Tuberculous Pleural Effusion-Derived Exosomal miR-130b-3p and miR-423-5p Promote the Proliferation of Lung Cancer Cells via Cyclin D1
Abstract
1. Introduction
2. Results
2.1. TPE-Derived Exosomes Induce the Invasion of Lung Cancer Cells
2.2. Identifying Exosomal miRNAs from TPE Involved in the Invasion of Lung Cancer Cells
2.3. GW4869 Inhibits the Migration and Invasion of TPE-Derived Exosome-Stimulated A549 Cells
2.4. Exosome-Mediated Transfer miR-130 and miR-423 from TPE Promotes the Invasion of Cancer Cells via Cyclin D
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Exosome Isolation and Quantification
4.3. Transmission Electron Microscopy
4.4. Small RNA Library Construction and miRNA Sequencing
4.5. Animal Experiments
4.6. Cell Culture and Reagent Treatment
4.7. Cellular Internalization of Exosomes
4.8. Cell Migration and Invasion Assays
4.9. Extraction of miRNA, Real-Time PCR, and Western Blotting
4.10. Transfection of miRNA Inhibitors
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, Y.; Zou, Y.Q.; Chen, X.; Huang, B.; Liu, J.; Xu, Y.M.; Li, J.; Zhang, J.; Yang, W.M.; et al. Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumour Biol. 2016, 37, 15835–15845. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shin, S.; Lee, K.A. Exosome-based detection of EGFR T790M in plasma and pleural fluid of prospectively enrolled non-small cell lung cancer patients after first-line tyrosine kinase inhibitor therapy. Cancer Cell Int. 2021, 21, 50. [Google Scholar] [CrossRef]
- Ahmadzada, T.; Kao, S.; Reid, G.; Clarke, S.; Grau, G.E.; Hosseini-Beheshti, E. Extracellular vesicles as biomarkers in malignant pleural mesothelioma: A review. Crit. Rev. Oncol. Hematol. 2020, 150, 102949. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhou, X.; Roizman, B.; Zhou, G.G. miRNAs Targeting ICP4 and Delivered to Susceptible Cells in Exosomes Block HSV-1 Replication in a Dose-Dependent Manner. Mol. Ther. 2018, 26, 1032–1039. [Google Scholar] [CrossRef]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020, 145, 102860. [Google Scholar] [CrossRef]
- Woodman, C.; Vundu, G.; George, A.; Wilson, C.M. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin. Cancer Biol. 2021, 69, 349–364. [Google Scholar] [CrossRef]
- Garcia, J.I.; Mambuque, E.; Nguenha, D.; Vilanculo, F.; Sacoor, C.; Sequera, V.G.; Fernandez-Quevedo, M.; Pierre, M.L.; Chiconela, H.; Faife, L.A.; et al. Mortality and risk of tuberculosis among people living with HIV in whom TB was initially ruled out. Sci. Rep. 2020, 10, 15442. [Google Scholar] [CrossRef]
- Luczynski, P.; Poulin, P.; Romanowski, K.; Johnston, J.C. Tuberculosis and risk of cancer: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0278661. [Google Scholar] [CrossRef] [PubMed]
- Abdeahad, H.; Salehi, M.; Yaghoubi, A.; Aalami, A.H.; Aalami, F.; Soleimanpour, S. Previous pulmonary tuberculosis enhances the risk of lung cancer: Systematic reviews and meta-analysis. Infect. Dis. 2022, 54, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.M.; Lee, C.S.; Wu, Y.C.; Shu, C.C.; Ho, C.H. Prior treated tuberculosis and mortality risk in lung cancer. Front. Med. 2023, 10, 1121257. [Google Scholar] [CrossRef]
- Nalbandian, A.; Yan, B.S.; Pichugin, A.; Bronson, R.T.; Kramnik, I. Lung carcinogenesis induced by chronic tuberculosis infection: The experimental model and genetic control. Oncogene 2009, 28, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Kim, Y.; Jung, H.; Lee, J.J.; Hong, J.Y. Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4-Autophagy Axis. Cancers 2021, 13, 687. [Google Scholar] [CrossRef]
