Solid Lipid Nanoparticles Incorporated with Retinol and Pentapeptide-18—Optimization, Characterization, and Cosmetic Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Qualitative Composition of Lipid Nanoparticle Dispersions
2.1.1. Lipid Screening—Selection of the Solid Lipid
2.1.2. Addition of Phosphatidylcholine as a Stabilizing Agent
2.1.3. Selection of the Surfactant System
2.2. Assessment of Encapsulation Efficiency and Loading Capacity of Retinol and Oligopeptide
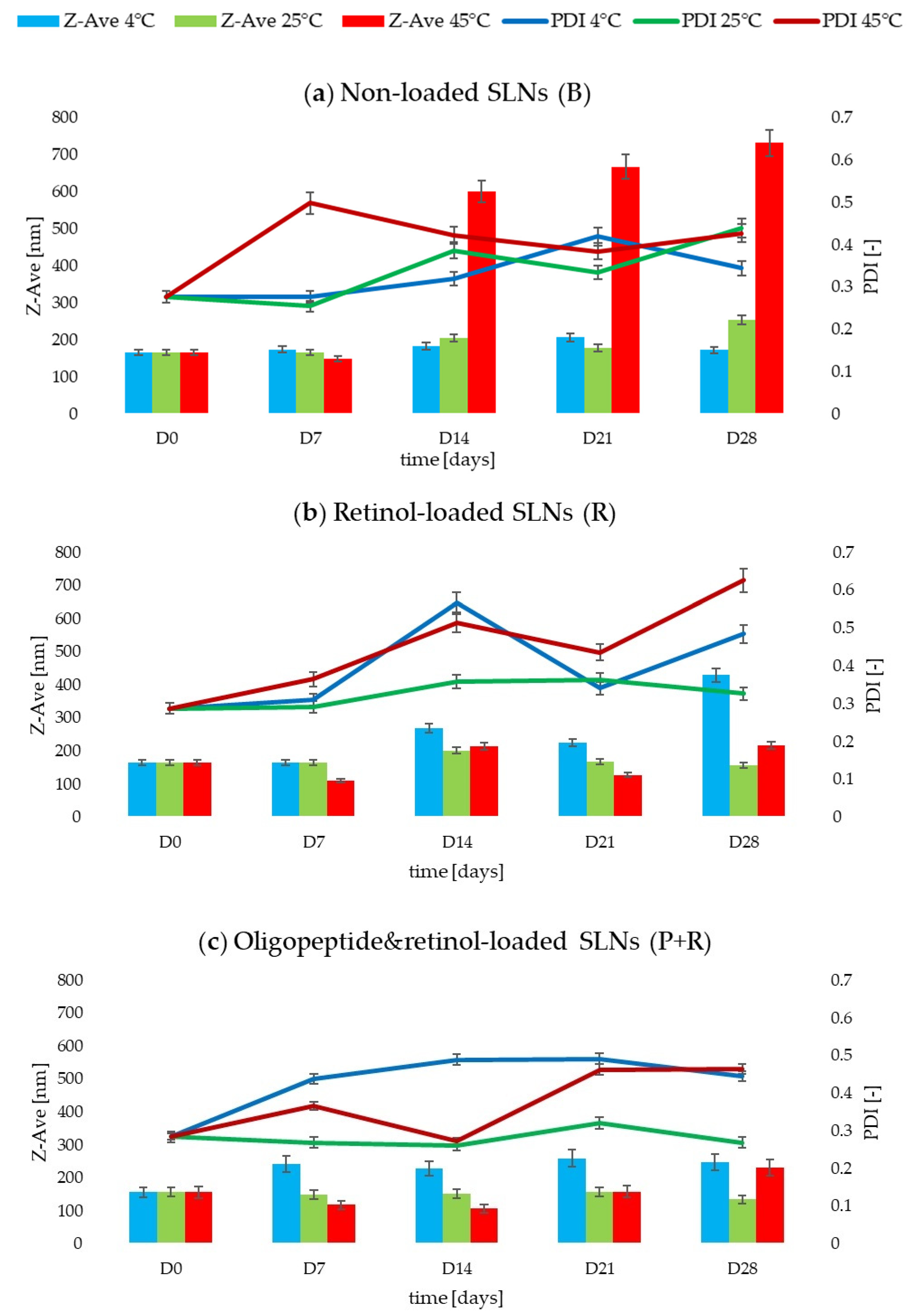
2.3. Evaluation of Stability
- The changes in the value of the mean particle size of the dispersion depended on the presence of the active substances. For non-incorporated lipid nanoparticles (B), over the assumed 28 days, the Z-Ave value increased depending on the storage temperature by 4% at 4 °C, by 35% at 25 °C and by 77% at 45 °C (p < 0.05) (Figure 2a). The retinol-loaded SLNs (R) recorded Z-Ave values 62% (4 °C) and 24% (45 °C) higher (p < 0.05) compared to the results obtained at D0 and D28. On the other hand, at 25 °C, there was a 4% decrease in the value from 164.0 ± 0.5 to 157.9 ± 1.6 nm (Figure 2b). For sample 3 (P+R) enriched with two active substances, the following values of Z-Ave at day 28 were obtained: 248.3 ± 7.4 nm (+37%), 134.7 ± 0.3 nm (−16%) and 231.1 ± 1.4 nm (+32%) for storage temperatures of 4, 25 and 45 °C, respectively (p < 0.05) (Figure 2c). Significantly, despite the noticeable changes in Z-Ave values for dispersion 3, regardless of storage conditions, the value of 300 nm indicative of stable dispersion in this case was not exceeded [38].
