Semen sEV tRF-Based Models Increase Non-Invasive Prediction Accuracy of Clinically Significant Prostate Cancer among Patients with Moderately Altered PSA Levels
Abstract
:1. Introduction
2. Results
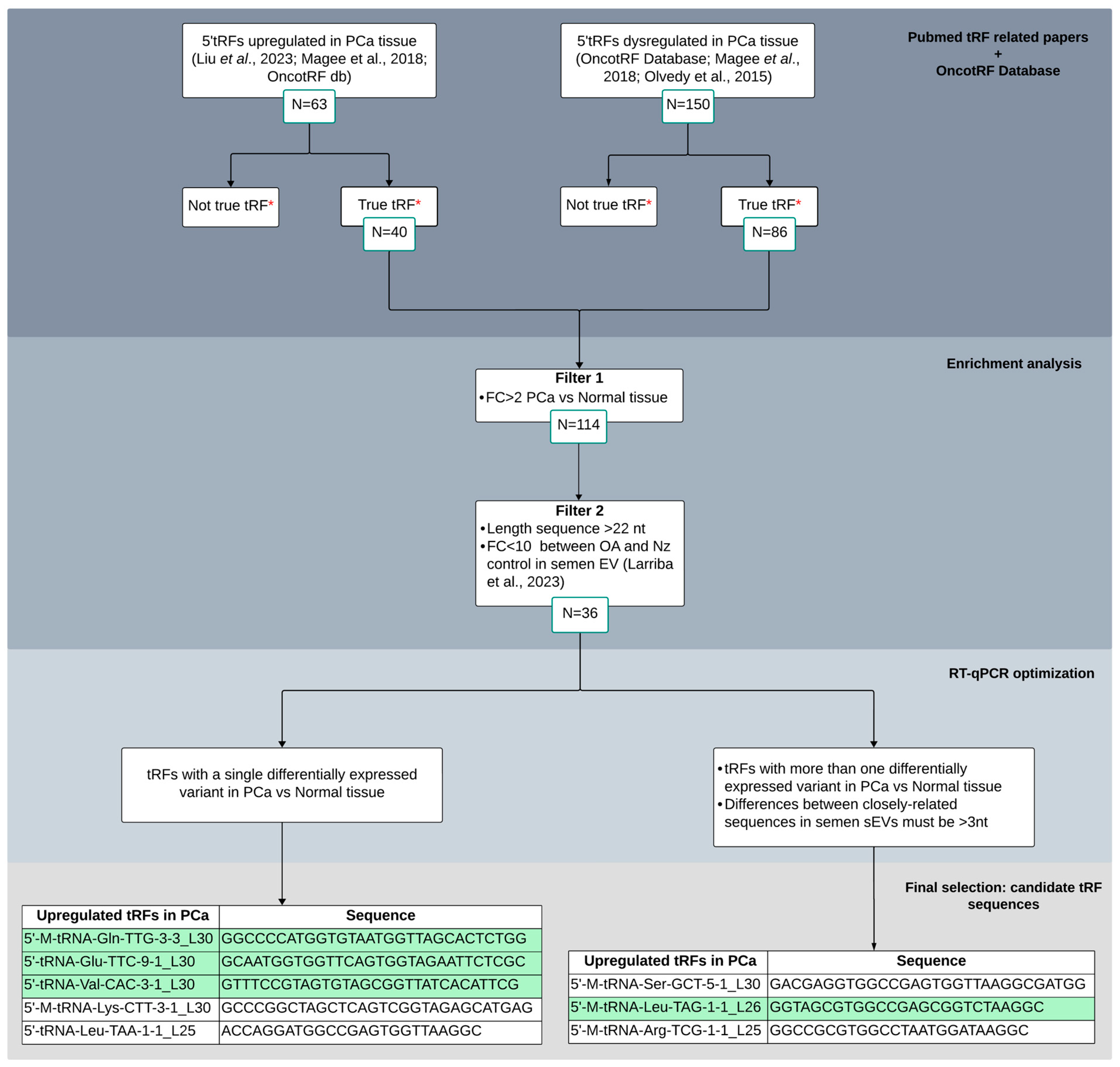
2.1. Selection of Candidate tRFs Differentially Expressed in PCa Tissue to Study in Semen sEVs
2.2. Clinical Assessment of the Individuals Included in the Study
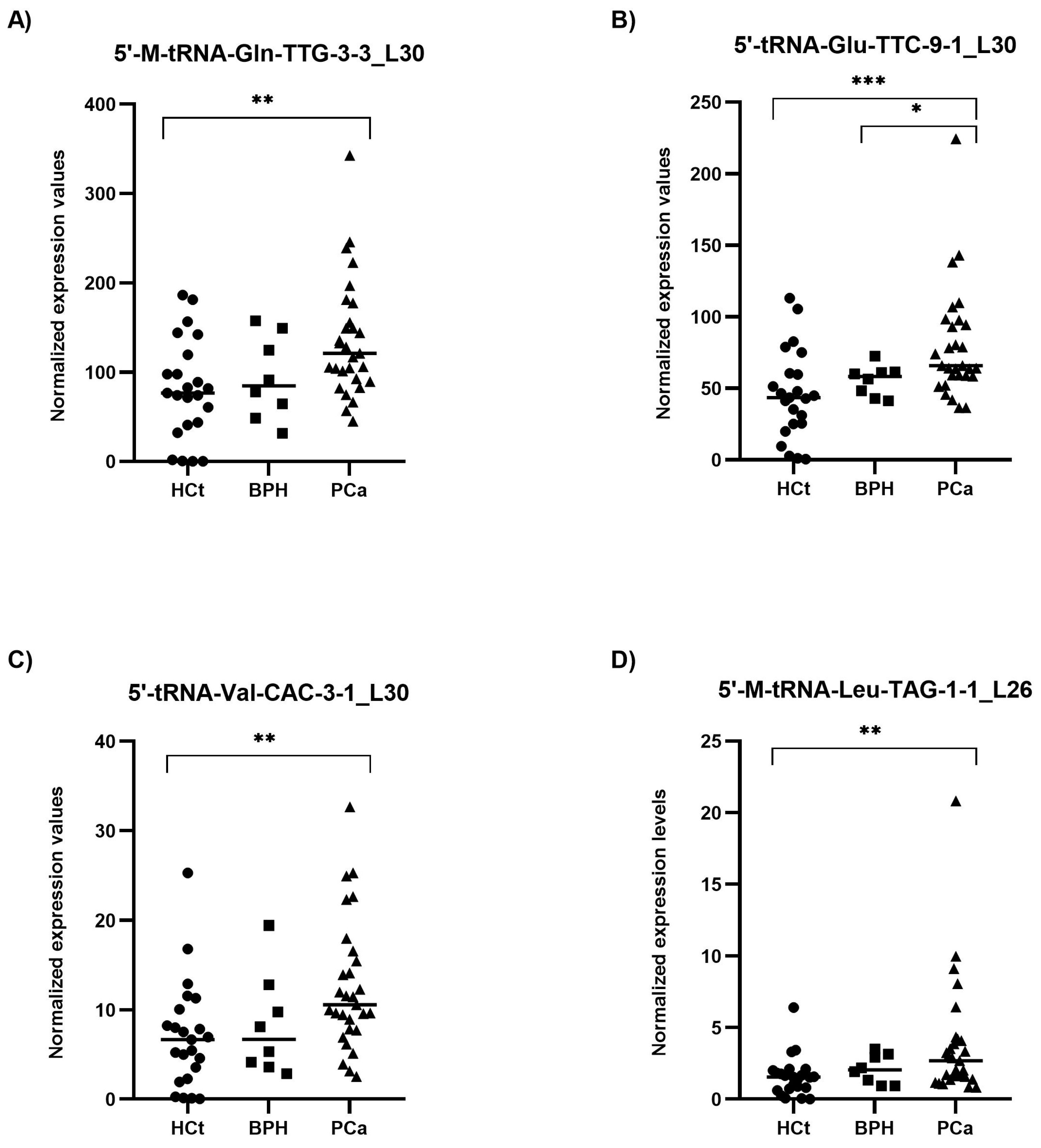
2.3. PCa-Associated tRFs Show Altered Levels in Semen sEVs from Men with Prostate Carcinogenesis
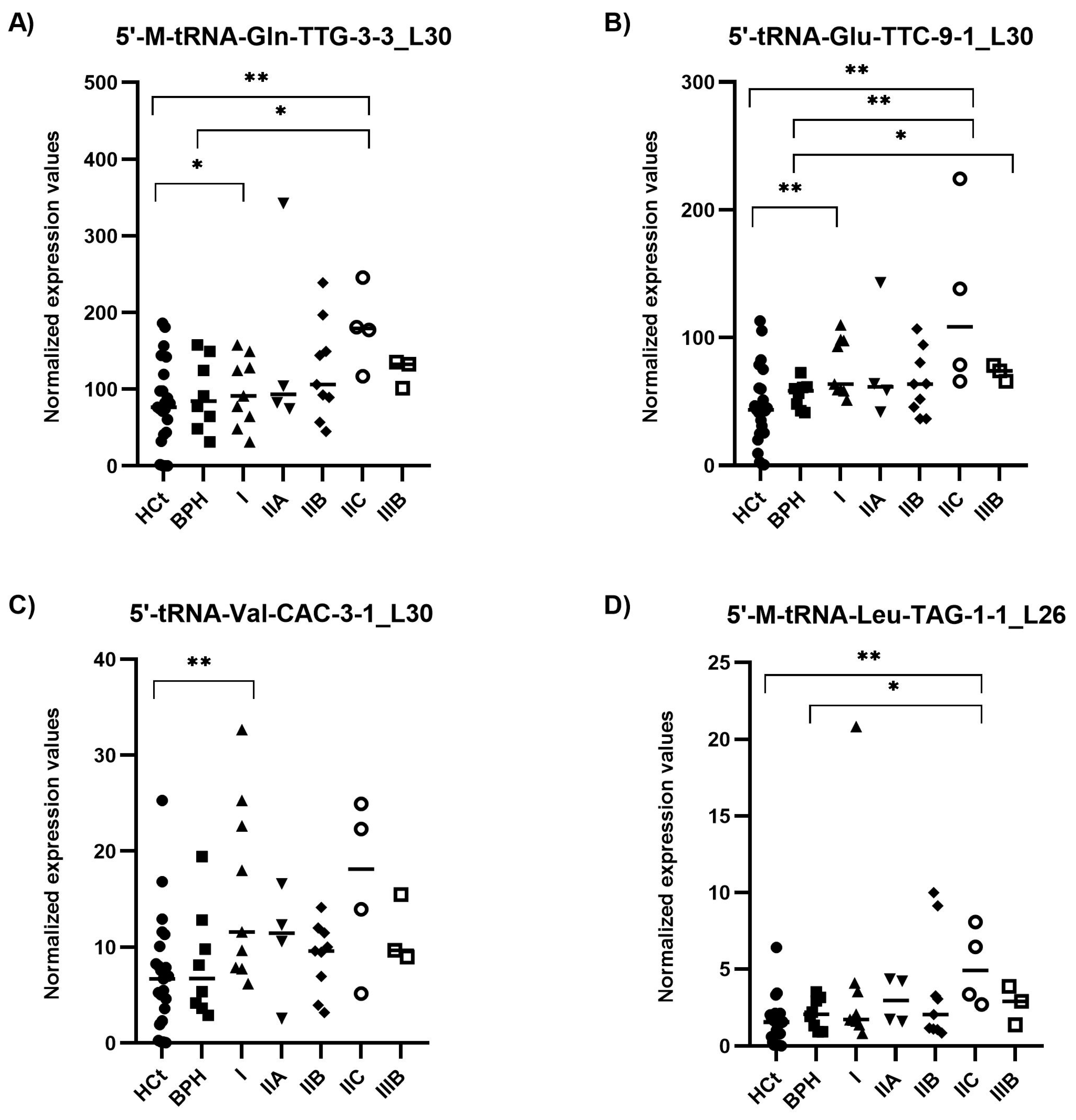
2.4. 5′tRF Levels in Semen sEVs Are Associated with PCa Clinical Risk/Severity
2.5. Prediction of the 5′tRF Target Genes
3. Discussion
4. Materials and Methods
4.1. Subjects of Study
4.2. Cell Culture and Reagents
4.3. Semen Samples and sEV Isolation
4.4. Small RNA-Containing Total RNA Isolation
4.5. tRF Quantification by miRPrimer2 RT-qPCR Strategy
4.6. Determining In Silico Target Genes of tsRNAs
4.7. Statistical Analysis
4.8. Data Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Roobol, M.J.; Carlsson, S.V. Risk stratification in prostate cancer screening. Nat. Rev. Urol. 2013, 10, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Oesterling, J.E. Prostate specific antigen: A critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J. Urol. 1991, 145, 907–923. [Google Scholar] [CrossRef]
- Roberts, M.J.; Schirra, H.J.; Lavin, M.F.; Gardiner, R.A. Metabolomics: A novel approach to early and noninvasive prostate cancer detection. Korean J. Urol. 2011, 52, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Selth, L.A.; Roberts, M.J.; Chow, C.W.; Marshall, V.R.; Doi, S.A.; Vincent, A.D.; Butler, L.M.; Lavin, M.F.; Tilley, W.D.; Gardiner, R.A. Human seminal fluid as a source of prostate cancer-specific microRNA biomarkers. Endocr. Relat. Cancer 2014, 21, L17–L21. [Google Scholar] [CrossRef] [PubMed]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Tewari, M. A functional extracellular transcriptome in animals? Implications for biology, disease and medicine. Genome Biol. 2015, 16, 47. [Google Scholar] [CrossRef]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef]
- Larriba, S.; Sánchez-Herrero, J.F.; Pluvinet, R.; López-Rodrigo, O.; Bassas, L.; Sumoy, L. Seminal extracellular vesicle sncRNA sequencing reveals altered miRNA/isomiR profiles as sperm retrieval biomarkers for azoospermia. Andrology 2024, 12, 137–156. [Google Scholar] [CrossRef]
- Barceló, M.; Castells, M.; Bassas, L.; Vigués, F.; Larriba, S. Semen miRNAs Contained in Exosomes as Non-Invasive Biomarkers for Prostate Cancer Diagnosis. Sci. Rep. 2019, 9, 13772. [Google Scholar] [CrossRef]
- Choy, K.H.K.; Chan, S.Y.; Lam, W.; Jin, J.; Zheng, T.; Law, T.Y.S.; Yu, S.S.; Wang, W.; Li, L.; Xie, G.; et al. The repertoire of testicular extracellular vesicle cargoes and their involvement in inter-compartmental communication associated with spermatogenesis. BMC Biol. 2022, 20, 78. [Google Scholar] [CrossRef]
