Intestinal Microbiota Interventions to Enhance Athletic Performance—A Review
Abstract
:1. Introduction
2. Materials and Methods
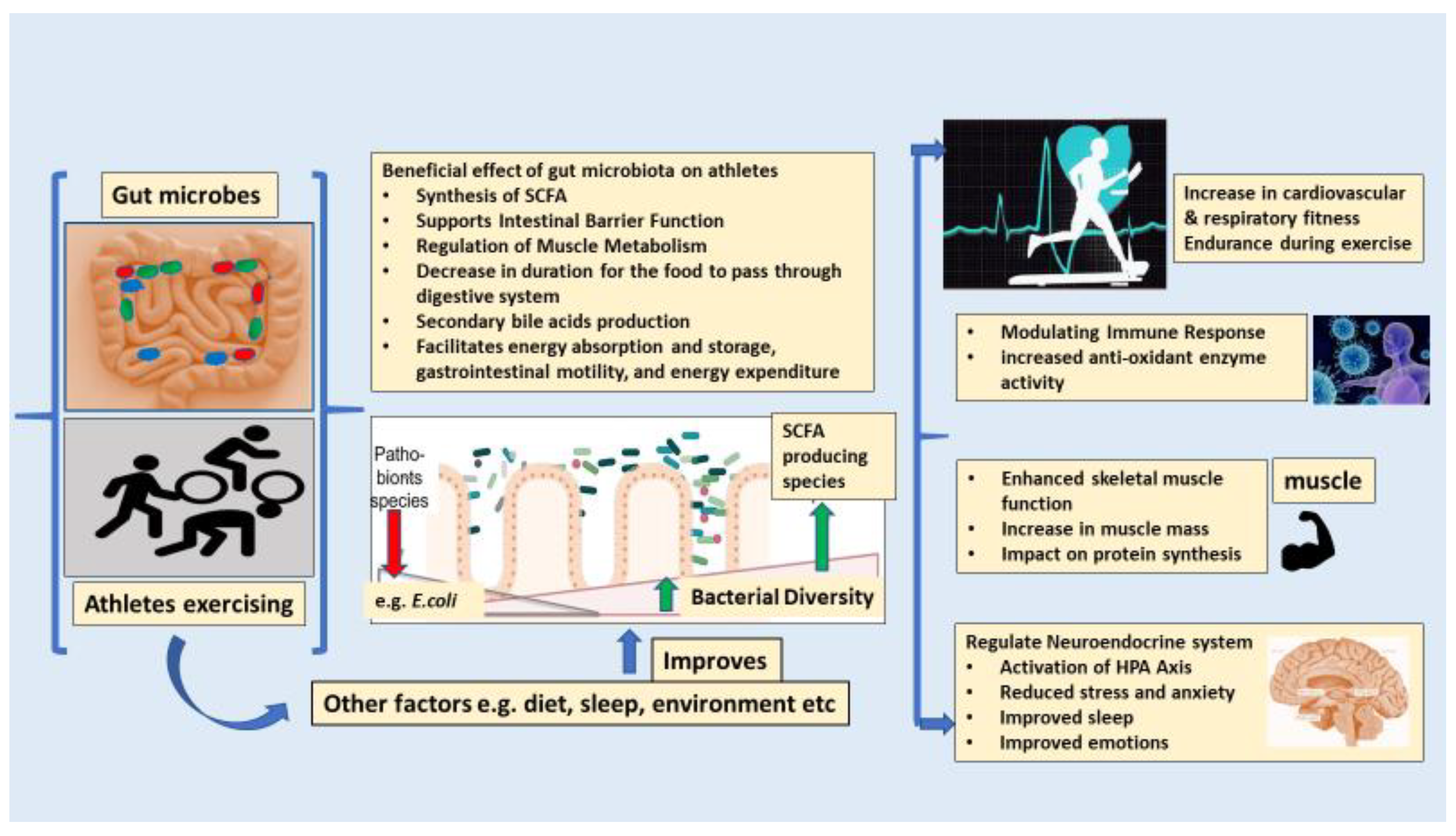
3. The Key Roles of the Gut Microbiota in Boosting the Overall Health and Performance of Athletes
4. The Correlation between Endurance Exercise and Intestinal Microbiota
5. Involvement of Gut–Brain Axis in Overall Athletic Performance
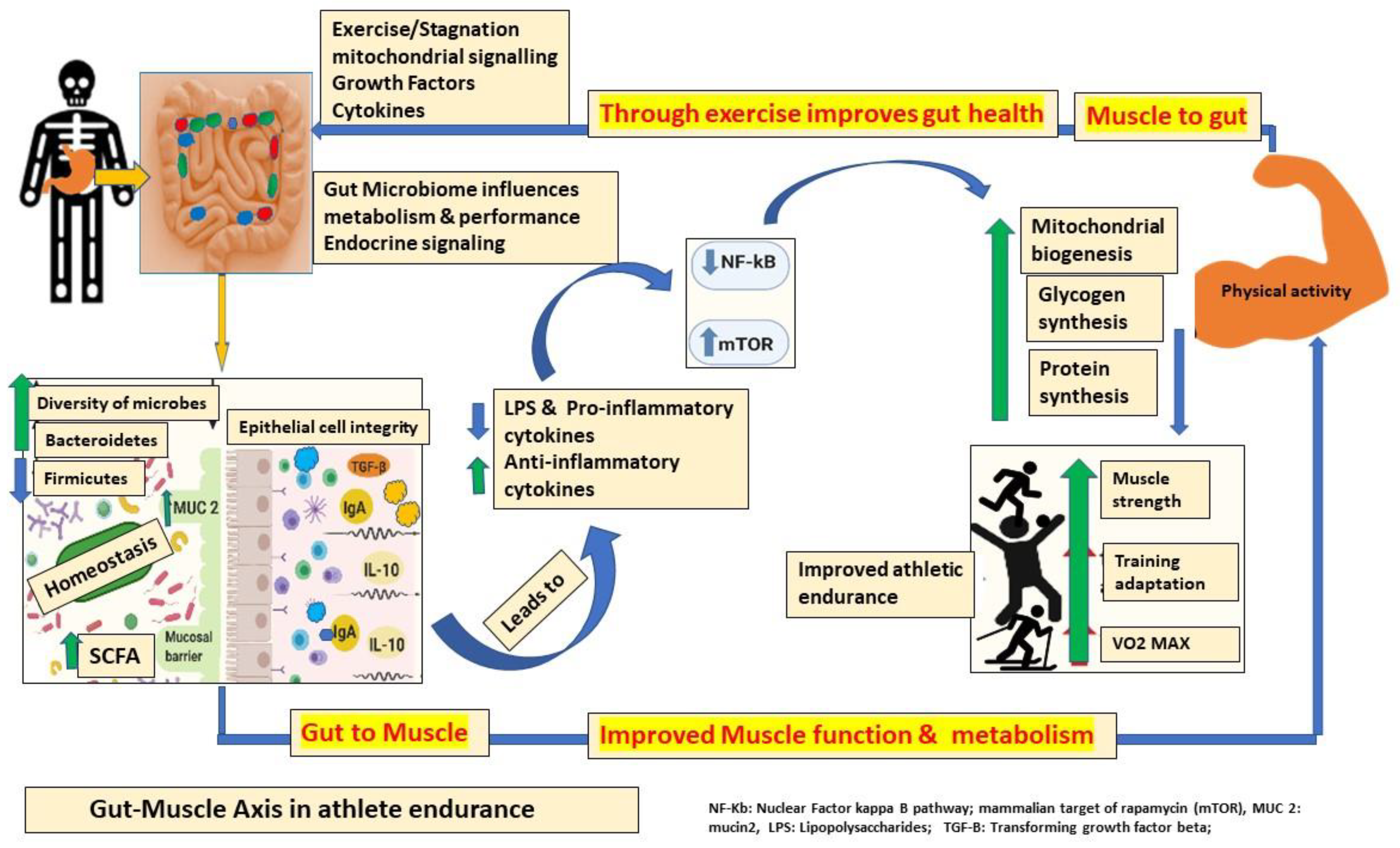
6. Bidirectional Communication between Intestinal Microbiota and Muscle (Gut–Muscle Axis) in Athletic Performance
7. Optimizing Athletic Performance with Microbiome Intervention Strategies
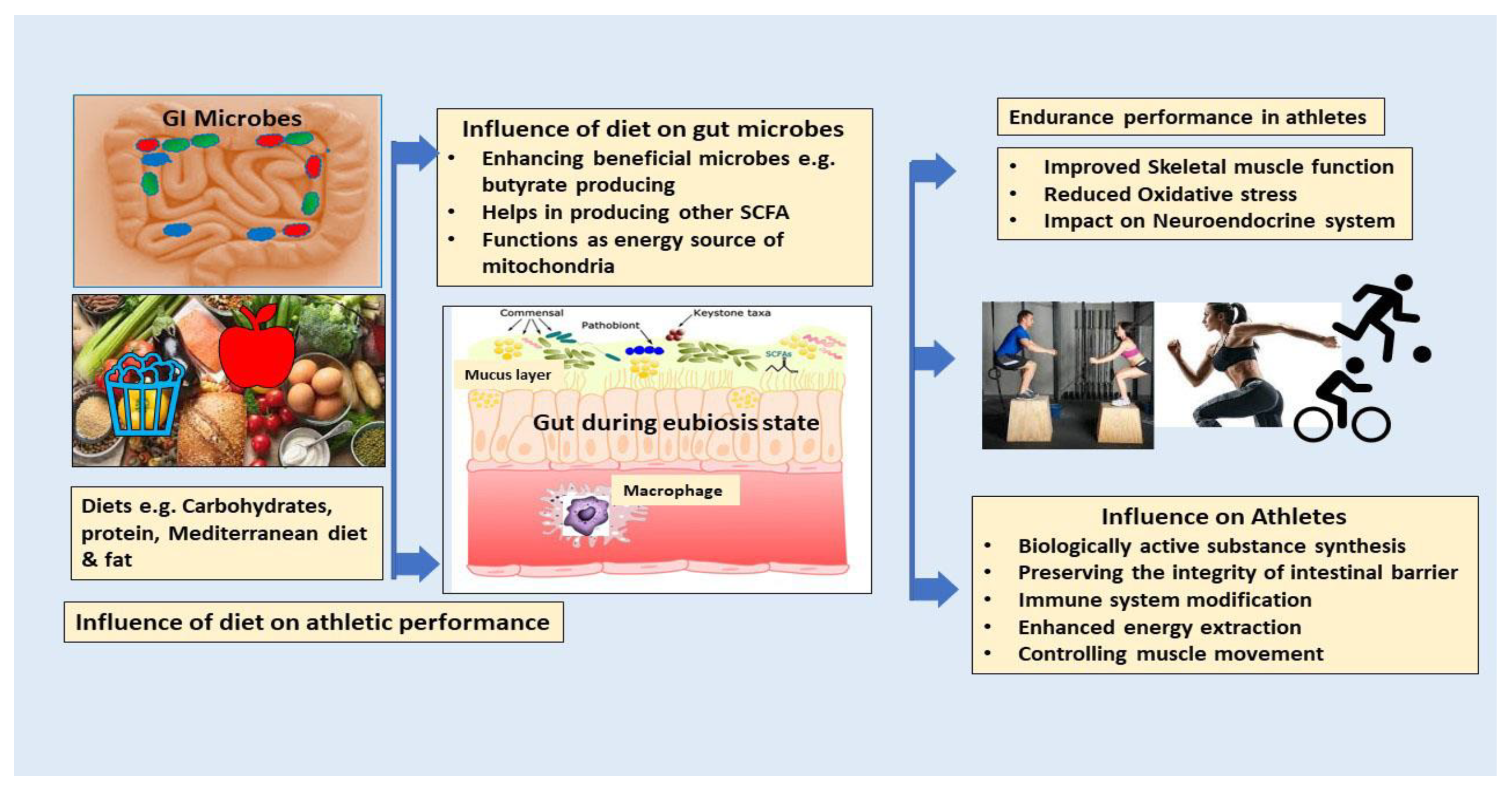
8. Diet as One of the Influencers in Athletic Endurance
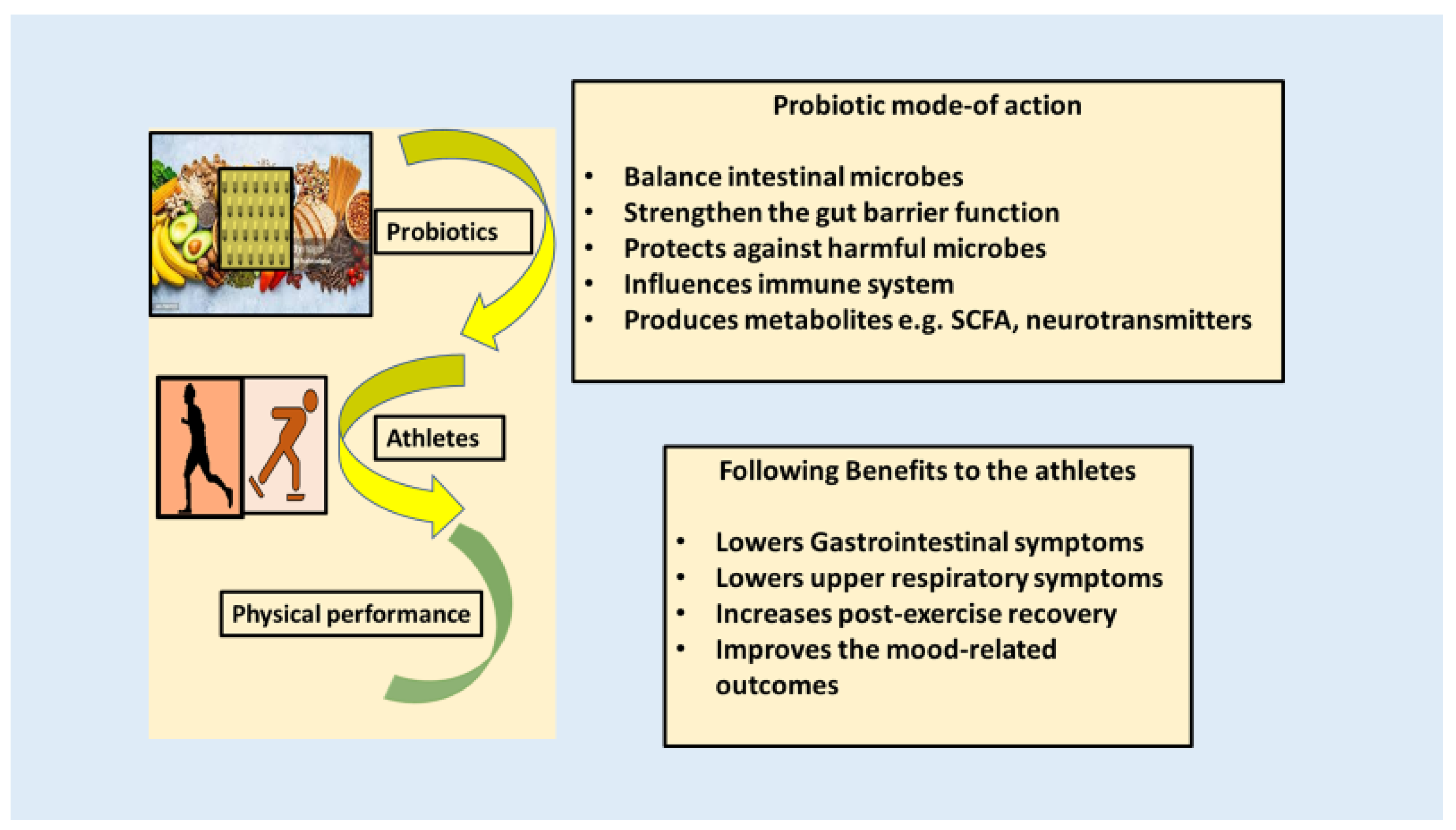
9. Prebiotics, Probiotics, Synbiotics, and Postbiotics in Athletic Endurance
10. Potential Mode of Action of Probiotics on an Athlete’s Endurance
11. Clinical Studies Performed on Athletes for Better Endurance Using Probiotics
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Janssens, Y.; Nielandt, J.; Bronselaer, A.; Debunne, N.; Verbeke, F.; Wynendaele, E.; Van Immerseel, F.; Vandewynckel, Y.-P.; De Tré, G.; De Spiegeleer, B. Disbiome database: Linking the microbiome to disease. BMC Microbiol. 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise sustains the hallmarks of health. J. Sport. Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; O’Sullivan, O.; Barton, W.; Cotter, P.D.; Molloy, M.G.; Shanahan, F. Gut microbiota: Implications for sports and exercise medicine. Br. J. Sports Med. 2017, 51, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Rowland, I.; Capurso, L.; Collins, K.; Cummings, J.; Delzenne, N.; Goulet, O.; Guarner, F.; Marteau, P.; Meier, R. Current level of consensus on probiotic science—Report of an expert meeting—London, 23 November 2009. Gut Microbes 2010, 1, 436–439. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The Athlete Gut Microbiome and its Relevance to Health and Performance: A Review. Sports Med. 2022, 52, 119–128. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2020 2023, 4, e420. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport. Health Sci. 2016, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Topi, S.; Bottalico, L. The Alcmaeon’s School of Croton: Philosophy and Science. Open Access Maced. J. Med. Sci. 2019, 7, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Neish, A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Boisseau, N.; Barnich, N.; Koechlin-Ramonatxo, C. The Nutrition-Microbiota-Physical Activity Triad: An Inspiring New Concept for Health and Sports Performance. Nutrients 2022, 14, 924. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Rivera-Brown, A.M.; Frontera, W.R. Principles of exercise physiology: Responses to acute exercise and long-term adaptations to training. PM&R 2012, 4, 797–804. [Google Scholar]
- Patil, H.R.; O’Keefe, J.H.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Cardiovascular damage resulting from chronic excessive endurance exercise. Mo. Med. 2012, 109, 312–321. [Google Scholar] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Stone, M.H. The Importance of Muscular Strength in Athletic Performance. Sports Med. 2016, 46, 1419–1449. [Google Scholar] [CrossRef]
- Montenegro, J.; Armet, A.M.; Willing, B.P.; Deehan, E.C.; Fassini, P.G.; Mota, J.F.; Walter, J.; Prado, C.M. Exploring the Influence of Gut Microbiome on Energy Metabolism in Humans. Adv. Nutr. 2023, 14, 840–857. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef] [PubMed]
- Basista Rabina, S.; Ravindra, P.V. The Interaction between Dietary Components, Gut Microbiome, and Endurance Performance. In Contemporary Advances in Sports Science; Redha, T., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 11. [Google Scholar]