- In the case of the polydispersity index (PDI), an increase in the value of this parameter was noted over time and under different storage conditions of the samples. The most noticeable changes in PDI values were noted for samples 1 (B) and 2 (R) stored at 45 °C for 28 days, changing by +35% and +54%, respectively (p < 0.05) (Figure 2a,b). Sample 3 (P+R) showed 36% and 39% increases in PDI at 4 °C and 45 °C, respectively (p < 0.05). The exception was sample 3, stored at 25 °C, for which the polydispersity index value decreased slightly (by 6%) from 0.284 ± 0.002 [−] (D0) to 0.269 ± 0.017 [−] (D28, p < 0.05) (Figure 2c). The PDI value for this sample did not exceed the value of 0.3 [-], considered in this study to be a value representing a stable dispersion [38].
- There were no statistically significant changes in the zeta potential values of the samples tested. There was also no correlation between the presence or absence of bioactive substances and variable storage conditions and the ZP results. In all three cases (B, R, P+R), the values were above the desired value of +30 mV, both at D0 and D28. After 28 days of stability testing, the parameters of sample 1 (B) stored at the three assumed temperatures were in the range 38.6 ± 0.4–44.6 ± 1.3 mV. The zeta potential values for sample 2 (R) were 39.9 ± 0.4 mV, 53.5 ± 5.2 mV, and 45.5 ± 