Crocin Protects the 661W Murine Photoreceptor Cell Line against the Toxic Effects of All-Trans-Retinal
Abstract
1. Introduction
2. Results
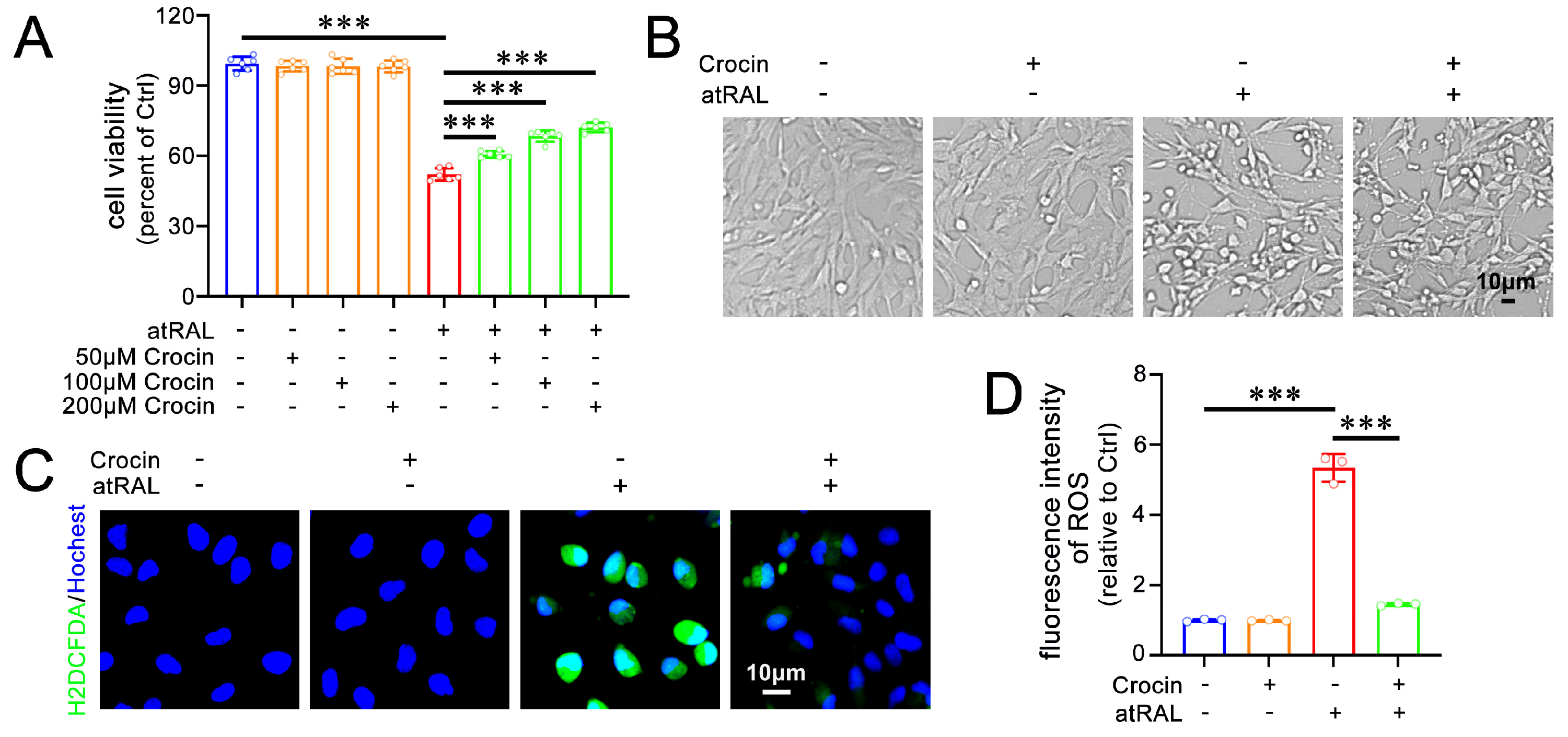
2.1. Crocin Enhances Cell Viability and Alleviates Oxidative Stress in atRAL-Loaded 661W Cells
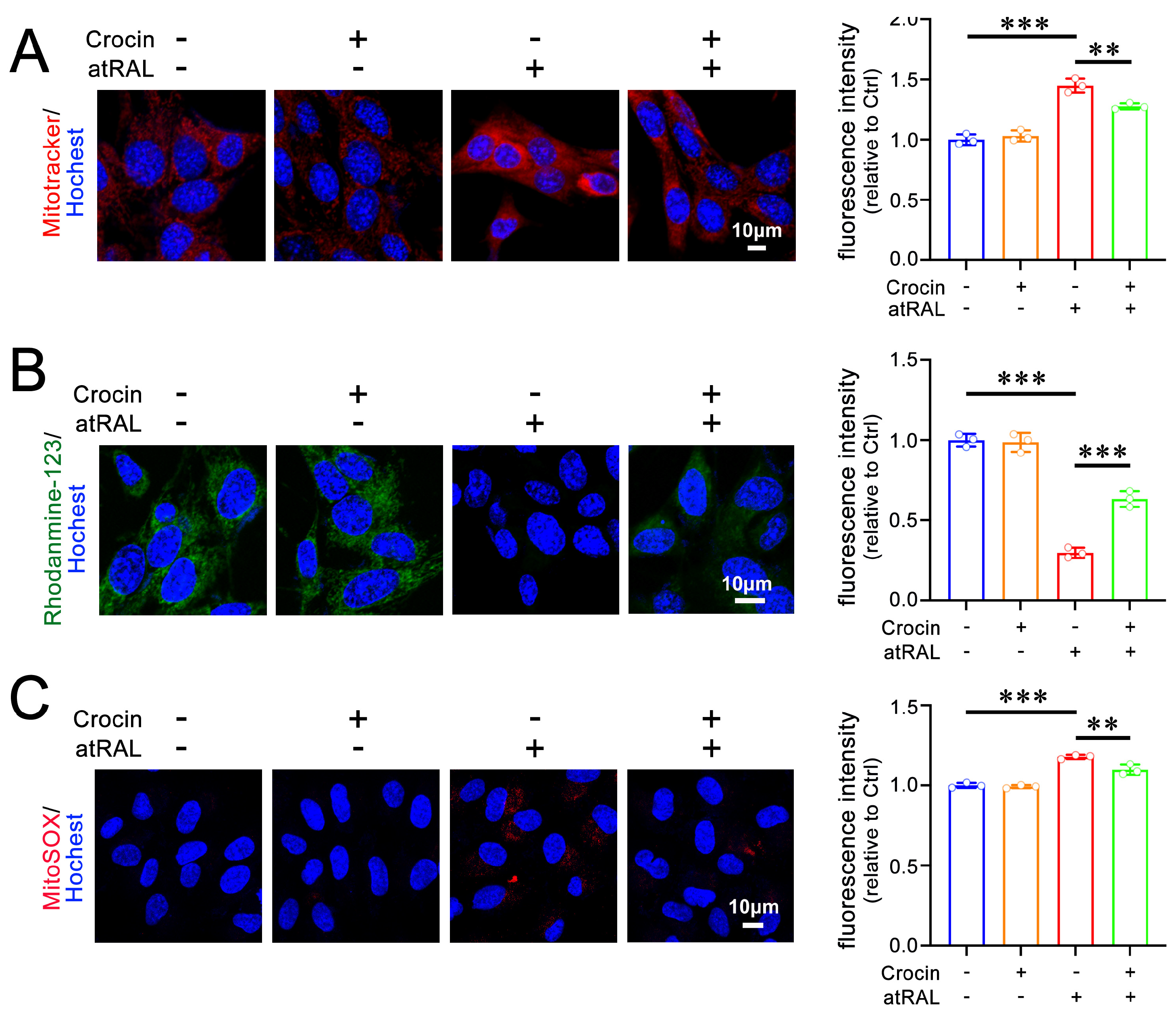
2.2. Crocin Mitigates Mitochondrial Damage in atRAL-Loaded 661W Cells
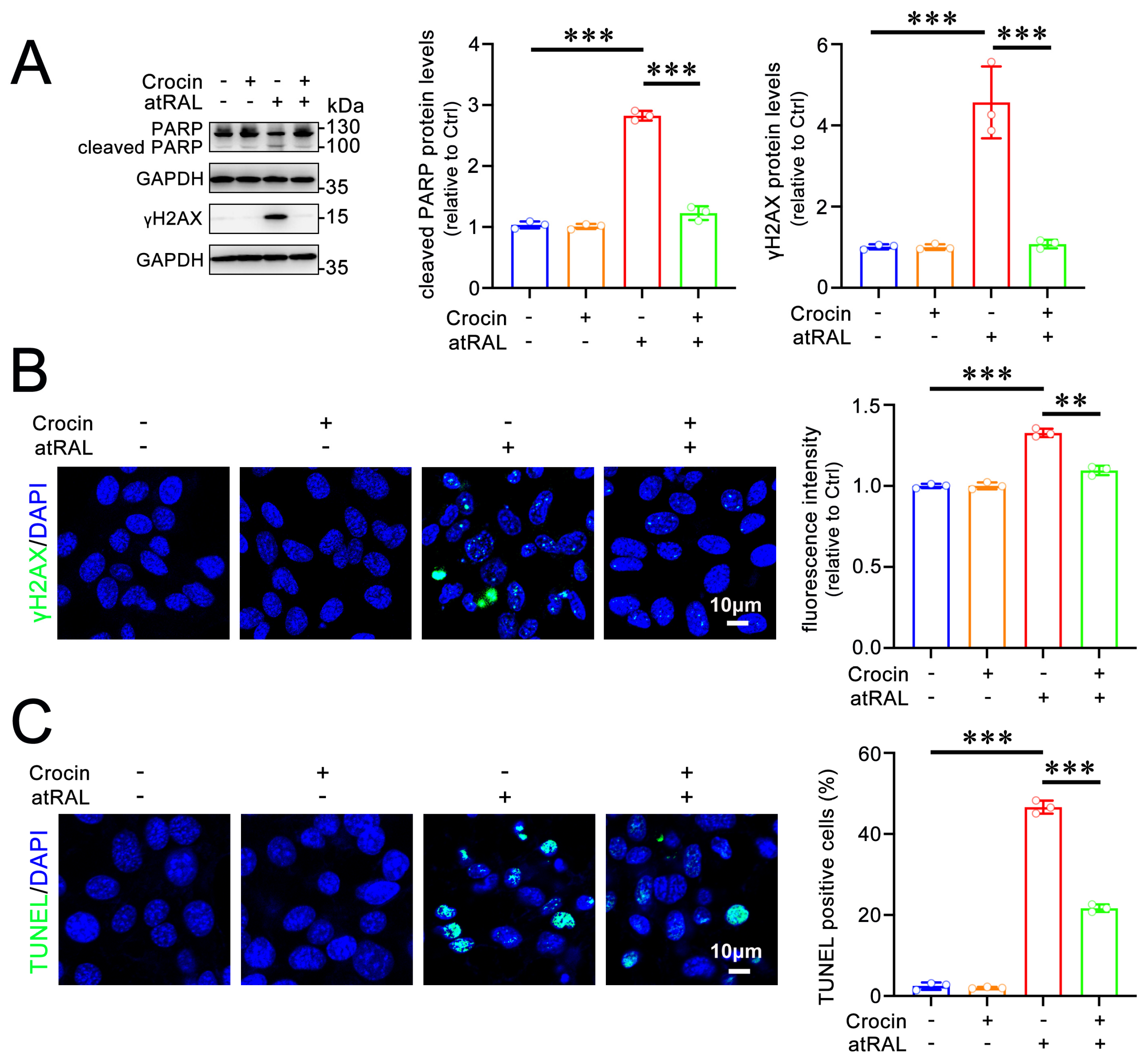
2.3. Crocin Protects 661W Cells from Apoptosis Induced by atRAL
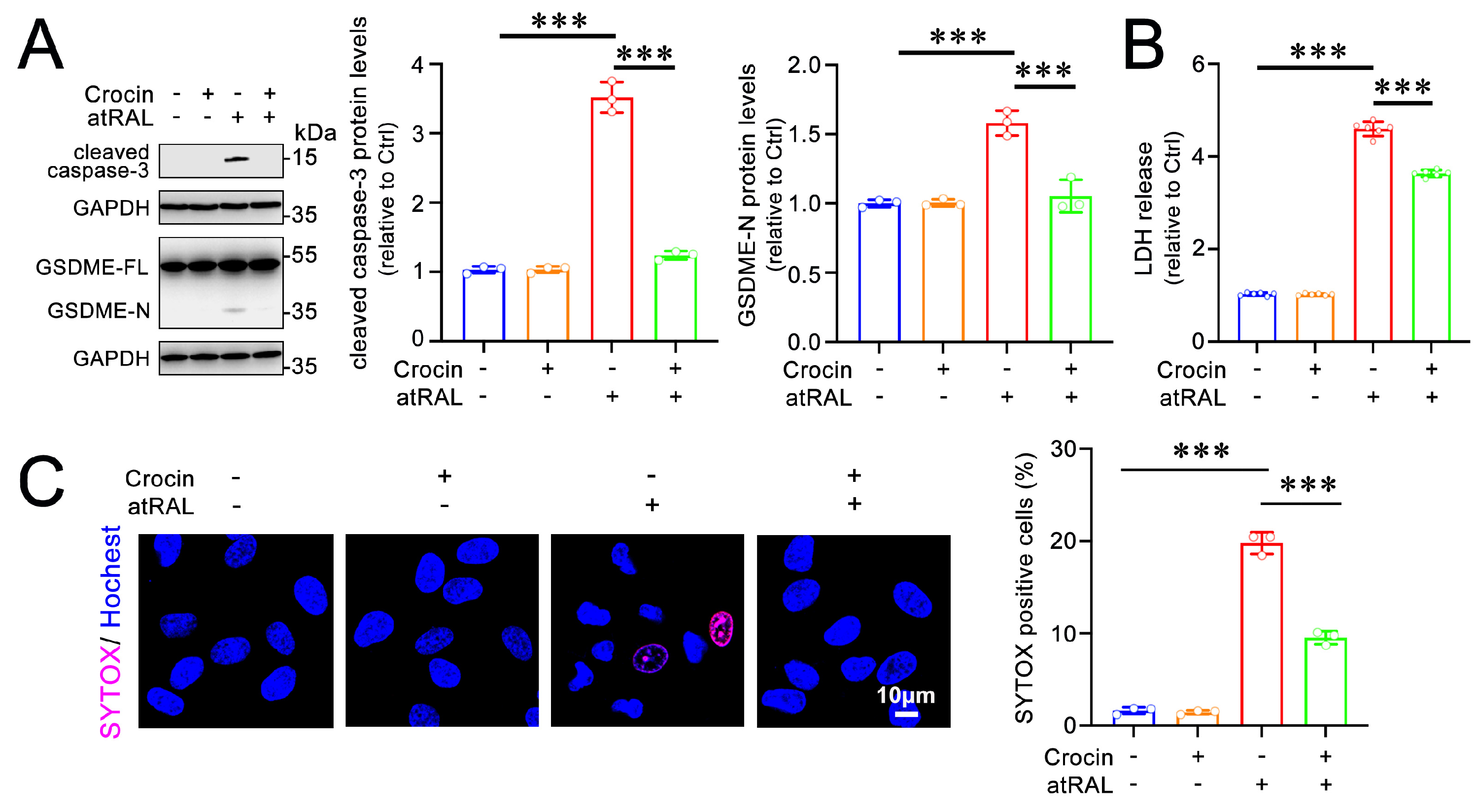
2.4. Crocin Protects 661W Cells from Pyroptosis Induced by atRAL
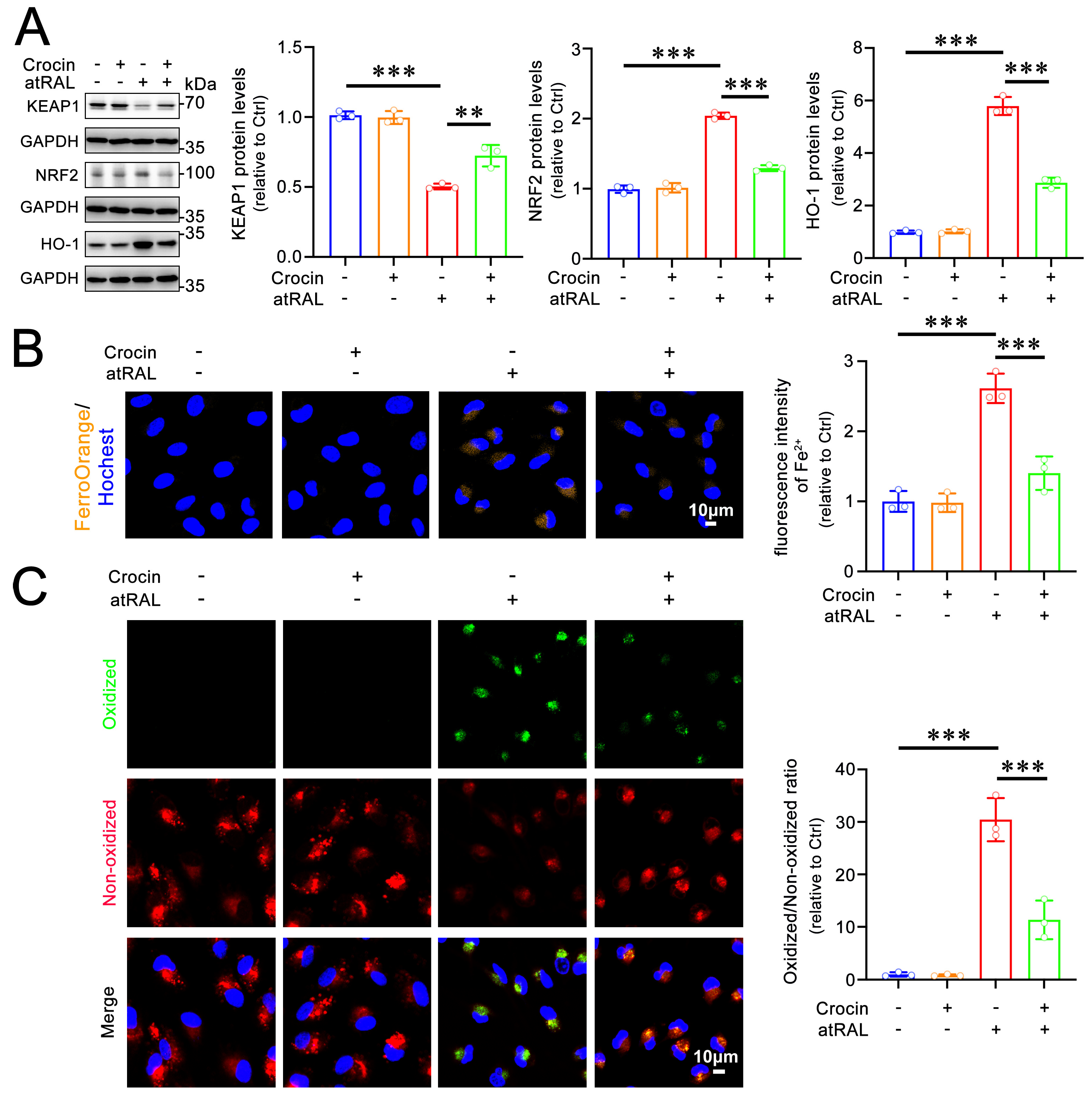
2.5. Crocin Protects 661W Cells from Ferroptosis Induced by atRAL
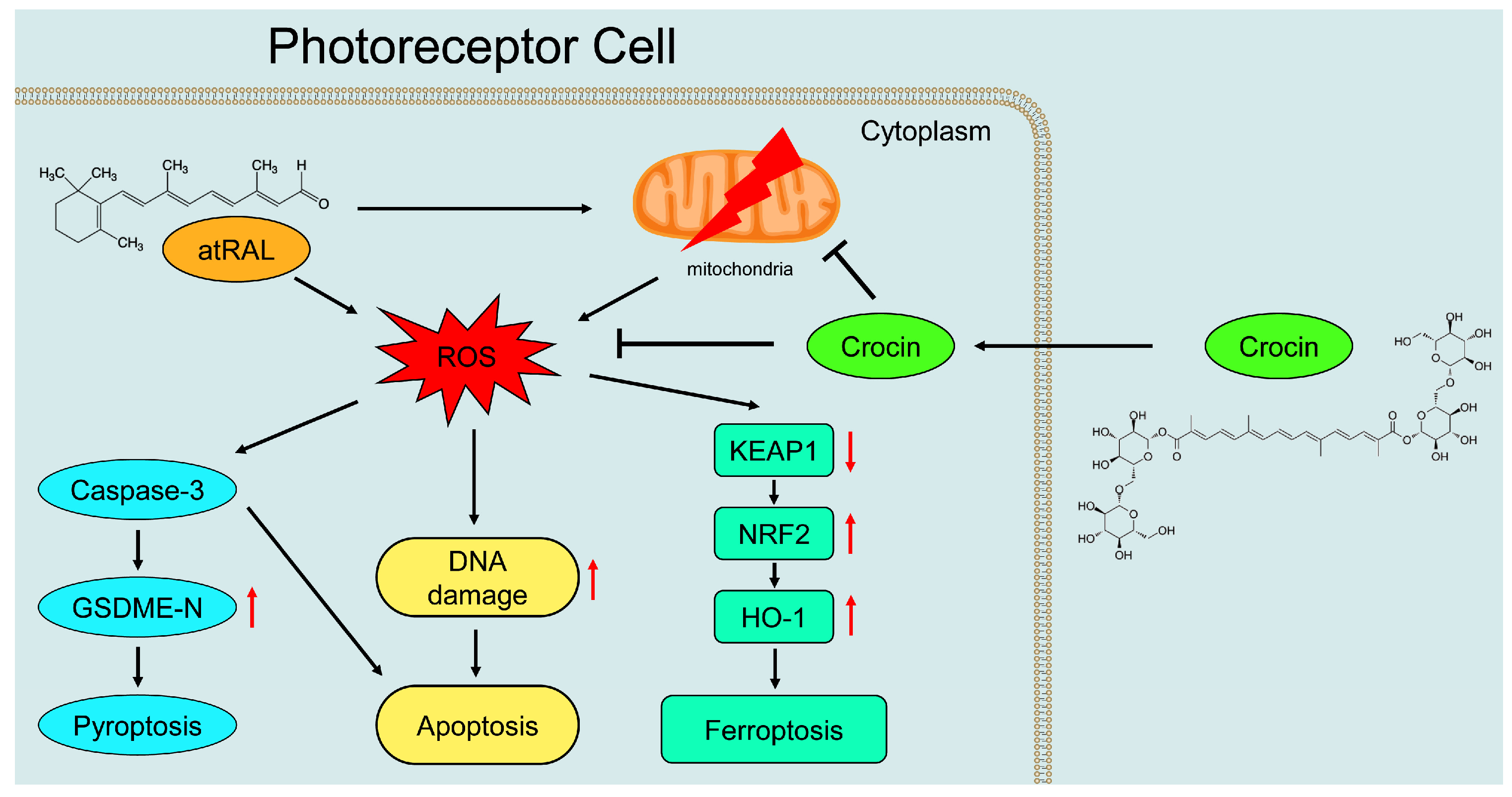
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability
4.4. Detection of ROS
4.5. Staining of Mitochondria
4.6. TUNEL Assay
4.7. LDH Assay
4.8. SYTOX Staining
4.9. Detection of Fe2+ Levels
4.10. Detection of Lipid Peroxidation
4.11. Immunofluorescence Staining
4.12. Western Blotting
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.D.