Abstract
Renovascular hypertension (RH), a secondary hypertension, can significantly impact heart health, resulting in heart damage and dysfunction, thereby elevating the risk of cardiovascular diseases. Coniferol (CA), which has vascular relaxation properties, is expected to be able to treat hypertension-related diseases. However, its potential effects on cardiac function after RH remain unclear. In this study, in combination with network pharmacology, the antihypertensive and cardioprotective effects of CA in a two-kidney, one-clip (2K1C) mice model and its ability to mitigate angiotensin II (Ang II)-induced hypertrophy in H9C2 cells were investigated. The findings revealed that CA effectively reduced blood pressure, myocardial tissue damage, and inflammation after RH. The possible targets of CA for RH treatment were screened by network pharmacology. The interleukin-17 (IL-17) and tumor necrosis factor (TNF) signaling pathways were identified using a Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. The inflammatory response was identified using a Gene Ontology (GO) enrichment analysis. Western blot analysis confirmed that CA reduced the expression of IL-17, matrix metallopeptidase 9 (MMP9), cyclooxygenase 2 (COX2), and TNF α in heart tissues and the H9C2 cells. In summary, CA inhibited cardiac inflammation and fibrohypertrophy following RH. This effect was closely linked to the expression of MMP9/COX2/TNF α/IL-17. This study sheds light on the therapeutic potential of CA for treating RH-induced myocardial hypertrophy and provides insights into its underlying mechanisms, positioning CA as a promising candidate for future drug development.
1. Introduction
Hypertension, a prevalent and incurable chronic disease, poses a significant threat to cardiac health [1]. Renovascular hypertension (RH) stands out as a type of secondary hypertension, primarily induced by arterial stenosis, which often manifests as unilateral or bilateral renal artery stenosis [2]. This stenosis triggers an increase in angiotensin II (Ang II) production and aldosterone release, activating the renin–angiotensin system, elevating blood pressure, and potentially leading to cardiac insufficiency [3,4]. It is important to note that RH carries a higher risk of heart disease compared to essential hypertension [5]. Recent studies have also shown enhanced cardiac inflammation in animal models of RH, resulting in more severe cardiac remodeling, fibrosis, and compromised cardiac function [6,7]. Therefore, research on treatment for RH is of great significance for preventing and controlling the progression of the aforementioned diseases.
Coniferyl alcohol (CA) is widely present in traditional Chinese herbal remedies, specifically in Picea neoveitchii Mast and Fructus Aurantii [8]. CA frequently serves as a precursor for synthesizing chemicals like ferulic acid, vanillin, and silybin, all known for their vasodilatory and antihypertensive properties [9,10,11]. Additionally, CA itself exhibits vasodilatory effects [12,13]. However, the therapeutic efficacy of CA in treating hypertension, as well as its potential to ameliorate hypertension-induced cardiac dysfunction, remains unexplored and awaits further investigation.
Network pharmacology, through the systematic integration and interpretation of biological systems, facilitates a comprehensive understanding of disease progression and evolution [14]. With an emphasis on revealing the mechanisms of action of drugs through multi-pathway and multi-target signaling pathways, this methodology provides many opportunities for the discovery of innovative therapeutic agents [15]. Network pharmacology is an innovation way in the research pathway of “diseases–targets–drugs” in medicine. It integrates and summarizes information from existing databases through a series of methods to determine effective drug intervention treatments for diseases [5]. The multi-target pattern of network pharmacology provides a more reasonable explanation for diseases and complications, facilitating disease identification and drug combination therapy [16,17]. Network pharmacology has been widely applied in cardiovascular diseases, such as for identifying the effective components and potential pharmacological mechanisms of Danshen Decoction in the treatment of cardiovascular diseases [18], clarifying the effect of QiShenYiQi Dripping Pills (T101) in the treatment of heart failure depending on multi-components, multi-targets, and multi-pathways [19], and revealing the efficacy and potential mechanism of anxia Baizhu Tianma Decoction in the treatment of hypertension [20]. Additionally, the integration of network pharmacology with molecular docking, a technique for evaluating the binding affinity between receptors and drug molecules, has emerged as a pivotal tool in drug discovery and development [21]. This combined approach holds great potential, particularly in situations where the targets of drugs therapy are not yet clear [22].
In this study, by applying the aforementioned methodologies, the intricate relationship between CA and RH was uncovered and the binding affinity between CA and its related targets was studied in detail. The protective efficacy and underlying mechanisms of CA against cardiac hypertrophy induced by RH were illuminated through experimental verification. This research presents novel perspectives and methodologies for the treatment of RH.
2. Results
2.1. The Protective Effect of CA on Cardiovascular Parameters and Cardiac Hypertrophy in RH Mice
In this study, the targets and signaling pathways for the CA treatment of RH myocardial hypertrophy were identified using network pharmacology. A total of 111 CA targets were obtained from TargetNet (scores > 0.1) (Supplementary Table S1), 88 CA targets were obtained from SuperPred (Supplementary Table S2), 25 CA targets were obtained from HERB (Supplementary Table S3), and 70 CA targets were obtained from SEA (scores ≥ 0.4) (Supplementary Table S4). By summarizing the results of the above four databases and removing duplicates, 227 CA targets were obtained (Supplementary Table S5). In total, 437 RH targets were obtained from GeneCards (Supplementary Table S6), 778 RH targets (scores ≥ 10) from CTD (Supplementary Table S7), 72 RH targets were obtained from OMIM (Supplementary Table S8), and 78 RH targets were obtained from DisGeNET (Supplementary Table S9). The results of the four databases were summarized, and 1227 RH targets were obtained by removing duplicates (Supplementary Table S10). A total of 52 intersection targets were obtained by comparing the CA and RH targets (Figure 1), and these targets are detailed in Table 1. Thus, CA may be useful for the treatment of RH. Therefore, we established a representative RH animal model using 2K1C mice for verification. Four weeks after surgery, the systolic and diastolic pressures of the 2K1C mice were obviously higher than those in the sham group, demonstrating the successful establishment of the RH mouse model (Figure 2A,B). Systolic and diastolic pressures were significantly decreased after three weeks of continuous administration of 10 mg/kg BENA and 20 and 40 mg/kg CA (Figure 2A,B). Without affecting body weight, 10 mg/kg BENA and 40 mg/kg CA significantly reduced the heart weight/body weight (HW/BW) ratio (Figure 2C,D). Echocardiographic indices were monitored simultaneously. As shown in Figure 3, the IVS, LVPW, EF, and FS indices of the heart showed obvious hypertrophy in the model group. These changes were relieved to varying degrees after the administration of 10 mg/kg BENA and 40 mg/kg CA. H&E staining of the heart tissues showed obvious myocardial cell injury, inflammatory penetration, and necrosis in the model group, which could be alleviated by 10 mg/kg BENA and 40 mg/kg CA (Figure 4A,B). These results indicated that CA and BENA had significant inhibitory effect on RH-induced cardiac inflammation and hypertrophy.