0.4 mV (D28) for temperatures of 4, 25, and 45 °C, respectively. In contrast, the ZP results for sample 3 (P+R) oscillated between 42.7 ± 1.2 and 44.5 ± 1.6 mV (D28). It was shown that the incorporation of active substances (oligopeptide and retinol) did not affect the ZP values determined for the samples of lipid nanoparticle dispersions. The cationic surfactant (CTAB), present in the composition of the dispersion, was primarily responsible for the positive zeta potential value [39].
- A comparison of the tested samples (B, R, P+R) containing different systems of encapsulated active substances indicated that the presence of these compounds influenced smaller variations in the values of parameters related to dispersion stability. This was, for example, evident by comparing the Z-Ave results obtained for samples stored at 25 °C, where sample 1(B) showed a 35% increase in mean particle size, while sample 2 (R) achieved an increase of 4%, and sample 3 (P+R) showed a 16% decrease in Z-Ave.
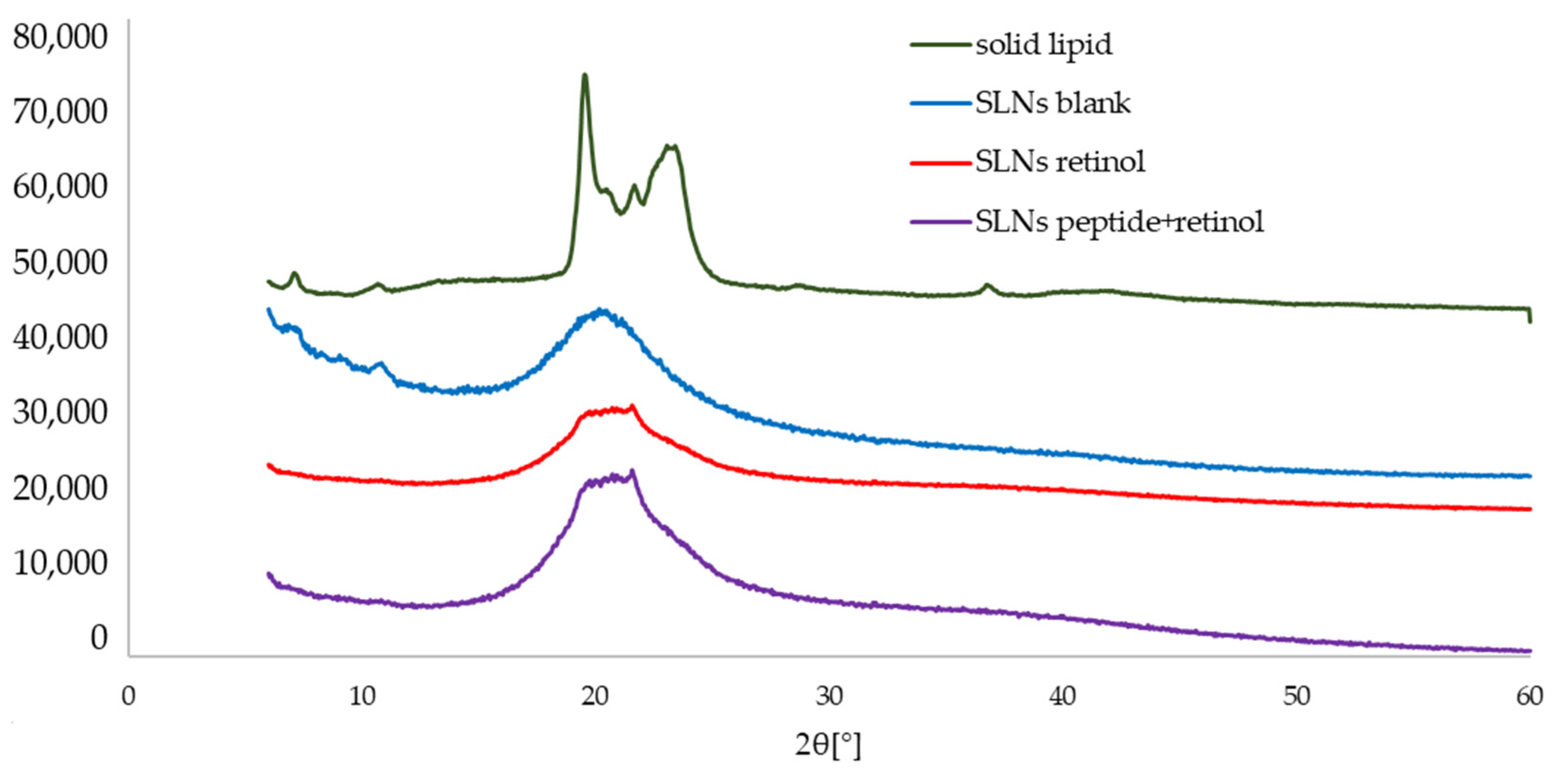
2.4. Characterization of Lipid Matrices
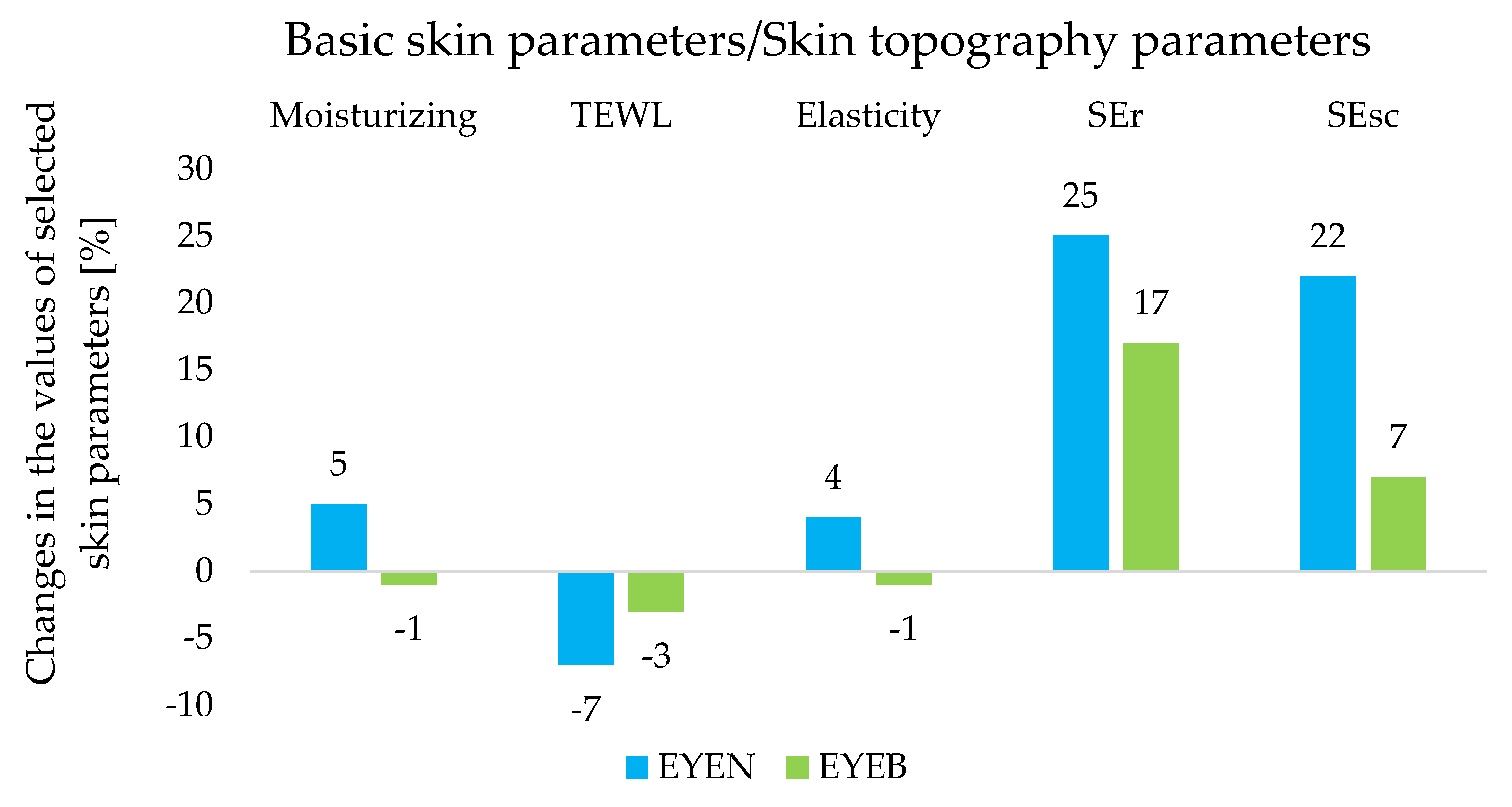
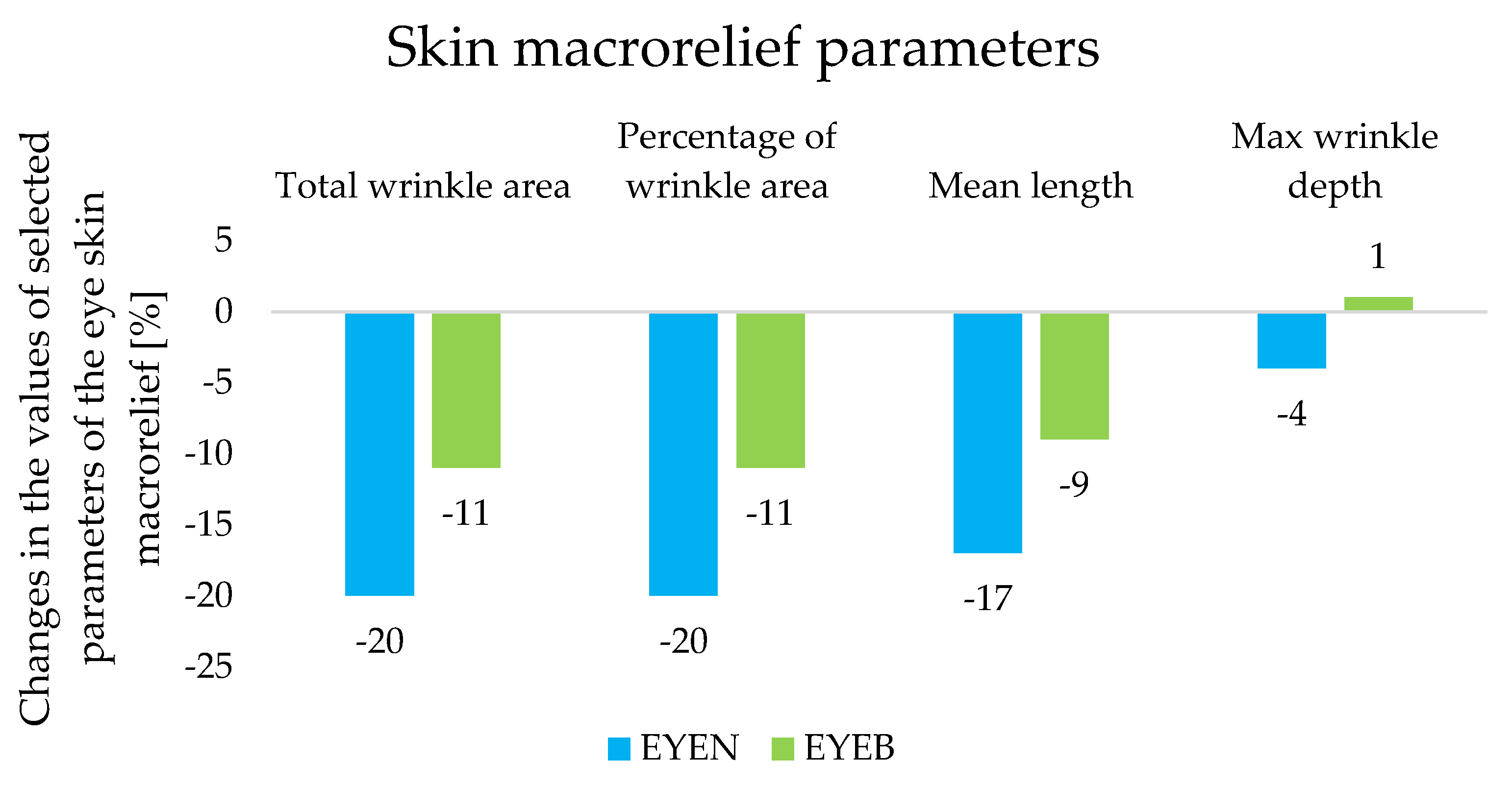
2.5. Assessment of the Effect of Cosmetic Products on Selected Skin Parameters
3. Materials and Methods
3.1. Materials
3.2. Optimization of the Composition of Lipid Nanoparticles—Selected Factors