- Crnčević, N.; Hukić, M.; Deumić, S.; Selimagić, A.; Dozić, A.; Gavrankapetanović, I.; Klepo, D.; Avdić, M. Gastrointestinal Tract Microbiome Effect and Role in Disease Development. Diseases 2022, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Cella, V.; Bimonte, V.M.; Sabato, C.; Paoli, A.; Baldari, C.; Campanella, M.; Lenzi, A.; Ferretti, E.; Migliaccio, S. Nutrition and Physical Activity-Induced Changes in Gut Microbiota: Possible Implications for Human Health and Athletic Performance. Foods 2021, 10, 3075. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Przewłócka, K.; Korewo-Labelle, D.; Berezka, P.; Karnia, M.J.; Kaczor, J.J. Current Aspects of Selected Factors to Modulate Brain Health and Sports Performance in Athletes. Nutrients 2024, 16, 1842. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. (Encinitas) 2018, 17, 28–32. [Google Scholar] [PubMed]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Akram, N.; Faisal, Z.; Irfan, R.; Shah, Y.A.; Batool, S.A.; Zahid, T.; Zulfiqar, A.; Fatima, A.; Jahan, Q.; Tariq, H.; et al. Exploring the serotonin-probiotics-gut health axis: A review of current evidence and potential mechanisms. Food Sci. Nutr. 2024, 12, 694–706. [Google Scholar] [CrossRef]
- Bonaz, B. Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction. Neurogastroenterol. Motil. 2022, 34, e14456. [Google Scholar] [CrossRef]
- Chew, W.; Lim, Y.P.; Lim, W.S.; Chambers, E.S.; Frost, G.; Wong, S.H.; Ali, Y. Gut-muscle crosstalk. A perspective on influence of microbes on muscle function. Front. Med. 2022, 9, 1065365. [Google Scholar] [CrossRef]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and "biotics" in exercise. J. Sport. Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Tabone, M.; Bressa, C.; García-Merino, J.A.; Moreno-Pérez, D.; Van, E.C.; Castelli, F.A.; Fenaille, F.; Larrosa, M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci. Rep. 2021, 11, 3558. [Google Scholar] [CrossRef]
