Abstract
Atherosclerosis is a chronic inflammatory disorder which remains the main cause of cardiovascular morbidity and mortality, with carotid atherosclerosis (CA) being a major cause of ischemic stroke. Epigenetic regulation plays a significant role in CA progression and stroke, yet the impact of circulating microRNA expression, associated with atherogenesis, has not been clearly defined. We included 81 patients with moderate–severe CA (mean age 67 ± 7 years, 53% male), 42% of whom had prior ipsilateral ischemic stroke (i.e., were symptomatic). A total of 24 miRs were identified and their plasma expression levels were measured. We observed that several microRNAs were up-regulated in stroke survivors, namely miR-200c-3p (30.6 vs. 29.7, p = 0.047), miR-106b-3p (31.01 vs. 30.25, p = 0.004), and miR-494-5p (39 vs. 33, p < 0.001), while others (miR183-3p [25.5 vs. 28.6, p < 0.001], miR-126-5p [35.6 vs. 37.1, p = 0.03], and miR-216-3p [12.34 vs. 16.2, p < 0.001]) had lower plasma levels in symptomatic patients. In a multivariable logistic regression model for symptomatic CA, the only miRs showing statistical significance were miR-106b-5p, miR-183-3p, miR-216-3p, and miR-494-5p. Cluster analysis demonstrated differential miR expression in CA patients depending on their stroke status. Epigenetic modulation, represented as complex interplay between circulating miRs of different atherogenic potential, may play a significant role in CA development and progression. In our study, we show possible candidates for future research regarding CA and stroke.
1. Introduction
Carotid atherosclerosis (CA) remains a major cause of ischemic stroke, comprising up to 20% of stroke cases [1]. Atherosclerosis is a chronic inflammatory disorder which involves the vessel wall of large and medium arteries, associated with the accumulation of lipids (forming plaques) and leading to the narrowing of their lumen (stenosis) and subsequent thrombosis [2].
The prevalence of CA in the general population is estimated to be >1.1 billion cases, with carotid stenosis (>50%) constituting 1.5% (approximately 58 million cases) [3]. The risk of stroke increases with the degree of carotid artery stenosis [4]; significant CA refers to a >50% narrowing of the carotid artery, with the term ‘symptomatic CA’ applied to patients who have ipsilateral