Freeze-Driven Adsorption of Oligonucleotides with polyA-Anchors on Au@Pt Nanozyme
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of Experiments
- (1)
- A linear polyA block consisting of 3, 5, 7, or 10 deoxyadenine residues (An), triple-branched A3 (3A3), or triple-branched A5 (3A5): The triple-branched structures were obtained using trebler phosphoramidite during oligonucleotide synthesis. The polyA block is necessary for attaching the oligonucleotide to the nanoparticle surface, as deoxyadenine has the highest affinity for gold [31,38]. A7 anchors are sufficient for robust attachment of oligonucleotides to [Au]NP surfaces when an additional polyT block (at least T5) is present at the opposite end [31]. In our study, the triple-branched A block was used as an anchor for the first time, and we hypothesized that it would provide better binding of the oligonucleotide to the nanoparticle surface.
- (2)
- A random sequence of 23 nucleotides: This length refers to the interval that covers most of the sequences used in molecular genetic analysis for unique hybridization interactions or selective enzymatic hydrolysis.
- (3)
- A polyT block consisting of seven linear deoxythymine residues (T7): The polyT block does not adsorb onto the nanoparticle surface due to its minimal affinity in the order of A > C > G > T [47,48]. However, several studies have shown that the presence of a polyT block as a tail at the opposite end of the polyA block, or even as a spacer immediately following the polyA block, significantly improves the adsorption of the oligonucleotide and stabilizes the conjugate [31,38].
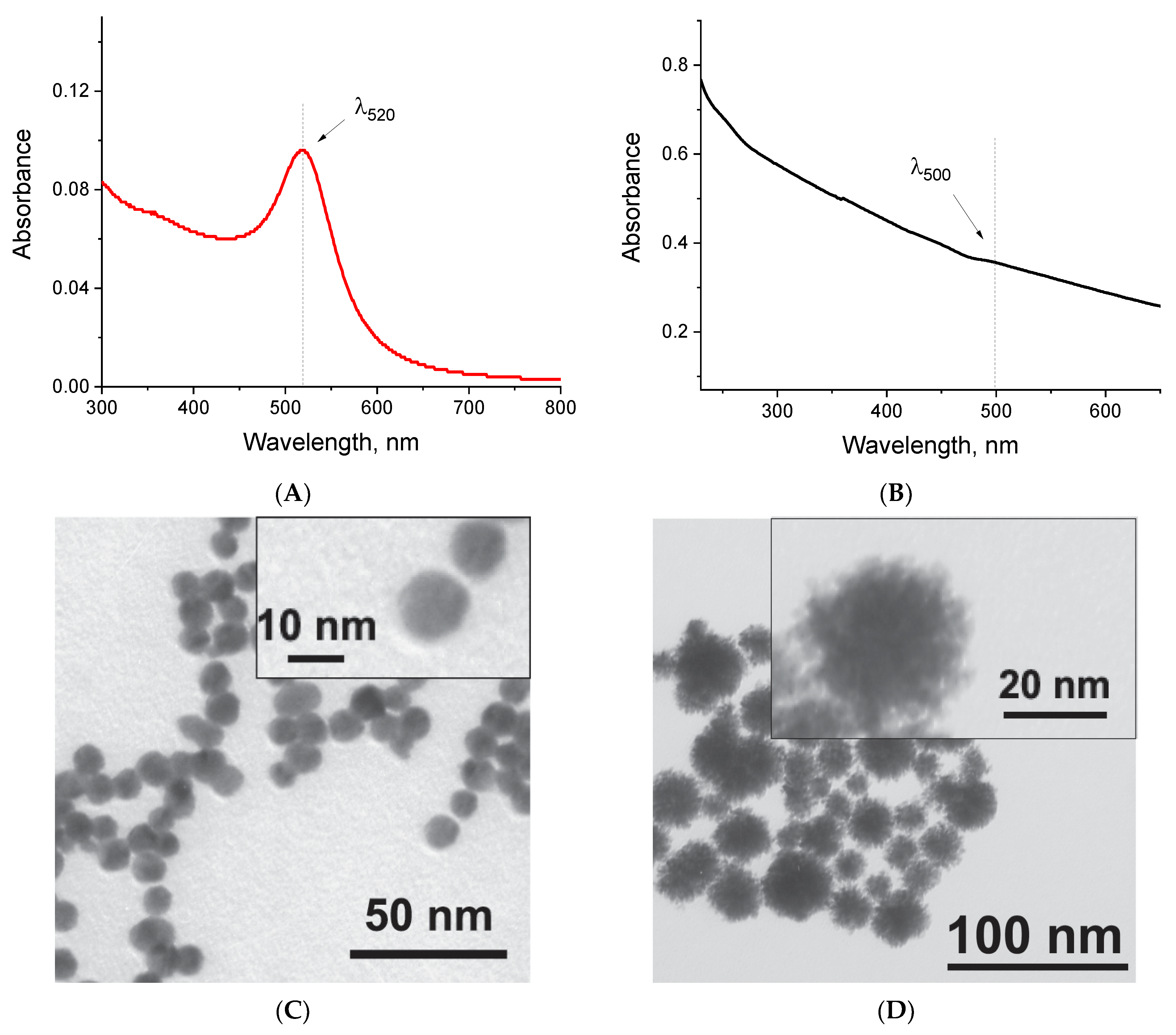
2.2. Synthesis and Characterization of Nanoparticles
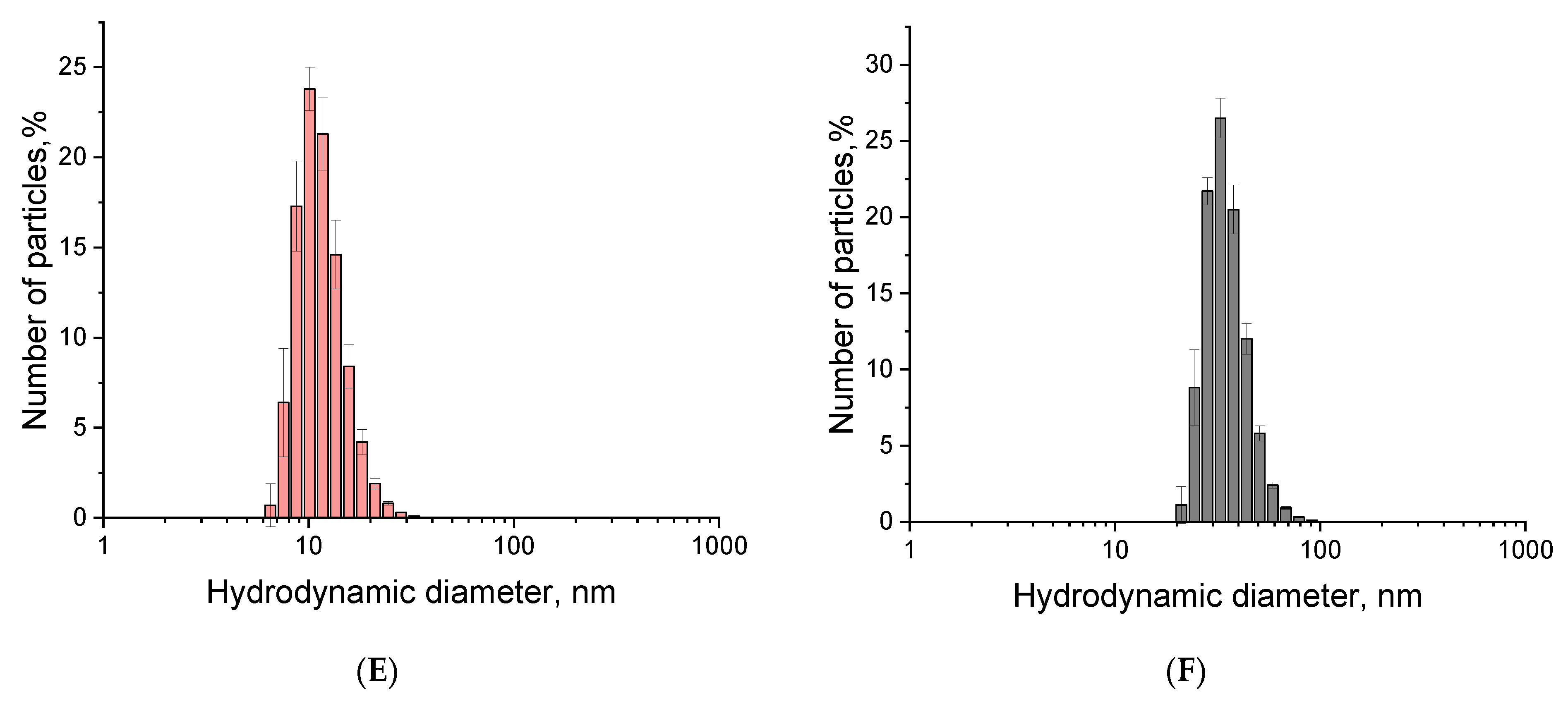
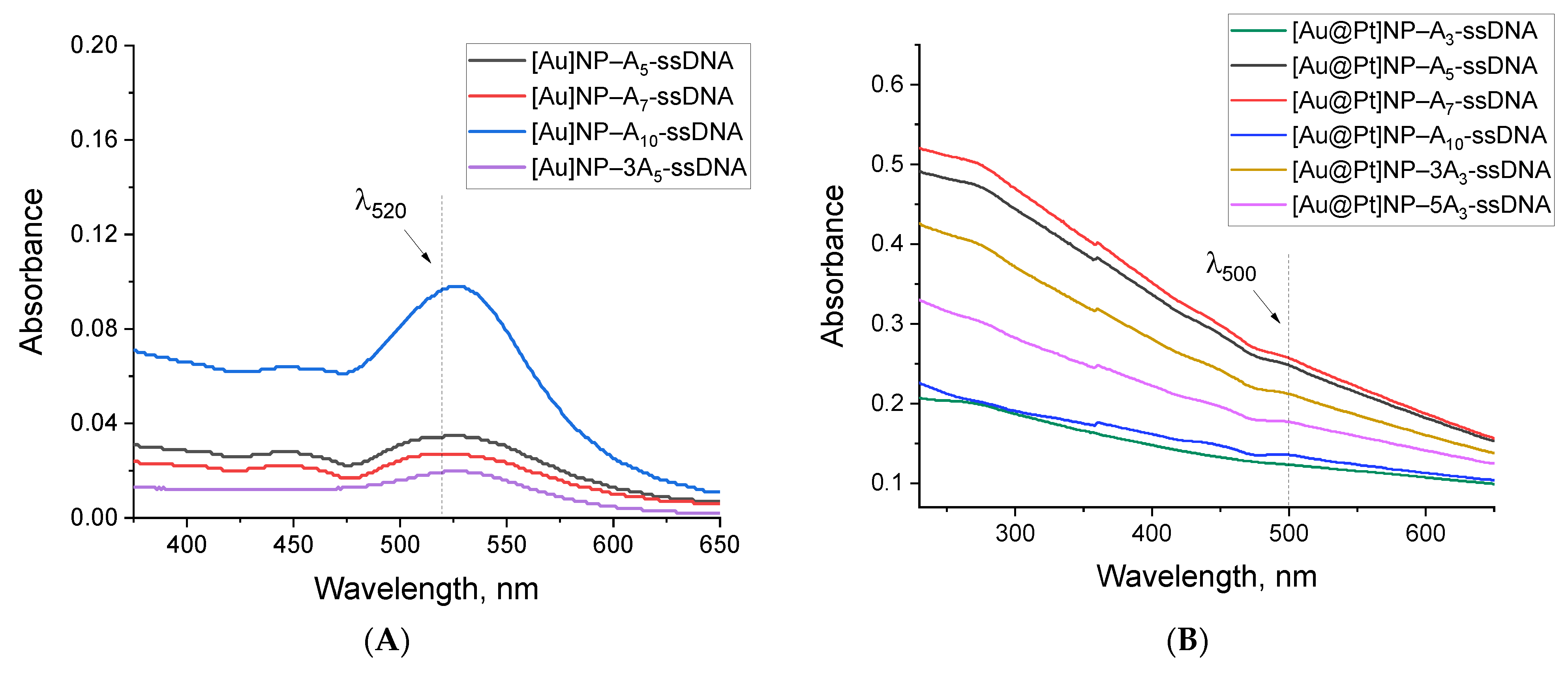
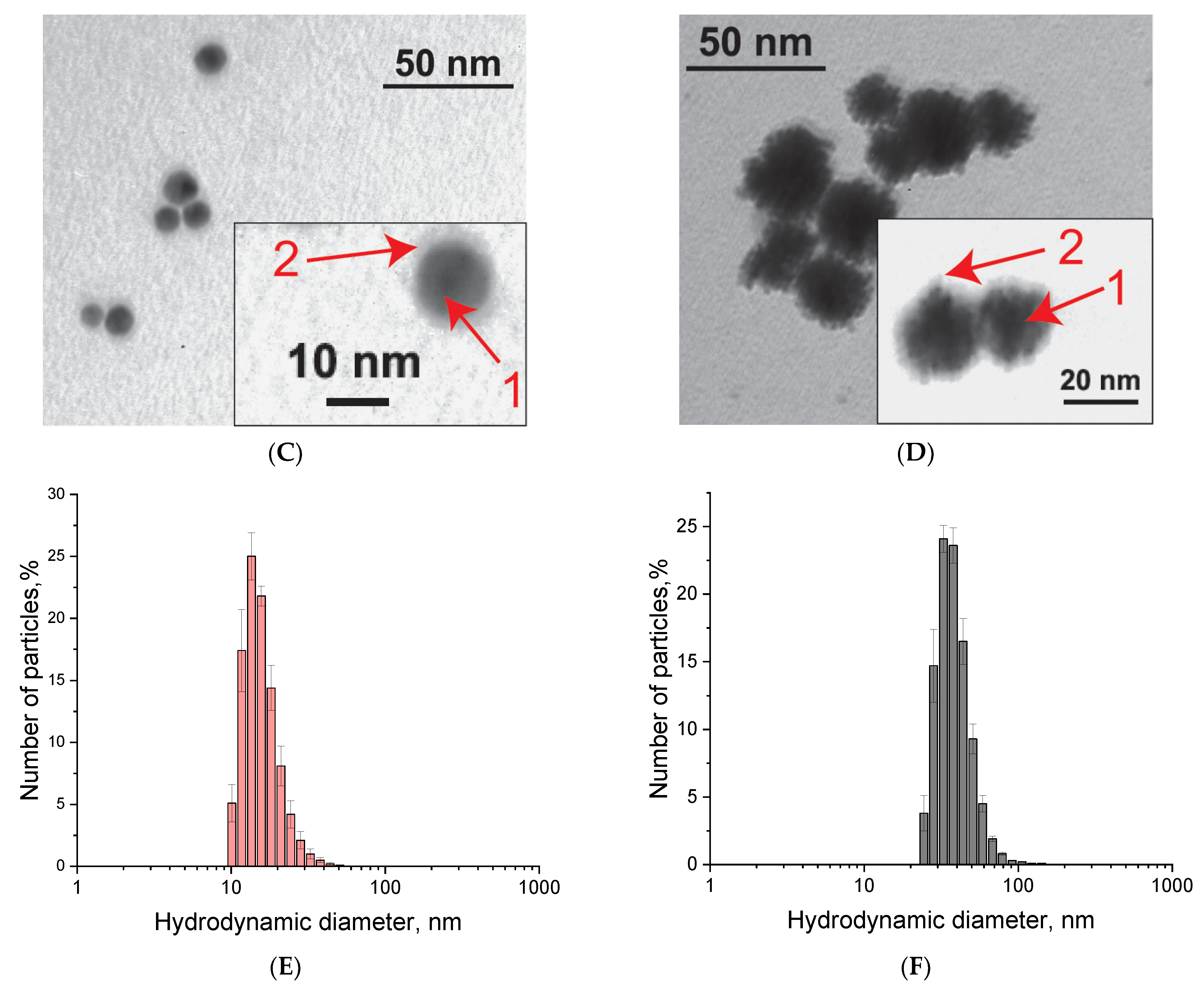