- Woo, S.J.; Kim, Y.; Kang, H.J.; Jung, H.; Youn, D.H.; Hong, Y.; Lee, J.J.; Hong, J.Y. Tuberculous pleural effusion-induced Arg-1(+) macrophage polarization contributes to lung cancer progression via autophagy signaling. Respir. Res. 2024, 25, 198. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Louloupi, A.; Ntini, E.; Liz, J.; Orom, U.A. Microprocessor dynamics shows co- and post-transcriptional processing of pri-miRNAs. RNA 2017, 23, 892–898. [Google Scholar] [CrossRef]
- Vanni, I.; Alama, A.; Grossi, F.; Dal Bello, M.G.; Coco, S. Exosomes: A new horizon in lung cancer. Drug Discov. Today 2017, 22, 927–936. [Google Scholar] [CrossRef]
- Goldie, B.J.; Dun, M.D.; Lin, M.; Smith, N.D.; Verrills, N.M.; Dayas, C.V.; Cairns, M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014, 42, 9195–9208. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The role of Exosomal miRNAs in cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Alix-Panabieres, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, P.P.; De Falco, V.; Giunta, E.F.; Ciardiello, D.; Cardone, C.; Vitale, P.; Zanaletti, N.; Borrelli, C.; Poliero, L.; Terminiello, M.; et al. Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience. Cancers 2019, 11, 1504. [Google Scholar] [CrossRef]
- Holla, S.; Ghorpade, D.S.; Singh, V.; Bansal, K.; Balaji, K.N. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-alpha-induced apoptosis. Mol. Cancer 2014, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Tripathi, D.; Kulkarni, S.; Rajan, M.G. Mycobacterium tuberculosis H37Rv infected THP-1 cells induce epithelial mesenchymal transition (EMT) in lung adenocarcinoma epithelial cell line (A549). Cell Immunol. 