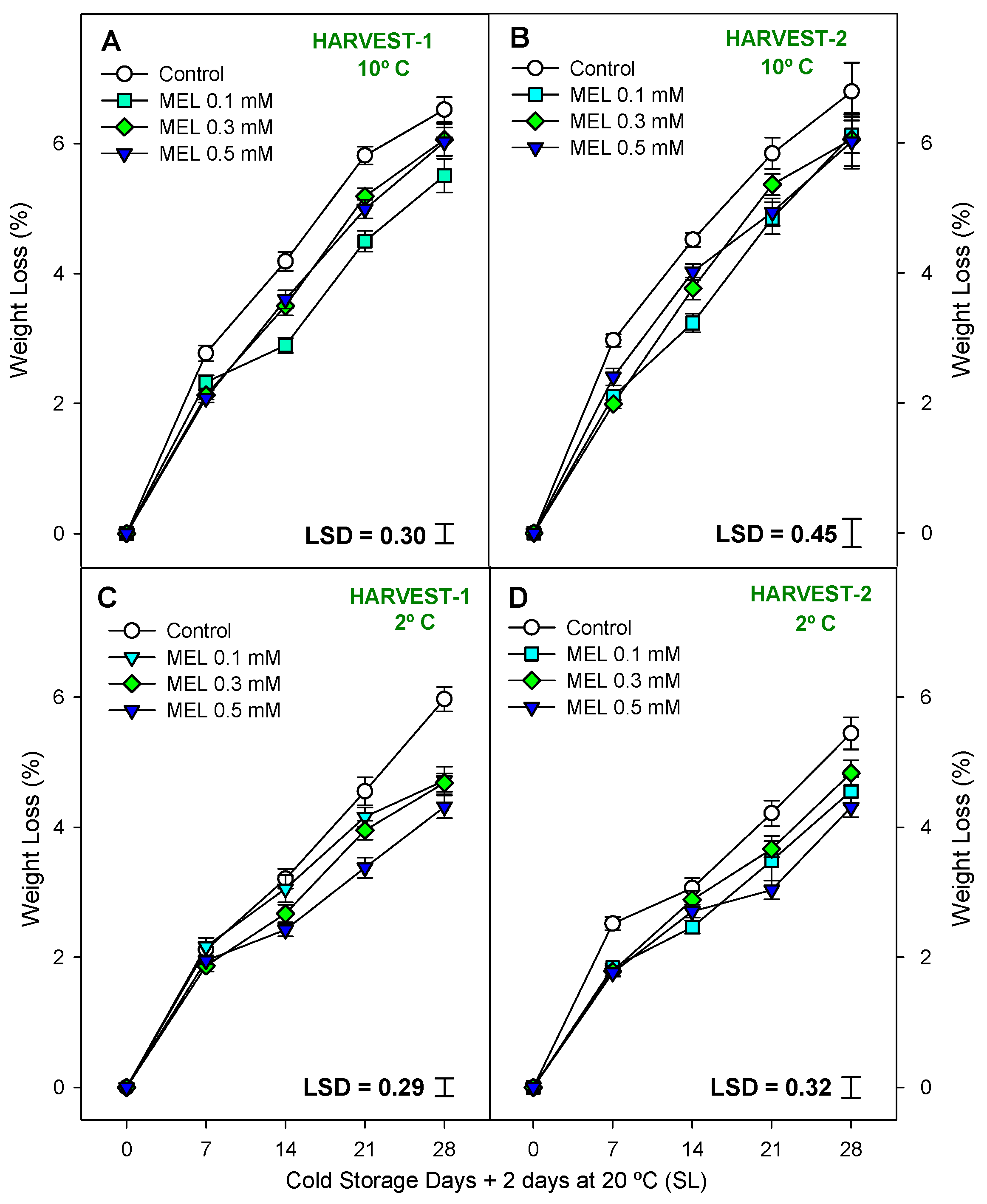
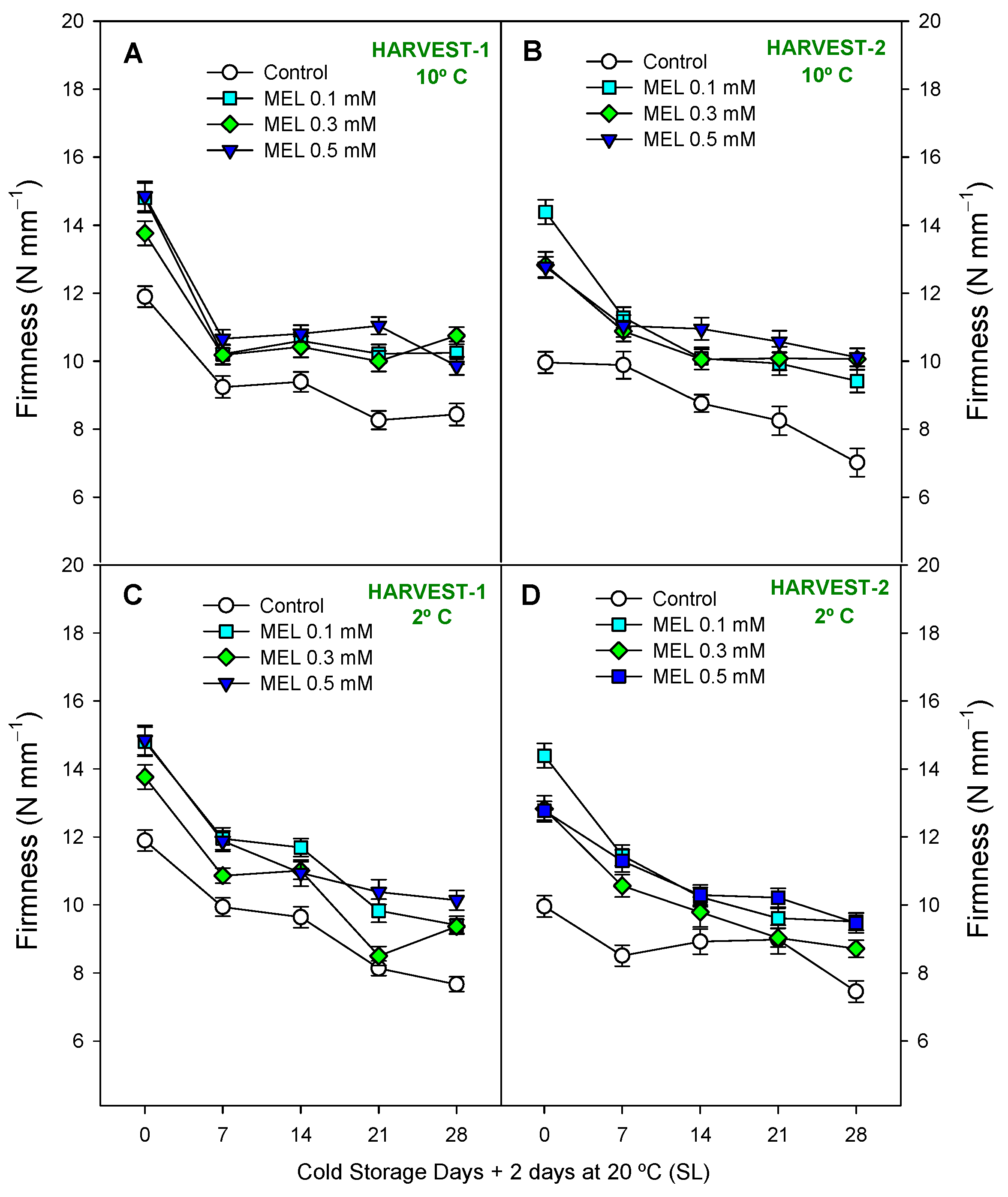
2.2. Fruit Quality Parameters
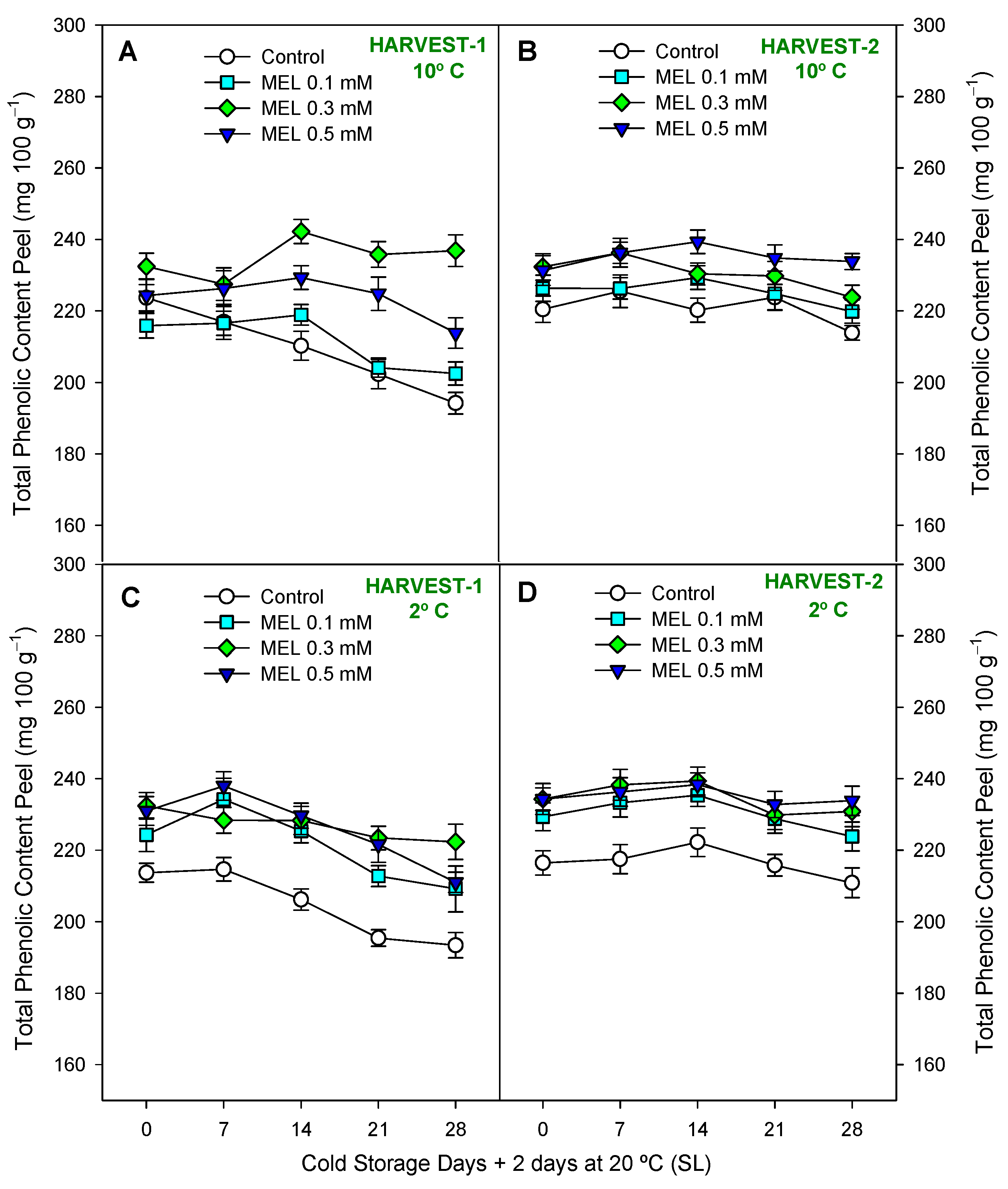
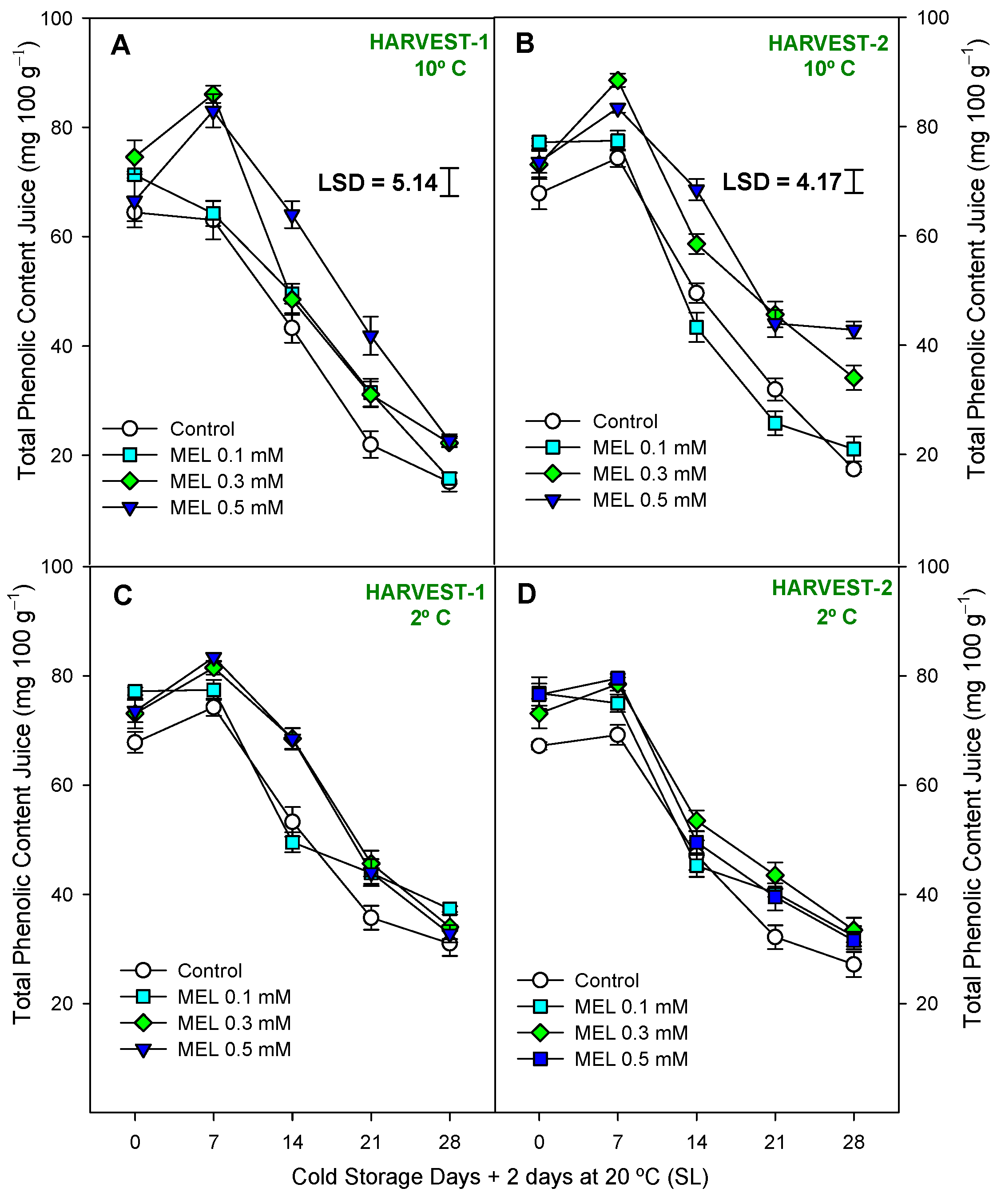
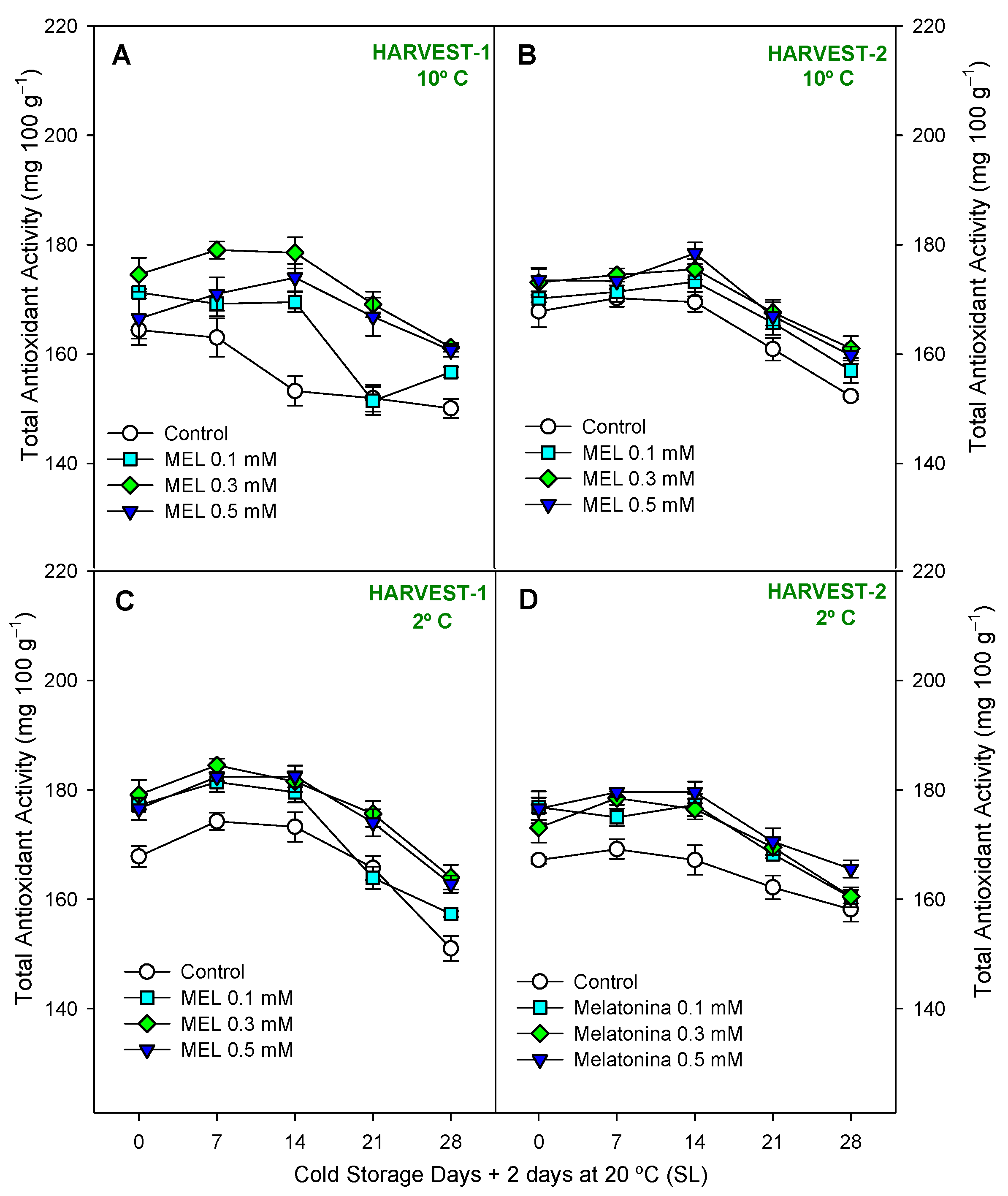
2.3. Fruit Functional Compounds and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material, Experimental Design and Storage Conditions
4.2. Physiological and Quality Parameters
4.3. Total Phenolics Content and Total Antioxidant Activity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Yarla, N.S.; Siddiqi, N.J.; de Lourdes Pereira, M.; Sharma, B. Features, pharmacological chemistry, molecular mechanism and health benefits of lemon. Med. Chem. 2021, 17, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Sania, R.; Mona, H.S.; Sharif, M.S.; Khurram, H.S.; Nageena, S.; Sumaira, P.; Maryam, M.; Muhammad, F. Biological attributes of lemon: A review. J. Addict. Med. Ther. Sci. 2020, 6, 030–034. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Simonne, A.H.; Ritenour, M.A. Citrus [Orange, Lemon, Mandarin, Grapefruit, Lime and Other citrus fruits]. In Health-Promoting Properties of Fruits and Vegetables; Terry, L.A., Ed.; CAB International: Oxfordshire, UK, 2011; pp. 90–117. [Google Scholar] [CrossRef]
- FAO World Crop Area and Production Statistics. Available online: https://www.fao.org/faostat/en#data/QC (accessed on 27 April 2024).