stroke/transient ischemic attack. However, the degree of stenosis appears to be not sufficient enough to accurately predict stroke risk—nearly half of strokes in the pivotal surgical trials for CA (NASCET [North American Symptomatic Carotid Endarterectomy Trial] and ECST [European Carotid Surgery Trial]) occurred in patients with <50% carotid stenosis [5]. Atherosclerotic plaque vulnerability (i.e., a ‘stable’ vs. ‘unstable’ phenotype) may be a promising surrogate marker, better at identifying high-risk asymptomatic patients for whom aggressive treatment would be beneficial.
Epigenetic regulation plays a significant role in atherosclerosis initiation and progression with non-coding RNAs as potential identifiable blood biomarkers [6]. MicroRNAs are small (20–40 nucleotides), relatively stable, and conserved molecules highly involved in atherogenesis [7]. Previously [8], we described a panel of microRNA involved in various pathological processes underlying atherosclerosis: inflammation, endothelial dysfunction, lipid/cholesterol metabolism, oxidative stress, the regulation of angiogenesis, and smooth muscle cell proliferation. In this study, we aimed to identify possible epigenetic markers (via circulating microRNA) of CA and elucidate their association with ischemic stroke.
2. Results
A brief summary of patient characteristics is presented in Table 1. There were no statistically significant differences between symptomatic and asymptomatic patients with CA by demographic and main clinical parameters. The median carotid stenosis levels were 75% in both groups, which corresponded to advanced CA. Nearly all patients had a history of arterial hypertension, and one-third had diabetes. Interestingly, low-density lipoprotein (LDL) levels were within the target range (<1.8 mmol/L) in patients with prior stroke, which could be explained by a potentially higher proportion of statin users in this group.

Table 1.
Patient characteristics.
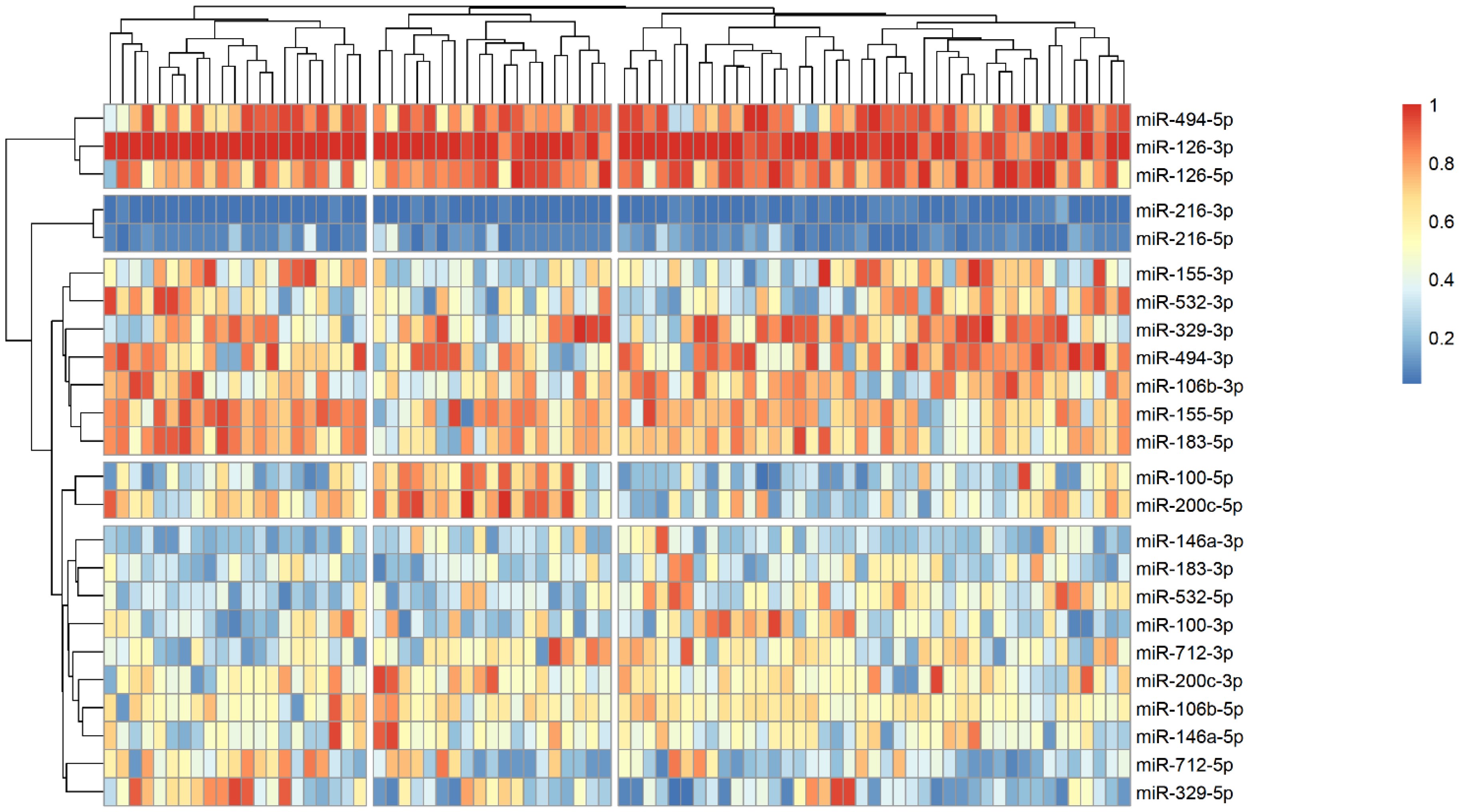
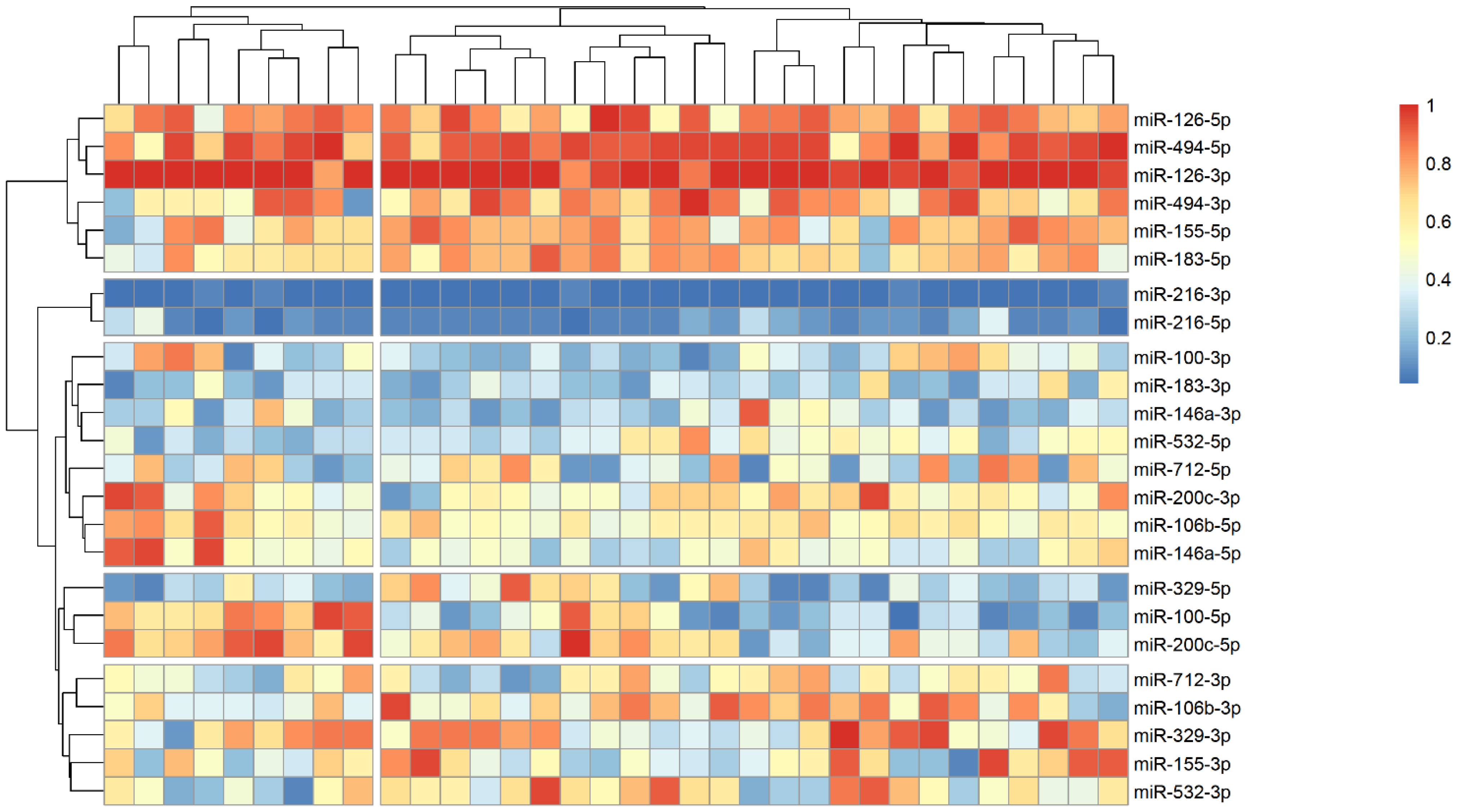
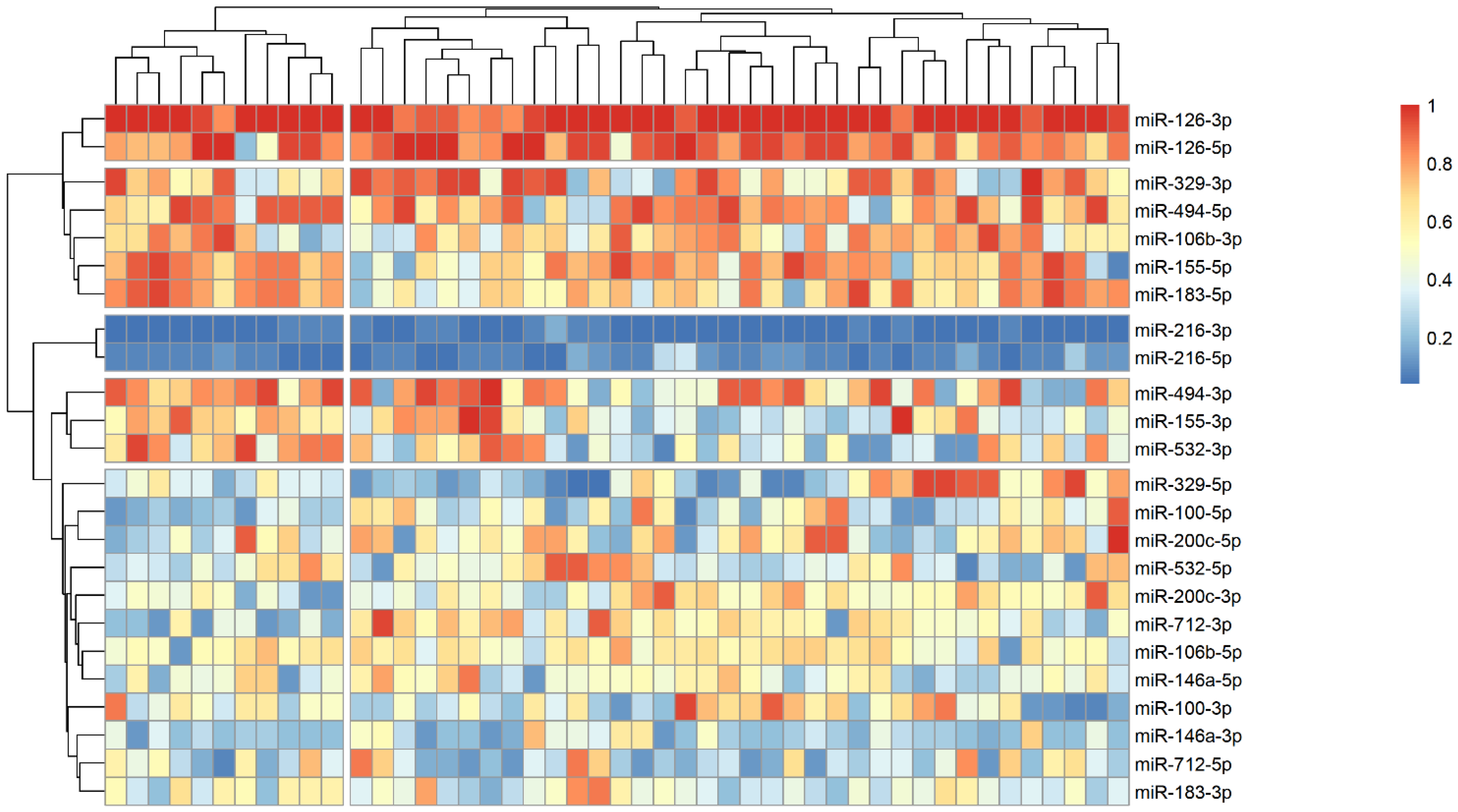