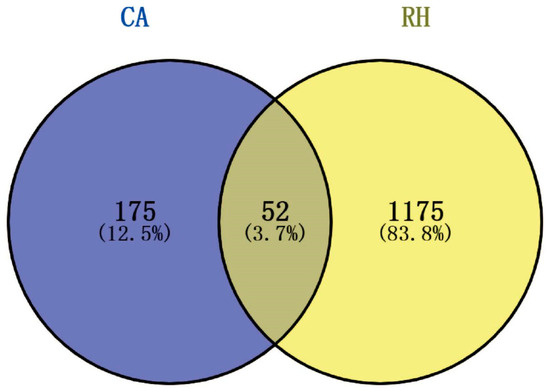
Figure 1.
Venn diagram of intersection targets of CA and RH. Blue represents the targets of CA and yellow represents the targets of RH. There were 52 intersecting targets.

Table 1.
Intersection targets of CA and RH.
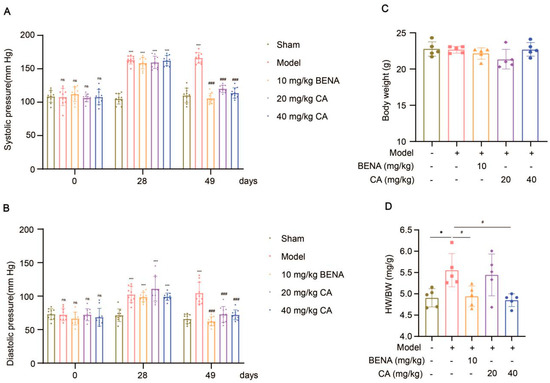
Figure 2.
Effects of CA on cardiovascular parameters in RH mice. Systolic (A) and diastolic (B) blood pressures were monitored before surgery, four weeks after surgery, and three weeks after administration (n = 10). After fasting for 12 h, the body weight of each mice was measured (C), and their hearts were weighed to obtain the HW/BW ratio (D) (n = 5). In the quantitative chart, green circles represent sham group, pink squares represent model group, orange triangles represent 10 mg/kg BENA group, purple rhombuses represent 20 mg/kg CA group, and blue hexagons represent 40 mg/kg CA group. The values are mean ± SD. * p < 0.05, and *** p < 0.001 vs. sham; # p < 0.05, and ### p < 0.001 vs. model. ns = p > 0.05.
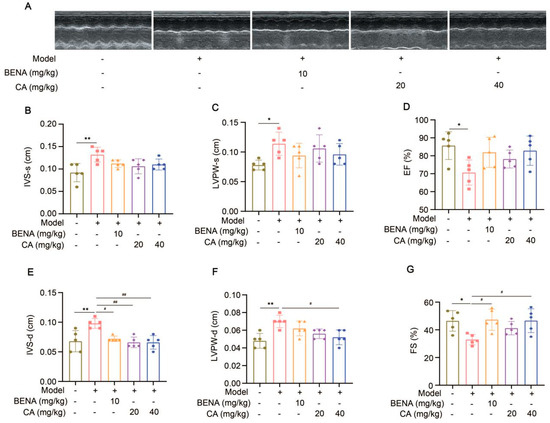
Figure 3.
Effects of CA on echocardiographic indexes in RH mice. Representations of echocardiographic images are shown (A). From left to right, they are the sham group, model group, 10 mg/kg BENA group, and 20 mg/kg and 40 mg/kg CA groups, respectively. Statistics for IVS-s (B), LVPW-s (C), EF (D), IVS-d (E), LVPW-d (F), and FS (G). In the quantitative chart, green circles represent sham group, pink squares represent model group, orange triangles represent 10 mg/kg BENA group, purple rhombuses represent 20 mg/kg CA group, and blue hexagons represent 40 mg/kg CA group. The values are mean ± SD, n = 5. * p < 0.05, and ** p < 0.01 vs. sham; # p < 0.05, and ## p < 0.01 vs. model.
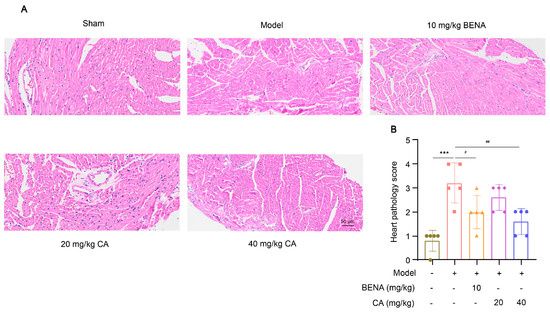
Figure 4.
Effect of CA on cardiac histological changes in RH mice. H&E staining was used to observe the pathological changes in heart tissues in the sham group, model group, 10 mg/kg BENA group, and 20 mg/kg and 40 mg/kg CA groups. Representative images of hearts (A) are shown. The statistics of cardiac pathology scores (B) are also displayed. The scale bar represents 50 µm. In the quantitative chart, green circles represent sham group, pink squares represent model group, orange triangles represent 10 mg/kg BENA group, purple rhombuses represent 20 mg/kg CA group, and blue hexagons represent 40 mg/kg CA group. The values are mean ± SD, n = 5. *** p < 0.001 vs. sham; # p < 0.05, and ## p < 0.01 vs. model.
2.2. Network Pharmacology and Molecular Docking Analysis
The mechanisms of CA in reducing blood pressure and alleviating myocardial inflammation and hypertrophy after RH were predicted using network pharmacology and molecular docking. The intersection targets were imported into Cytoscape to establish a “drug–target–disease” network (Figure 5A). The information on core targets (degree > 20) is shown in Table 2. A GO analysis was used to identify the top ten molecular functions (MFs) (Figure 5B), biological processes (BPs) (Figure 5C), and cellular components (CCs) (Figure 5D). A KEGG analysis enriched the disease pathways, including the IL-17 and TNF signaling pathways (Figure 5E). The IL-17 and TNF signaling pathways play key roles in the regulation of disease inflammation [23,24]. Based on the above results showing that CA alleviated cardiac inflammation after RH, the KEGG results also indicated that the IL-17 and TNF signaling pathways were closely related to the core targets. Therefore, we conducted the molecular docking of CA with the core targets and IL-17. The specific docking mechanisms between CA and the core targets are shown in Figure 6. Those with a lower docking binding energy were MMP9 (−6.02 kcal/mol), TNF α (−6.01 kcal/mol), COX2 (−5.04 kcal/mol), and IL-17 (−5.07 kcal/mol), which were associated with inflammation. It was speculated that CA might affect myocardial inflammation and hypertrophy in RH models by regulating the expression of key proteins in the IL-17 and TNF signaling pathways.