- MAPA Aforo De La Cosecha Nacional Del Sector De Cítricos En España En 2022/2024. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/frutas-y-hortalizas/plan_medidas_sector_citricos.aspx (accessed on 27 April 2024).
- Aguilar-Hernández, M.G.; Núñez-Gómez, D.; Forner-Giner, M.Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality parameters of Spanish lemons with commercial interest. Foods 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Singh, Z.; Tokala, V.Y.; Heather, B. Harvest maturity stage and cold storage period influence lemon fruit quality. Sci. Hortic. 2019, 249, 322–328. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Admane, N.; Genovese, F.; Di Renzo, G.C. Advance in citrus postharvest management: Diseases, cold storage and quality evaluation. In Citrus Pathology; Harsimran, G., Harsh, G., Eds.; IntechOpen: London, UK, 2017; Volume 10. [Google Scholar] [CrossRef]
- Di Matteo, A.; Simeone, G.D.R.; Cirillo, A.; Rao, M.A.; Di Vaio, C. Morphological characteristics, ascorbic acid and antioxidant activity during fruit ripening of four lemon (Citrus limon (L.) Burm. F.) cultivars. Sci. Hortic. 2021, 276, 109741. [Google Scholar] [CrossRef]
- Rovetto, E.I.; La Spada, F.; Aloi, F.; Riolo, M.; Pane, A.; Garbelotto, M.; Cacciola, S.O. Green solutions and new technologies for sustainable management of fungus and oomycete diseases in the citrus fruit supply chain. J. Plant Pathol. 2024, 106, 411–437. [Google Scholar] [CrossRef]
- Caicedo-López, L.H.; Villagómez, A.L.; Espinoza-Márquez, E.; Gómez, C.E.Z.; Sáenz, D.; Zepeda, H.R. Elicitors: Bioethical implications for agriculture and human health. Rev. Bioet. 2021, 29, 76–86. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Farooq, M.S.; Uzair, M.; Raza, A.; Habib, M.; Xu, Y.; Yousuf, M.; Yang, S.H.; Ramzan Khan, M. Uncovering the research gaps to alleviate the negative impacts of climate change on food security: A Review. Front. Plant Sci. 2022, 13, 927535. [Google Scholar] [CrossRef]
- Badiche, F.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Preharvest use of γ-aminobutyric acid (GABA) as an innovative treatment to enhance yield and quality in lemon fruit. Horticulturae 2023, 9, 93. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehleers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Back, K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 2012, 53, 385–389. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Qadir, A.; Anwar, M.; Shakoor, A.; Hayat, F. Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol. Plant. 2020, 64, 604–615. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Y.; Yan, R.; Liu, Y.; Huan, C.; Zheng, X. Preharvest spray with melatonin improves postharvest disease resistance in cherry tomato fruit. Postharvest Biol. Technol. 2022, 193, 112055. [Google Scholar] [CrossRef]
- Abd El-Naby, S.K.M.; Mohamed, A.A.A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’ apricot fruits. Acta Sci. Pol. Hortorum Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Sun, H.-L.; Wang, X.-Y.; Shang, Y.; Wang, X.-Q.; Du, G.D.; Lü, D.G. Preharvest application of melatonin induces anthocyanin accumulation and related gene upregulation in red pear (Pyrus ussuriensis). J. Integr. Agric. 2021, 20, 2126–2137. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of melatonin treatment on sweet cherry tree yield and fruit quality. Agronomy 2022, 12, 3. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Zapata, P.J.; Valverde, J.M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin treatment of apricot trees leads to maintenance of fruit quality attributes during storage at chilling and non-chilling temperatures. Agronomy 2021, 11, 917. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Guillén, F.; Ilea, M.I.M.; Ruíz-Aracil, M.C.; Valero, D.; Castillo, S.; Serrano, M. Melatonin treatments reduce chilling injury and delay ripening, leading to maintenance of quality in cherimoya fruit. Int. J. Mol. Sci. 2023, 24, 3787. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.-X.; et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin pre-harvest treatments leads to maintenance of sweet cherry quality during storage by increasing antioxidant systems. Front. Plant Sci. 2022, 13, 863467. [Google Scholar] [CrossRef]
- Sharafi, Y.; Jannatizadeh, A.; Fard, J.R.; Aghdam, M.S. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 2021, 288, 110304. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Guillén, F.; Zapata, P.J.; Agulló, V.; Castillo, S.; Serrano, M.; Valero, D. Melatonin: A new tool to increase yield and quality at harvest and to extend postharvest shelf-life of pomegranate. In IV International Symposium on Pomegranate and Minor Mediterranean Fruits; Bartual, J., Badenes, M.L., Eds.; ISHS Acta Horticulturae: Elche, Spain, 2019; pp. 289–294. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Serradilla, M.J.; Velardo-Micharet, B. Optimal preharvest melatonin applications to enhance endogenous melatonin content, harvest and postharvest quality of Japanese plum. Agriculture 2023, 13, 1318. [Google Scholar] [CrossRef]
- Okatan, V.; Aşkın, M.A.; Polat, M.; Bulduk, I.; Çolak, A.M.; Güçlü, S.F.; Kahramanoğlu, İ.; Tallarita, A.V.; Caruso, G. Effects of melatonin dose on fruit yield, quality, and antioxidants of strawberry cultivars grown in different crop systems. Agriculture 2023, 13, 71. [Google Scholar] [CrossRef]
- Meng, J.-F.; Xu, T.-F.; Song, C.-Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.-W.; Xi, Z.-M. Melatonin treatment of pre-veraison grape berries to increase size and synchronicity of berries and modify wine aroma components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef]
- El-Shieny, A.-H.A.H.; Abd-Elkarim, N.A.; Elsadek, M.A. Melatonin pre-harvest foliar application improves pepper fruit yield and postharvest fruit quality. Seybold Rep. 2022, 17, 193–207. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator. Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Posmyk, M.M. Melatonin—A new plant biostimulator? J. Elem. 2016, 21, 1187–1198. [Google Scholar] [CrossRef]
- Sati, H.; Chinchkar, A.V.; Kataria, P.; Pareek, S. The role of phytomelatonin in plant homeostasis, signaling, and crosstalk in abiotic stress mitigation. Physiol. Plant. 2024, 176, e14413. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Guddimalli, R.; Kulkarni, J.; Singam, P.; Somanaboina, A.K.; Nandimandalam, T.; Patil, S.; Polavarapu, R.; Suravajhala, P.; Sreenivasulu, N.; et al. Impact of climate change on altered fruit quality with organoleptic, health benefit, and nutritional attributes. J. Agric. Food Chem. 2023, 71, 17510–17527. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Mechanisms and modelling approaches to weight loss in fresh fruit: A review. Technol. Hortic. 2024, 4, e006. [Google Scholar] [CrossRef]
- Hassan, Z.H.; Lesmayati, S.; Qomariah, R.; Hasbianto, A. Effects of wax coating applications and storage temperatures on the quality of tangerine citrus (Citrus reticulata) var. Siam Banjar. Int. Food Res. J. 2014, 21, 641–648. [Google Scholar]