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Liu, Z.; Li, J.; Yao, K.; Wu, Y. Potential Therapeutic Agents Against Retinal Diseases Caused by Aberrant Metabolism of Retinoids. Invest. Ophthalmol. Vis. Sci. 2016, 57, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Okano, K.; Maeda, T.; Chauhan, V.; Golczak, M.; Maeda, A.; Palczewski, K. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 2012, 287, 5059–5069. [Google Scholar] [CrossRef]
- Liao, C.; Cai, B.; Feng, Y.; Chen, J.; Wu, Y.; Zhuang, J.; Liu, Z.; Wu, Y. Activation of JNK signaling promotes all-trans-retinal-induced photoreceptor apoptosis in mice. J. Biol. Chem. 2020, 295, 6958–6971. [Google Scholar] [CrossRef]
- He, D.; Tao, L.; Cai, B.; Chen, X.; Wang, Y.; Li, S.; Liao, C.; Chen, Y.; Chen, J.; Liu, Z.; et al. eIF2alpha incites photoreceptor cell and retina damage by all-trans-retinal. J. Biol. Chem. 2023, 299, 104686. [Google Scholar] [CrossRef]
- Cai, B.; Liao, C.; He, D.; Chen, J.; Han, J.; Lu, J.; Qin, K.; Liang, W.; Wu, X.; Liu, Z.; et al. Gasdermin E mediates photoreceptor damage by all-trans-retinal in the mouse retina. J. Biol. Chem. 2022, 298, 101553. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wang, Y.; Liu, Z.; Wu, Y. Ferroptosis drives photoreceptor degeneration in mice with defects in all-trans-retinal clearance. J. Biol. Chem. 2021, 296, 100187. [Google Scholar] [CrossRef]