- Speer, J.; Gehrke, C.W.; Kuo, K.C.; Waalkes, T.P.; Borek, E. tRNA breakdown products as markers for cancer. Cancer 1979, 44, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Balatti, V.; Pekarsky, Y.; Croce, C.M. Role of the tRNA-Derived Small RNAs in Cancer: New Potential Biomarkers and Target for Therapy. Adv. Cancer Res. 2017, 135, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mudunuri, S.B.; Anaya, J.; Dutta, A. tRFdb: A database for transfer RNA fragments. Nucleic Acids Res. 2015, 43, D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lee, I.; Ren, J.; Ajay, S.S.; Lee, Y.S.; Bao, X. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ptashkin, R.N.; Chen, Y.; Cheng, Z.; Liu, G.; Phan, T.; Deng, X.; Zhou, J.; Lee, I.; Lee, Y.S.; et al. Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1622–1629. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhao, D.; Cui, W.; Wu, Y.; Zhang, C.; Duan, C. tRNA-derived small RNAs: Novel regulators of cancer hallmarks and targets of clinical application. Cell Death Discov. 2021, 7, 249. [Google Scholar] [CrossRef]
- Olvedy, M.; Scaravilli, M.; Hoogstrate, Y.; Visakorpi, T.; Jenster, G.; Martens-Uzunova, E.S. A comprehensive repertoire of tRNA-derived fragments in prostate cancer. Oncotarget 2016, 7, 24766–24777. [Google Scholar] [CrossRef]
- Magee, R.G.; Telonis, A.G.; Loher, P.; Londin, E.; Rigoutsos, I. Profiles of miRNA Isoforms and tRNA Fragments in Prostate Cancer. Sci. Rep. 2018, 8, 5314. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Yan, W.; Huang, C.; Ding, Z.; Yang, J.; Jiang, S.; Sun, L. Clinical Significance of High Expression of tRF-Glu-TTC-2 in Prostate Carcinoma and its Effect on Growth. Am. J. Men’s Health 2022, 16, 15579883221135970. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, M.; Cheng, S.; Zhou, X.; Li, J.; Lu, Y.; Liu, P.; Ding, S. tRNA-Derived RNA Fragments Are Novel Biomarkers for Diagnosis, Prognosis, and Tumor Subtypes in Prostate Cancer. Curr. Oncol. 2023, 30, 981–999. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Sun, X.; Zhou, L.; Amanullah, M.; Pan, X.; Liu, Y.; Liang, M.; Liu, P.; Lu, Y. OncotRF: An online resource for exploration of tRNA-derived fragments in human cancers. RNA Biol. 2020, 17, 1081–1091. [Google Scholar] [CrossRef]
- Buyyounouski, M.K.; Choyke, P.L.; McKenney, J.K.; Sartor, O.; Sandler, H.M.; Amin, M.B.; Kattan, M.W.; Lin, D.W. Prostate cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 245–253. [Google Scholar] [CrossRef]
- Ferre, A.; Santiago, L.; Sánchez-Herrero, J.F.; López-Rodrigo, O.; Sánchez-Curbelo, J.; Sumoy, L.; Bassas, L.; Larriba, S. 3′IsomiR Species Composition Affects Reliable Quantification of miRNA/isomiR Variants by Poly(A) RT-qPCR: Impact on Small RNA-Seq Profiling Validation. Int. J. Mol. Sci. 2023, 24, 5436. [Google Scholar] [CrossRef] [PubMed]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Larriba, S.; Vigués, F.; Bassas, L. Using Small Non-Coding RNAs in Extracellular Vesicles of Semen as Biomarkers of Male Reproductive System Health: Opportunities and Challenges. Int. J. Mol. Sci. 2023, 24, 5447. [Google Scholar] [CrossRef]