The analysis of microRNA expression between groups revealed several statistically significant differences (Table 2). We observed that several microRNAs are up-regulated in stroke survivors, namely miR-200c-3p, miR-106b-3p and miR-494-5p, while others (miR183-3p, miR-126-5p and miR-216-3p) have lower plasma levels in symptomatic patients. In order to identify possible clusters of similar microRNA expression, we performed a hierarchical clustering analysis visualized by heatmaps (Figure 1, Figure 2 and Figure 3). When looking at all patients with CA, we see a cluster of almost uniformly up-regulated (miR-494-5p and miR-126-5p/3p) and down-regulated (miR-216-5p/-3p) microRNAs, with all others showing different expression across clusters. In symptomatic CA patients (Figure 2), the cluster of up-regulated microRNAs shows several more markers (i.e., miR-155-5p and miR-183-5p), while asymptomatic CA patients (Figure 3) show two more clusters of up-regulated microRNAs (namely miR-155-3p, miR-532-3p). MiR-100-5p and miR-200c-5p demonstrate overexpression in a cluster of patients (Figure 1, middle group of columns), corresponding to symptomatic CA patients.

Table 2.
MicroRNA expression depending on stroke occurrence.
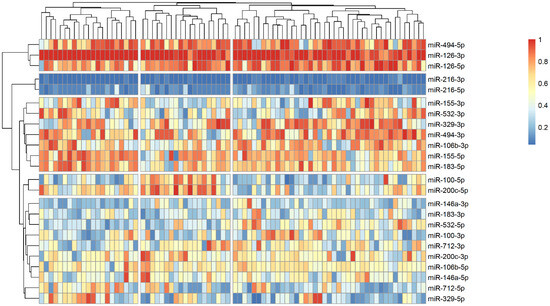
Figure 1.
Heatmap showing relative microRNA levels in all patients with CA; clusters visualized via hierarchical clustering. Columns represent individual patients, rows—relative miR expression (with larger values on the color legend corresponding to higher relative levels). Dendrograms were calculated via the optimal leaf ordering algorithm; both row and column clusters are separated by narrow white spaces.
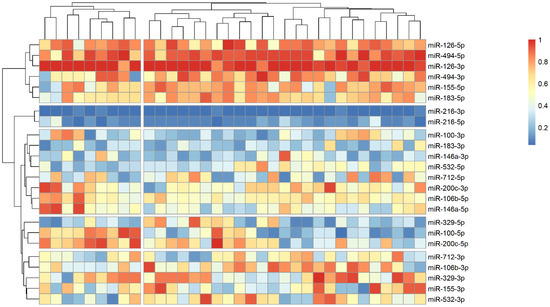
Figure 2.
Heatmap showing relative microRNA levels in stroke CA patients; clusters visualized via hierarchical clustering. Columns represent individual patients, rows—relative miR expression (with larger values on the color legend corresponding to higher relative levels). Dendrograms were calculated via the optimal leaf ordering algorithm; both row and column clusters are separated by narrow white spaces.
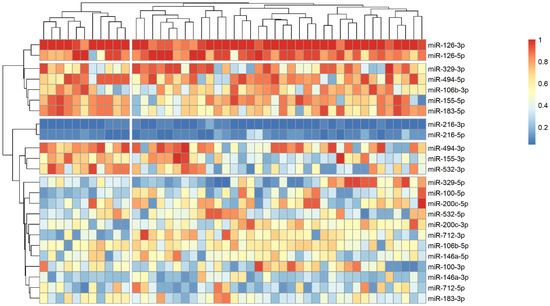
Figure 3.
Heatmap showing relative microRNA levels in asymptomatic CA patients; clusters visualized via hierarchical clustering. Columns represent individual patients, rows—relative miR expression (with larger values on the color legend corresponding to higher relative levels). Dendrograms were calculated via the optimal leaf ordering algorithm; both row and column clusters are separated by narrow white spaces.