2.3. Synthesis and Characterization of Nanoparticle–Oligonucleotide Conjugates
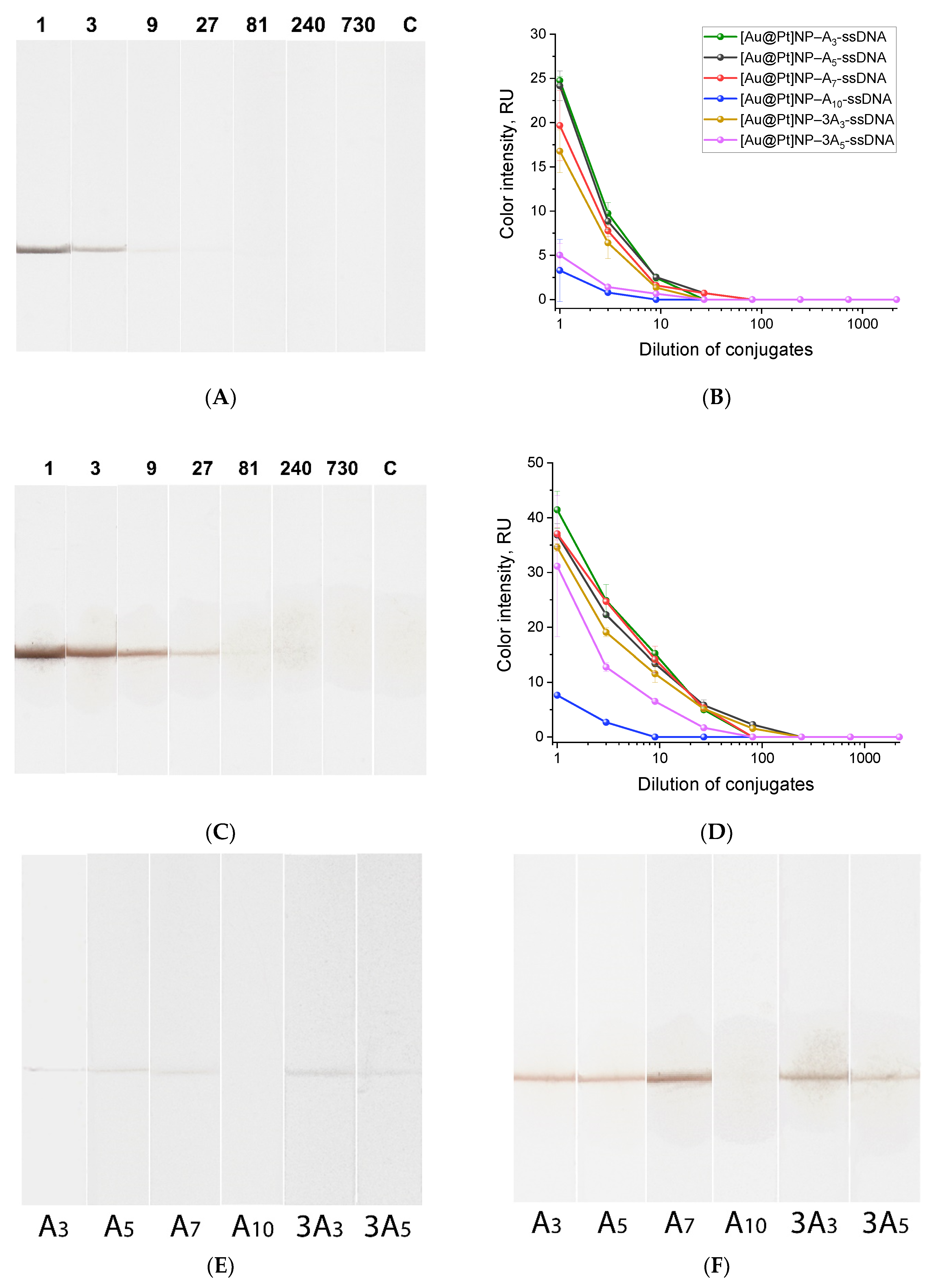
2.4. Comparison of the Conjugates Using Lateral Flow Test Strips
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of Gold Nanoparticles
3.3. Preparation of Bimetallic Au@Pt Nanoparticles
3.4. Nanoparticle Characterization
3.5. Synthesis of Oligonucleotide–Nanoparticle Conjugates
3.6. Fabrication of Lateral Flow Test Strips
3.7. Conjugate Characterization
3.8. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [PubMed]
- Huang, Y.Y.; Ren, J.S.; Qu, X.G. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.; Stevens, M.M. The nanozyme revolution: Enhancing the performance of medical biosensing platforms. Adv. Mater. 2023, 36, 2300184. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, K.; Cheng, N. Recent advances in nanozyme-mediated strategies for pathogen detection and control. Int. J. Mol. Sci. 2023, 24, 13342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, L.; Zhang, K. Breakthroughs in nanozyme-inspired application diversity. Mater. Chem. Front. 2023, 7, 44–64. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.-N.; Liang, X.; Sun, H.-b.; Xin, H.; Liang, Q. Recent Advances in Nanozymes: From Matters to Bioapplications. Adv. Funct. Mater. 2022, 32, 2110432. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Fu, R.; Ma, Z.; Zhao, H.; Jin, H.; Tang, Y.; He, T.; Ding, Y.; Zhang, J.; Ye, D. Research Progress in Iron-Based Nanozymes: Catalytic Mechanisms, Classification, and Biomedical Applications. Anal. Chem. 2023, 95, 10844–10858. [Google Scholar] [CrossRef]
- Jin, C.; Fan, S.; Zhuang, Z.; Zhou, Y. Single-atom nanozymes: From bench to bedside. Nano Res. 2023, 16, 1992–2002. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, B.; Pan, X.; Wang, H.; Wu, Q.; Li, S.; Jiang, B.; Liu, H. Carbon-based nanozymes: Design, catalytic mechanism, and bioapplication. Coord. Chem. Rev. 2023, 475, 214896. [Google Scholar] [CrossRef]