- Marullo, A.L.; O’Halloran, K.D. Microbes, metabolites and muscle: Is the gut–muscle axis a plausible therapeutic target in Duchenne muscular dystrophy? Exp. Physiol. 2023, 108, 1132–1143. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2020, 6, 191. [Google Scholar] [CrossRef]
- Koppel, N.; Balskus, E.P. Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem. Biol. 2016, 23, 18–30. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Albarrati, A.; Albratty, M.; Najmi, A.; Meraya, A.M.; Bungau, S. The road to precision medicine: Eliminating the "One Size Fits All" approach in Alzheimer’s disease. Biomed. Pharmacother. 2022, 153, 113337. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Nolte, S.; Krüger, K.; Lenz, C.; Zentgraf, K. Optimizing the Gut Microbiota for Individualized Performance Development in Elite Athletes. Biology 2023, 12, 1491. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Chuang, H.L.; Hsieh, P.S.; Ho, H.H.; Chen, W.L.; Chiu, Y.S.; Huang, C.C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Sung, H.C.; Huang, C.C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Sun, W. Sports performance prediction based on chaos theory and machine learning. Wirel. Commun. Mob. Comput. 2022, 2022, 3916383. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Marlicz, W.; Loniewski, I. The effect of exercise and diet on gut microbial diversity. Gut 2015, 64, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Ghazzawi, H.A.; Hussain, M.A.; Raziq, K.M.; Alsendi, K.K.; Alaamer, R.O.; Jaradat, M.; Alobaidi, S.; Al Aqili, R.; Trabelsi, K.; Jahrami, H. Exploring the Relationship between Micronutrients and Athletic Performance: A Comprehensive Scientific Systematic Review of the Literature in Sports Medicine. Sports 2023, 11, 109. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in Ultra-Endurance: State of the Art. Nutrients 2018, 10, 1995. [Google Scholar] [CrossRef]