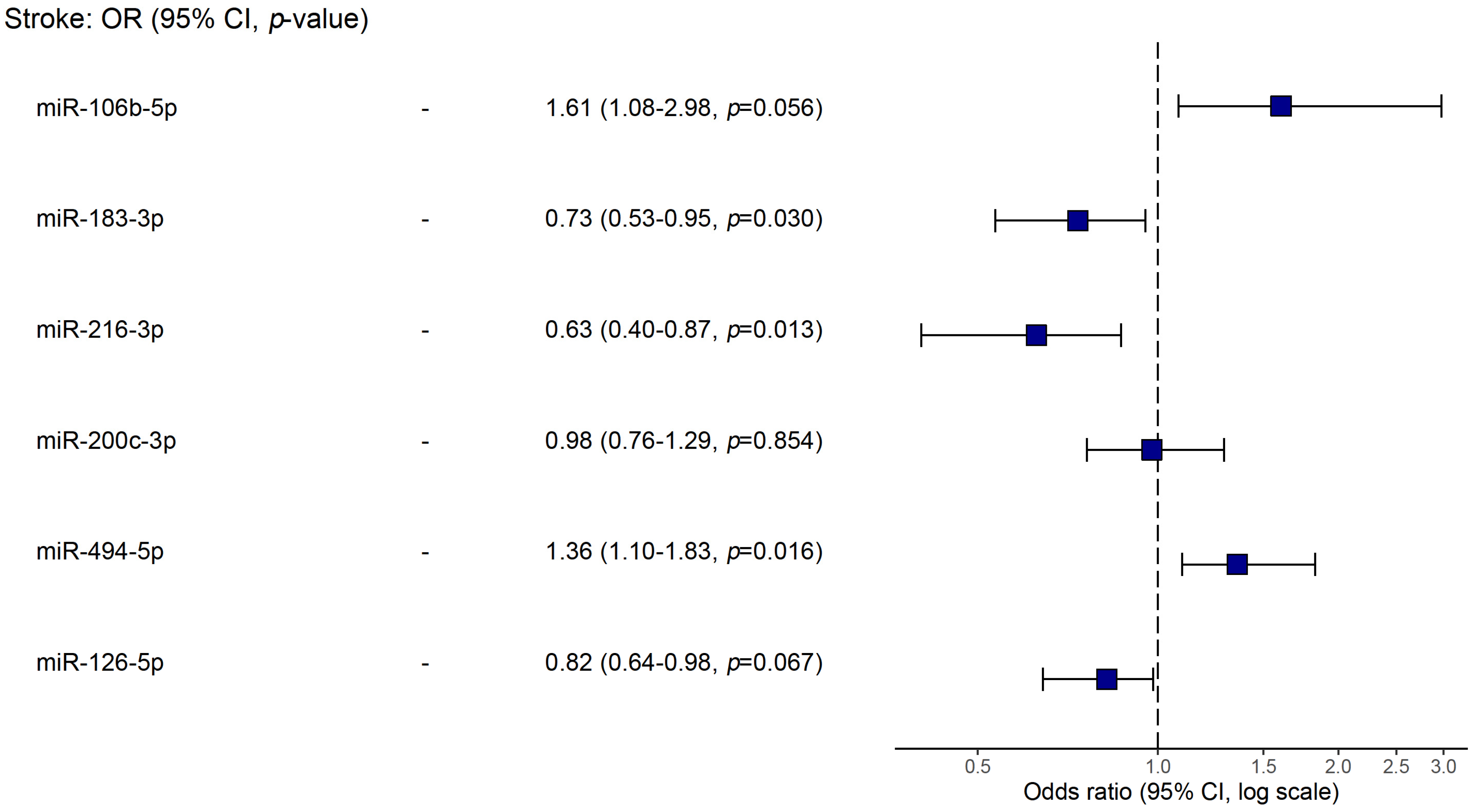
Using logistic regression, we analyzed possible associations of studied biomarkers (microRNAs, level of stenosis, age and LDL levels) with stroke (Table 3). In a model of univariable regression, we found that the up-regulation of miR-106b-5p, miR-200c-3p, and miR-494-5p and the down-regulation of miR-183-3p, miR-216-3p, and miR-126-5p were linked to stroke occurrence (p < 0.05). Yet, in a multivariable model, the only miRs showing statistical significance were miR-106b-5p, miR-183-3p, miR-216-3p, and miR-494-5p. The latter are plotted in Figure 4.

Table 3.
Regression analysis for factors associated with symptomatic CA.
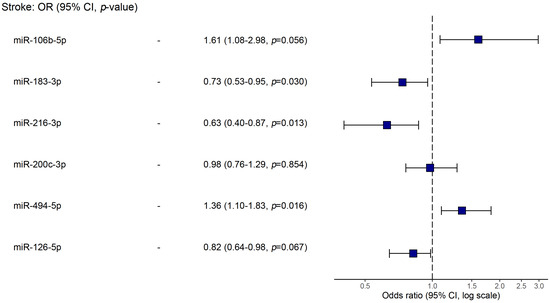
Figure 4.
Forest plot of the multivariable logistic regression for associations with stroke.
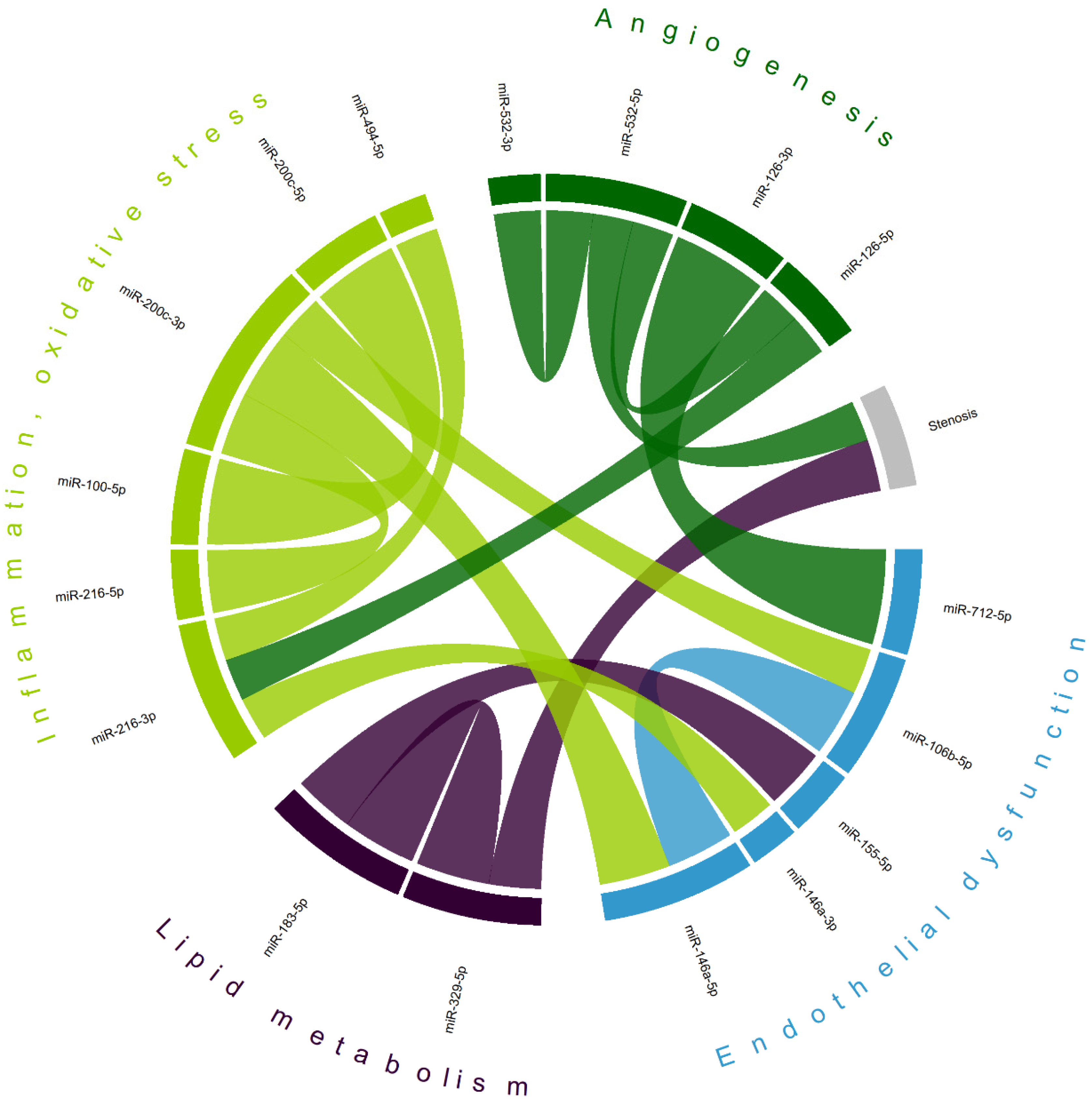
Correlation analysis (Table S1) has demonstrated a complex interplay between various microRNAs in patients with CA (Figure 5), not only within clusters of pathological processes involved in atherosclerosis, but also between clusters. The levels of two microRNAs were found to be correlated with carotid stenosis measurements (miR-329-5p [r = 0.391] and miR-532-5p [r = −0.303]), which may indicate their potential as biomarkers of CA progression. The largest absolute correlation was observed between miR-126-3p (of the ‘angiogenesis’ domain) and miR-712-5p (associated with ‘endothelial dysfunction’), r = 0.713. A previously described cluster of overexpressed miR-183-5p and miR-155-5p in CA stroke patients also shows a moderate positive correlation (r = 0.456). Intradomain correlations, including miR-100-5p and miR-200c-5p (r = 0.643, also observed as a separate cluster in symptomatic CA patients)—miRs involved in inflammation and oxidative stress—reveal a complex interplay of various factors associated with atherogenesis.