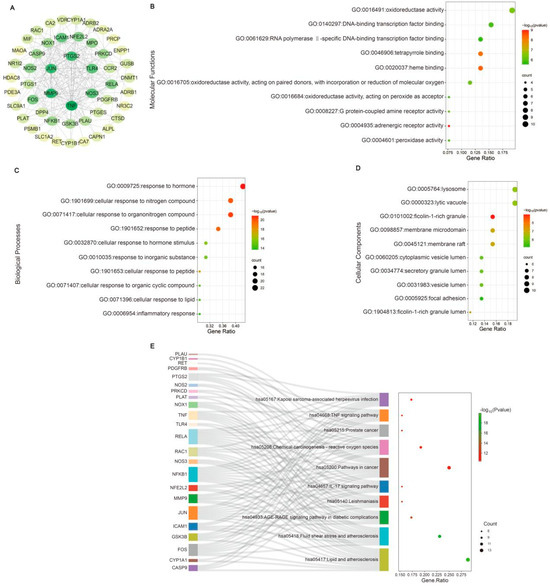
Figure 5.
Network pharmacology analysis of CA in alleviating cardiac hypertrophy in mice with RH. A PPI network was established. The innermost nodes represent the CA–RH intersection targets with a degree of >20 (A). The top ten GO enrichment items of CA anti-RH core targets are reflected in MFs (B), BPs (C), and CCs (D). The top ten KEGG enrichment terms of core targets for CA against RH (E) are also shown.

Table 2.
Information on core targets.
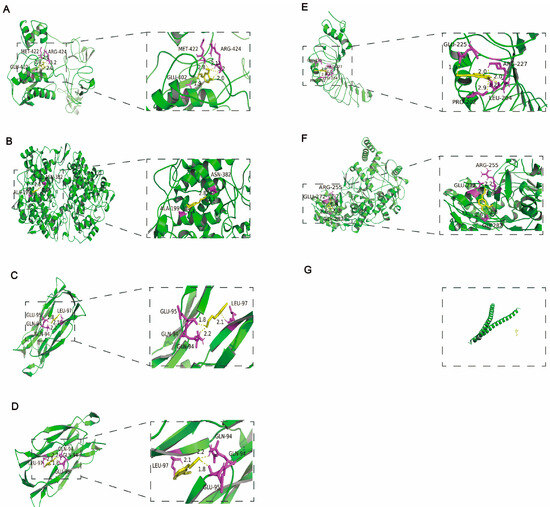
Figure 6.
Molecular docking of CA with the core targets. Molecular docking of CA with the top six intersection targets. CA interacts with MMP9 via GLU-402, MET-422, and ARG-424 (A). CA interacts with COX2 via ALA-199, and ASN-382 (B). CA interacts with TNF α via GLU-94, GLU-95, and LEU-97 (C). CA interacts with IL-17 via GLU-94, GLU-95, and LEU-97 (D). CA interacts with TLR4 via GLU-225, ARG-227, PRO-202, and LEU-204 (E). CA interacts with NOS3 via GLU-272, ARG-255, and GLY-282 (F). CA and JUN have no interaction (G).
2.3. CA Alleviated RH-Induced Myocardial Inflammation and Hypertrophy by Decreasing the Expression of MMP9/COX2/TNF α/IL-17
CA may attenuate cardiac inflammation and myocardial hypertrophy in the RH model through the TNF and IL-17 signaling pathways. Thus, we examined the protein expressions of the main targets of the inflammatory signaling pathways in cardiac tissues before and after CA treatment. The serum levels of TNF α and IL-17 were significantly increased in the model group compared to the sham group (Figure 7A,B). The expressions of TNF α, IL-17, COX2, and MMP9 in the heart tissues of the RH group were obviously enhanced by Western blot analysis (Figure 7C–F). The 40 mg/kg CA treatment significantly suppressed the expressions of these proteins (Figure 7C–F). These results suggest that CA regulated cardiac inflammation and hypertrophy in the RH mice by down-regulating MMP9/COX2/TNFα/IL-17, thereby inhibiting the TNF and IL-17 signaling pathways.
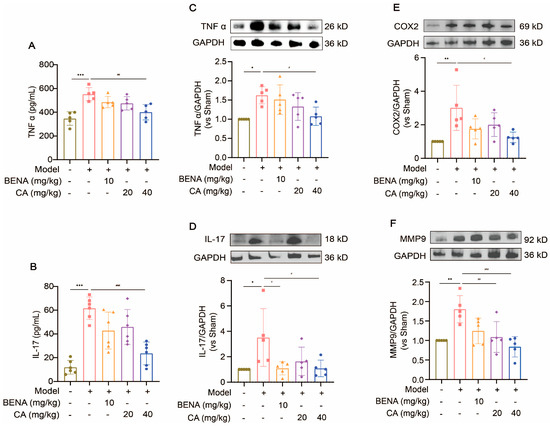
Figure 7.
CA inhibited the expressions of TNF and IL-17 signaling pathway proteins. The serum levels of TNF α (A) and IL-17 (B) were measured using commercially available ELLSA kits after the administration of 10 mg/kg of BENA, 20 mg/kg of CA, and 40 mg/kg of CA, respectively, to the RH mice. The expressions of TNF α (C), IL-17 (D), COX2 (E), and MMP9 (F) were detected by Western blot analysis after the administration of 10 mg/kg of BENA, 20 mg/kg of CA, and 40 mg/kg of CA, respectively, to the RH mice. The quantification of normalized TNF α, IL-17, COX2, and MMP9 was performed. In the quantitative chart, green circles represent sham group, pink squares represent model group, orange triangles represent 10 mg/kg BENA group, purple rhombuses represent 20 mg/kg CA group, and blue hexagons represent 40 mg/kg CA group. The values are mean ± SD, n = 5. * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. sham; # p < 0.05, ## p < 0.01, and ### p < 0.001 vs. model.