3.3. Lipid Nanoparticles—Method of Production
3.4. Lipid Nanoparticles—Physicochemical Parameters
3.5. Encapsulation Efficiency and Loading Capacity
3.6. Stability Test
3.7. X-ray Diffraction (XRD) and Differential Scanning Calorimetry (DSC) Analysis
3.8. Cosmetic Products/Eye Creams—Preparation
3.8.1. EYEN (Anti-Aging Night Eye Cream Containing 1.0 wt.% of Lipid Nanoparticles Loaded with Retinol and Pentapeptide-18)
3.8.2. EYEB (Anti-Aging Night Eye Cream without Lipid Nanoparticles)
3.9. Evaluation of the Effectiveness of Cosmetic Products—In Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Anil, L.; Kannan, K. Microemulsion as drug delivery system for peptides and proteins. J. Pharm. Sci. Res. 2020, 10, 16–25. [Google Scholar]
- Czerwonka, W.; Puchalska, D.; Lipińska, M.; Habrat, A. Mechanizmy i metody przenikaniasubstancji czynnych przez barierę lipidową skóry. Kosmetol. Estet. 2018, 7, 667–670. [Google Scholar]
- Jaworska, M.; Sikora, E.; Ogonowski, J. Czynniki Wpływające Na Penetrację Składników Aktywnych Przez Skórę Factors Influencing the Percutaneous Penetration of Active Ingerdients. Wiadomości Chem. 2011, 65, 3–4. [Google Scholar]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 4265. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Charoenputtakhun, P.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. All-trans retinoic acid-loaded lipid nanoparticles as a transdermal drug delivery carrier. Pharm. Dev. Technol. 2014, 19, 164–172. [Google Scholar] [CrossRef]
- Zasada, M.; Adamczyk, A.; Witamina, A. Budowa i mechanizm działania. Vitamin A. Structure and mechanism of action. Kosmetologia estetyczna. Kosmetol. Estet. 2018, 7, 517–521. [Google Scholar]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Resende, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of synthetic peptides in cosmetics for sensitive skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef] [PubMed]
- SCCS/1576/16; Scientific Committee on Consumer Safety SCCS. 2016. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_199.pdf (accessed on 16 September 2024).
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 13. [Google Scholar] [CrossRef] [PubMed]
- Limbert, G.; Masen, M.A.; Pond, D.; Graham, H.K.; Sherratt, M.J.; Jobanputra, R.; McBride, A. Biotribology of the ageing skin—Why we should care. Biotribology 2018, 17, 75–90. [Google Scholar] [CrossRef]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef]
- Errante, F.; Ledwoń, P.; Latajka, R.; Rovero, P.; Papini, A.M. Cosmeceutical Peptides in the Framework of Sustainable Wellness Economy. Front. Chem. 2020, 8, 572923. [Google Scholar] [CrossRef] [PubMed]
- Information material from Lipotec®. Leuphasyl. 2020; pp. 1–15.