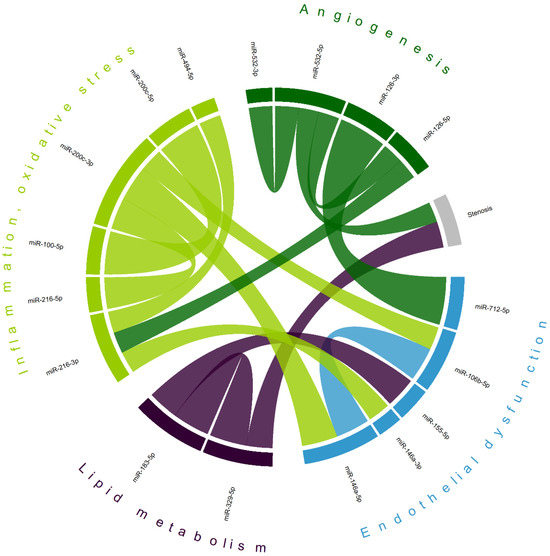
Figure 5.
Chord diagram of most correlated microRNAs; matching colors represent groups of microRNAs primarily associated with different mechanisms of atherogenesis. Shown are only statistically significant correlations with the absolute value of Pearson’s coefficient > 0.3; most correlations are positive (exceptions—stenosis/miR-532-5p and miR-216-3p/miR-494-5p); the width of each chord is proportional to Pearson’s coefficient.
In order to identify possible ‘miR’–‘target gene’ interactions, we performed a network analysis via MIENTURNET (see ‘Statistics’ section), limiting the threshold for the minimum number of miRNA–target interactions to three and the FDR to 0.1 (Figure S1). A number of common target genes were observed, including PHF14, UBQLN1, MYLK, HIF1A, FLT1, PTEN, KRAS, SIRT1, PDCD4, CXCR4, TCF4, and CXCL12.
3. Discussion
Epigenetic regulation plays an important role in atherosclerotic cerebrovascular disease, with microRNAs as potential biomarkers for CA progression and stroke occurrence. In our study, we show that the differentiated expression of certain miRNA clusters may affect stroke susceptibility in CA patients with hemodynamically significant carotid stenosis.
miR-200c-3p plasma levels were higher in atherothrombotic stroke survivors—this is a microRNA closely related to (and up-regulated by) oxidative stress and reactive oxygen species formation [9]. The higher expression of miR-200c in stroke models [10], given a possible neuroprotective effect of anti-miR-200c treatment [11], may suggest a putative role of this microRNA in ischemic stroke. It has also been regarded as a promising biomarker of carotid atherosclerotic plaque instability [12], possibly mediated by promoting oxidized LDL-induced endothelial to mesenchymal transition [13]. Both miR-200c-3p and miR-100-5p have the FLT1 gene as a predicted target—this gene is closely involved in vascular integrity and is regarded as a candidate atherosclerosis gene [14].
Stroke patients with CA had up-regulated levels of miR-106b-5p—a microRNA which was also positively correlated with miR-200c-3p. In a study of patients with atherosclerosis, miR-106b levels were higher compared to control patients [15]; stroke patients had consistent elevation of miR-106b (according to [16]). Antagonism to miR-106b in a mouse model of stroke proved to be neuroprotective [17]. A common target of miR-106b-5p and miR-155-5p is hypoxia-induced factor 1A gene (HIF1A), which is regarded as proatherogenic [18].
Another significantly up-regulated microRNA in CA stroke patients was miR-494-5p—an miR highly expressed in unstable carotid atherosclerosis plaques [19]. Interestingly, miR-494 is a part of the 14q32 miR gene cluster, the hypomethylation of which has been shown to be implicated with the development of atherosclerosis [20]. miR-494 targets inflammatory genes, and its inhibition in mice resulted in a reduction in atherosclerotic lesions and an increase in plaque stability (including via an increase in collagen content) [21]. Circulating miR-494 was elevated in patients with acute ischemic stroke, which corroborates our current findings; moreover, it was a predictor of poor long-term prognosis [22].
Several miRs were down-regulated in CA stroke patients—notably, miR 183-3p, which had a different expression profile than its 5p counterpart. Experimental data show that miR-183-3p may regulate the proliferation of vascular smooth muscle cells binding to KCNQ1OT1, a long-coding RNA, up-regulated in atherosclerosis [23]. In the stroke setting, a possible role for miR-183 lies in negatively regulating PTEN gene expression—it has been shown that following ischemia in mice, the levels of miR-183 were reduced, while transfection with agomiR-183-5p resulted in reduced cerebral ischemic injury and apoptosis [24]. Another important target for miR-183 is FOXO1, which has been found to be associated with cell apoptosis, cell cycle progression, and oxidative stress—a study has shown the mediating role of miR-183 in cerebral ischemia–reperfusion injury [25].
Another down-regulated microRNA was miR-216, which plays an important role in vascular endothelial senescence, inflammation, and macrophage regulation in atherosclerosis [26]. The plasma expression of miR-216 was higher in unstable coronary disease patients [27], and in CA, it was demonstrated that miR-216 may promote endothelial inflammation and monocyte adhesion via the Smad7/IκBα pathway [28]. However, in stroke models, the up-regulation of miR-216 is shown to be beneficial for inducing neuroprotection against cerebral ischemia by targeting JAK2 [29]. This latter hypothesis agrees with our data where stroke patients had significantly lower levels of circulating miR-216. But overall, in our cohort of patients, miR-216-3p/-5p were the most underexpressed miR (as visualized by heatmap as shown in Figure 1, Figure 2 and Figure 3), forming a cluster of their own. Along with miR-200c-3p, they share an important anti-inflammatory target gene—SIRT1—which regulates endothelial activation, platelet aggregation and coagulation, along with oxidative stress and thrombosis [30].