- Meseguer, S. MicroRNAs and tRNA-Derived Small Fragments: Key Messengers in Nuclear-Mitochondrial Communication. Front. Mol. Biosci. 2021, 8, 643575. [Google Scholar] [CrossRef]
- Suresh, P.S.; Thankachan, S.; Venkatesh, T. Landscape of Clinically Relevant Exosomal tRNA-Derived Non-coding RNAs. Mol. Biotechnol. 2023, 65, 300–310. [Google Scholar] [CrossRef]
- Brokāne, A.; Bajo-Santos, C.; Zayakin, P.; Belovs, A.; Jansons, J.; Lietuvietis, V.; Martens-Uzunova, E.S.; Jenster, G.W.; Linē, A. Validation of potential RNA biomarkers for prostate cancer diagnosis and monitoring in plasma and urinary extracellular vesicles. Front. Mol. Biosci. 2023, 10, 1279854. [Google Scholar] [CrossRef]
- Sobala, A.; Hutvagner, G. Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013, 10, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ragavi, R.; Muthukumaran, P.; Nandagopal, S.; Ahirwar, D.K.; Tomo, S.; Misra, S.; Guerriero, G.; Shukla, K.K. Epigenetics regulation of prostate cancer: Biomarker and therapeutic potential. Urol. Oncol. 2023, 41, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.S.; Falconer, A.; Dodson, A.R.; Norman, A.R.; Dennis, N.; Fletcher, A.; Southgate, C.; Dowe, A.; Dearnaley, D.; Jhavar, S.; et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene 2004, 23, 5871–5879. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.Y.; Feber, A.; Edwards, S.; Te Poele, R.; Giddings, I.; Merson, S.; Cooper, C.S. Role of E2F3 expression in modulating cellular proliferation rate in human bladder and prostate cancer cells. Oncogene 2007, 26, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, H.; Ha, S.; Wang, B.D. Deregulated microRNAs Involved in Prostate Cancer Aggressiveness and Treatment Resistance Mechanisms. Cancers 2023, 15, 3140. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, X.; Narwade, N.; Lim, M.G.L.; Chen, Z.; Tennakoon, C.; Guan, P.; Chan, U.I.; Zhao, Z.; Deng, M.; et al. Single-cell analysis reveals androgen receptor regulates the ER-to-Golgi trafficking pathway with CREB3L2 to drive prostate cancer progression. Oncogene 2021, 40, 6479–6493. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Seo, J.H.; Beshiri, M.L.; Wankowicz, S.; Liu, D.; Cheung, A.; Li, J.; Qiu, X.; Hong, A.L.; Botta, G.; et al. CREB5 Promotes Resistance to Androgen-Receptor Antagonists and Androgen Deprivation in Prostate Cancer. Cell Rep. 2019, 29, 2355–2370.e6. [Google Scholar] [CrossRef]
- Sunkel, B.; Wu, D.; Chen, Z.; Wang, C.M.; Liu, X.; Ye, Z.; Horning, A.M.; Liu, J.; Mahalingam, D.; Lopez-Nicora, H.; et al. Integrative analysis identifies targetable CREB1/FoxA1 transcriptional co-regulation as a predictor of prostate cancer recurrence. Nucleic Acids Res. 2016, 44, 4105–4122. [Google Scholar] [CrossRef]
- Meng, D.; Yang, S.; Wan, X.; Zhang, Y.; Huang, W.; Zhao, P.; Li, T.; Wang, L.; Huang, Y.; Li, T.; et al. A transcriptional target of androgen receptor, miR-421 regulates proliferation and metabolism of prostate cancer cells. Int. J. Biochem. Cell Biol. 2016, 73, 30–40. [Google Scholar] [CrossRef]