2.4. CA Reduced Ang II-Induced Hypertrophy of H9C2 Cells by Decreasing the Expression of MMP9/COX2/TNF α/IL-17
H9C2 cells were treated with different concentrations of Ang II (0.04, 0.2, 1, 5, and 25 μM) for 48 h. Their cell viability decreased significantly at concentrations above 5 μM (Figure 8A). The exposure of the H9C2 cells to CA (0.5, 5, and 50 μM) for 24 h did not affect their cell viability (Figure 8B), but 50 μM CA significantly alleviated the decreased cell viability caused by 5 μM Ang II (Figure 8C). Further validation of the animal experimental results was achieved by stimulating the H9C2 cells with Ang II in vitro. This stimulation markedly up-regulated α-smooth muscle actin (α-SMA) expression, indicating successful hypertrophy induction by Ang II (Figure 9A). Consistent with the in vivo results, the expressions of TNF α, IL-17, COX2, and MMP9 were up-regulated in the H9C2 cells stimulated by Ang II at 5 μM and were significantly down-regulated by CA at 50 μM (Figure 9B–E).
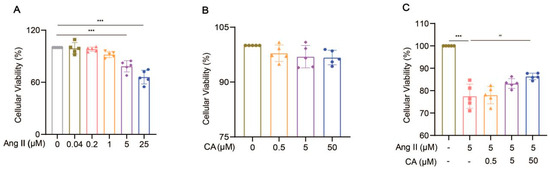
Figure 8.
Effect of CA on the viability of H9C2 cells induced Ang II. The cell viability of the H9C2 cells treated with Ang II (0.04, 0.2, 1, 5, and 25 μM) (A) and CA (0.5, 5, and 50 μM) (B) was analyzed by CCK8. The H9C2 cells were treated with CA (0.5, 5, and 50 μM) in the presence of 5 μM Ang II (C). The cell viability of the 5 μM Ang II-induced H9C2 cells treated with CA (0.5, 5, and 50 μM) was detected by CCK8 assay. In quantitative (A), gray circles represent control, green squares represent 0.04 μM Ang II, pink upright triangles represent 0.02 μM Ang II, orange inverted triangles represent 1 μM Ang II, purple rhombuses represent 5 μM Ang II, and blue hexagons represent 25 μM Ang II. In quantitative (B), green circles represent control, orange triangles represent 0.5 μM CA, purple rhombuses represent 5 μM CA, and blue hexagons represent 50 μM CA. In quantitative (C), green circles represent control, pink squares represent 5 μM Ang II, orange triangles represent 5 μM Ang II + 0.5 μM CA, purple rhombuses represent 5 μM Ang II + 5 μM CA, and blue hexagons represent 5 μM Ang II + 50 μM CA. The values are mean ± SD, n = 5. *** p < 0.001 vs. control; ## p < 0.01 vs. 5 μM Ang II.
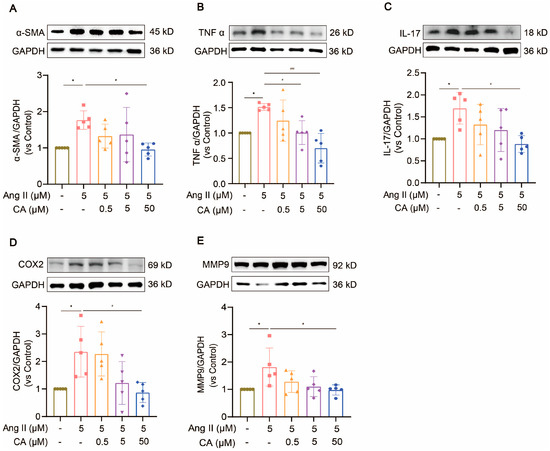
Figure 9.
The inhibitory effect of CA on Ang II-stimulated hypertrophy of H9C2 cells. H9C2 cells were exposed to different concentrations of CA (0.5, 5, and 50 μM) under the stimulation of 5 μM Ang II. The expressions of α-SMA (A), TNF α (B), IL-17 (C), COX2 (D), and MMP9 (E) were detected by Western blot analysis. The quantification of normalized α-SMA, TNF α, IL-17, COX2, and MMP9 was performed. In the quantitative chart, green circles represent control, pink squares represent 5 μM Ang II, orange triangles represent 5 μM Ang II + 0.5 μM CA, purple rhombuses represent 5 μM Ang II + 5 μM CA, and blue hexagons represent 5 μM Ang II + 50 μM CA The values are mean ± SD, n = 5. * p < 0.05 vs. control; # p < 0.05, and ### p < 0.001 vs. 5 μM Ang II.
2.5. Effects of CA Toxicity on Brain, Liver, Lungs, and Spleen of Mice
After administrating CA for three weeks, the brain, liver, lung, and spleen tissues were stained with H&E. As shown in Figure 10, there was no inflammatory infiltration or cell necrosis in the brain, liver, lungs, or spleen tissues of any group, suggesting that CA itself did not cause damage to other organs.
Figure 10.
H&E staining of brain, liver, lungs, and spleen after CA treatment. Pathological changes in brain, liver, lungs, and spleen tissues from the sham group, model group, and 20 mg/kg and 40 mg/kg CA groups were evaluated by H&E staining. Representative images of the brain (A), liver (B), lungs (C), and spleen (D) are shown. The scale bar represents 50 µm.
3. Discussion
In this study, based on reports of CA vasodilation [12], we demonstrated, for the first time, that CA had the effect of lowering blood pressure and alleviating cardiac inflammation in an RH model through the combination of a network pharmacological analysis and experimental testing. We found that CA ameliorated cardiac inflammation and myocardial hypertrophy after RH by regulating the expressions of IL-17, TNF α, MMP9, and COX2 in both the IL-17 and TNF signaling pathways. Moreover, similar verification was further obtained with H9C2 cells in vitro.
We conducted a comprehensive investigation into the antihypertensive and cardioprotective effects of CA within an RH model. Experimental data indicated that both CA and BENA (positive control) significantly reduced diastolic and systolic blood pressures in the RH model. It is worth noting that 10 mg/kg of BENA, as well as 20 and 40 mg/kg of CA, had no significant impact on the weight of the mice, while 10 mg/kg of BENA and 40 mg/kg of CA strikingly reduced the HW/BW ratio. Further echocardiographic analysis and H&E staining of heart tissues indicated that 10 mg/kg of BENA and 40 mg/kg of CA were effective in alleviating the cardiac inflammation and hypertrophy induced by RH. These results clearly indicate that CA can reduce RH-induced cardiac inflammation and hypertrophy while reducing blood pressure. Previous reports have indicated that BENA can reduce the incidence of cardiovascular events in the treatment of hypertension [25]. A similar conclusion was also obtained in this study.