Interestingly, most miRs demonstrated positive correlations between each other, with only one—miR-535-5p—negatively associated with carotid stenosis degree. In a study by Sun H. et al. [31], serum miR-532-5p was inversely related to carotid intima media thickness, which corroborates our findings. The authors showed that, in vitro, the overexpression of miR-532-5p inhibited the proliferation and migration of vascular smooth muscle cells—a crucial step in atherogenesis. Consequently, patients with more severe CA exhibited lower plasma levels of circulating miR-532-5p. Among other targets, miR-532-5p is linked to programmed cell death 4 (PDCD4) gene expression—which acts as a regulator of apoptosis in vascular smooth muscle cells [32]. This gene is also a target for miR-183-5p, another highly expressed miR in CA patients.
Our target interaction analysis revealed several possible mediators involved in CA; e.g., miR-155-5p and miR-200c-3p share two significant target genes—MYLK (myosin light chain kinase) and UBQLN1 (ubiquilin-1). The former is one of the key regulators of endothelial cytoskeletal organization [33] and thus may be involved in endothelial dysfunction and atherosclerosis initiation. The latter is closely related to ischemic stroke pathophysiology as ubiquitin seems to be protective against oxidative stress and ischemic/reperfusion injury [34]. A protooncogene—KRAS (Kirsten rat sarcoma virus)—was found to be a target of miR-126-3p, miR-200c-3p, and miR-155-5p, confirming emerging evidence of its critical role in atherosclerosis and ischemic stroke [35]. We also elucidated a cluster of chemokine signaling regulation via microRNAs involving CXCL12 and CXCR4 (miR-126-5p/-3p, miR-146a-5p/-3p and miR-494-5p)—factors closely associated with atherosclerosis development and progression [36].
4. Materials and Methods
4.1. Study Population
We included in this cross-sectional prospective study 81 consecutive patients (mean age 67 ± 7 years, 53% male) with carotid atherosclerosis (CA) admitted to the angioneurology department of our clinic (Research Center of Neurology, Moscow, Russia) during the period of 2023–2024. All patients had moderate–advanced carotid stenosis (>50%), and 42% were symptomatic (i.e., had prior [>6 months] ipsilateral ischemic stroke). We excluded patients with decompensated somatic (e.g., cardiovascular, renal, hepatic) disease, a history or current signs of neoplasm, concurrent infectious, autoimmune or rheumatic disorder, or those who had undergone major surgery less than 3 months prior. All patients gave informed consent.
All patients underwent a thorough clinical and neurological examination, routine blood analyses (including low-density lipoproteins [LDLs] and total cholesterol [TC]), ultrasound examination of the extracranial carotid arteries (with luminal stenosis measured according to the NASCET criteria [37]).
4.2. Laboratory Analysis
Blood collection was carried out in the morning, on an empty stomach in vacutest tubes (with clot activator). Since it is known from the literature that the level of microRNAs can increase due to hemolysis, serum samples with its presence were not used [38].
A total of 24 microRNAs were analyzed (hsa-miR-200c-3p, hsa-miR-200c-5p, hsa-miR-146a-3p, hsa-miR-146b-5p, hsa-miR-155-3p, hsa-miR-155-5p, hsa-miR-106b-3p, hsa-miR-106b-5p, hsa-miR-183-3p, hsa-miR-183-5p, hsa-miR-126-3p, hsa-miR-126-5p, hsa-miR-712-3p, hsa-miR-712-5p, hsa-miR-532-3p, hsa-miR-532-5p, hsa-miR-494-3p, hsa-miR-494-5p, hsa-miR-329-3p, hsa-miR-329-5p, hsa-miR-100-3p, hsa-miR-100-5p, hsa-miR-216-3p, hsa-miR-216-5p).
RNA (including micro–RNA) was isolated from 200 µL of serum using the miRNeasy Serum/Plasma Advanced Kit—microRNA Isolation (Qiagen, Hilden, Germany) in accordance with the manufacturer’s protocol. The RNA concentration was evaluated using a Qubit 4 fluorimeter (Thermo Fisher Scientific, Waltham, MA, USA). Exogenous synthetic cel-miR-39-3p (0.2 nM long microRNA C. elegans) was used to normalize microRNA levels. miR-16-5p was chosen as an endogenous normalizer of circulating microRNA expression due to its relative stability in serum/plasma shown by various studies and its widespread use as a reference in the association of circulating microRNAs with various pathologies [39,40,41,42].
RNA samples (final volume 20 µL) were stored at a temperature of −80 °C until the stage of cDNA synthesis in the reverse transcription reaction.
For the reverse transcription and determination of microRNA transcripts, sets of ALMIR reagents (Algimed Techno RT–PCR, Belarus, Russia) containing optimized enzyme mixtures for reverse transcription and PCR and balanced solutions of oligonucleotides for the analysis of a specific miRNA molecule were used: an RT primer, a pair of PCR primers, and a fluorescently labeled probe for amplification detection [43].
The volume of the microRNA sample for cDNA synthesis was 2 µL, in accordance with the manufacturer’s instructions.
The quantitative determination of microRNA transcripts was performed by real-time PCR using the CFX96 C1000 Touch amplifier (BioRad, California, CA, USA) according to the following program: activation of the enzyme—20 c at 95 °C; 45 cycles, denaturation—1 c at 95 °C, annealing/elongation—20 c at 60 °C. The relative microRNA level was calculated using the 2−ΔΔCt method using the appropriate CFX Manager (version 3.1) software.
4.3. Statistical Analysis
Statistical analysis and visualization were performed using R programming language (version 4.4.1) in RStudio environment (version 2024.04.2), utilizing the following packages: ‘tidyverse’, ‘sjPlot’, ‘corrplot’, ‘finalfit’, ‘circlize’, ‘gtsummary’, ‘ggstatsplot’, and ‘pheatmap’. A comparison of continuous variables between two independent groups was performed using the Wilcoxon–Mann–Whitney test (rank-sum test), and for discrete variables, we used Pearson’s Chi-squared test or Fisher’s exact test. The Pearson correlation coefficient was used to identify possible associations between continuous variables; then, the matrix of coefficients was further visualized via a chord diagram using only statistically significant correlations with the absolute value of the coefficient > 0.3. Logistic regression (first univariable, then multivariable) was performed with ‘stroke’ as the dependent variable and most continuous variables as independent (explanatory). A heatmap using the expression of microRNAs was constructed with clustering using the ‘optimal leaf ordering’ algorithm (https://www.mathworks.com/help/stats/optimalleaforder.html; accessed on 1 August 2024); relative expression was calculated using the ‘percentize’ function; the color scheme represents relative expression; columns are individual cases. Missing data were treated as missing at random; they were dealt with according to the CART (classification and regression trees) method of the ‘mice’ package. Possible ‘MiR’–‘target’ interactions were analyzed using the MIENTURNET tool [44] with the miRTarBase database. All statistical tests were two-sided; the alpha-level was 0.05.