- Almeida, A.J.; Runge, S.; Müller, R.H. Peptide-loaded solid lipid nanoparticles (SLN): Influence of production parameters. Int. J. Pharm. 1997, 149, 255–265. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Lasoń, E.; Ogonowski, J. Stałe Nanocząsteczki Lipidowe-charakterystyka, zastosowanie i otrzymywanie. Chemik 2011, 65, 960–967. [Google Scholar]
- Wosicka, J. Stałe nanocząstki lipidowe i nanostrukturalne nośniki lipidowe w nowoczesnych kosmetykach. Pol. J. Cosmetol. 2009, 12, 23–37. [Google Scholar]
- Yadav, N.; Khatak, S.; Vir, U.; Sara, S. Solid lipid nanoparticles—A review. Int. J. Appl. Pharm. 2013, 5, 8–18. [Google Scholar]
- Banerjee, J.; Pillai, S. Solid lipid matrix mediated nanoarchitectonics for improved oral bioavailability of drugs. Expert Opin. Drug Metab. Toxicol. 2019, 15, 499–515. [Google Scholar] [CrossRef]
- Abdelbary, G.; Fahmy, R.H. Diazepam-Loaded solid lipid nanoparticles: Design and characterization. AAPS PharmSciTech 2009, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Runge, S.A.; Ravelli, V.; Thünemann, A.F.; Mehnert, W.; Souto, E.B. Cyclosporine-loaded solid lipid nanoparticles (SLN®): Drug-lipid physicochemical interactions and characterization of drug incorporation. Eur. J. Pharm. Biopharm. 2008, 68, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schafer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2022, 66, 115–126. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Souto, E.B. Experimental factorial design applied to mucoadhesive lipid nanoparticles via multiple emulsion process. Colloids Surf. B Biointerfaces 2012, 100, 84–89. [Google Scholar] [CrossRef]
- Molet-Rodriguez, A.; Martin-Belloso, O.; Salvia-Trujillo, L. Formation and Stabilization of W1/O/W2 Emulsions with Gelled Lipid Phases. Molecules 2021, 26, 312. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials 2022, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Vanic, Z. Phospholipid vesicles for enhanced drug delivery in dermatology. J. Drug Discov. Dev. Deliv. 2015, 2, 8. [Google Scholar]
- Saupe, A.; Rades, T. Nanocarrier Technologies; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Dąbrowska, M.; Souto, E.B.; Nowak, I. Lipid nanoparticles loaded with iridoid glycosides: Development and optimization using experimental factorial design. Molecules 2021, 26, 3161. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Charged liposomes as carriers to enhance the permeation through the skin. Drug Deliy 2011, 8, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Müller, R.H. Lipid Nanoparticles: Effect on Bioavailability and Pharmacokinetic Changes; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Guimarães, K.L.; Ré, M.I. Lipid Nanoparticles as Carriers for Cosmetic Ingredients: The First (SLN) and the Second Generation (NLC); Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Boskabadi, M.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Hashemi, S.M.H.; Babaei, A. Topical Gel of Vitamin A Solid Lipid Nanoparticles: A Hopeful Promise as a Dermal Delivery System. Adv. Pharm. Bull. 2021, 11, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.A. Optymalizacja właściwości fizykochemicznych oraz aplikacyjnych formulacji kosmetycznych zawierających wybrane glikozydy irydoidowe. Ph.D. Thesis, AMU Poznań, Poznan, Poland, 2019; p. 167. [Google Scholar]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the physical characterization of lipid nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.; Nowak, I. Lipid nanoparticles loaded with selected iridoid glycosides as effective components of hydrogel formulations. Materials 2021, 14, 4090. [Google Scholar] [CrossRef]
- Saupe, A.; Wissing, S.A.; Lenk, A.; Schmidt, C.; Müller, R.H. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC)—Structural investigations on two different carrier systems. Bio-Med. Mater. Eng. 2005, 15, 393–402. [Google Scholar]
- Averina, E.S.; Seewald, G.; Müller, R.H.; Radnaeva, L.D.; Popov, D.V. Nanostructured lipid carriers (NLC) based on Siberian pine (Pinus sibirica) seed oil. Pharmazie 2010, 65, 25–31. [Google Scholar]
- Silva, A.; González-Mira, E.; García, M.; Egea, M.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef]
- Kilpatrick-Liverman, L.; Mattai, J.; Tinsley, R.; Wu, J. Mechanisms of Skin Hydration. In Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2014; Chapter 7; pp. 81–92. [Google Scholar]
- Adamski, Z.; Kaszuba, A. Dermatologia dla kosmetologów; Edra Urban & Partner: Wrocław, Poland, 2019; ISBN 9788376091471. [Google Scholar]
- Noszczyk, M. Kosmetologia pielęgnacyjna i lekarska; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2012; ISBN 9788320061062. [Google Scholar]
- Machado, M.; Salgado, T.M.; Hadgraft, J.; Lane, M.E. The relationship between transepidermal water loss and skin permeability. Int. J. Pharm. 2010, 384, 73–77. [Google Scholar] [CrossRef]
- Manual Instruction from Couriage+Khazaka®. Visioscan VC 98. 2017.