- Cursi, L.; Mirra, G.; Boselli, L.; Pompa, P.P. Metrology of platinum nanozymes: Mechanistic insights and analytical issues. Adv. Funct. Mater. 2024, 34, 2315587. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Tian, X.; Jiang, Y.; Ma, H.-Y.; Wang, W.; Zhang, W.-S.; Martin, J.S.; Yan, Y.; Qin, D.-D.; Han, D.-X.; et al. Advances in bimetallic materials and bimetallic oxide nanozymes: Synthesis, classification, catalytic mechanism and application in analytical chemistry. TrAC Trends Anal. Chem. 2024, 176, 117757. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, H.; Wu, S.; Zhang, J.; Cheng, Y.; Lei, L.; Qin, Y.; Wei, H.; Yu, C.-Y. Spherical nucleic acids: Emerging amplifiers for therapeutic nanoplatforms. Nanoscale 2024, 16, 4392–4406. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, S.; Lu, Y. From structure to application: The evolutionary trajectory of spherical nucleic acids. Small 2024, 2310026. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, J.; Wang, E. DNA-encoded nanomaterials with controllable properties. Anal. Sens. 2023, 3, e202200067. [Google Scholar] [CrossRef]
- Lei, Y.; He, X.; Zeng, Y.; Wang, X.; Yang, L.; Liu, X.; Qing, Z. Pt–S bond stabilized DNAzyme nanosensor with thiol-resistance enabling high-fidelity biosensing. Talanta 2024, 276, 126187. [Google Scholar] [CrossRef]
- Qing, Z.; Luo, G.; Xing, S.; Zou, Z.; Lei, Y.; Liu, J.; Yang, R. Pt–S Bond-Mediated Nanoflares for High-Fidelity Intracellular Applications by Avoiding Thiol Cleavage. Angew. Chem. Int. Ed. 2020, 59, 14044–14048. [Google Scholar] [CrossRef]
- Chen, G.; Jin, M.; Yan, M.; Cui, X.; Wang, Y.; Zheng, W.; Qin, G.; Zhang, Y.; Li, M.; Liao, Y.; et al. Colorimetric bio-barcode immunoassay for parathion based on amplification by using platinum nanoparticles acting as a nanozyme. Mikrochim. Acta 2019, 186, 339. [Google Scholar] [CrossRef]
