Exogenous Melatonin Alleviates NaCl Injury by Influencing Stomatal Morphology, Photosynthetic Performance, and Antioxidant Balance in Maize
Abstract
:1. Introduction
2. Results
2.1. Effects of MT on the Growth of Maize Seedlings under Salt Stress
2.2. Effects of MT on Photosynthetic Parameters of Maize Seedlings under Salt Stress
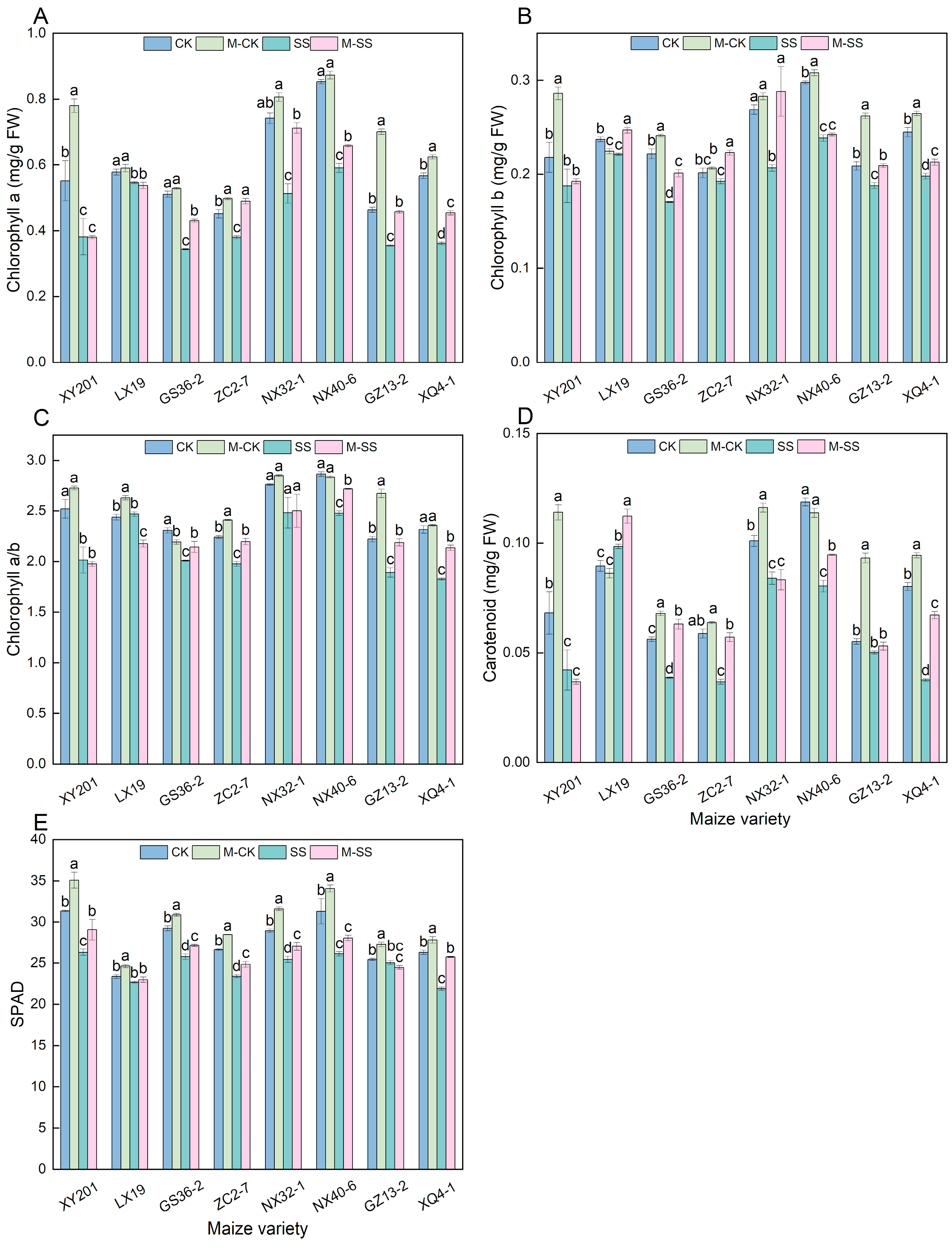
2.3. Effects of MT on Chlorophyll Accumulation of Maize Seedlings under Salt Stress
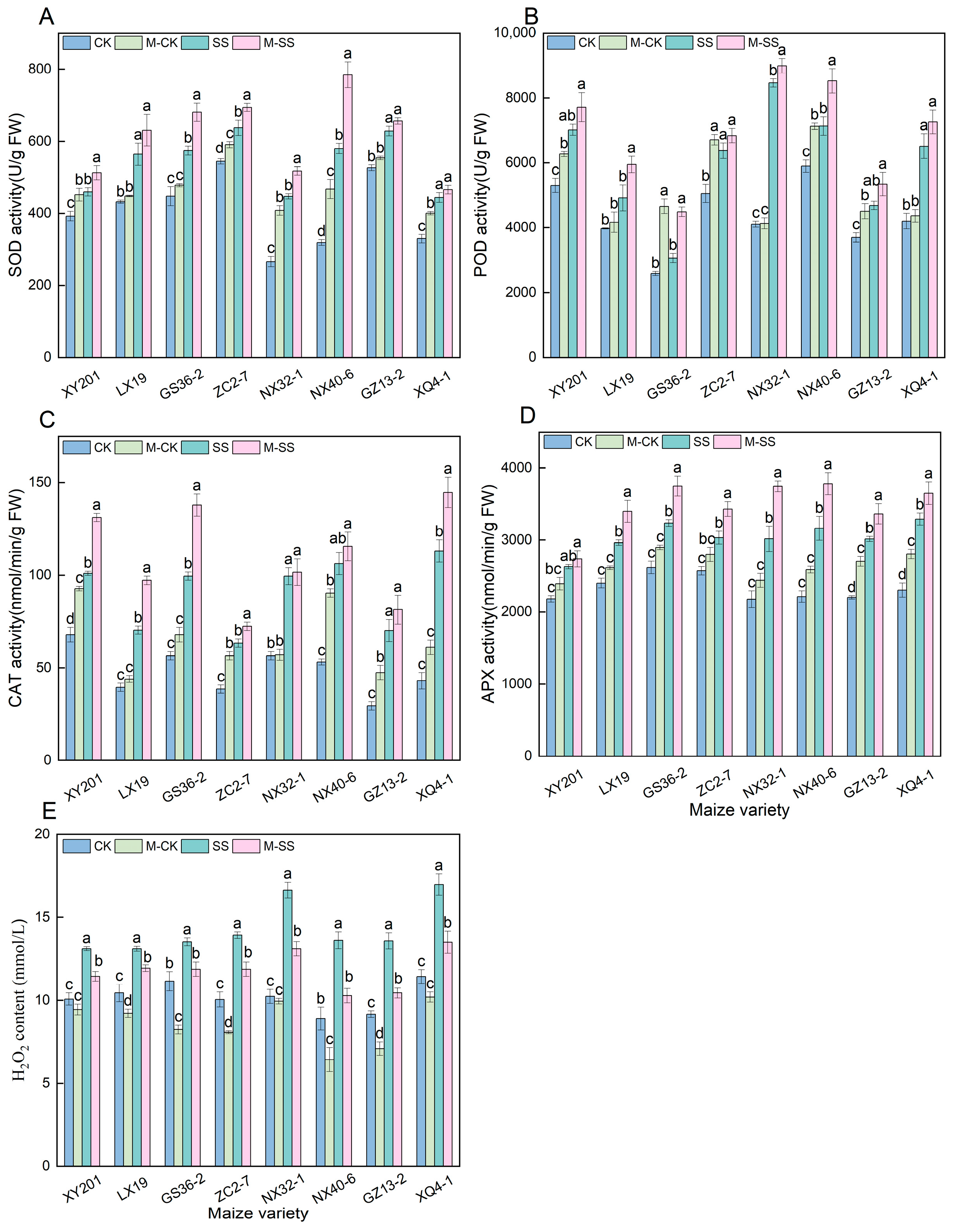
2.4. Effects of MT on Antioxidant Enzyme Activities and H2O2 Content of Maize Seedlings under Salt Stress
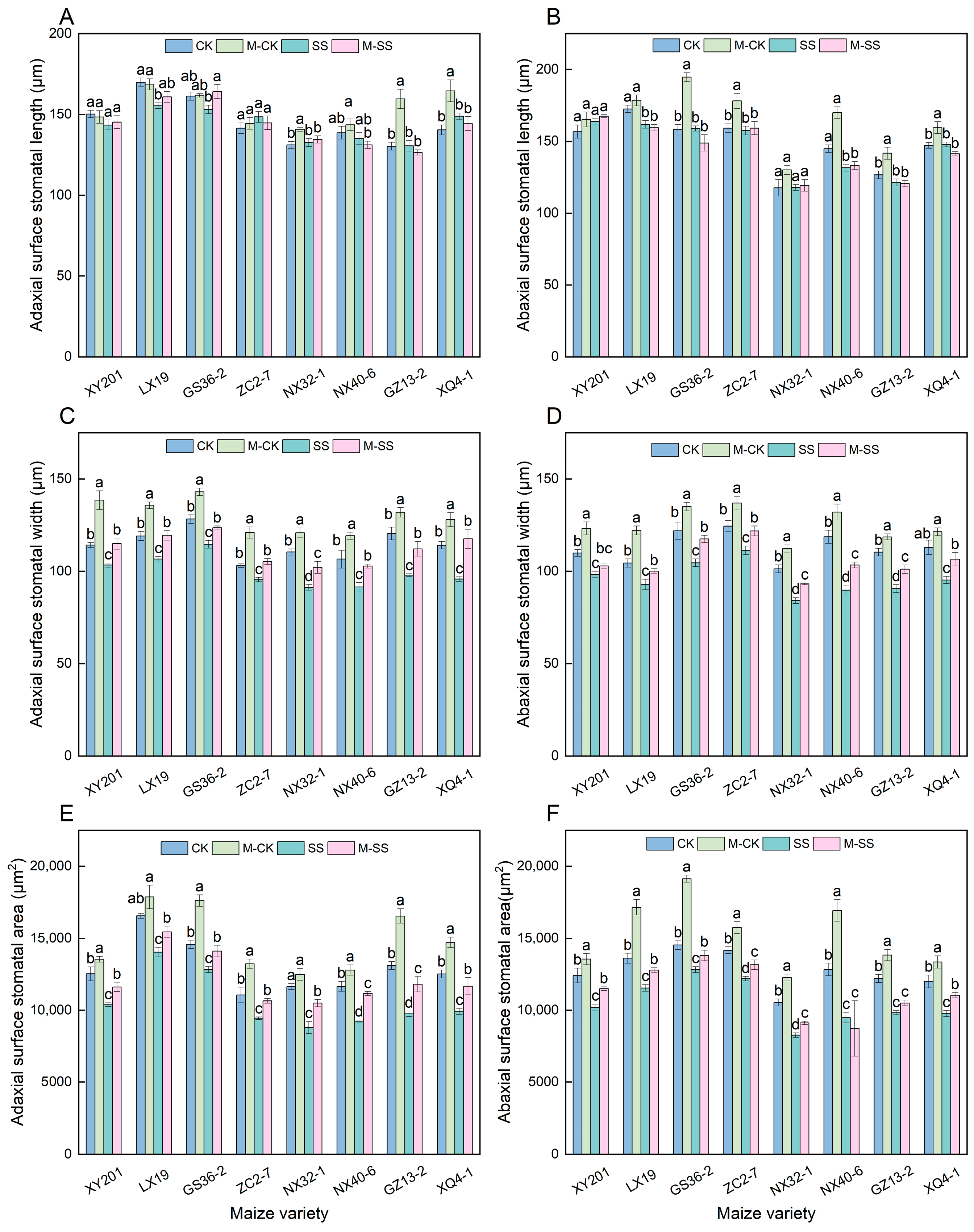
2.5. Effects of MT on Stomatal Morphology of Maize Seedlings under Salt Stress
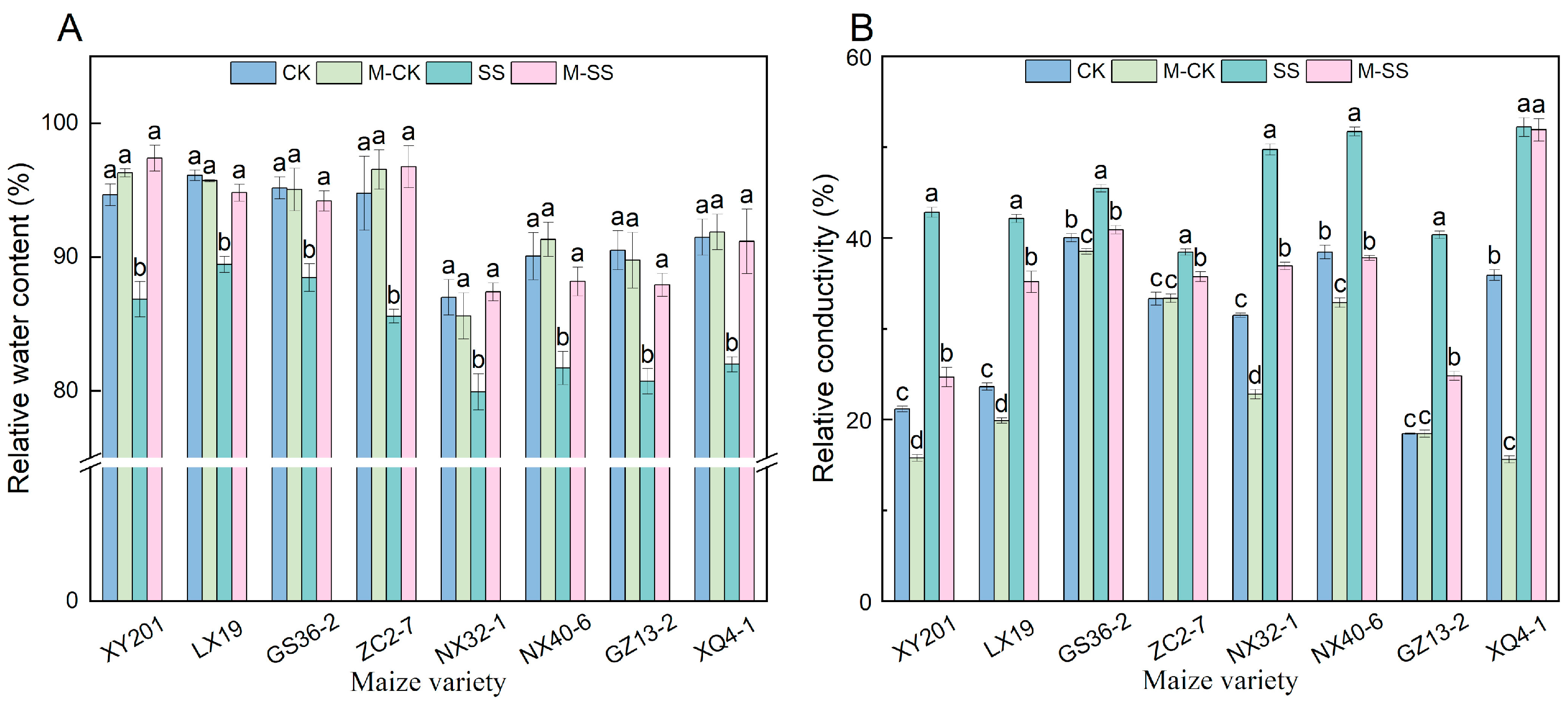
2.6. Effects of MT on Relative Water Content and Membrane Characteristics of Maize Seedlings under Salt Stress
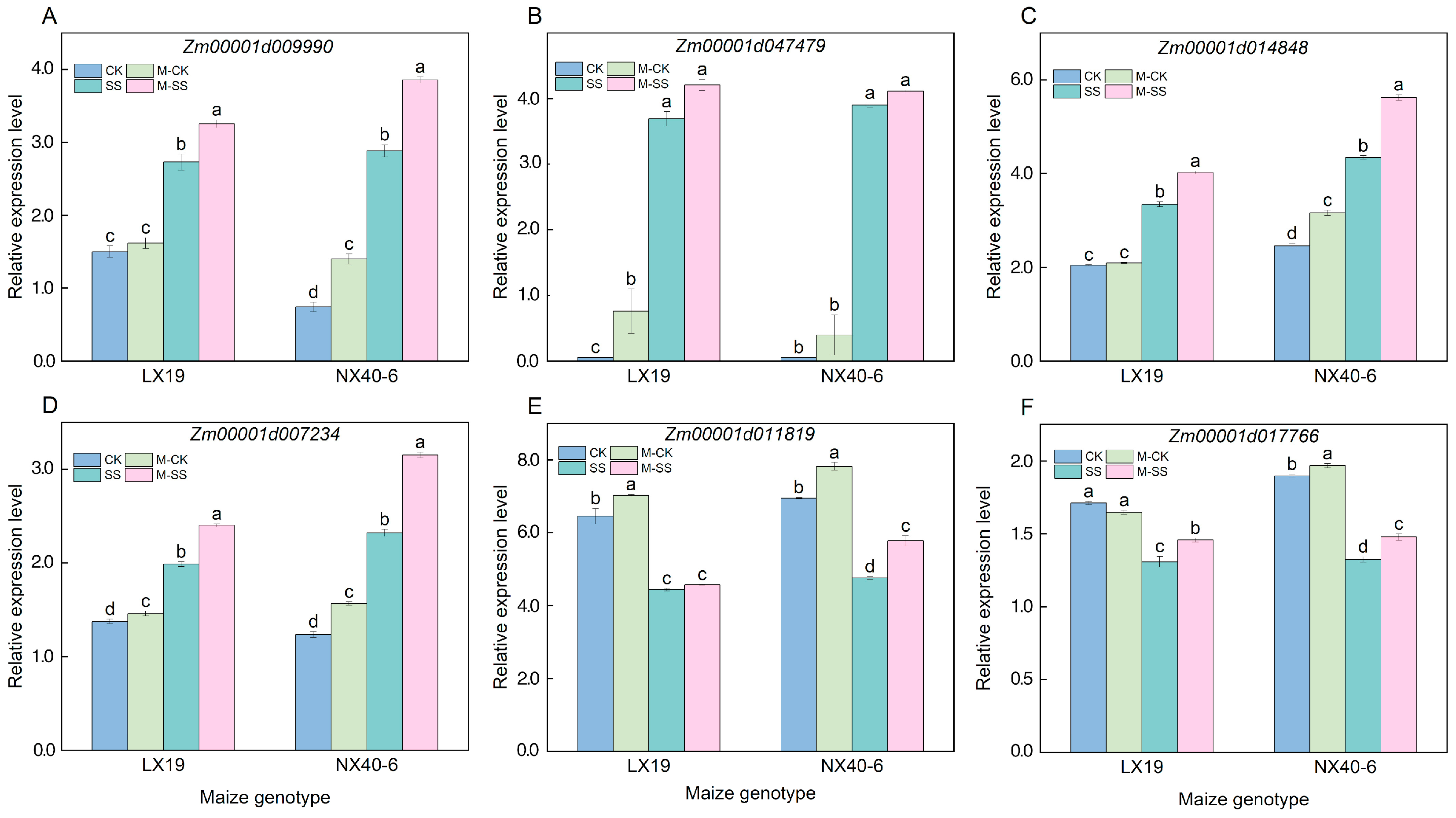
2.7. Expression Analysis of Six Candidate Genes
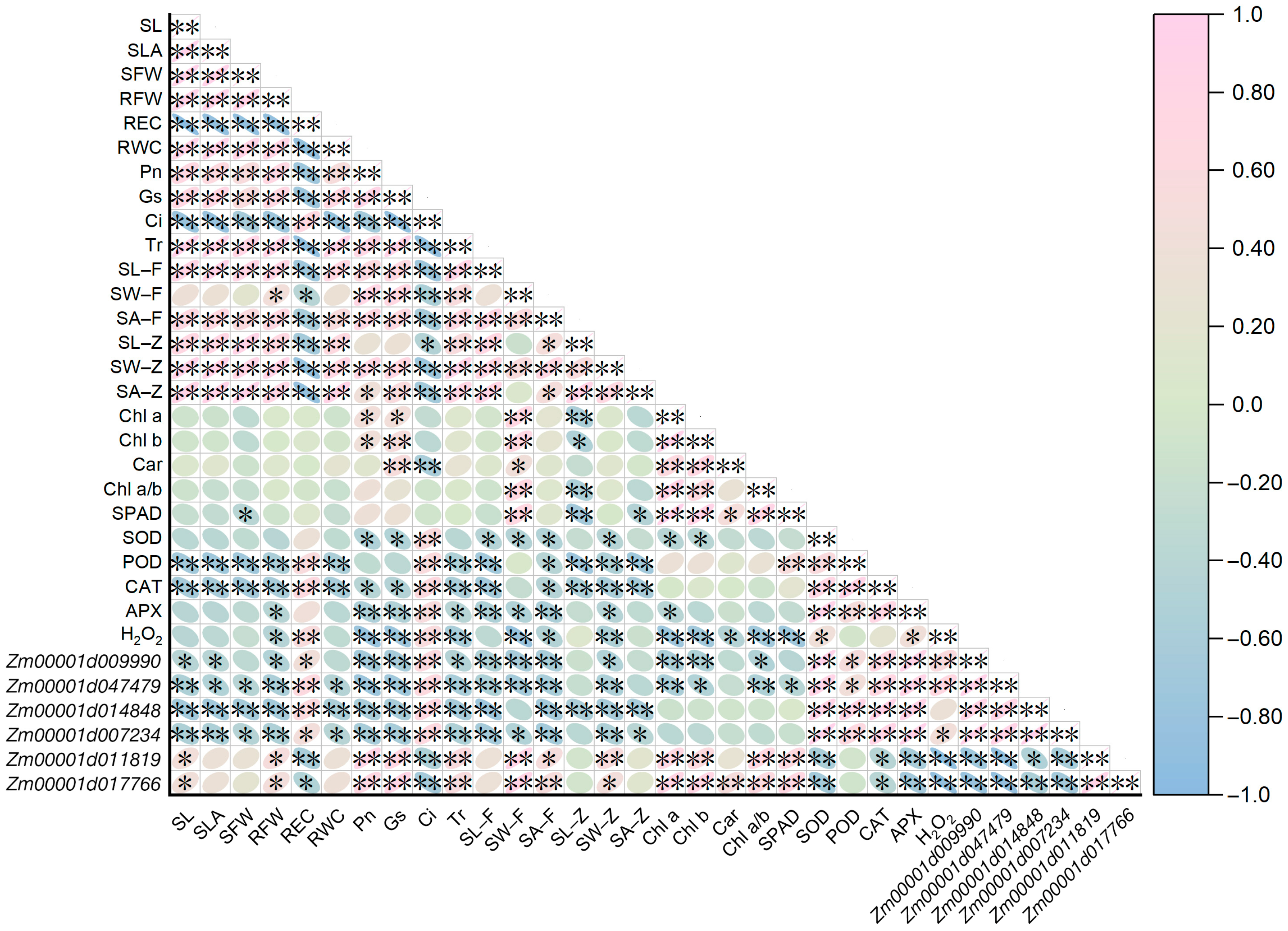
2.8. Correlation Relationships among Traits and Candidate Genes of Maize Seedlings under All Treatments
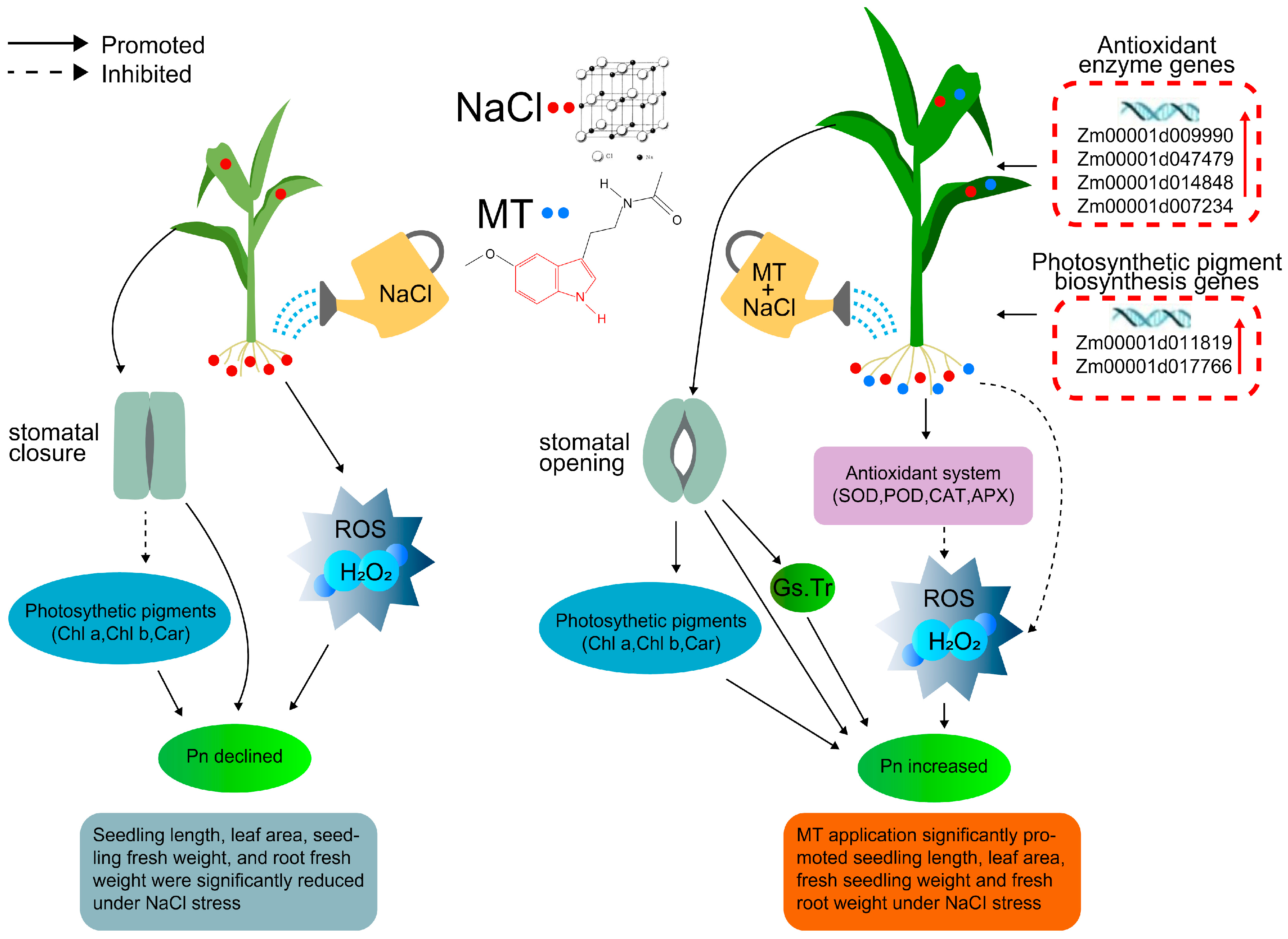
3. Discussion
4. Materials and Methods
4.1. Maize Materials and Treatments
4.2. Growth Performances Observation
4.3. Determination of Chlorophyll and Photosynthetic Parameters
4.4. Determination of Antioxidant Enzyme Activity and H2O2 Content
4.5. Characterization of Stomatal Morphology
4.6. Determination of Relative Water Content and Relative Conductivity