- Chen, C.; Yang, K.; He, D.; Yang, B.; Tao, L.; Chen, J.; Wu, Y. Induction of ferroptosis by HO-1 contributes to retinal degeneration in mice with defective clearance of all-trans-retinal. Free Radic. Biol. Med. 2023, 194, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Alugoju, P.; Krishna Swamy, V.K.D.; Anthikapalli, N.V.A.; Tencomnao, T. Health benefits of astaxanthin against age-related diseases of multiple organs: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10709–10774. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Mamoulakis, C.; Tsarouhas, K.; Georgiadis, G.; Lazopoulos, G.; Tsatsakis, A.; Shojaei Asrami, E.; Rezaee, R. Crocin: A fighter against inflammation and pain. Food Chem. Toxicol. 2020, 143, 111521. [Google Scholar] [CrossRef]
- Ahmed, S.; Hasan, M.M.; Heydari, M.; Rauf, A.; Bawazeer, S.; Abu-Izneid, T.; Rebezov, M.; Shariati, M.A.; Daglia, M.; Rengasamy, K.R. Therapeutic potentials of crocin in medication of neurological disorders. Food Chem. Toxicol. 2020, 145, 111739. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Jung, W.W.; Choi, E.M. Crocin attenuates methylglyoxal-induced osteoclast dysfunction by regulating glyoxalase, oxidative stress, and mitochondrial function. Food Chem. Toxicol. 2019, 124, 367–373. [Google Scholar] [CrossRef]