- Bojarowicz, H.; Płowiec, A. Wpływ witaminy A na kondycję skóry. Probl. Hig. Epidemiol. 2010, 91, 352–356. [Google Scholar]
- Czarnota, A. Retinoidy. Mechanizm działania, właściwości oraz zakres stosowania w dermatologii i kosmetologii. Kosmetol. Estet. 2018, 7, 371–376. [Google Scholar]
- Suligowska, I. Pielęgnowanie oczu i ich oprawy. Poradnik dla ucznia; Instytut Technologii Eksploatacji-Państwowy Instytut Badawczy: Radom, Poland, 2006. [Google Scholar]
- Carreira, A.C.; Santos, T.C.; Lone, M.A.; Zupančič, E.; Lloyd-Evans, E.; de Almeida, R.F.M.; Hornemann, T.; Silva, L.C. Mammalian sphingoid bases: Biophysical, physiological and pathological properties. Prog. Lipid Res. 2018, 75, 100988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, X.; Miao, Y.; Wang, X.; Yang, Y.; Zhang, X.; Gan, Y. Effect of phosphatidylcholine on the stability and lipolysis of nanoemulsion drug delivery systems. Int. J. Pharm. 2020, 583, 119354. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tian, B.; Han, J.; Zhang, J.; Shi, Y.; Lv, Q.; Li, K. Enhanced transdermal delivery of lornoxicam by nanostructured lipid carrier gels modified with polyarginine peptide for the treatment of carrageenan-induced rat paw edema. Int. J. Nanomed. 2019, 2, 6135–6150. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Dąbek, P.; Witkowski, A.; Monedeiro, F.; Pomastowski, P.; Buszewski, B.; Nowak, I. Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles. Pharmaceutics 2022, 14, 1171. [Google Scholar] [CrossRef] [PubMed]

| Solid Lipid | Solubility | |||||
|---|---|---|---|---|---|---|
| 0 min | 15 min | 30 min | 1 h | 24 h | 72 h | |
| Compritol® 888 ATO | + | + | + | + | + | + |
| Imwitor® 900 K | + | + | + | + | + | + |
| Precirol® ATO 5 | − | − | − | − | − | − |
| Witepsol® H15 | + | +/− | +/− | +/− | − | − |
| Surfactant System | Results | D0 | D14 | |
|---|---|---|---|---|
| 1. | Tween® 80 | Z-Ave [nm] | 165.1 ± 0.5 | 173.9 ± 0.9 |
| sodium cholate | PDI [−] | 0.379 ± 0.001 | 0.286 ± 0.002 | |
| CTAB | ZP 1 [mV] | 42.3 ± 1.3 | 42.7 ± 0.4 | |
| 2. | Tween® 80 | Z-Ave [nm] | 200.0 ± 0.7 | 185.7 ± 1.5 |
| − | PDI [−] | 0.403 ± 0.004 | 0.417 ± 0.013 | |
| CTAB | ZP 1 [mV] | 45.6 ± 0.2 | 43.9 ± 2.3 | |
| 3. | Lutrol® F68 | Z-Ave [nm] | 203.5 ± 2.4 | 475.8 ± 19.8 |
| sodium cholate | PDI [−] | 0.456 ± 0.012 | 0.489 ± 0.042 | |
| CTAB | ZP 1 [mV] | 43.6 ± 1.1 | 39.6 ± 5.2 | |
| 4. | Lutrol® F68 | Z-Ave [nm] | 192.1 ± 0.4 | 180.9 ± 0.5 |
| − | PDI [−] | 0.396 ± 0.005 | 0.374 ± 0.019 | |
| CTAB | ZP 1 [mV] | 39.7 ± 0.4 | 38.1 ± 0.7 | |
| 5. | Lutrol® F68 | Z-Ave [nm] | 5618.0 ± 33.0 | − |
| sodium cholate | PDI [−] | 0.380 ± 0.022 | − | |
| − | ZP 1 [mV] | − | − |
| Temperature | D0 | D7 | D14 | D21 | D28 | ||
|---|---|---|---|---|---|---|---|
| 1. (B) blank | Z-Ave [nm] | 4 °C | 173.5 ± 0.9 | 183.5 ± 0.4 | 206.3 ± 0.5 | 172.3 ± 1.1 | |
| 25 °C | 165.1 ± 1.1 | 165.9 ± 0.4 | 204.5 ± 1.2 | 177.8 ± 0.5 | 253.1 ± 9.8 | ||
| 45 °C | 147.8 ± 0.8 | 600.8 ± 12.3 | 667.4 ± 9.8 | 731.6 ± 7.4 | |||
| PDl [−] | 4 °C | 0.276 ± 0.008 | 0.319 ± 0.023 | 0.419 ± 0.02 | 0.344 ± 0.007 | ||
| 25 °C | 0.379 ± 0.007 | 0.255 ± 0.006 | 0.386 ± 0.032 | 0.334 ± 0.008 | 0.439 ± 0.013 | ||