To gain a deeper understanding of how CA exerts its antihypertensive and cardioprotective effects, we conducted a detailed investigation of its mechanisms using network pharmacology. The findings revealed that TNF, IL-17, COX2, and MMP9 were the primary genes involved in CA’s action against RH. Further KEGG analysis highlighted the critical roles of the TNF and IL-17 signaling pathways in this process. Molecular docking techniques also demonstrated an excellent binding affinity between CA and these core targets. Hypertension is often accompanied by systemic inflammation, triggering the excessive secretion of proinflammatory cytokines, which can lead to functional abnormalities such as cardiac hypertrophy [26]. Notably, when cells sense inflammatory stimuli, COX2 exhibits a high level of responsiveness to various pro-inflammatory mediators and cytokines [27]. Meanwhile, pro-inflammatory cells in the heart release MMP9, and its activation not only intensifies the inflammatory response, but also disrupts the balance of the extracellular matrix (ECM), ultimately impairing cardiac function [22]. In our study, we observed that 40 mg/kg of CA significantly down-regulated the expression levels of TNF α, IL-17, COX2, and MMP9 in heart tissues, but 10 mg/kg of BENA did not significantly reduce their expression. These results suggest that CA alleviated RH-induced cardiac inflammation and myocardial hypertrophy by reducing the expression of MMP9/COX2/TNF α/IL-17, and that the therapeutic mechanism of 10 mg/kg of BENA did not depend on them. These results strongly indicate that CA improves cardiac inflammation and myocardial hypertrophy in RH by regulating the expression of MMP9/COX2/TNF α/IL-17 in the TNF and IL-17 signaling pathways.
Previous studies on small-molecule drugs in diseases have shown that drugs often affect protein stability by binding to proteins and affecting their abundance, which are more common in inflammatory, tumor, and autoimmune diseases. It is common for drugs to regulate protein expression by interacting with proteins to induce ubiquitination, phosphorylation, and acetylation, or to reduce protein abundance to affect stability [28,29,30]. Therefore, CA, a small-molecule drug derived from traditional Chinese medicine, may have a similar pattern of action to other small molecules. In cardiac inflammation caused by renovascular hypertension, CA may interact with TNF α, IL-17, COX2, and MMP9 to affect their protein stability and reduce their expressions, thus playing a role in improving inflammation. The interaction between CA and TNF α, IL-17, COX2, and MMP9 needs to be further explored through pull-down, surface plasmon resonance analyses, and other experiments, which will be the primary problem to be solved in our subsequent research.
Excessive Ang II in RH has been proven to induce cardiac hypertrophy [31]. Current research indicates that Ang II is a central factor causing myocardial hypertrophy [32]. To further investigate this phenomenon, we successfully established an AngII-stimulated hypertrophy model of H9C2 cells in vitro [33]. The degree of cardiomyocyte hypertrophy was assessed by observing the expression of α-SMA [34]. The results showed that CA can effectively reduce the Ang II-induced hypertrophy of H9C2 cells and significantly down-regulate the expression of inflammation-related proteins such as TNF α, IL-17, COX2, and MMP9. These findings suggest that CA alleviates the hypertrophy of cardiomyocytes by reducing the expressions of these inflammatory proteins.
4. Materials and Methods
4.1. Reagents and Materials
CA (CAS number: 458-35-5) was obtained from Adamas (Shanghai, China). A tumor necrosis factor α (TNF α) assay kit (SEKM-0034) was obtained from Solarbio (Beijing, China). An interleukin-17 (IL-17) kit (orb775012) was purchased from Biorbyt (Cambridge, UK). Hematoxylin and eosin (H&E) staining was performed using Baso Cell (Zhuhai, China). Antibodies against TNF α (AF7014) and IL-17 (DF6127) were obtained from Affinity Biosciences (Changzhou, China). Antibodies against cyclooxygenase 2 (COX2) (sc-514489) and matrix metallopeptidase 9 (MMP9) (sc-393859) were procured from Santa Cruz Biotechnology (Santa Cruz, TX, USA). The GAPDH antibody (60004-1-Ig), goat anti-rabbit IgG-HRP (SA00001-2), and goat anti-mouse IgG-HRP (SA00001-1) were bought from Proteintech (Wuhan, China).
4.2. Prediction of Targets of CA and RH
CA targets were obtained from TargetNet (http://targetnet.scbdd.com, accessed on 13 July 2023), SuperPred (https://prediction.charite.de/, accessed on 13 July 2023), a high-throughput experiment and reference-guided database (HERB) (http://herb.ac.cn, accessed on 13 July 2023), and a similarity ensemble approach (SEA) (https://sea.bkslab.org/, accessed on 13 July 2023) [35].
RH targets were acquired from GeneCards (https://www.genecards.org/, accessed on 10 July 2023), a comparative toxicogenomics database (CTD) (http://ctdbase.org/, accessed on 10 July 2023), online mendelian inheritance in man (OMIM) (https://www.omim.org/, accessed on 10 July 2023), and the disease gene network (DisGeNET) (https://www.disgenet.org/, accessed on 10 July 2023).
4.3. Animals and Experimental Design
For animal experiments, 6–8-week-old male C57BL/6 mice were obtained from the SPF company (license number: SCXK jing 2019-0010). They were housed in a room with a 12 h light/12 h dark cycle (23 ± 2 °C), where food and water were freely available. The experimental design was approved by the Ethics Committee of West China Hospital, Sichuan University (Ethics record number: 20220302077).
After adaptation, the animals were divided into five groups: sham (saline), model (saline), 10 mg/kg benazepril (BENA), 20 mg/kg CA, and 40 mg/kg CA. CA and BENA were administered intragastrically once daily for three weeks.
4.4. 2K1C Hypertension Model
RH was induced in the mice using a two-kidney, one-clip (2K1C) operation, as previously described [36,37]. The mice were anesthetized with isoflurane. After disinfecting the skin on the back, the right kidney was exposed via an incision. The right renal artery was divided and silver clips were placed around it to constrict the blood flow. Finally, the incision was carefully sutured.
4.5. Blood Pressure Measurement
The mice were monitored for systolic and diastolic blood pressure before surgery, postoperatively, and after administration using a tail-cuff non-invasive blood pressure analysis system (BP-2000 system, VisiTech Systems, Singapore) [38]. Blood pressure was measured three times under rest state and the average values were obtained [39].