4.7. Data Statistical Analyses
4.8. RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello, S.K.; Alayafi, A.H.; ALSolaimani, S.G.; Abo–Elyousr, K.A. Mitigating soil salinity stress with gypsum and bio-organic amendments: A review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Mahmood, S.; Al-Solaimani, S.G.; Shams, S.; Naveed, S.; Haider, B.; Naveed, M.; Ali, R.; Waqas, M. Silicon and biochar synergistically stimulate nutrients uptake, photosynthetic pigments, gaseous exchange and oxidative defense to improve maize growth under salinity. Water Air Soil Pollut. 2024, 235, 413. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Etesami, H.; Noori, F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants. In Saline Soil–Based Agriculture by Halotolerant Microorganisms; Springer: Singapore, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Handayani, D.P.; Bidara, I.S.; Himawati, S.; Saryanah, N.A.; Reninta, R. The Application of endophytic halotolerant bacteria in modulating the development of maize seedlings under salinity. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 012042. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Li, Z.Y.; Zhang, Z.Q.; You, L.R.; Xu, L.F.; Huang, H.Y.; Wang, X.P.; Gao, Y.; Cui, X.J. Treatment of the saline-alkali soil with acidic corn stalk biochar and its effect on the sorghum yield in western songnen plain. Sci. Total Environ. 2021, 797, 149190. [Google Scholar] [CrossRef]