2016, 300, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Antony, V.B. Immunological mechanisms in pleural disease. Eur. Respir. J. 2003, 21, 539–544. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, Y.; Gu, L.; Jian, Z.; Stary, C.M.; Xiong, X. Extracellular vesicle-derived miRNA as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis. J. Transl. Med. 2021, 19, 202. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal 2023, 21, 77. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.; Sun, L.; Yan, Q.; Zhang, Y.; Zhang, Z.; Su, Y.; Wang, C. MicroRNA-130b targets PTEN to induce resistance to cisplatin in lung cancer cells by activating Wnt/beta-catenin pathway. Cell Biochem. Funct. 2018, 36, 194–202. [Google Scholar] [CrossRef]
- Sekino, Y.; Sakamoto, N.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. MiR-130b Promotes Sunitinib Resistance through Regulation of PTEN in Renal Cell Carcinoma. Oncology 2019, 97, 164–172. [Google Scholar] [CrossRef]
- Miao, Y.; Zheng, W.; Li, N.; Su, Z.; Zhao, L.; Zhou, H.; Jia, L. MicroRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3K/Akt signaling pathway. Sci. Rep. 2017, 7, 41942. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Lei, B.; Gao, H.; Jia, L.; Luo, D.; Han, J.; Jia, B. MiR-130b suppresses the invasion and migration of prostate cancer via inhibiting DLL1 and regulating the PI3K/Akt pathways. Exp. Ther. Med. 2022, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Sharova, E.; Grassi, A.; Marcer, A.; Ruggero, K.; Pinto, F.; Bassi, P.; Zanovello, P.; Zattoni, F.; D’Agostino, D.M.; Iafrate, M.; et al. A circulating miRNA assay as a first-line test for prostate cancer screening. Br. J. Cancer 2016, 114, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, T.; Fucci, A.; Votino, C.; Sabatino, L.; Pancione, M.; Laudanna, C.; Binaschi, M.; Bigioni, M.; Maggi, C.A.; Parente, D.; et al. MicroRNA-130b promotes tumor development and is associated with poor prognosis in colorectal cancer. Neoplasia 2013, 15, 1086–1099. [Google Scholar] [CrossRef]