- Traynor, P.; McGlynn, L.M.; Mukhergee, R.; Grimsley, S.J.; Bartlett, J.M.; Edwards, J. An increase in N-Ras expression is associated with development of hormone refractory prostate cancer in a subset of patients. Dis. Markers 2008, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Assessment of the prognostic value of the 8th AJCC staging system for patients with clinically staged prostate cancer; A time to sub-classify stage IV? PLoS ONE 2017, 12, e0188450. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, M.; Mata, A.; Bassas, L.; Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum. Reprod. 2018, 33, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, S.; Guo, C.; Guan, H.; Xiong, C. Cell-free seminal mRNA and microRNA exist in different forms. PLoS ONE 2012, 7, e34566. [Google Scholar] [CrossRef] [PubMed]
- Balcells, I.; Cirera, S.; Busk, P.K. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, H.; Cui, Q.; Zhou, Y. tRFTar: Prediction of tRF-target gene interactions via systemic re-analysis of Argonaute CLIP-seq datasets. Methods 2021, 187, 57–67. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, C.; Lang, X.; Guo, G.; Chen, J.; Su, X. Small noncoding RNA discovery and profiling with sRNAtools based on high-throughput sequencing. Brief. Bioinform. 2021, 22, 463–473. [Google Scholar] [CrossRef]
- Soutschek, M.; Gross, F.; Schratt, G.; Germain, P.L. scanMiR: A biochemically based toolkit for versatile and efficient microRNA target prediction. Bioinformatics 2022, 38, 2466–2473. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Lawrence, M.; Huber, W.; Pagès, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Durinck, S.; Moreau, Y.; Kasprzyk, A.; Davis, S.; De Moor, B.; Brazma, A.; Huber, W. BioMart and Bioconductor: A powerful link between biological databases and microarray data analysis. Bioinformatics 2005, 21, 3439–3440. [Google Scholar] [CrossRef] [PubMed]




| tRF | ID | RNA Sequence | Chromosome (tRNA Number) | miRPrimer2 Forward Primer | miRPrimer2 Reverse Primer |
|---|---|---|---|---|---|
| 5′-M-tRNA-Gln-TTG-3-3_L30 | tRF-30- -6RJ89O9NF5W8 | GGCCCCAUGGUGUAAUGGUUAGCACUCUGG | 6 (tRNA130); 6 (tRNA173); 6 (tRNA174) | ccccatggtgtaatggttag | cagtttttttttttttttccagagtg |
| 5′-tRNA-Glu-TTC- -9-1_L30 | tRF-30- -PER8YP9LON4V | GCAAUGGUGGUUCAGUGGUAGAAUUCUCGC | 2 (tRNA17) | aatggtggttcagtggtaga | ccagtttttttttttttttgcgaga |
| 5′-tRNA-Val-CAC- -3-1_L30 | tRF-30- -79MP9PMNH5IS | GUUUCCGUAGUGUAGCGGUUAUCACAUUCG | 19 (tRNA13) | cgtagtgtagcggttatcac | gtccagtttttttttttttttcgaatg |
| 5′-M-tRNA-Leu-TAG-1-1_L26 | tRF-26- -RPM830MMUKD | GGUAGCGUGGCCGAGCGGUCUAAGGC | 17 (tRNA42) | gtagcgtggccgag | ccagtttttttttttttttgccttag |
| hsa-miR-30e-3p | tRF-30- -6RJ89O9NF5W8 | CUUUCAGUCGGAUGUUUACAGC | * gcagctttcagtcggatgt | * tccagtttttttttttttttgctgt |
| Markers | AUC (p-Value) | 95% CI | Sensitivity % | Specificity % | PPV % | NPV % |
|---|---|---|---|---|---|---|