- Fernandez-Albarral, J.A.; Ramirez, A.I.; de Hoz, R.; Lopez-Villarin, N.; Salobrar-Garcia, E.; Lopez-Cuenca, I.; Licastro, E.; Inarejos-Garcia, A.M.; Almodovar, P.; Pinazo-Duran, M.D.; et al. Neuroprotective and Anti-Inflammatory Effects of a Hydrophilic Saffron Extract in a Model of Glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, L.; Zhang, L.; Liu, W.B.; Chen, X.Y.; Yang, X.G. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp. Eye Res. 2013, 107, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of Crocin on Diabetic Maculopathy: A Placebo-Controlled Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef]
- Hou, J.; Hsu, J.-M.; Hung, M.-C. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol. Cell 2021, 81, 4579–4590. [Google Scholar] [CrossRef]
- Privitera, G.; Rana, N.; Armuzzi, A.; Pizarro, T.T. The gasdermin protein family: Emerging roles in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 366–387. [Google Scholar] [CrossRef]
- Ma, F.; Ghimire, L.; Ren, Q.; Fan, Y.; Chen, T.; Balasubramanian, A.; Hsu, A.; Liu, F.; Yu, H.; Xie, X.; et al. Gasdermin E dictates inflammatory responses by controlling the mode of neutrophil death. Nat. Commun. 