5. Conclusions
For the first time (to our knowledge), a broad spectrum of circulating microRNAs associated with atherogenesis was analyzed in a cohort of moderate–severe carotid atherosclerosis patients (both symptomatic and asymptomatic). A number of miRs (incl. miR-183, miR-216, miR-106b, and miR-494) was independently associated with symptomatic carotid stenosis; they may be chosen as key markers for future prospective studies. CA patients exhibit a differential expression pattern of studied miRs based on their stroke status. Epigenetic modulation, represented as complex interplay between circulating miRs of different atherogenic potential, may play a significant role in CA development and progression.
6. Limitations
The study has several limitations, of which its design (no follow-up) and relatively small sample size may be the most important. Both arose due to the pilot nature of this study—in most studies on microRNAs (in patients), the research is primarily focused on a handful of microRNAs, and the samples are usually not as large as for other biomarker studies. In our work, we decided to analyze a large number of potentially relevant microRNAs with the goal of identifying the most promising ones for future research. The choice of the microRNA panel presents potential bias as we probably may have omitted many relevant microRNAs—in our previous manuscript, we outlined the methodology underlying our strategy, but, as miRs have multiple targets, their partition (i.e., as in Figure 5) is largely arbitrary and serves a more graphic purpose. Selection bias arose due to the single-center nature of this study and may be a significant confounding factor (firstly, because severe stroke associated with CA has high mortality rates, and thus, these patients could not have been included; secondly, because most patients were on the best medical treatment for CA, which could have influenced miR expression). No in vitro or in vivo experiments were possible to confirm possible miRNA–target interactions, which prompted us to use publicly available databases, which is a limitation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms251810026/s1.
Author Contributions
Conceptualization, M.M.T. and A.A.R.; methodology, A.A.S.; software, A.A.R.; investigation, A.A.R., P.I.K., A.S.M. and V.A.A.; data curation, A.A.R.; writing—original draft preparation, A.A.R. and P.I.K.; writing—review and editing, M.M.T.; visualization, A.A.R.; supervision, M.M.T.; project administration, M.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian Science Foundation (grant No. 23-25-00477).
Institutional Review Board Statement
The study was approved by the local ethics committee (protocol No. 11-4/19, 20 November 2019) and was conducted according to the Declaration of Helsinki.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- van Velzen, T.J.; Kuhrij, L.S.; Westendorp, W.F.; van de Beek, D.; Nederkoorn, P.J. Prevalence, predictors and outcome of carotid stenosis: A sub study in the Preventive Antibiotics in Stroke Study (PASS). BMC Neurol. 2021, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; I Fowkes, F.J.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, M.; Kokubo, Y.; Arafa, A.; Kashima, R.; Nakao, Y.M.; Sheerah, H.A.; Kataoka, H. Common Carotid Artery Stenosis Degree as a Predictor of Cardiovascular Disease in a General Population: The Suita Study. J. Am. Heart Assoc. 2024, 13, e030828. [Google Scholar] [CrossRef]
- Carballo-Perich, L.; Puigoriol-Illamola, D.; Bashir, S.; Terceño, M.; Silva, Y.; Gubern-Mérida, C.; Serena, J. Clinical Parameters and Epigenetic Biomarkers of Plaque Vulnerability in Patients with Carotid Stenosis. Int. J. Mol. Sci. 2022, 23, 5149. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zeng, Z.; Shang, C.; Li, Q.; Liu, J. Epigenetic Regulation in Pathology of Atherosclerosis: A Novel Perspective. Front. Genet. 2021, 12, 810689. [Google Scholar] [CrossRef]
- Tao, J.; Xia, L.; Cai, Z.; Liang, L.; Chen, Y.; Meng, J.; Wang, Z. Interaction Between microRNA and DNA Methylation in Atherosclerosis. DNA Cell Biol. 2021, 40, 101–115. [Google Scholar] [CrossRef]
- Tanashyan, M.M.; Rakurazhev, A.A.; Kuznetsova, P.I.; Mazur, A.S.; Shabalina, A.A. MicroRNA detection in carotid atherosclerosis: Prospects for clinical use. Ann. Clin. Exp. Neurol. 2023, 17, 69–74. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Arvola, O.; Griffiths, B.; Rao, A.; Xu, L.; Pastroudis, I.-A.; Stary, C.M. Expression of miR-200c corresponds with increased reactive oxygen species and hypoxia markers after transient focal ischemia in mice. Neurochem. Int. 2021, 149, 105146. [Google Scholar] [CrossRef]
- Griffiths, B.; Xu, L.; Sun, X.; Greer, M.; Murray, I.; Stary, C. Inhibition of microRNA-200c preserves astrocyte sirtuin-1 and mitofusin-2, and protects against hippocampal neurodegeneration following global cerebral ischemia in mice. Front. Mol. Neurosci. 2022, 15, 1014751. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Sileno, S.; D’agostino, M.; Persiani, F.; Beji, S.; Paolini, A.; Camilli, D.; Platone, A.; Capogrossi, M.C.; Furgiuele, S. Atherosclerotic plaque instability in carotid arteries: miR-200c as a promising biomarker. Clin. Sci. 2018, 132, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Jiang, L. MiR-200c-3p promotes ox-LDL-induced endothelial to mesenchymal transition in human umbilical vein endothelial cells through SMAD7/YAP pathway. J. Physiol. Sci. 2021, 71, 30. [Google Scholar] [CrossRef] [PubMed]
- Mukushkina, D.; Aisina, D.; Pyrkova, A.; Ryskulova, A.; Labeit, S.; Ivashchenko, A. In silico Prediction of miRNA Interactions With Candidate Atherosclerosis Gene mRNAs. Front. Genet. 2020, 11, 605054. [Google Scholar] [CrossRef] [PubMed]
- Telkoparan-Akillilar, P.; Cevik, D. Identification of miR-17, miR-21, miR-27a, miR-106b and miR-222 as endoplasmic reticulum stress-related potential biomarkers in circulation of patients with atherosclerosis. Mol. Biol. Rep. 2021, 48, 3503–3513. [Google Scholar] [CrossRef]
- Dewdney, B.; Trollope, A.; Moxon, J.; Manapurathe, D.T.; Biros, E.; Golledge, J. Circulating MicroRNAs as Biomarkers for Acute Ischemic Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 522–530. [Google Scholar] [CrossRef]