4.6. Cardiac Function Assessment
After three weeks of continuous administration, all animals were fasted for 12 h. After isoflurane anesthesia, the interventricular septum (IVS), left ventricular posterior wall (LVPW), ejection fraction (EF), and fractional shortening (FS) were measured using a color ultrasonic Doppler diagnostic system (Mindray-M9Vet) [40,41].
4.7. Tissue and Blood Sample Collection
After fasting for 12 h, the mice were intraperitoneally anesthetized with 1% pentobarbital. Subsequently, blood samples were quickly drawn from the abdominal aorta. The heart, liver, spleen, lungs, and brain tissues were rapidly extracted. Some heart tissues were preserved in liquid nitrogen, whereas others were immobilized with 10% formaldehyde for further analysis [42].
4.8. Enrichment and Establishment of Drug–Targets–Disease Networks
The intersection targets of CA and RH were analyzed using Venny. A protein–protein interaction (PPI) network was built by using the STRING database. Common targets in the PPI network were visualized using Cytoscape. The pathways were identified using GO and KEGG analyses [14].
4.9. Molecular Docking
The target structure of the anti-RH mechanism was downloaded from the Protein Data Bank (PDB). The binding capacity between CA and its targets was determined using the AutoDock 1.5.7 software. The results were visualized using PyMol [15]. The crystal structures of MMP9 (ID: 1L6J), TNF α (ID: 3L9J), COX2 (ID: 5IKR), IL-17 (ID: 7AMA), toll-like receptor 4 (TLR4) (ID: 2Z62), nitric oxide synthase 3 (NOS3) (ID: 1M9M), and the jun proto-oncogene, AP-1 transcription factor subunit (JUN) (ID: 5FV8), were acquired from the PDB database.
4.10. Histopathological Analysis
Heart, liver, and other tissues were cleaned with physiological saline, immobilized in 10% formaldehyde solution, and embedded in paraffin. Slices (3 μm) were cut and stained with hematoxylin and eosin [43]. Histomorphological changes in various tissues were observed by optical microscopy.
4.11. ELLSA
Blood was centrifuged at 3000× g/5 min to obtain serum. The contents of TNF α and IL-17 were quantitated by an enzyme-linked immunosorbent assay (ELISA) kit on the basis of the manufacturer’s protocol [44].
4.12. Cell Culturing
H9C2 cells were purchased from the American Type Culture Collection (ATCC) and were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (PS). The cells were maintained in a 37 °C thermostatic incubator with 5% CO2 [45].
4.13. CCK-8 Assay
The H9C2 cells were seeded in 96-well plates at a density of 3000 cells/well and incubated overnight [42]. Ang II and CA were added with different concentrations and incubated for 24 h. Cell viability was measured after adding a cell counting kit-8 (CCK-8) solution for 1.5 h.
4.14. Western Blot Analysis
RIPA solution containing protease inhibitors was used to lyse the heart tissue and H9C2 cells to obtain the total proteins. A BCA kit was used to determine protein concentrations. Subsequently, the protein sample was mixed with the loading buffer and boiled for denaturation. Proteins were isolated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to PVDF membranes. Then, the membranes were sealed in 5% skim milk at room temperature for 1 h. Primary antibodies against MMP9 (dilution at 1:1000), TNF α (dilution at 1:1000), COX2 (dilution at 1:1000), and IL-17 (dilution at 1:1000) were used to incubate the membranes at 4 °C overnight. After incubation with the corresponding secondary antibody at room temperature for 1 h, the protein bands were observed using enhanced chemiluminescence (ECL) reagents and analyzed using Image Lab 6.1 software [46,47].
4.15. Statistical Analysis
GraphPad Prism 8.0 analyzed all data, and all results are presented as mean ± standard deviation (SD). One-way ANOVA and the Mann–Whitney test were used to analyze significant differences. p < 0.05 represents statistical significance.
5. Conclusions
In this study, we explored the efficacy of CA in treating cardiac inflammation and hypertrophy caused by RH. Through the integrated application of network pharmacology and experimental validation, we elucidated that CA can effectively prevent RH-induced myocardial hypertrophy by suppressing inflammatory responses. Our study not only provides a theoretical basis for the CA treatment of myocardial hypertrophy caused by RH, but also provides a possibility for the discovery of novel drugs with the dual functions of reducing blood pressure and alleviating myocardial hypertrophy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms251810063/s1.
Author Contributions
Q.W.: writing—original draft, writing—review and editing, visualization, validation, methodology, investigation, formal analysis, conceptualization, data curation. Q.Z.: visualization, validation, methodology, formal analysis. C.W.: validation, methodology, investigation, conceptualization. G.X. and T.W.: visualization, investigation, conceptualization. Y.G., T.L. and X.Y.: methodology, investigation. B.Z.: supervision, resources, funding acquisition. W.H.: writing—review and editing, supervision, resources, funding acquisition. Q.W. and Q.Z. contributed equally to this work. B.Z. and W.H. are corresponding authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovative Chinese Medicine and Health Products Research Academician Workstation of Academicians Boli Zhang and Beiwei Zhu of the West China Hospital, Sichuan University (No. HXYS19001, HXYS19002), the 135 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYXY21002), and the Innovative Chinese Medicine Preclinical Research Fund of “Liqing No.2”, West China Hospital, Sichuan University (No. 161200012).
Institutional Review Board Statement
The animal experimental procedures used in this study were approved by the Ethics Committee of West China Hospital, Sichuan University (Ethics Record Number: 20220302077).
Data Availability Statement
Data will be provided upon request in the Supplementary Materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships.
Abbreviations
RH: renovascular hypertension; 2K1C, two-kidney, one-clip; CA, coniferyl alcohol; Ang II, angiotensin II; PPI, protein–protein interaction; H&E, hematoxylin and eosin; MMP9, matrix metallopeptidase 9; COX2, cyclooxygenase-2; TNF α, tumor necrosis factor α; IL-17, interleukin 17A; TLR4, toll-like receptor 4, NOS3, nitric oxide synthase 3; and JUN, jun proto-oncogene, AP-1 transcription factor subunit.