- Subramanian, S.; Han, L.; Dutilleul, P.; Smith, D.L. Computed tomography scanning can monitor the effects of soil medium on root system development: An example of salt stress in corn. Front. Plant Sci. 2015, 6, 256. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Gao, J.; Liu, N.G.; Wang, X.Q.; Niu, Z.Y.; Liao, Q.; Ding, R.S.; Du, T.S.; Kang, S.Z.; Tong, L. Maintaining grain number by reducing grain abortion is the key to improve water use efficiency of maize under deficit irrigation and salt stress. Agric. Water Management. 2024, 294, 108727. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shi, J.; Niu, Y.N.; Lu, P.N.; Chen, X.J.; Mao, T.T. 24-epibrassinolide alleviates aluminum toxicity by improving leaf chlorophyll fluorescence and photosynthetic performance and root antioxidant-oxidant balance and ascorbate-glutathione cycle in maize. Russ. J. Plant Physiol. 2022, 69, 99. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identifed by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.; Shafig, M.; Naz, S.; Ali, S.; Ahmad, R.; Ejaz, S. Enhancing drought tolerance in broccoli (Brassica oleracea L.) through melatonin application: Physiological and biochemical insights into growth, photosynthesis, and antioxidant defense mechanisms. Biocatal. Agric. Biotechnol. 2024, 59, 103256. [Google Scholar] [CrossRef]
- Wang, J.J.; Yan, D.; Lu, Z.Z.; Liu, R.; Hong, Y.; Wang, Y.; Peng, Z.; Yu, C.X.; Gao, Y.R.; Liu, Z.Y.; et al. Integration of the metabolome and transcriptome reveals diurnal variability in the effects of melatonin on salt tolerance in maize seedlings. J. Plant Growth Regul. 2024, 43, 1672–1688. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 2017, 203, 286–294. [Google Scholar] [CrossRef]