2024, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- von Mässenhausen, A.; Zamora Gonzalez, N.; Maremonti, F.; Belavgeni, A.; Tonnus, W.; Meyer, C.; Beer, K.; Hannani, M.T.; Lau, A.; Peitzsch, M.; et al. Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor-induced dipeptidase-1 expression and glutathione depletion. Sci. Adv. 2022, 8, eabl8920. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, H.; Xiao, Y.; Guo, Y.; Wan, X.; Li, X.; Li, M.; Liang, J.; Zhai, Y.; Liu, W.; et al. xCT regulates redox homeostasis and promotes photoreceptor survival after retinal detachment. Free. Radic. Biol. Med. 2020, 158, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Lu, H.; Ma, D.; Liu, J.; Luo, L.; Wang, L.; Qin, Y. Melatonin protects photoreceptor cells against ferroptosis in dry AMD disorder by inhibiting GSK-3B/Fyn-dependent Nrf2 nuclear translocation. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166969. [Google Scholar] [CrossRef] [PubMed]
- Getter, T.; Suh, S.; Hoang, T.; Handa, J.T.; Dong, Z.; Ma, X.; Chen, Y.; Blackshaw, S.; Palczewski, K. The selective estrogen receptor modulator raloxifene mitigates the effect of all-trans-retinal toxicity in photoreceptor degeneration. J. Biol. Chem. 2019, 294, 9461–9475. [Google Scholar] [CrossRef]