References
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Poasakate, A.; Maneesai, P.; Potue, P.; Bunbupha, S.; Tong-Un, T.; Settheetham-Ishida, W.; Khamseekaew, J.; Pakdeechote, P. Genistein alleviates renin-angiotensin system mediated vascular and kidney alterations in renovascular hypertensive rats. Biomed. Pharmacother. 2022, 146, 112601. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Muiesan, M.L.; Porteri, E.; Salvetti, M.; Castellano, M.; Bettoni, G.; Tiberio, G.; Giulini, S.M.; Monteduro, C.; Garavelli, G.; et al. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J. Am. Coll. Cardiol. 1998, 32, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.Y.; Zhao, Y.; Eirin, A.; Liu, L.; Ferguson, C.M.; Tang, H.; Lerman, A.; Lerman, L.O. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic Res. Cardiol. 2020, 115, 16. [Google Scholar] [CrossRef]
- Farahani, R.A.; Yu, S.; Ferguson, C.M.; Zhu, X.-Y.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Herrmann, S.M.; Chade, A.R.; Lerman, A.; et al. Renal revascularization attenuates myocardial mitochondrial damage and improves diastolic function in pigs with metabolic syndrome and renovascular hypertension. J. Cardiovasc. Transl. Res. 2022, 15, 15–26. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, X.; Wu, D.; Du, G.; Zhou, J.; Chen, J. Improving bioconversion of eugenol to coniferyl alcohol by in situ eliminating harmful H2O2. Bioresour. Technol. 2018, 267, 578–583. [Google Scholar] [CrossRef]
- Overhage, J.; Steinbüchel, A.; Priefert, H. Highly efficient biotransformation of eugenol to ferulic acid and further conversion to vanillin in recombinant strains of Escherichia coli. Appl. Environ. Microbiol. 2003, 69, 6569–6576. [Google Scholar] [CrossRef]
- Althagafy, H.S.; Meza-Aviña, M.E.; Oberlies, N.H.; Croatt, M.P. Mechanistic study of the biomimetic synthesis of Flavonolignan Diastereoisomers in Milk Thistle. J. Org. Chem. 2013, 78, 7594–7600. [Google Scholar] [CrossRef]
- El-Bassossy, H.; Badawy, D.; Neamatallah, T.; Fahmy, A. Ferulic acid, a natural polyphenol, alleviates insulin resistance and hypertension in fructose fed rats: Effect on endothelial-dependent relaxation. Chem.-Biol. Interact. 2016, 254, 191–197. [Google Scholar] [CrossRef]
- Fukuda, T.; Kuroda, T.; Kono, M.; Hyoguchi, M.; Tanaka, M.; Matsui, T. Augmentation of ferulic acid-induced vasorelaxation with aging and its structure importance in thoracic aorta of spontaneously hypertensive rats. Naunyn-Schmiedeb. Arch. Pharmacol. 2015, 388, 1113–1117. [Google Scholar] [CrossRef]
- Chanda, J.; Mukherjee, P.K.; Biswas, R.; Singha, S.; Kar, A.; Haldar, P.K. Lagenaria siceraria and it’s bioactive constituents in carbonic anhydrase inhibition: A bioactivity guided LC-MS/MS approach. Phytochem. Anal. 2021, 32, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, Z.; Yousaf, Z.; Anjum, I.; Bilal, M.; Yasin, H.; Aftab, A.; Booker, A.; Ullah, R.; Bari, A. Network pharmacology and molecular docking: Combined computational approaches to explore the antihypertensive potential of Fabaceae species. Bioresour. Bioprocess. 2024, 11, 53. [Google Scholar] [CrossRef]
- Wang, M.L.; Yang, Q.Q.; Ying, X.H.; Li, Y.Y.; Wu, Y.S.; Shou, Q.Y.; Ma, Q.X.; Zhu, Z.W.; Chen, M.L. Network pharmacology-based approach uncovers the mechanism of GuanXinNing Tablet for treating thrombus by MAPKs signal pathway. Front. Pharmacol. 2020, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Kovács, I.A.; Barabási, A.-L. Network-based prediction of drug combinations. Nat. Commun. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Sarno, F.; Benincasa, G.; List, M.; Barabasi, A.-L.; Baumbach, J.; Ciardiello, F.; Filetti, S.; Glass, K.; Loscalzo, J.; Marchese, C.; et al. Clinical epigenetics settings for cancer and cardiovascular diseases: Real-life applications of network medicine at the bedside. Clin. Epigenet. 2021, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Z.; Ouyang, Y.; Chen, M.; Guo, X.; Mazhar, M.; Kang, J.; Zhou, H.; Wu, Q.; Yang, S. Material basis and integrative pharmacology of danshen decoction in the treatment of cardiovascular diseases. Phytomedicine 2023, 108, 154503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Z.; Jia, X.; Li, X.; Wang, X.; Rong, H.; Liang, Y.; Zeng, W.; Jia, W.; Ma, X. Integrated network pharmacology and metabolomics to reveal the mechanism of QiShenYiQi Dripping Pills (T101) against cardiac structural and functional abnormalities. Front. Pharmacol. 2022, 13, 1017433. [Google Scholar]
- Lin, J.; Wang, Q.; Xu, S.; Zhou, S.; Zhong, D.; Tan, M.; Zhang, X.; Yao, K. Banxia baizhu tianma decoction, a Chinese herbal formula, for hypertension: Integrating meta-analysis and network pharmacology. Front. Pharmacol. 2022, 13, 1025104. [Google Scholar] [CrossRef]
- Huang, S.J. Efficient analysis of toxicity and mechanisms of environmental pollutants with network toxicology and molecular docking strategy: Acetyl tributyl citrate as an example. Sci. Total Environ. 2023, 905, 167904. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Hu, C.; Song, Q.; Li, Y.; Da, X.; Yu, Y.; Li, H.; Clark, I.M.; Chen, Q.; Wang, Q.K. ADAMTS16 activates latent TGF-β, accentuating fibrosis and dysfunction of the pressure-overloaded heart. Cardiovasc. Res. 2020, 116, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Molecular biochemical aspects of salt (sodium chloride) in inflammation and immune response with reference to hypertension and type 2 diabetes mellitus. Lipids Health Dis. 2021, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.; Briasoulis, A.; Dahlof, B.; Jamerson, K.; Weber, M.A.; Kelly, R.Y.; Hester, A.; Hua, T.; Zappe, D.; Pitt, B. Comparison of benazepril plus amlodipine or hydrochlorothiazide in high-risk patients with hypertension and coronary artery disease. Am. J. Cardiol. 2013, 112, 255–259. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, hypertension, and cardiac dysfunction: Novel roles of immunometabolism in macrophage activation and inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Nie, X.B.; Deng, W.; Zhou, H.; Wang, Z.G. Long noncoding RNA MCM3AP-AS1 attenuates sepsis-induced cardiomyopathy by improving inflammation, oxidative stress, and mitochondrial function through mediating the miR-501-3p/CADM1/STAT3 axis. Int. Immunopharmacol. 2024, 128, 111500. [Google Scholar] [CrossRef]