- Jia, W.; Yu, T.; An, Q.; Cao, X.; Pan, H. MicroRNA-423-5p inhibits colon cancer growth by promoting caspase-dependent apoptosis. Exp. Ther. Med. 2018, 16, 1225–1231. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, G. MiR-423-5p aggravates lung adenocarcinoma via targeting CADM1. Thorac. Cancer 2021, 12, 210–217. [Google Scholar] [CrossRef]
- Jorge, K.T.O.S.; Souza, R.P.; Assis, M.T.A.; Arújo, M.G.; Locati, M.; Jesus, A.M.R.; Baptista, I.M.F.D.; Lima, C.X.; Teixeira, A.L.; Teixeira, M.M.; et al. Characterization of MicroRNA Expression Profiles and Identification of Potential Biomarkers in Leprosy. J. Clin. Microbiol. 2017, 55, 1516–1525. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef]
- Bandi, N.; Zbinden, S.; Gugger, M.; Arnold, M.; Kocher, V.; Hasan, L.; Kappeler, A.; Brunner, T.; Vassella, E. MiR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009, 69, 5553–5559. [Google Scholar] [CrossRef] [PubMed]
- Bonci, D.; Coppola, V.; Musumeci, M.; Addario, A.; Giuffrida, R.; Memeo, L.; D’Urso, L.; Pagliuca, A.; Biffoni, M.; Labbaye, C.; et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008, 14, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Krappmann, D.; Eichten, A.; Heder, A.; Scheidereit, C.; Strauss, M. NF-kappaB function in growth control: Regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell Biol. 1999, 19, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Zhao, X.; Li, Y.; Yu, L.; Li, Y.; Zhou, X.; Gong, Q. miR-423 Promotes Breast Cancer Invasion by Activating NF-kappaB Signaling. OncoTargets Ther. 2020, 13, 5467–5478. [Google Scholar] [CrossRef]