- Zhang, J.; Kiser, P.D.; Badiee, M.; Palczewska, G.; Dong, Z.; Golczak, M.; Tochtrop, G.P.; Palczewski, K. Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J. Clin. Investig. 2015, 125, 2781–2794. [Google Scholar] [CrossRef]
- Maeda, T.; Maeda, A.; Matosky, M.; Okano, K.; Roos, S.; Tang, J.; Palczewski, K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4917–4925. [Google Scholar] [CrossRef]
- Travis, G.H.; Golczak, M.; Moise, A.R.; Palczewski, K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 469–512. [Google Scholar] [CrossRef]
- Lee, M.; Li, S.; Sato, K.; Jin, M. Interphotoreceptor Retinoid-Binding Protein Mitigates Cellular Oxidative Stress and Mitochondrial Dysfunction Induced by All-trans-Retinal. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1553–1562. [Google Scholar] [CrossRef]
- Zhang, T.; Gillies, M.; Wang, Y.; Shen, W.; Bahrami, B.; Zeng, S.; Zhu, M.; Yao, W.; Zhou, F.; Murray, M.; et al. Simvastatin protects photoreceptors from oxidative stress induced by all-trans-retinal, through the up-regulation of interphotoreceptor retinoid binding protein. Br. J. Pharmacol. 