- Lu, H.; Wu, Z.; Wang, Y.; Zhao, D.; Zhang, B.; Hong, M. Study on inhibition of Britannin on triple-negative breast carcinoma through degrading ZEB1 proteins. Phytomedicine 2022, 104, 154291. [Google Scholar] [CrossRef]
- Yang, N.; Liang, Y.; Yang, P.; Ji, F. Propofol suppresses LPS-induced nuclear accumulation of HIF-1α and tumor aggressiveness in non-small cell lung cancer. Oncol. Rep. 2017, 37, 2611–2619. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, M.Y.; Xiao, S.; Sun, B.; Li, Y.M.; Yang, L.Y. Downregulation of castor zinc finger 1 predicts poor prognosis and facilitates hepatocellular carcinoma progression via MAPK/ERK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 45. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Gao, T.; Huang, Y.; Xue, J.; Xie, M.L. Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lα in rats. Food Funct. 2016, 7, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Senbonmatsu, T.; Ichihara, S.; Price, E.; Gaffney, A.; Inagami, T. Evidence for angiotensin II type 2 receptor—Mediated cardiac myocyte enlargement during in vivo pressure overload. J. Clin. Investig. 2000, 106, R25–R29. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.J.; Luo, W.; Khan, Z.A.; Wu, G.J.; Xuan, L.N.; Shan, P.R.; Lin, K.; Chen, T.W.; Wang, J.Y.; Hu, X.; et al. Celastrol attenuates angiotensin II—Induced cardiac remodeling by targeting STAT3. Circ. Res. 2022, 126, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.L.; Chu, P.M.; Cheng, H.C.; Huang, Y.T.; Chou, W.C.; Tsai, K.L.; Chan, S.H. Dapagliflozin mitigates doxorubicin-caused myocardium damage by regulating AKT-mediated oxidative stress, cardiac remodeling, and inflammation. Int. J. Mol. Sci. 2022, 23, 10146. [Google Scholar] [CrossRef]
- Wan, X.; Cui, X.; Wang, X.; Feng, M.; Wei, S.; Yu, J.; Cheng, S.; Luo, H.; Hu, J. Di-n-butyl phthalate induces toxicity in male fetal mouse testicular development by regulating the MAPK signaling pathway. Toxicol. Appl. Pharmacol. 2024, 486, 116933. [Google Scholar] [CrossRef]
- De Sousa Lima, E.B.; de Oliveira, L.C.S.; Cardoso, S.; Telles, P.V.N.; Lima, C.; Sousa, J.F.R.E.; Araújo, R.P.N.; de Oliveira, A.P.; de Santos, R.F.; de Santos, A.A.; et al. Moderate-intensity exercise and renin angiotensin system blockade improve the renovascular hypertension (2K1C)-induced gastric dysmotility in rats. Life Sci. 2018, 210, 55–64. [Google Scholar] [CrossRef]
- Du, X.; Ma, X.; Tan, Y.; Shao, F.; Li, C.; Zhao, Y.; Miao, Y.; Han, L.; Dang, G.; Song, Y.; et al. B cell-derived anti-beta 2 glycoprotein I antibody mediates hyperhomocysteinemia-aggravated hypertensive glomerular lesions by triggering ferroptosis. Signal Transduct. Target. Ther. 2023, 8, 103. [Google Scholar] [CrossRef]
- Wolak, T.; Kim, H.J.; Ren, Y.; Kim, J.; Vaziri, N.D.; Nicholas, S.B. Osteopontin modulates angiotensin II—Induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int. 2009, 76, 32–43. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Sheng, H.; Lv, X.; Li, J.; Han, B.; Wang, S.; Liu, K.; Zhang, C.; Zhang, W.; et al. Study on the mechanism of stir-fried Fructus Tribuli in enhancing the essential hypertension treatment by an integrated “spectrum-effect relationship-network pharmacology-metabolomics” strategy. Biomed. Pharmacother. 2023, 165, 115160. [Google Scholar] [CrossRef]
- Liao, Z.; Li, D.; Chen, Y.; Li, Y.; Huang, R.; Zhu, K.; Chen, H.; Yuan, Z.; Zheng, X.; Zhao, H.; et al. Early moderate exercise benefits myocardial infarction healing via improvement of inflammation and ventricular remodelling in rats. J. Cell. Mol. Med. 2019, 23, 8328–8342. [Google Scholar] [CrossRef]
- Kellogg, A.P.; Converso, K.; Wiggin, T.; Stevens, M.; Pop-Busui, R. Effects of cyclooxygenase-2 gene inactivation on cardiac autonomic and left ventricular function in experimental diabetes. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H453–H461. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Xie, R.; Bai, Y.; Duan, Y.; Sun, C.; Wang, Y.; Cao, R.; Ling, Z.; Qu, X. The mechanism actions of stragaloside IV prevents the progression of hypertensive heart disease based on network pharmacology and experimental pharmacology. Front. Pharmacol. 2021, 12, 755653. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hao, Z.; An, N.; Han, Y.; Miao, W.; Storey, K.B.; Lefai, E.; Liu, X.; Wang, J.; Liu, S.; et al. Integrated transcriptomics and metabolomics reveal protective effects on heart of hibernating Daurian ground squirrels. J. Cell. Physiol. 2023, 238, 2724–2748. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, X.-Y.; Dang, W.-Z.; Jiang, B.; Zou, J.; Shen, X.-Y. Total glucosides of paeony protects against collagen-induced mouse arthritis via inhibiting follicular helper T cell differentiation. Phytomedicine 2019, 65, 153091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ji, L.; Liu, X.; Tu, D.; Shi, N.; Yangqu, W.; Chen, S.; Gao, P.; Zhu, H.; Ruan, C. AIFM1, negatively regulated by miR-145-5p, aggravates hypoxia-induced cardiomyocyte injury. Biomed. J. 2021, 45, 870–882. [Google Scholar] [CrossRef]
- Chan, Y.H.; Tsai, F.C.; Chang, G.J.; Lai, Y.J.; Chang, S.H.; Chen, W.J.; Yeh, Y.H. CD44 regulates Epac1-mediated β-adrenergic-receptor-induced Ca2+-handling abnormalities: Implication in cardiac arrhythmias. J. Biomed. Sci. 2023, 30, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Li, H.; Wang, M.; Qiu, Y.; Lu, L. miR-29b-3p regulates cardiomyocytes pyroptosis in CVB3-induced myocarditis through targeting DNMT3A. Cell. Mol. Biol. Lett. 2024, 29, 55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).