- Mao, K.Q.; Li, J.L.; Wu, S.H.; Qian, J.J.; Liao, Y.Y.; Zeng, L.T.; Gu, D.C. Melatonin treatment promotes cold adaptation and spring growth of tea plants. Ind. Crops Prod. 2023, 200, 116834. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.W.; Liang, D.; Liu, C.H.; Yin, L.H.; Jia, D.F.; Fu, M.Y.; Ma, F.W. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Shahani, A.A.; Abbas, A.; Hameed, R.; Iqbal, A.; Chen, S.; Liu, Q.; Liu, Y.; Zhang, D.; Zhu, R.; Fayyaz, A.; et al. Melatonin in plants: A pleiotropic molecule for abiotic stresses and pathogens infection. Sci. Hortic. 2023, 322, 112387. [Google Scholar] [CrossRef]
- Talaat, N.B. Drought stress alleviator melatonin reconfigures water-stressed barley (Hordeum vulgare L.) plants’ photosynthetic efficiency, antioxidant capacity, and endogenous phytohormone profile. Int. J. Mol. Sci. 2023, 24, 16228. [Google Scholar] [CrossRef]
- Park, H.S.; Kazerooni, E.A.; Kang, S.M.; Al–Sadi, A.M.; Lee, I.J. Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. under saline conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Zhang, T.G.; Wang, J.; Sun, Y.P.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2021, 1–17. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the stress: Breeding for climate resilience in maize for the tropical rainfed environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.W.; Zhang, F.F.; Huang, Y.H. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants. 2022, 3, 512. [Google Scholar] [CrossRef] [PubMed]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Muhammad, A.; Neelofar, H.; Zainul, A.; Faisal, Z.; Anam, M.; Muhammad, N.; Ali, E.K. Exogenous melatonin application stimulates growth, photosynthetic pigments and antioxidant potential of white beans under salinity stress. S. Afr. J. Bot. 2023, 160, 219–228. [Google Scholar] [CrossRef]
- Sezer, I.; Kiremit, M.S.; Öztürk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376. [Google Scholar] [CrossRef]
- Jiang, C.Q.; Cui, Q.R.; Feng, K.; Xu, D.F.; Li, C.F.; Zheng, Q.S. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Xu, C.P.; Huang, B.R. Differential proteomic responses to water stress induced by PEG in two creeping bentgrass cultivars differing in stress tolerance. J. Plant Physiol. 2010, 167, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Sun, S.S.; Wen, D.; Yang, W.Y.; Meng, Q.F.; Shi, Q.H.; Gong, B. Overexpression of cafeic acid o-methyltransferase 1 (COMT1) increases melatonin level and salt stress tolerance in tomato plant. J. Plant Growth Regul. 2020, 39, 1221–1235. [Google Scholar] [CrossRef]
- Ullah, A.; Al-Rajhi, R.S.; Al-Sadi, A.M.; Farooq, M. Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2378–2391. [Google Scholar] [CrossRef]
- Al Hinai, M.S.; Ullah, A.; Al-Rajhi, R.S.; Farooq, M. Proline accumulation, ion homeostasis and antioxidant defence system alleviate salt stress and protect carbon assimilation in bread wheat genotypes of Omani origin. Environ. Exp. Bot. 2022, 193, 104687. [Google Scholar] [CrossRef]