| A. (HCt + BPH) vs. PCa | ||||||
| 5′-M-tRNA-Gln-TTG-3-3_L30 | 0.736 (0.002) | 0.610–0.862 | 55.2 | 71 | 64 | 62.8 |
| 5′-tRNA-Glu-TTC-9-1_L30 | 0.766 (0.000 *) | 0.647–0.886 | 65.5 | 80.6 | 76 | 71.4 |
| 5′-tRNA-Val-CAC-3-1_L30 | 0.727 (0.003) | 0.599–0.855 | 51.7 | 77.4 | 68.2 | 63.1 |
| 5′-M-tRNA-Leu-TAG-1-1_L26 | 0.711 (0.005) | 0.582–0.840 | 51.7 | 80.6 | 71.4 | 64.1 |
| B. BPH vs. PCa | ||||||
| PSA | 0.580 (0.495) | 0.371–0.789 | 100 | 0 | 78.4 | 0 |
| 5′-M-tRNA-Gln-TTG-3-3_L30 | 0.694 (0.097) | 0.475–0.913 | 100 | 12.5 | 76.3 | 1 |
| 5′-tRNA-Glu-TTC-9-1_L30 | 0.737 (0.042) | 0.574–0.901 | 93.1 | 0 | 77.1 | 0 |
| 5′-tRNA-Val-CAC-3-1_L30 | 0.681 (0.121) | 0.465–0.897 | 100 | 0 | 78.4 | 0 |
| 5′-M-tRNA-Leu-TAG-1-1_L26 | 0.625 (0.285) | 0.426–0.824 | 100 | 0 | 78.4 | 0 |
| Combined PSA-tRF model (PSA + Gln + Glu) | 0.759 (0.027) | 0.591–0.927 | 96.6 | 25 | 82.3 | 66.6 |
| C. (HCt + BPH + PCa_GS6) vs. (PCa GS7 + GS8) | ||||||
| 5′-M-tRNA-Gln-TTG-3-3_L30 | 0.7 (0.018) | 0.555–0.846 | 12.5 | 95.5 | 50 | 75 |
| 5′-tRNA-Glu-TTC-9-1_L30 | 0.698 (0.020) | 0.551–0.846 | 12.5 | 97.7 | 67 | 75.5 |
| 5′-tRNA-Val-CAC-3-1_L30 | 0.617 (0.168) | 0.468–0.767 | 0 | 100 | 0 | 73.3 |
| 5′-M-tRNA-Leu-TAG-1-1_L26 | 0.666 (0.05) | 0.505–0.827 | 0 | 97.7 | 0 | 72.8 |
| Combined tRF model (Gln + Glu + Val) | 0.658 (0.064) | 0.505–0.810 | 12.5 | 97.7 | 66.7 | 75.4 |
| D. (BPH + PCa_GS6) vs. (PCa_GS7 + GS8) | ||||||
| PSA | 0.670 (0.081) | 0.487–0.852 | 31.3 | 76.2 | 50 | 59.2 |
| 5′-M-tRNA-Gln-TTG-3-3_L30 | 0.628(0.187) | 0.443–0.813 | 18.8 | 90.5 | 60 | 59.4 |
| 5′-tRNA-Glu-TTC-9-1_L30 | 0.616 (0.232) | 0.421–0.811 | 18.8 | 90.5 | 60 | 59.4 |
| 5′-tRNA-Val-CAC-3-1_L30 | 0.504 (0.963) | 0.316–0.693 | 0 | 100 | 0 | 56.7 |
| 5′-M-tRNA-Leu-TAG-1-1_L26 | 0.571 (0.462) | 0.377–0.766) | 6.3 | 95.2 | 50 | 57.1 |
| Combined tRF model (Glu + Val) | 0.673 (0.075) | 0.494–0.851 | 37.5 | 85.7 | 55.5 | 60.7 |
| Combined PSA-tRF model (PSA + Glu + Val) | 0.732 (0.017) | 0.553–0.911 | 43.8 | 85.7 | 70 | 66.6 |
| Combined PSA-tRF model (PSA + Gln + Glu + Val+ Leu) | 0.780 (0.004) | 0.615–0.944 | 50 | 85.7 | 72.7 | 69.2 |
| E. (BPH + PCa_I) vs. (PCa_IIA + IIB + IIC + IIIB) | ||||||
| PSA | 0.629 (0.180) | 0.447–0.812 | 50 | 70.6 | 66.7 | 54.5 |
| 5′-M-tRNA-Gln-TTG-3-3_L30 | 0.606 (0.273) | 0.422–0.789 | 60 | 47.1 | 57.1 | 50 |
| 5′-tRNA-Glu-TTC-9-1_L30 | 0.604 (0.279) | 0.416–0.793 | 70 | 52.9 | 63.6 | 60 |
| 5′-tRNA-Val-CAC-3-1_L30 | 0.507 (0.939) | 0.312–0.703) | 90 | 23.5 | 58.1 | 66.7 |
| 5′-M-tRNA-Leu-TAG-1-1_L26 | 0.606 (0.273) | 0.421–0.791 | 100 | 0 | 54.1 | 0 |
| Combined tRF model (Glu + Val) | 0.697 (0.041) | 0.527–0.867 | 65 | 52.9 | 61.9 | 56.2 |
| Combined PSA-tRF model (PSA + Glu + Val) | 0.756 (0.008) | 0.592–0.920 | 70 | 76.5 | 77.8 | 68.4 |
| tRF | Target Gene | Ensembl ID (Human Gene) | Description | Molecular Function | |||||
|---|---|---|---|---|---|---|---|---|---|
| A. tRFtar: tRF-target gene interaction prediction | |||||||||