- Shao, N.; Han, X.; Song, Y.; Zhang, P.; Qin, L. CRISPR-Cas12a Coupled with Platinum Nanoreporter for Visual Quantification of SNVs on a Volumetric Bar-Chart Chip. Anal. Chem. 2019, 91, 12384–12391. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric in Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-Induced Hot Carriers in Metallic Nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, W.; Wang, M.; Zhang, L.; Li, P. Nanozyme-assisted amplification-free CRISPR/Cas system realizes visual detection. Front. Bioeng. Biotechnol. 2023, 11, 1327498. [Google Scholar] [CrossRef] [PubMed]
- Zare, I.; Choi, D.; Zhang, J.; Yaraki, M.T.; Ghaee, A.; Nasab, S.Z.; Taheri-Ledari, R.; Maleki, A.; Rahi, A.; Fan, K.; et al. Modulating the catalytic activities of nanozymes for molecular sensing. Nano Today 2024, 56, 102276. [Google Scholar] [CrossRef]
- Tao, Y.; Yi, K.; Wang, H.; Kim, H.-W.; Li, K.; Zhu, X.; Li, M. CRISPR-Cas12a-regulated DNA adsorption and metallization on MXenes as enhanced enzyme mimics for sensitive colorimetric detection of hepatitis B virus DNA. J. Colloid. Interf. Sci. 2022, 613, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Pebdeni, A.; Hosseini, M. Fast and selective whole cell detection of Staphylococcus aureus bacteria in food samples by paper based colorimetric nanobiosensor using peroxidase-like catalytic activity of DNA-Au/Pt bimetallic nanoclusters. Microchem. J. 2020, 159, 105475. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Luo, G.; Jiao, J. DNA-functionalized gold nanoparticles: Modification, characterization, and biomedical applications. Front. Chem. 2022, 10, 1095488. [Google Scholar] [CrossRef]