- Cui, X.; Kong, C.; Zhu, Y.; Zeng, Y.; Zhang, Z.; Liu, X.; Zhan, B.; Piao, C.; Jiang, Z. MiR-130b, an onco-miRNA in bladder cancer, is directly regulated by NF-kappaB and sustains NF-kappaB activation by decreasing Cylindromatosis expression. Oncotarget 2016, 7, 48547–48561. [Google Scholar] [CrossRef]
- Brock, M.; Haider, T.J.; Vogel, J.; Gassmann, M.; Speich, R.; Trenkmann, M.; Ulrich, S.; Kohler, M.; Huber, L.C. The hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int. J. Biochem. Cell Biol. 2015, 61, 129–137. [Google Scholar] [CrossRef]
- Lin, J.; Huang, S.; Wu, S.; Ding, J.; Zhao, Y.; Liang, L.; Tian, Q.; Zha, R.; Zhan, R.; He, X. MicroRNA-423 promotes cell growth and regulates G(1)/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis 2011, 32, 1641–1647. [Google Scholar] [CrossRef]
- Light, R.W.; Macgregor, M.I.; Luchsinger, P.C.; Ball, W.C., Jr. Pleural effusions: The diagnostic separation of transudates and exudates. Ann. Intern. Med. 1972, 77, 507–513. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
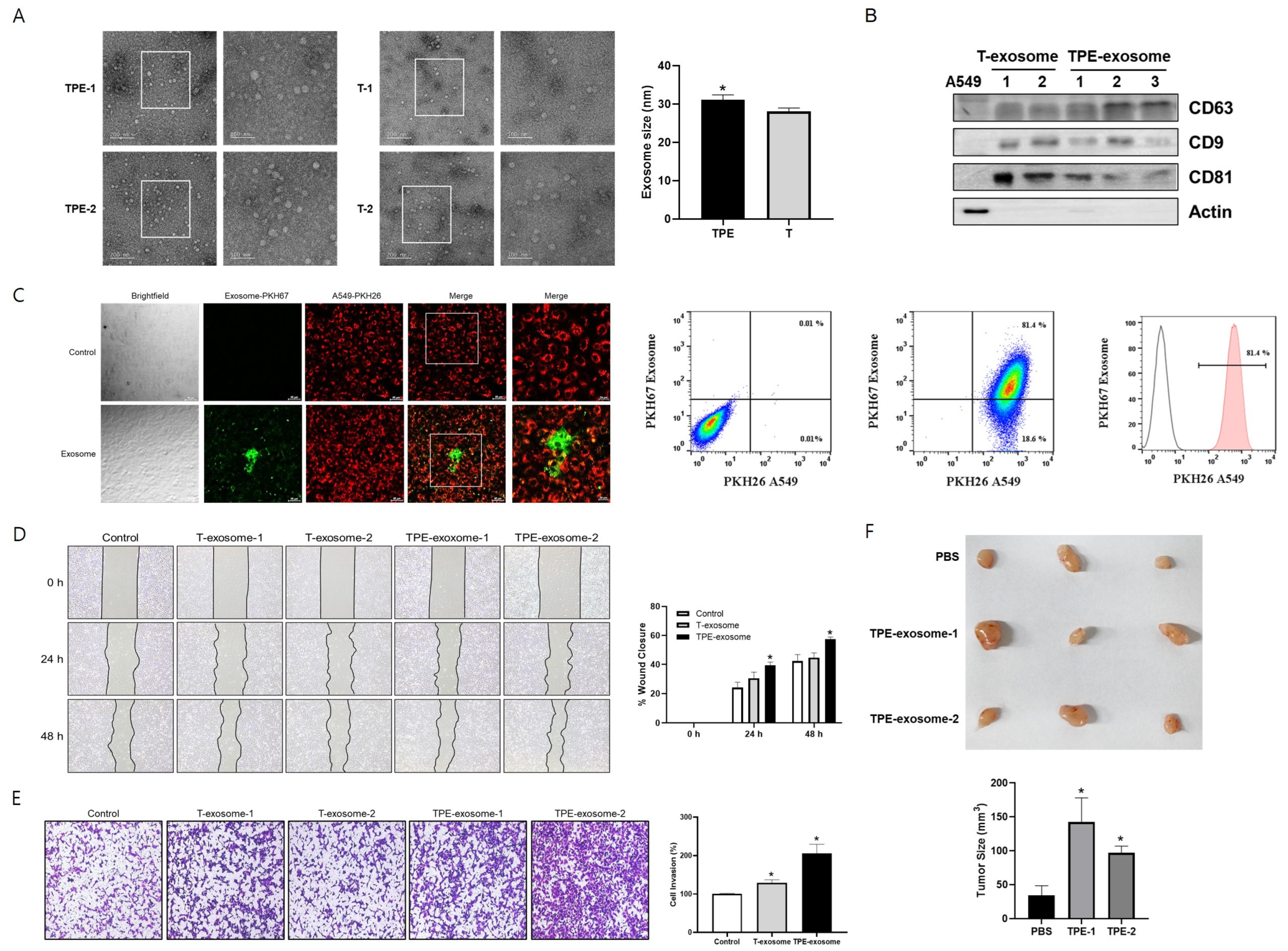
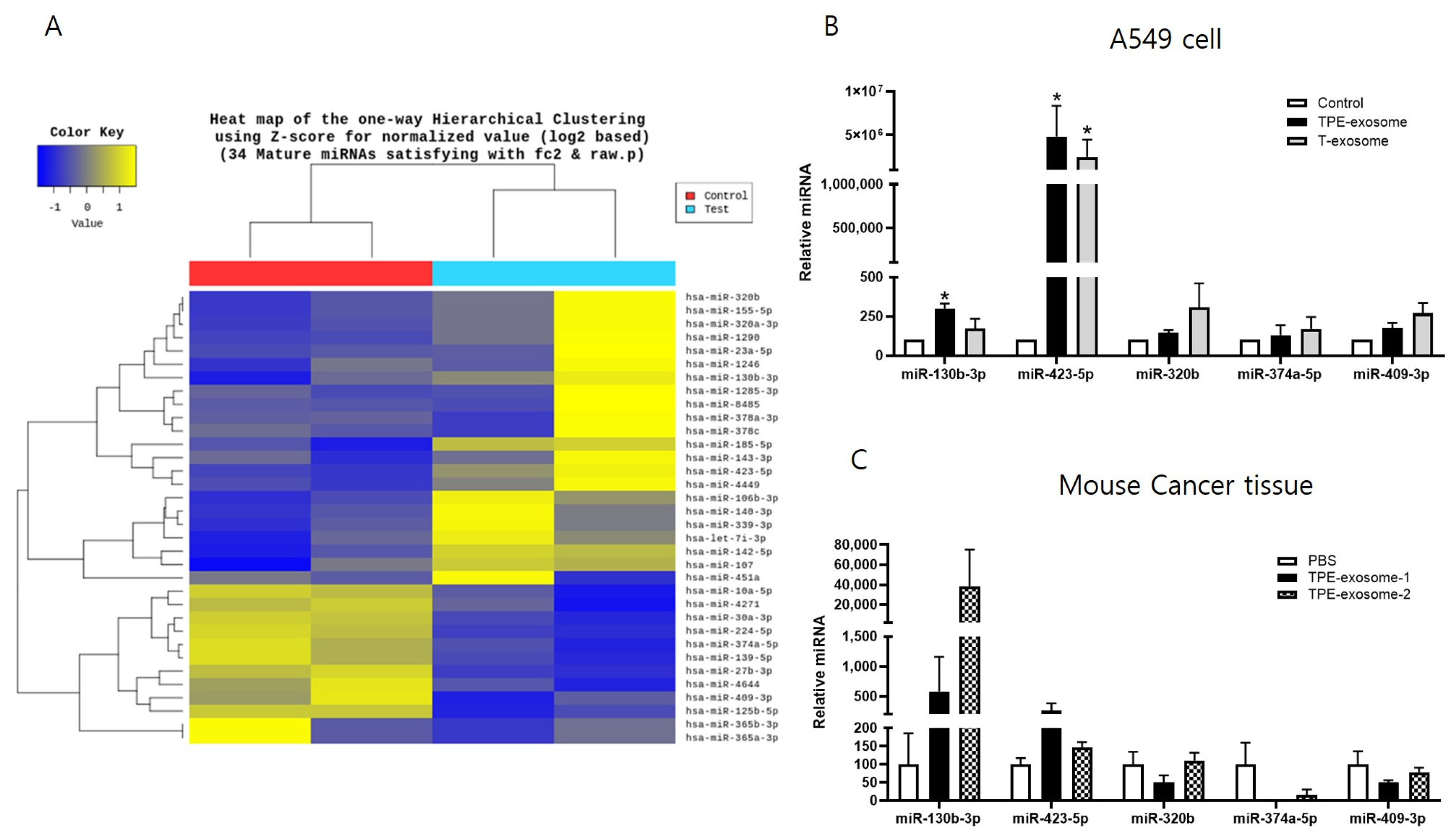
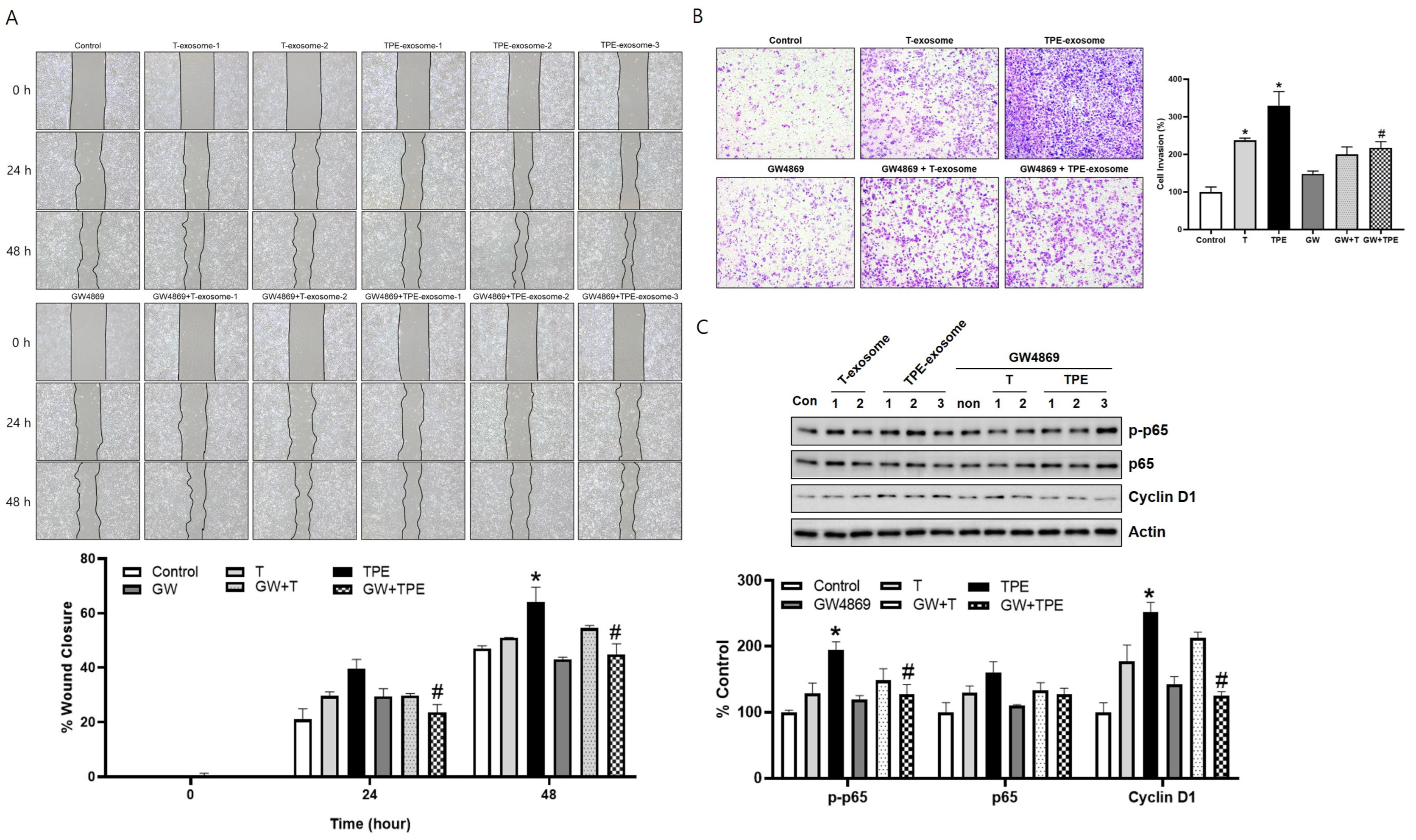
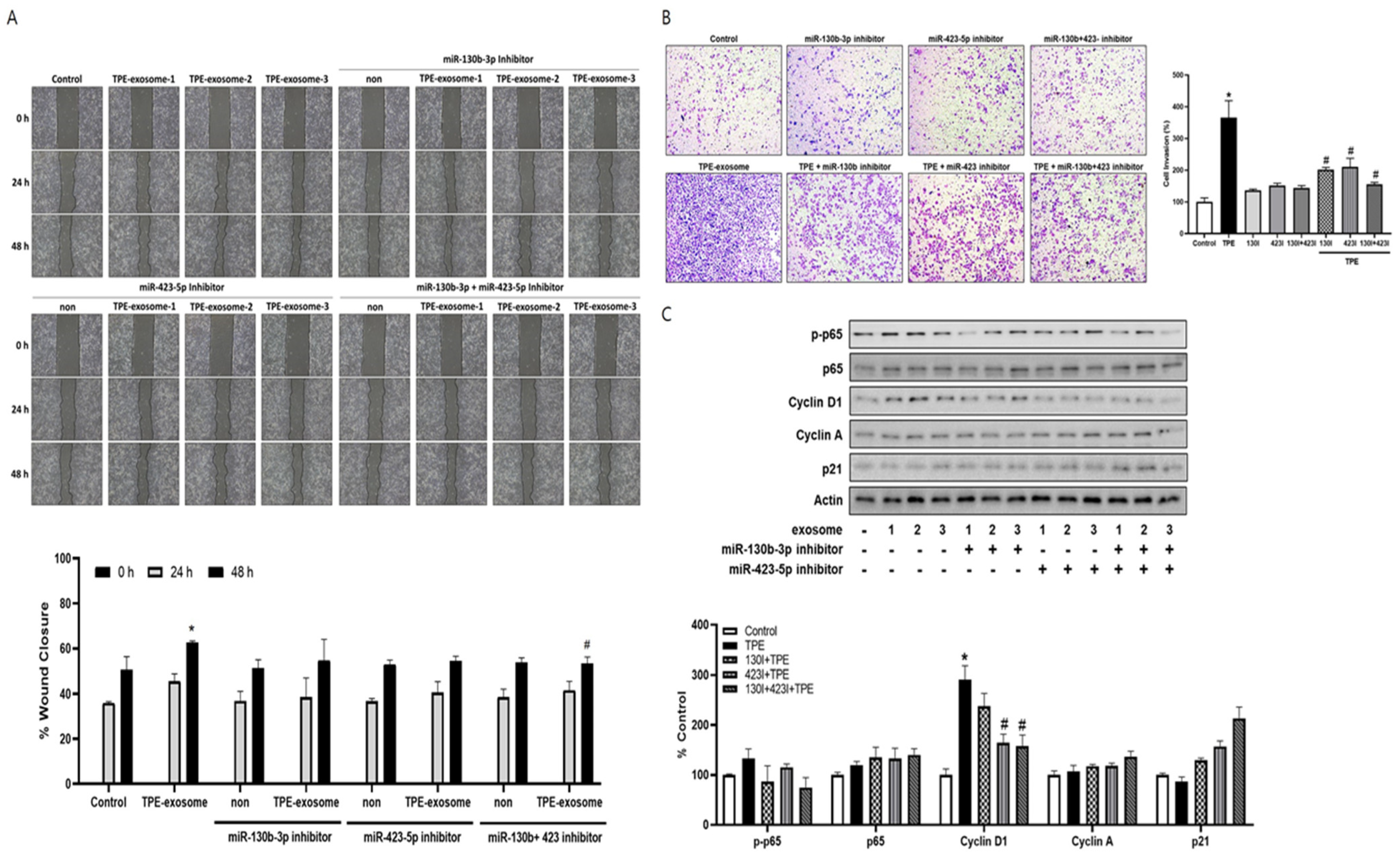
| Characteristics | TPE1 | TPE2 | T1 | T2 |
|---|---|---|---|---|
| Age | 23 | 77 | 51 | 51 |
| Sex | Male | Male | Male | Male |
| Comorbidity | None | HTN, CAOD | Cirrhosis, CKD | Wernicke’s encephalopathy |
| Pleural fluid analysis | ||||
| Leukocyte (/mm3) | 288 | 1530 | 576 | 46 |
| Total protein (g/dL) | 5.4 | 4.7 | 3 | <2.0 |
| Albumin | 3.6 | 2.5 | 1.8 | 1.2 |
| LDH (IU/L) | 1613 | 178 | 76 | 61 |
| CRP (mg/L) | 6.7 | 6.2 | <4.0 | 4.8 |
| ADA (IU/L) | 102.5 | 77.5 | 29 | 6 |
| Neutrophil (%) | 20 | 20 | 60 | 5 |