| 5′-M-tRNA-Leu-TAG-1-1_L26 | AR | ENSG00000169083 | androgen receptor [KO:K08557] | Steroid-hormone activated transcription factor | |||||
| ERBB2 | ENSG00000141736 | erb-b2 receptor tyrosine kinase 2 [KO:K05083] [EC:2.7.10.1] | Bind tightly to other ligand-bound EGF receptor family members to form a heterodimer, and enhancing kinase-mediated activation of downstream signalling pathways | ||||||
| GSTP1 | ENSG00000084207 | glutathione S-transferase pi 1 [KO:K23790] [EC:2.5.1.18] | Catalyses the conjugation of many hydrophobic and electrophilic compounds with reduced glutathione | ||||||
| MAP2K1 | ENSG00000169032 | mitogen-activated protein kinase 1 [KO:K04368] [EC:2.7.12.2] | It is a mitogen-activated protein (MAP) kinase involved in many cellular processes such as proliferation, differentiation, transcription regulation and development | ||||||
| MTOR | ENSG00000198793 | mechanistic target of rapamycin kinase [KO:K07203] [EC:2.7.11.1] | Kinase which mediates cellular responses to stresses such as DNA damage and nutrient deprivation. | ||||||
| B. miRNA-target gene prediction tools | |||||||||
| TargetScan | miRDB | miRanda | RNA-Hybrid | scan-MiR | |||||
| 5′-M-tRNA-Gln-TTG-3-3_L30 * 5′-M-tRNA-Leu-TAG-1-1_L26 # | PDPK1 | ENSG00000140992 | 3-phosphoinositide dependent protein kinase 1 [KO:K06276] [EC:2.7.11.1] | Involved in cell surface receptor signalling pathway; regulation of protein kinase activity; and regulation of signal transduction | Yes * | Yes # | |||
| IKBKG | ENSG00000269335 | inhibitor of nuclear factor kappa B kinase regulatory subunit gamma [KO:K07210] | The regulatory subunit of the inhibitor of kappaB kinase (IKK) complex, which activates NF-kappaB resulting in activation of genes involved in inflammation, immunity, cell survival, and other pathways. | Yes *,# | |||||
| 5′-M-tRNA-Gln-TTG-3-3_L30 * 5′-tRNA-Val-CAC-3-1_L30 $ | IGF1R | ENSG00000140443 | insulin like growth factor 1 receptor [KO:K05087] [EC:2.7.10.1] | This receptor binds insulin-like growth factor with a high affinity. It has tyrosine kinase activity. The IGF1R plays a critical role in transformation events. | Yes *,$ | Yes $ | Yes *,$ | ||
| 5′-tRNA-Glu-TTC-9-1_L30 a 5′-tRNA-Val-CAC-3-1_L30 $ | CREB3L2 | ENSG00000182158 | cAMP responsive element binding protein 3 like 2 [KO:K09048] | Transcriptional activator | Yes a,$ | Yes a,$ | Yes $ | ||
| 5′-tRNA-Glu-TTC-9-1_L30 a 5′-M-tRNA-Leu-TAG-1-1_L26 # | BRAF | ENSG00000157764 | B-Raf proto-oncogene, serine/threonine kinase [KO:K04365] [EC:2.7.11.1] | Regulates the MAP kinase/ERK signalling pathway, which affects cell division, differentiation, and secretion | Yes # | Yes a | |||
| 5′-M-tRNA-Gln-TTG-3-3_L30 | FOXO1 | ENSG00000150907 | forkhead box O1 [KO:K07201] | Transcription factor which may play a role in myogenic growth and differentiation | Yes | ||||
| TMPRSS2 | ENSG00000184012 | transmembrane serine protease 2 [KO:K09633] [EC:3.4.21.122] | Up-regulated by androgenic hormones in prostate cancer cells and down-regulated in androgen-independent prostate cancer tissue | Yes | |||||