2019, 176, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Boozari, M.; Hosseinzadeh, H. Crocin molecular signaling pathways at a glance: A comprehensive review. Phytother. Res. 2022, 36, 3859–3884. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef]
- Sokar, S.S.; Alkabbani, M.A.; Akool, E.-S.; Abu-Risha, S.E.-S. Hepatoprotective effects of carvedilol and crocin against leflunomide-induced liver injury. Int. Immunopharmacol. 2022, 113, 109297. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ruan, J.; Wu, R.; Zhao, M.; Zhao, T.; Qi, M.; Yau, S.S.Y.; Yao, G.; Zhang, H.; Hu, Y.; et al. A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling. J. Adv. Res. 2023, 43, 219–231. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Lu, W.; Xu, L.; Kuang, L.; Hua, D. ROS-sensitive Crocin-loaded chitosan microspheres for lung targeting and attenuation of radiation-induced lung injury. Carbohydr. Polym. 2023, 307, 120628. [Google Scholar] [CrossRef]
- Laabich, A.; Vissvesvaran, G.P.; Lieu, K.L.; Murata, K.; McGinn, T.E.; Manmoto, C.C.; Sinclair, J.R.; Karliga, I.; Leung, D.W.; Fawzi, A.; et al. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3156–3163. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Elias, E.E.; Lyons, B.; Muruve, D.A. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol. 2023, 19, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Tang, Z.; Ju, Y.; Dai, X.; Ni, N.; Liu, Y.; Zhang, D.; Gao, H.; Sun, H.; Zhang, J.; Gu, P. HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Yang, K.; Chen, J.; Wu, Y. Crocin Protects the 661W Murine Photoreceptor Cell Line against the Toxic Effects of All-Trans-Retinal. Int. J. Mol. Sci. 2024, 25, 10124. https://doi.org/10.3390/ijms251810124
Yang B, Yang K, Chen J, Wu Y. Crocin Protects the 661W Murine Photoreceptor Cell Line against the Toxic Effects of All-Trans-Retinal. International Journal of Molecular Sciences. 2024; 25(18):10124. https://doi.org/10.3390/ijms251810124
Chicago/Turabian StyleYang, Bo, Kunhuan Yang, Jingmeng Chen, and Yalin Wu. 2024. "Crocin Protects the 661W Murine Photoreceptor Cell Line against the Toxic Effects of All-Trans-Retinal" International Journal of Molecular Sciences 25, no. 18: 10124. https://doi.org/10.3390/ijms251810124
APA StyleYang, B., Yang, K., Chen, J., & Wu, Y. (2024). Crocin Protects the 661W Murine Photoreceptor Cell Line against the Toxic Effects of All-Trans-Retinal. International Journal of Molecular Sciences, 25(18), 10124. https://doi.org/10.3390/ijms251810124