- Li, P.; Shen, M.; Gao, F.; Wu, J.; Zhang, J.; Teng, F.; Zhang, C. An Antagomir to MicroRNA-106b-5p Ameliorates Cerebral Ischemia and Reperfusion Injury in Rats Via Inhibiting Apoptosis and Oxidative Stress. Mol. Neurobiol. 2016, 54, 2901–2921. [Google Scholar] [CrossRef]
- Mylonas, K.S.; Sarantis, P.; Kapelouzou, A.; Karamouzis, M.V.; Kapetanakis, E.I.; Kontzoglou, K.; Iliopoulos, D.C.; Nikiteas, N.; Schizas, D. Mechanosensitive Stem-Cell Genes and Klotho in Atherosclerotic Aortas: Regulating Spatially Deranged Expression Patterns Using Colchicine Regimens. J. Clin. Med. 2022, 11, 6465. [Google Scholar] [CrossRef]
- van Ingen, E.; Foks, A.C.; Kröner, M.J.; Kuiper, J.; Quax, P.H.A.; Bot, I.; Nossent, A.Y. Antisense Oligonucleotide Inhibition of MicroRNA-494 Halts Atherosclerotic Plaque Progression and Promotes Plaque Stabilization. Mol Ther Nucleic Acids. 2019, 18, 638–649. [Google Scholar] [CrossRef]
- Aavik, E.; Lumivuori, H.; Leppänen, O.; Wirth, T.; Häkkinen, S.-K.; Bräsen, J.-H.; Beschorner, U.; Zeller, T.; Braspenning, M.; van Criekinge, W.; et al. Global DNA methylation analysis of human atherosclerotic plaques reveals extensive genomic hypomethylation and reactivation at imprinted locus 14q32 involving induction of a miRNA cluster. Eur. Hear. J. 2014, 36, 993–1000. [Google Scholar] [CrossRef]
- Wezel, A.; Welten, S.M.J.M.; Razawy, W.M.; Lagraauw, H.M.M.; de Vries, M.R.; Goossens, E.A.C.B.; Boonstra, M.C.; Hamming, J.F.; Kandimalla, E.R.; Kuiper, J.; et al. Inhibition of MicroRNA-494 Reduces Carotid Artery Atherosclerotic Lesion Development and Increases Plaque Stability. Ann. Surg. 2015, 262, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, G.; Zhang, S.; Li, F.; Wang, R.; Tao, Z.; Ma, Q.; Han, Z.; Yan, F.; Fan, J.; et al. Inhibition of histone deacetylase 3 by MiR-494 alleviates neuronal loss and improves neurological recovery in experimental stroke. J. Cereb. Blood Flow Metab. 2019, 39, 2392–2405. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Hu, X.; Luo, L.; Liang, C.; Wang, H.; Chen, C. Phoenixin-14 regulates proliferation and apoptosis of vascular smooth muscle cells by modulation of KCNQ1OT1/miR-183-3p/CTNNB1 axis. Environ. Toxicol. Pharmacol. 2021, 86, 103655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, X.; Li, S.; Liu, J.; Yang, J.; Fan, X.; Zhou, S. miR-183-5p attenuates cerebral ischemia injury by negatively regulating PTEN. Mol. Med. Rep. 2020, 22, 3944–3954. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Liu, X.; Huo, Y.; Gao, Y.; Yang, Y. Suppression of lncRNA SNHG15 protects against cerebral ischemia-reperfusion injury by targeting miR-183-5p/FOXO1 axis. Am. J. Transl. Res. 2020, 12, 6250–6263. [Google Scholar]
- Yang, S.; Mi, X.; Chen, Y.; Feng, C.; Hou, Z.; Hui, R.; Zhang, W. MicroRNA-216a induces endothelial senescence and inflammation via Smad3/IκBα pathway. J. Cell. Mol. Med. 2018, 22, 2739–2749. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Chen, Y.; Zhang, S.; Feng, C.; Hou, Z.; Cai, J.; Wang, Y.; Hui, R.; Lv, B.; et al. MicroRNA-216a promotes M1 macrophages polarization and atherosclerosis progression by activating telomerase via the Smad3/NF-κB pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1865, 1772–1781. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Mi, X.; Zhang, S.; Yang, Y.; Hui, R.; Zhang, W. MicroRNA-216a Promotes Endothelial Inflammation by Smad7/IκBα Pathway in Atherosclerosis. Dis. Markers 2020, 2020, 8864322. [Google Scholar] [CrossRef]
- Tian, Y.S.; Zhong, D.; Liu, Q.Q.; Zhao, X.L.; Sun, H.X.; Jin, J.; Wang, H.N.; Li, G.Z. Upregulation of miR-216a exerts neuroprotective effects against ischemic injury through negatively regulating JAK2/STAT3-involved apoptosis and inflammatory pathways. J. Neurosurg. 2019, 130, 977–988. [Google Scholar] [CrossRef]
- Bettiol, A.; Urban, M.L.; Emmi, G.; Galora, S.; Argento, F.R.; Fini, E.; Borghi, S.; Bagni, G.; Mattioli, I.; Prisco, D.; et al. SIRT1 and thrombosis. Front. Mol. Biosci. 2024, 10, 1325002. [Google Scholar] [CrossRef]
- Sun, H.; Wu, S.; Sun, B. MicroRNA-532-5p protects against atherosclerosis through inhibiting vascular smooth muscle cell proliferation and migration. Cardiovasc. Diagn. Ther. 2020, 10, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xu, Z.; Yuan, M.; Zhang, Y.; Zhao, B.; Wang, J.; Zhang, A.; Li, G. MicroRNA-16 suppresses the activation of inflammatory macrophages in atherosclerosis by targeting PDCD4. Int. J. Mol. Med. 2016, 37, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kim, S.; Patterson, N.; Rooney, K.; Searles, C.D. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am. J. Physiol. Circ. Physiol. 2014, 306, H1192–H1203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, F.; Wang, H. Enhanced Proteostasis in Post-ischemic Stroke Mouse Brains by Ubiquilin-1 Promotes Functional Recovery. Cell. Mol. Neurobiol. 2016, 37, 1325–1329. [Google Scholar] [CrossRef]
- Ban, R.; Huo, C.; Wang, J.; Zhang, G.; Zhao, X. Exploration of the Shared Gene Signatures and Molecular Mechanisms Between Ischemic Stroke and Atherosclerosis. Int. J. Gen. Med. 2024, 17, 2223–2239. [Google Scholar] [CrossRef]
- Murad, H.A.S.; Rafeeq, M.M.; Alqurashi, T.M.A. Role and implications of the CXCL12/CXCR4/CXCR7 axis in atherosclerosis: Still a debate. Ann. Med. 2021, 53, 1598–1612. [Google Scholar] [CrossRef]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.K.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef]
- Zhelankin, A.V.; Stonogina, D.A.; Akselrod, A.S. Circulating platelet-derived microRNAs as potential biomarkers of STEMI in patients with hypertension. Res. Results Biomed. 2024, 10, 351–373. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef]
- Chen, S.-S.; Tang, C.-H.; Chie, M.-J.; Tsai, C.-H.; Fong, Y.-C.; Lu, Y.-C.; Chen, W.-C.; Lai, C.-T.; Wei, C.-Y.; Tai, H.-C.; et al. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019, 10, 31. [Google Scholar] [CrossRef]
- Binderup, H.G.; Madsen, J.S.; Heegaard, N.H.H.; Houlind, K.; Andersen, R.F.; Brasen, C.L. Quantification of microRNA levels in plasma—Impact of preanalytical and analytical conditions. PLoS ONE 2018, 13, e0201069. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.D.R.; Margiotti, K.; Mesoraca, A.; Giorlandino, C. Quantification of circulating microRNAs by droplet digital PCR for cancer detection. BMC Res. Notes 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform. 2019, 20, 545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).