| BCL2 | ENSG00000171791 | BCL2 apoptosis regulator [KO:K02161] | Involved in the inhibition of apoptosis | Yes | |||||
| KLK3 | ENSG00000142515 | kallikrein related peptidase 3 [KO:K01351] [EC:3.4.21.77] | A protease (PSA) which is synthesized in the epithelial cells of the prostate gland | Yes | |||||
| NKX3-1 | ENSG00000167034.9 | NK3 homeobox 1 [KO:K09348] | Negative regulator of epithelial cell growth in prostate tissue | Yes | |||||
| AKT2 | ENSG00000105221.16 | AKT serine/threonine kinase 2 [KO:K04456] [EC:2.7.11.1] | Protein kinase involved in signalling pathways as oncogene | Yes | |||||
| CREBBP | ENSG00000005339.14 | CREB binding protein [KO:K04498] [EC:2.3.1.48] | Involved in the transcriptional coactivation of many different transcription factors | Yes | |||||
| NFKBIA | ENSG00000100906 | NFKB inhibitor alpha [KO:K04734] | Interacts with REL dimers to inhibit NF-kappa-B/REL complexes which are involved in inflammatory responses | Yes | |||||
| MAPK3 | ENSG00000102882.11 | mitogen-activated protein kinase 3 [KO:K04371] [EC:2.7.11.24] | Regulates cell proliferation, differentiation, and cell cycle progression in response to a variety of extracellular signals | Yes | |||||
| 5′-tRNA-Glu-TTC-9-1_L30 | E2F3 | ENSG00000112242 | E2F transcription factor 3 [KO:K06620] | Regulate the expression of genes involved in the cell cycle | Yes | Yes | |||
| PTEN | ENSG00000171862 | phosphatase and tensin homolog [KO:K01110] [EC:3.1.3.16 3.1.3.48 3.1.3.67] | Negatively regulates intracellular levels of phosphatidylinositol-3,4,5-trisphosphate in cells and functions as a tumor suppressor by negatively regulating AKT/PKB signalling pathway | Yes | Yes | Yes | |||
| NRAS | ENSG00000213281 | NRAS proto-oncogene, GTPase [KO:K07828] | Membrane protein with GTPase activity. Oncogene | Yes | Yes | Yes | |||
| CREB5 | ENSG00000146592 | cAMP responsive element binding protein 5 [KO:K09047] | Specifically binds to CRE as a homodimer or a heterodimer with c-Jun or CRE-BP1, and functions as a CRE-dependent trans-activator | Yes | Yes | ||||
| CREB1 | ENSG00000118260 | cAMP responsive element binding protein 1 [KO:K05870] | Transcription factor that induces transcription of genes in response to hormonal stimulation of the cAMP pathway | Yes | Yes | ||||
| CASP9 | ENSG00000132906.17 | caspase 9 [KO:K04399] [EC:3.4.22.62] | Plays a central role in apoptosis. Tumor suppressor | Yes | |||||
| PIK3CA | ENSG00000121879 | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha [KO:K00922] [EC:2.7.1.153] | Catalytic subunit of PIK3. Oncogenic gene | Yes | |||||
| KRAS | ENSG00000133703 | KRAS proto-oncogene, GTPase [KO:K07827] | Member of the small GTPase superfamily. Proto-oncogene | Yes | |||||
| AR | ENSG00000169083 | androgen receptor [KO:K08557] | Steroid-hormone activated transcription factor | Yes | |||||
| 5′-tRNA-Val-CAC-3-1_L30 | PIK3R2 | ENSG00000105647 | phosphoinositide-3-kinase regulatory subunit 2 [KO:K02649] | Lipid kinase that phosphorylates phosphatidylinositol and similar compounds, creating second messengers important in growth signalling pathways | Yes | ||||