| 45 °C | 0.498 ± 0.039 | 0.422 ± 0.027 | 0.384 ± 0.023 | 0.427 ± 0.022 | |||
| ZP 1 [mV] | 4 °C | 43.0 ± 1.1 | 40.3 ± 0.4 | 39.5 ± 0.9 | 38.6 ± 0.4 | ||
| 25 °C | 42.7 ± 0.9 | 41.1 ± 0.9 | 42.3 ± 0.8 | 42.7 ± 0.8 | 33.1 ± 0.3 | ||
| 45 °C | 41.0 ± 0.6 | 45.0 ± 0.3 | 43.5 ± 0.5 | 44.6 ± 1.3 | |||
| 2. (R) retinol | Z-Ave [nm] | 4 °C | 163.9 ± 0.4 | 268.4 ± 0.5 | 224.8 ± 5.4 | 429.4 ± 12.3 | |
| 25 °C | 164.0 ± 0.5 | 164.5 ± 1.1 | 202.1 ± 1.2 | 168.6 ± 0.3 | 157.9 ± 1.6 | ||
| 45 °C | 111.3 ± 1.4 | 213.4 ± 4.4 | 128.0 ± 0.23 | 216.7 ± 6.4 | |||
| PDl [−] | 4 °C | 0.311 ± 0.018 | 0.566 ± 0.022 | 0.341 ± 0.011 | 0.485 ± 0.036 | ||
| 25 °C | 0.287 ± 0.008 | 0.292 ± 0.008 | 0.358 ± 0.011 | 0.364 ± 0.015 | 0.327 ± 0.020 | ||
| 45 °C | 0.365 ± 0.005 | 0.515 ± 0.014 | 0.436 ± 0.012 | 0.626 ± 0.017 | |||
| ZP 1 [mV] | 4 °C | 37.6 ± 0.7 | 44.5 ± 0.1 | 38.1 ± 0.8 | 39.9 ± 0.4 | ||
| 25 °C | 43.6 ± 1.4 | 42.1 ± 1.2 | 42.6 ± 0.3 | 39.6 ± 1.3 | 53.5 ± 5.2 | ||
| 45 °C | 41.1 ± 0.3 | 43.1 ± 0.4 | 35.6 ± 1.1 | 45.5 ± 0.4 | |||
| 3. (P+R) oligopeptide retinol | Z-Ave [nm] | 4 °C | 242.3 ± 7.2 | 228.3 ± 1.4 | 259.3 ± 8.7 | 248.3 ± 7.4 | |
| 25 °C | 156.5 ± 0.8 | 148.2 ± 0.8 | 151.8 ± 0.7 | 157.4 ± 1.8 | 134.7 ± 0.3 | ||
| 45 °C | 117.7 ± 0.9 | 107.2 ± 0.4 | 158.5 ± 2.9 | 231.1 ± 1.4 | |||
| PDl [−] | 4 °C | 0.438 ± 0.009 | 0.489 ± 0.019 | 0.491 ± 0.022 | 0.445 ± 0.022 | ||
| 25 °C | 0.284 ± 0.002 | 0.269 ± 0.006 | 0.261 ± 0.002 | 0.320 ± 0.034 | 0.269 ± 0.017 | ||
| 45 °C | 0.366 ± 0.003 | 0.272 ± 0.004 | 0.463 ± 0.007 | 0.464 ± 0.006 | |||
| ZP 1 [mV] | 4 °C | 40.9 ± 0.4 | 43.3 ± 0.5 | 44.5 ± 0.6 | 43.8 ± 0.3 | ||
| 25 °C | 45.6 ± 0.2 | 44.7 ± 0.7 | 45.8 ± 0.3 | 44.0 ± 1.9 | 42.7 ± 1.2 | ||
| 45 °C | 38.1 ± 2.1 | 45.1 ± 0.2 | 37.2 ± 0.9 | 44.5 ± 1.6 |
| Sample | Melting Peak [°C] | Onset [°C] | Enthalpy [J/g] |
|---|---|---|---|
| Imwitor® 900 K | 65.4 | 54.7 | 164.5 |
| (B) blank | 62.3 | 51.7 | 26.8 |
| (R) retinol | 61.6 | 50.7 | 32.1 |
| (P+R) oligopeptide + retinol | 61.7 | 50.9 | 33.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, M.; Marzec, M.; Jankowiak, W.; Nowak, I. Solid Lipid Nanoparticles Incorporated with Retinol and Pentapeptide-18—Optimization, Characterization, and Cosmetic Application. Int. J. Mol. Sci. 2024, 25, 10078. https://doi.org/10.3390/ijms251810078
Pawłowska M, Marzec M, Jankowiak W, Nowak I. Solid Lipid Nanoparticles Incorporated with Retinol and Pentapeptide-18—Optimization, Characterization, and Cosmetic Application. International Journal of Molecular Sciences. 2024; 25(18):10078. https://doi.org/10.3390/ijms251810078
Chicago/Turabian StylePawłowska, Małgorzata, Marta Marzec, Waldemar Jankowiak, and Izabela Nowak. 2024. "Solid Lipid Nanoparticles Incorporated with Retinol and Pentapeptide-18—Optimization, Characterization, and Cosmetic Application" International Journal of Molecular Sciences 25, no. 18: 10078. https://doi.org/10.3390/ijms251810078
APA StylePawłowska, M., Marzec, M., Jankowiak, W., & Nowak, I. (2024). Solid Lipid Nanoparticles Incorporated with Retinol and Pentapeptide-18—Optimization, Characterization, and Cosmetic Application. International Journal of Molecular Sciences, 25(18), 10078. https://doi.org/10.3390/ijms251810078