- Zhao, X.Q.; He, F.Q.; Qi, G.X.; Sun, S.Q.; Shi, Z.Z.; Niu, Y.N.; Wu, Z.F. Transcriptomic and physiological studies unveil that brassinolide maintains the balance of maize’s multiple metabolisms under low-temperature stress. Int. J. Mol. Sci. 2024, 25, 9396. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.A.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Zhao, C.; Niu, Y.N.; Chao, W.; He, W.; Wang, Y.F.; Bai, X.D. Understanding and comprehensive evaluation of cold resistance in the seedlings of multiple maize–genotypes. Plants 2022, 11, 1881. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Xiong, Z.; Zhang, Z.L.; Wei, Y.B.; Xiong, D.L.; Wang, F.; Huang, J.L. Exploration of chlorophyll fluorescence characteristics gene regulatory in rice (Oryza sativa L.): A genome-wide association study. Front. Plant Sci. 2023, 14, 1234866. [Google Scholar] [CrossRef] [PubMed]

| Maize Variety | Treatment | SL(cm) | SLA (cm2) | SFW (g) | RFW (g) |
|---|---|---|---|---|---|
| XY201 | CK | 20.47 ± 0.22 ab | 17.78 ± 0.19 ab | 1.13 ± 0.05 b | 1.57 ± 0.07 a |
| M–CK | 22.23 ± 0.79 a | 19.26 ± 0.86 a | 1.33 ± 0.04 a | 1.69 ± 0.11 a | |
| SS | 16.13 ± 0.58 c | 13.06 ± 0.28 c | 0.80 ± 0.03 c | 1.27 ± 0.05 b | |
| M–SS | 19.37 ± 0.43 b | 16.90 ± 0.22 b | 1.13 ± 0.06 b | 1.46 ± 0.09 ab | |
| LX19 | CK | 26.80 ± 0.42 b | 21.07 ± 0.96 b | 1.52 ± 0.06 b | 1.86 ± 0.18 b |
| M–CK | 29.97 ± 0.96 a | 24.58 ± 0.46 a | 1.89 ± 0.09 a | 2.46 ± 0.19 a | |