- Henselmans, M.; Bjørnsen, T.; Hedderman, R.; Vårvik, F.T. The effect of carbohydrate intake on strength and resistance training performance: A systematic review. Nutrients 2022, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Hammad, A.A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Saoud, H.A.; et al. Athletes’ nutritional demands: A narrative review of nutritional requirements. Front. Nutr. 2024, 10, 1331854. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’Alicandro, G. The biological role of vitamins in athletes’ muscle, heart and microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M. Dietary protein and muscle mass: Translating science to application and health benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet-Microbe-Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Butteiger, D.N.; Hibberd, A.A.; McGraw, N.J.; Napawan, N.; Hall-Porter, J.M.; Krul, E.S. Soy Protein Compared with Milk Protein in a Western Diet Increases Gut Microbial Diversity and Reduces Serum Lipids in Golden Syrian Hamsters. J. Nutr. 2016, 146, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Noh, H.J.; Park, J.M.; Kwon, Y.J.; Kim, K.; Park, S.Y.; Kim, I.; Lim, J.H.; Kim, B.K.; Kim, B.Y. Immunostimulatory Effect of Heat-Killed Probiotics on RAW264.7 Macrophages. J. Microbiol. Biotechnol. 2022, 32, 638–644. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Moon, J.M.; Jäger, R. It’s Dead! Can Postbiotics Really Help Performance and Recovery? A Systematic Review. Nutrients 2024, 16, 720. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Lee, C.C.; Lee, M.C.; Hsu, H.Y.; Lin, J.S.; Huang, C.C.; Watanabe, K. Effects of heat-killed Lactiplantibacillus plantarum TWK10 on exercise performance, fatigue, and muscle growth in healthy male adults. Physiol. Rep. 2023, 11, e15835. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. The current trends and future perspectives of prebiotics research: A review. 3 Biotech. 2012, 2, 115–125. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Li, L. Effects of Probiotic Supplementation on Exercise and the Underlying Mechanisms. Foods 2023, 12, 1787. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.; Wessjohann, L.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Song, Z.; Liu, Y.; Zhang, X. Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota. Foods 2024, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.S.; Student, A.S.R.M.; West, N.P.; Lancha, A.H., Jr. Probiotics and sports: A new magic bullet? Nutrition 2019, 60, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential health effects on inflammatory, immunological and stress parameters in professional soccer players and sedentary individuals after consuming a synbiotic. A triple-blinded, randomized, placebo-controlled pilot study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. -Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current status of probiotic and related health benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Kim, W.-G.; Kim, H.I.; Kwon, E.K.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018, 9, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Townsend, J.R.; Bender, D.; Vantrease, W.C.; Sapp, P.A.; Toy, A.M.; Woods, C.A.; Johnson, K.D. Effects of probiotic (Bacillus subtilis DE111) supplementation on immune function, hormonal status, and physical performance in division I baseball players. Sports 2018, 6, 70. [Google Scholar] [CrossRef]
- Jäger, R.; Shields, K.A.; Lowery, R.P.; De Souza, E.O.; Partl, J.M.; Hollmer, C.; Purpura, M.; Wilson, J.M. Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ 2016, 4, e2276. [Google Scholar] [CrossRef]