- Gameel, A.F.A. Reducing Chilling Injury of Eureka Lemon Fruits during Cold Storage. Ph.D. Thesis, Faculty of Agriculture, Tanta University, Tanta, Egypt, 2012. [Google Scholar]
- Vázquez, D.E.; Meier, G.E.; Ponte, M. Influence of postharvest curing on marsh grapefruit quality during long-term storage. In V International Postharvest Symposium; ISHS Acta Horticultura: Verona, Italy, 2004; Volume 682, pp. 1257–1264. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Al-Qurashi, A.D.; Awad, M.A. Effect of exogenous melatonin and chitosan treatments on quality and biochemical changes of ‘Balady Banzahir’ limes during shelf life. J. Anim. Plant Sci. 2023, 33, 310–319. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Establés-Ortíz, B.; González-Candelas, L. Insights into the molecular events that regulate heat-induced chilling tolerance in citrus fruits. Front. Plant Sci. 2014, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.N.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Liao, L.; Li, J.; Lan, X.; Li, Y.; Li, Y.; Huang, Z.; Jin, Z.; Yang, Y.; Wang, X.; Zhang, M.; et al. Exogenous melatonin and interstock treatments confer chilling tolerance in citrus fruit during cold storage. Sci. Hortic. 2024, 327, 112802. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Hilali, F.B.-E.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin postharvest treatment in leafy ‘Fino’ lemon maintains quality and bioactive compounds. Foods 2023, 12, 2979. [Google Scholar] [CrossRef]
- Tijero, V.; Muñoz, P.; Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Lu, X.; Huang, M.; Yu, W.; Li, C. The role of melatonin in delaying senescence and maintaining quality in postharvest horticultural products. Plant Biol. 2024; in press. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Mcharek, N.; Hanchi, B. Maturational effects on phenolic constituents, antioxidant activities and LC-MS/MS profiles of lemon (Citrus limon) peels. J. Appl. Bot. Food Qual. 2017, 90, 1–9. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badiche-El Hilali, F.; García-Pastor, M.E.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Melatonin as an Efficient and Eco-Friendly Tool to Increase Yield and to Maintain Quality Attributes during Lemon Storage. Int. J. Mol. Sci. 2024, 25, 10025. https://doi.org/10.3390/ijms251810025
Badiche-El Hilali F, García-Pastor ME, Valverde JM, Castillo S, Valero D, Serrano M. Melatonin as an Efficient and Eco-Friendly Tool to Increase Yield and to Maintain Quality Attributes during Lemon Storage. International Journal of Molecular Sciences. 2024; 25(18):10025. https://doi.org/10.3390/ijms251810025
Chicago/Turabian StyleBadiche-El Hilali, Fátima, María E. García-Pastor, Juan Miguel Valverde, Salvador Castillo, Daniel Valero, and María Serrano. 2024. "Melatonin as an Efficient and Eco-Friendly Tool to Increase Yield and to Maintain Quality Attributes during Lemon Storage" International Journal of Molecular Sciences 25, no. 18: 10025. https://doi.org/10.3390/ijms251810025
APA StyleBadiche-El Hilali, F., García-Pastor, M. E., Valverde, J. M., Castillo, S., Valero, D., & Serrano, M. (2024). Melatonin as an Efficient and Eco-Friendly Tool to Increase Yield and to Maintain Quality Attributes during Lemon Storage. International Journal of Molecular Sciences, 25(18), 10025. https://doi.org/10.3390/ijms251810025