- He, Q.; Wu, Q.; Feng, X.; Liao, Z.; Peng, W.; Liu, Y.; Peng, D.; Liu, Z.; Mo, M. Interfacing DNA with nanoparticles: Surface science and its applications in biosensing. Int. J. Biol. Macromol. 2020, 151, 757–780. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Methods for preparing DNA-functionalized gold nanoparticles, a key reagent of bioanalytical chemistry. Anal. Methods 2017, 9, 2633–2643. [Google Scholar] [CrossRef]
- Shang, Z.; Deng, Z.; Yi, X.; Yang, M.; Nong, X.; Lin, M.; Xia, F. Construction and bioanalytical applications of poly-adenine-mediated gold nanoparticle-based spherical nucleic acids. Anal. Methods 2023, 15, 5564–5576. [Google Scholar] [CrossRef]
- Kushalkar, M.P.; Liu, B.; Liu, J. Promoting DNA adsorption by acids and polyvalent cations: Beyond charge screening. Langmuir 2020, 36, 11183–11195. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Li, Y.; Huang, K.; Cheng, N. Towards rational design: Developing universal freezing routes for anchoring DNA onto gold nanoparticles. J. Colloid. Interf. Sci. 2024, 655, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Yi, X.; Xiao, Y.; Zhang, J.; Lin, M.; Xia, F. Programming the dynamic range of nanobiosensors with engineering poly-adenine-mediated spherical nucleic acid. Talanta 2023, 256, 124278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, J.; Du, Y.; Zhou, X.; Liang, H.; Jiang, W. Enhancing the stability of single-stranded DNA on gold nanoparticles as molecular machines through salt and acid regulation. J. Mat. Chem. B 2019, 7, 5554–5562. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, Z.; An, H.; Yang, E.; Wu, M.; Huang, Z.; Duan, Y. Poly-adenine regulated DNA density on AuNPs to construct efficient DNA walker for microRNA-21 detection. Talanta 2020, 217, 121056. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, P.A.; Ramasanoff, R.R.; Gabrusenok, P.V.; Baryshev, A.V.; Kasyanenko, N.A. Hybridization-driven adsorption of polyadenine DNA onto gold nanoparticles. Langmuir 2022, 38, 15776–15781. [Google Scholar] [CrossRef]