| SS | 20.83 ± 0.52 c | 15.84 ± 0.13 c | 1.03 ± 0.13 c | 1.35 ± 0.07 b | |
| M–SS | 25.30 ± 0.57 b | 20.95 ± 0.29 b | 1.26 ± 0.06 bc | 1.69 ± 0.15 b | |
| GS36-2 | CK | 24.07 ± 0.85 b | 19.36 ± 0.55 b | 1.29 ± 0.02 b | 1.38 ± 0.05 b |
| M–CK | 26.60 ± 0.87 a | 25.32 ± 0.77 a | 1.68 ± 0.05 a | 1.86 ± 0.11 a | |
| SS | 17.63 ± 0.75 d | 12.65 ± 0.17 d | 0.85 ± 0.04 d | 1.06 ± 0.13 c | |
| M–SS | 20.67 ± 0.39 c | 15.76 ± 0.67 c | 1.08 ± 0.06 c | 1.32 ± 0.05 bc | |
| ZC2-7 | CK | 24.70 ± 0.49 ab | 19.58 ± 0.97 b | 1.47 ± 0.07 a | 2.01 ± 0.09 a |
| M–CK | 26.40 ± 0.61 a | 22.89 ± 1.22 a | 1.48 ± 0.03 a | 2.05 ± 0.04 a | |
| SS | 20.26 ± 1.16 c | 14.14 ± 0.35 c | 0.95 ± 0.01 b | 1.49 ± 0.09 b | |
| M–SS | 22.73 ± 0.74 bc | 16.96 ± 0.93 bc | 1.10 ± 0.11 b | 1.67 ± 0.09 b | |
| NX32-1 | CK | 18.30 ± 0.61 a | 13.53 ± 0.28 b | 0.71 ± 0.03 a | 1.11 ± 0.04 b |
| M–CK | 19.66 ± 0.39 a | 15.51 ± 0.27 a | 0.76 ± 0.02 a | 1.30 ± 0.05 a | |
| SS | 14.20 ± 0.42 c | 8.23 ± 0.28 d | 0.44 ± 0.01 c | 0.81 ± 0.04 c | |
| M–SS | 16.50 ± 0.62 b | 11.17 ± 0.71 c | 0.56 ± 0.06 b | 0.97 ± 0.06 b | |
| NX40-6 | CK | 21.70 ± 0.58 b | 14.63 ± 0.42 b | 0.86 ± 0.02 ab | 1.51 ± 0.15 ab |
| M–CK | 23.76 ± 0.85 a | 18.29 ± 0.75 a | 0.98 ± 0.08 a | 1.78 ± 0.13 a | |
| SS | 16.10 ± 0.25 d | 8.25 ± 0.19 d | 0.51 ± 0.05 c | 0.81 ± 0.11 c | |
| M–SS | 19.43 ± 0.49 c | 10.62 ± 0.36 c | 0.77 ± 0.06 b | 1.19 ± 0.08 bc | |
| GZ13-2 | CK | 23.63 ± 0.64 b | 19.20 ± 0.28 b | 1.06 ± 0.06 b | 1.98 ± 0.17 ab |