| Lymphocyte (%) | 80 | 80 | 40 | 95 |
| Serum | ||||
| Total protein (g/dL) | 7.1 | 6.4 | 6.5 | 5.2 |
| Albumin (g/dL) | 4.6 | 3.4 | 3.6 | 3.1 |
| LDH (IU/L) | 225 | 229 | 176 | 177 |
| Light criteria | ||||
| Pleural protein/serum protein > 0.5 | Yes | Yes | No | No |
| Pleural LDH/serum LDH > 0.6 | Yes | Yes | No | No |
| Pleural LDH > 2/3 of serum LDH | Yes | Yes | No | No |
| Positive pleural fluid TB culture | Yes | Yes | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.-J.; Yun, S.; Shin, S.-H.; Youn, D.H.; Son, G.-H.; Lee, J.J.; Hong, J.Y. Tuberculous Pleural Effusion-Derived Exosomal miR-130b-3p and miR-423-5p Promote the Proliferation of Lung Cancer Cells via Cyclin D1. Int. J. Mol. Sci. 2024, 25, 10119. https://doi.org/10.3390/ijms251810119
Kang H-J, Yun S, Shin S-H, Youn DH, Son G-H, Lee JJ, Hong JY. Tuberculous Pleural Effusion-Derived Exosomal miR-130b-3p and miR-423-5p Promote the Proliferation of Lung Cancer Cells via Cyclin D1. International Journal of Molecular Sciences. 2024; 25(18):10119. https://doi.org/10.3390/ijms251810119
Chicago/Turabian StyleKang, Hyun-Jung, Sangho Yun, Seung-Ho Shin, Dong Hyuk Youn, Ga-Hyun Son, Jae Jun Lee, and Ji Young Hong. 2024. "Tuberculous Pleural Effusion-Derived Exosomal miR-130b-3p and miR-423-5p Promote the Proliferation of Lung Cancer Cells via Cyclin D1" International Journal of Molecular Sciences 25, no. 18: 10119. https://doi.org/10.3390/ijms251810119
APA StyleKang, H.-J., Yun, S., Shin, S.-H., Youn, D. H., Son, G.-H., Lee, J. J., & Hong, J. Y. (2024). Tuberculous Pleural Effusion-Derived Exosomal miR-130b-3p and miR-423-5p Promote the Proliferation of Lung Cancer Cells via Cyclin D1. International Journal of Molecular Sciences, 25(18), 10119. https://doi.org/10.3390/ijms251810119