- Toubanaki, D.K.; Karagouni, E. Oligonucleotide-conjugated gold nanoparticles for application on lateral flow biosensors: Evaluation and optimization of low pH and salt-aging conjugation methods. Anal. Lett. 2016, 49, 2833–2850. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y.; Song, L.; Deng, Z. Flash synthesis of spherical nucleic acids with record DNA density. J. Am. Chem. Soc. 2021, 143, 3065–3069. [Google Scholar] [CrossRef]
- Huang, M.; Xiong, E.; Wang, Y.; Hu, M.; Yue, H.; Tian, T.; Zhu, D.; Liu, H.; Zhou, X. Fast microwave heating-based one-step synthesis of DNA and RNA modified gold nanoparticles. Nat. Commun. 2022, 13, 968. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Freezing directed construction of bio/nano interfaces: Reagentless conjugation, denser spherical nucleic acids, and better nanoflares. J. Am. Chem. Soc. 2017, 139, 9471–9474. [Google Scholar] [CrossRef]
- Dong, H.; Zhong, L.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Guo, Y.; Kawasaki, H. A study on the PEG-assisted stability of spherical nucleic acid constructed by the freezing method. Coll. Surf. A 2024, 686, 133349. [Google Scholar] [CrossRef]
- Ye, Y.; Hou, S.; Wu, X.; Cheng, X.; He, S. Freeze-driven adsorption of poly-A DNA on gold nanoparticles: From a stable biointerface to plasmonic dimers. Langmuir 2022, 38, 4625–4632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Zhang, S.; Deng, L. An ultrasensitive fluorescence detection template of pathogenic bacteria based on dual catalytic hairpin DNA walker@gold nanoparticles enzyme-free amplification. Spectrochim. Acta A 2022, 277, 121259. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yuan, C.; Tian, T.; Wang, X.; Sun, J.; Xiong, E.; Zhou, X. Single-step, salt-aging-free, and thiol-free freezing construction of AuNP-based bioprobes for advancing CRISPR-based diagnostics. J. Am. Chem. Soc. 2020, 142, 7506–7513. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, T.; Huang, Z.; Liu, Y.; Liu, J. Freezing-directed stretching and alignment of DNA oligonucleotides. Angew. Chem. Int. Ed. 2019, 58, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Li, X.; Deng, J.; Chen, W.; Zou, Z.; Chen, K.; Wu, L.; Liu, J.; Qing, Z. Pt-Decorated gold nanoflares for high-fidelity phototheranostics: Reducing side-effects and enhancing cytotoxicity toward target cells. Angew. Chem. Int. Ed. 2024, 63, e202402881. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, W.; Zhang, Y.; Zheng, S.; Liao, J.; Shan, H.; Tian, B.; Wu, T.; Zhang, L.; Tu, Z.; et al. Simple and rapid co-freezing construction of SERS signal probes for the sensitive detection of pathogens. Chem. Eng. J. 2023, 466, 143066. [Google Scholar] [CrossRef]
- Kimura-Suda, H.; Petrovykh, D.Y.; Tarlov, M.J.; Whitman, L.J. Base-dependent competitive adsorption of single-stranded DNA on gold. J. Am. Chem. Soc. 2003, 125, 9014–9015. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Interface-driven hybrid materials based on DNA-functionalized gold nanoparticles. Matter 2019, 1, 825–847. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Urchin peroxidase-mimicking Au@Pt nanoparticles as a label in lateral flow immunoassay: Impact of nanoparticle composition on detection limit of Clavibacter michiganensis. Microchim. Acta 2020, 187, 268. [Google Scholar] [CrossRef]
- Tymchenko, E.; Glova, V.; Soldatova, A.; Chikhirzhina, E.; Polyanichko, A. FTIR Study of the Secondary Structure of DNA in Complexes with Platinum Coordination Compounds. J. Phys. Conf. Ser. 2019, 1400, 033004. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. The steadfast Au@Pt soldier: Peroxide-tolerant nanozyme for signal enhancement in lateral flow immunoassay of peroxidase-containing samples. Talanta 2021, 225, 121961. [Google Scholar] [CrossRef] [PubMed]