| M–CK | 27.50 ± 0.95 a | 20.57 ± 0.58 a | 1.35 ± 0.06 a | 2.26 ± 0.14 a | |
| SS | 17.23 ± 0.20 c | 12.37 ± 0.20 d | 0.61 ± 0.03 c | 1.34 ± 0.06 c | |
| M–SS | 19.06 ± 0.38 c | 15.82 ± 0.20 c | 0.77 ± 0.08 c | 1.72 ± 0.16 bc | |
| XQ4-1 | CK | 25.83 ± 0.33 b | 19.99 ± 0.33 b | 1.30 ± 0.07 a | 1.80 ± 0.10 b |
| M–CK | 28.27 ± 0.36 a | 22.66 ± 0.67 a | 1.45 ± 0.03 a | 2.15 ± 0.06 a | |
| SS | 19.96 ± 0.29 d | 13.25 ± 0.49 d | 0.69 ± 0.04 c | 1.26 ± 0.05 c | |
| M–SS | 23.50 ± 0.51 c | 17.28 ± 0.59 c | 1.02 ± 0.06 b | 1.62 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Zhao, X.; Qi, G.; Sun, S.; Shi, Z.; Niu, Y.; Wu, Z.; Zhou, W. Exogenous Melatonin Alleviates NaCl Injury by Influencing Stomatal Morphology, Photosynthetic Performance, and Antioxidant Balance in Maize. Int. J. Mol. Sci. 2024, 25, 10077. https://doi.org/10.3390/ijms251810077
He F, Zhao X, Qi G, Sun S, Shi Z, Niu Y, Wu Z, Zhou W. Exogenous Melatonin Alleviates NaCl Injury by Influencing Stomatal Morphology, Photosynthetic Performance, and Antioxidant Balance in Maize. International Journal of Molecular Sciences. 2024; 25(18):10077. https://doi.org/10.3390/ijms251810077
Chicago/Turabian StyleHe, Fuqiang, Xiaoqiang Zhao, Guoxiang Qi, Siqi Sun, Zhenzhen Shi, Yining Niu, Zefeng Wu, and Wenqi Zhou. 2024. "Exogenous Melatonin Alleviates NaCl Injury by Influencing Stomatal Morphology, Photosynthetic Performance, and Antioxidant Balance in Maize" International Journal of Molecular Sciences 25, no. 18: 10077. https://doi.org/10.3390/ijms251810077