- Soares, A.D.N.; Wanner, S.P.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.S.; Cardoso, V.N. Supplementation with Saccharomyces boulardii increases the maximal oxygen consumption and maximal aerobic speed attained by rats subjected to an incremental-speed exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Huang, W.C.; Pan, C.H.; Wei, C.C.; Huang, H.Y. Lactobacillus plantarum PS128 Improves Physiological Adaptation and Performance in Triathletes through Gut Microbiota Modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef]
- Roberts, J.D.; Suckling, C.A.; Peedle, G.Y.; Murphy, J.A.; Dawkins, T.G.; Roberts, M.G. An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the Effects of a Multi-Strain Probiotic/Prebiotic, Antioxidant Intervention. Nutrients 2016, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Chuang, H.L.; Huang, Y.T.; Wu, C.C.; Chou, G.T.; Wang, S.; Tsai, Y.C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, B.K.; Patel, K.H.; Lee, C.N.; Moochhala, S. Intestinal Microbiota Interventions to Enhance Athletic Performance—A Review. Int. J. Mol. Sci. 2024, 25, 10076. https://doi.org/10.3390/ijms251810076
Patel BK, Patel KH, Lee CN, Moochhala S. Intestinal Microbiota Interventions to Enhance Athletic Performance—A Review. International Journal of Molecular Sciences. 2024; 25(18):10076. https://doi.org/10.3390/ijms251810076
Chicago/Turabian StylePatel, Bharati Kadamb, Kadamb Haribhai Patel, Chuen Neng Lee, and Shabbir Moochhala. 2024. "Intestinal Microbiota Interventions to Enhance Athletic Performance—A Review" International Journal of Molecular Sciences 25, no. 18: 10076. https://doi.org/10.3390/ijms251810076
APA StylePatel, B. K., Patel, K. H., Lee, C. N., & Moochhala, S. (2024). Intestinal Microbiota Interventions to Enhance Athletic Performance—A Review. International Journal of Molecular Sciences, 25(18), 10076. https://doi.org/10.3390/ijms251810076