| Name | Sequence 5′-3′ |
|---|---|
| A3-ssDNA | AAACCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM * |
| A5-ssDNA | AAAAACCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM |
| A7-ssDNA | AAAAAAACCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM |
| A10-ssDNA | AAAAAAAAAACCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM |
| 3A3-ssDNA | (AAA)3[TREBLER]CCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM ** |
| 3A5-ssDNA | (AAAAA)3[TREBLER]CCTCCAAGAGTTAGATCATACAGTTTTTTT-FAM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapshinov, N.E.; Pridvorova, S.M.; Zherdev, A.V.; Dzantiev, B.B.; Safenkova, I.V. Freeze-Driven Adsorption of Oligonucleotides with polyA-Anchors on Au@Pt Nanozyme. Int. J. Mol. Sci. 2024, 25, 10108. https://doi.org/10.3390/ijms251810108
Lapshinov NE, Pridvorova SM, Zherdev AV, Dzantiev BB, Safenkova IV. Freeze-Driven Adsorption of Oligonucleotides with polyA-Anchors on Au@Pt Nanozyme. International Journal of Molecular Sciences. 2024; 25(18):10108. https://doi.org/10.3390/ijms251810108
Chicago/Turabian StyleLapshinov, Nikita E., Svetlana M. Pridvorova, Anatoly V. Zherdev, Boris B. Dzantiev, and Irina V. Safenkova. 2024. "Freeze-Driven Adsorption of Oligonucleotides with polyA-Anchors on Au@Pt Nanozyme" International Journal of Molecular Sciences 25, no. 18: 10108. https://doi.org/10.3390/ijms251810108
APA StyleLapshinov, N. E., Pridvorova, S. M., Zherdev, A. V., Dzantiev, B. B., & Safenkova, I. V. (2024). Freeze-Driven Adsorption of Oligonucleotides with polyA-Anchors on Au@Pt Nanozyme. International Journal of Molecular Sciences, 25(18), 10108. https://doi.org/10.3390/ijms251810108