| MAPK1 | ENSG00000100030 | mitogen-activated protein kinase 1 [KO:K04371] [EC:2.7.11.24] | Regulates cell proliferation, differentiation, transcription regulation and development | Yes | |||||
| 5′-M-tRNA-Leu-TAG-1-1_L26 | AKT1 | ENSG00000142208 | AKT serine/threonine kinase 1 [KO:K04456] [EC:2.7.11.1] | Regulates cell proliferation, survival, metabolism, and angiogenesis | Yes | ||||
| BAD | ENSG00000002330 | BCL2 associated agonist of cell death [KO:K02158] | Positively regulates cell apoptosis | Yes | |||||
| CCND1 | ENSG00000110092 | cyclin D1 [KO:K04503] | Required for cell cycle G1/S transition. Interact with tumor suppressor protein Rb | ||||||
| RAF1 | ENSG00000132155 | Raf-1 proto-oncogene, serine/threonine kinase [KO:K04366] [EC:2.7.11.1] | MAP kinase kinase kinase (MAP3K) involved in the cell division cycle, apoptosis, cell differentiation and cell migration | Yes | |||||
| CREB3 | ENSG00000107175 | cAMP responsive element binding protein 3 [KO:K09048] | Binds to the cAMP-response element and regulates cell proliferation and tumor suppression | Yes | |||||
| TCF7L2 | ENSG00000148737 | transcription factor 7 like 2 [KO:K04491] | Transcription factor involved in the Wnt signalling pathway | Yes | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferre-Giraldo, A.; Castells, M.; Sánchez-Herrero, J.F.; López-Rodrigo, O.; de Rocco-Ponce, M.; Bassas, L.; Vigués, F.; Sumoy, L.; Larriba, S. Semen sEV tRF-Based Models Increase Non-Invasive Prediction Accuracy of Clinically Significant Prostate Cancer among Patients with Moderately Altered PSA Levels. Int. J. Mol. Sci. 2024, 25, 10122. https://doi.org/10.3390/ijms251810122
Ferre-Giraldo A, Castells M, Sánchez-Herrero JF, López-Rodrigo O, de Rocco-Ponce M, Bassas L, Vigués F, Sumoy L, Larriba S. Semen sEV tRF-Based Models Increase Non-Invasive Prediction Accuracy of Clinically Significant Prostate Cancer among Patients with Moderately Altered PSA Levels. International Journal of Molecular Sciences. 2024; 25(18):10122. https://doi.org/10.3390/ijms251810122
Chicago/Turabian StyleFerre-Giraldo, Adriana, Manel Castells, José Francisco Sánchez-Herrero, Olga López-Rodrigo, Maurizio de Rocco-Ponce, Lluís Bassas, Francesc Vigués, Lauro Sumoy, and Sara Larriba. 2024. "Semen sEV tRF-Based Models Increase Non-Invasive Prediction Accuracy of Clinically Significant Prostate Cancer among Patients with Moderately Altered PSA Levels" International Journal of Molecular Sciences 25, no. 18: 10122. https://doi.org/10.3390/ijms251810122
APA StyleFerre-Giraldo, A., Castells, M., Sánchez-Herrero, J. F., López-Rodrigo, O., de Rocco-Ponce, M., Bassas, L., Vigués, F., Sumoy, L., & Larriba, S. (2024). Semen sEV tRF-Based Models Increase Non-Invasive Prediction Accuracy of Clinically Significant Prostate Cancer among Patients with Moderately Altered PSA Levels. International Journal of Molecular Sciences, 25(18), 10122. https://doi.org/10.3390/ijms251810122





