Biomarker Discovery in Liver Disease Using Untargeted Metabolomics in Plasma and Saliva
Abstract
:1. Introduction
2. Results
2.1. Saliva Metabolites Associated with Liver Disease
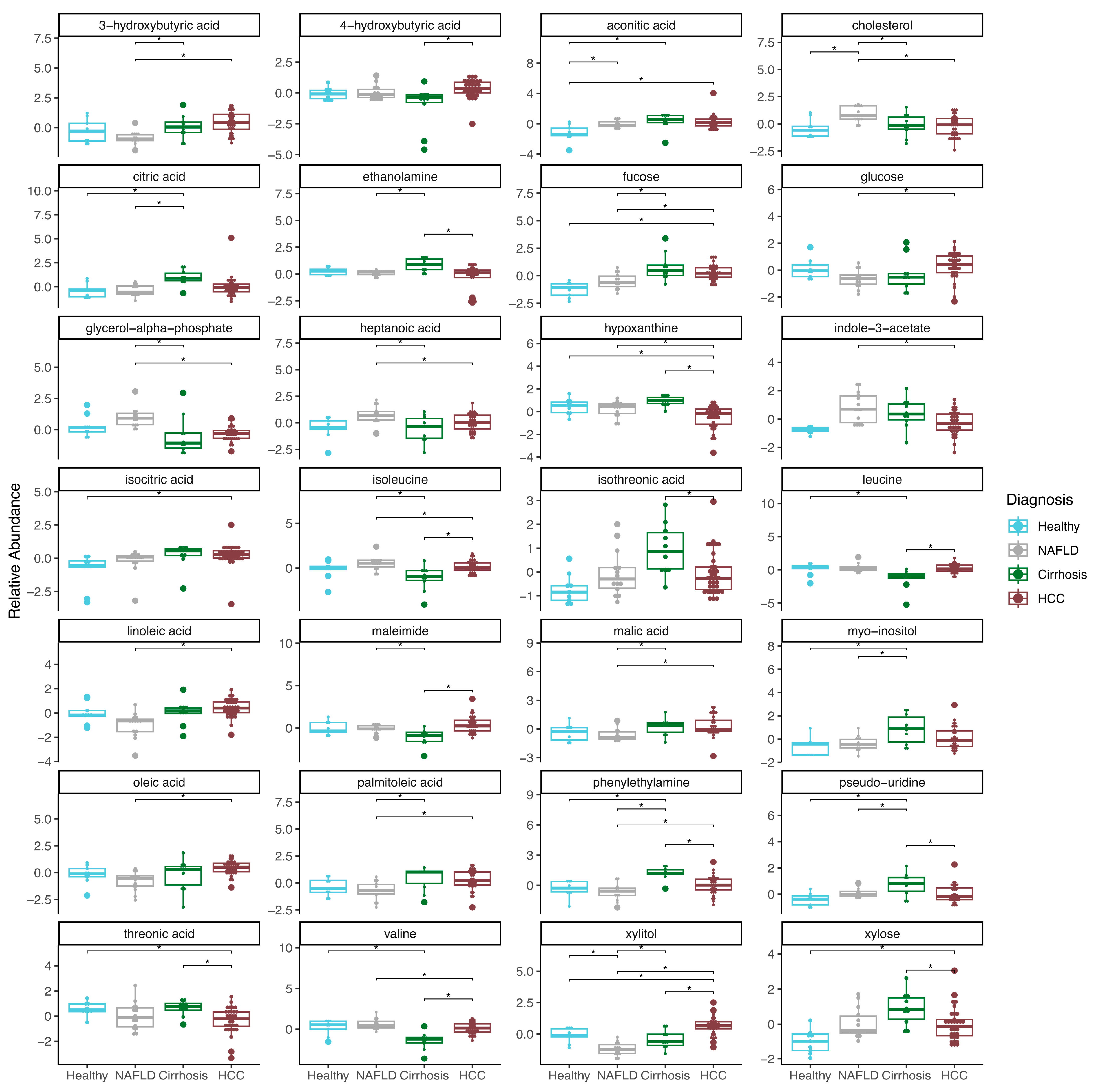
2.2. Plasma Metabolites Associated with Liver Disease
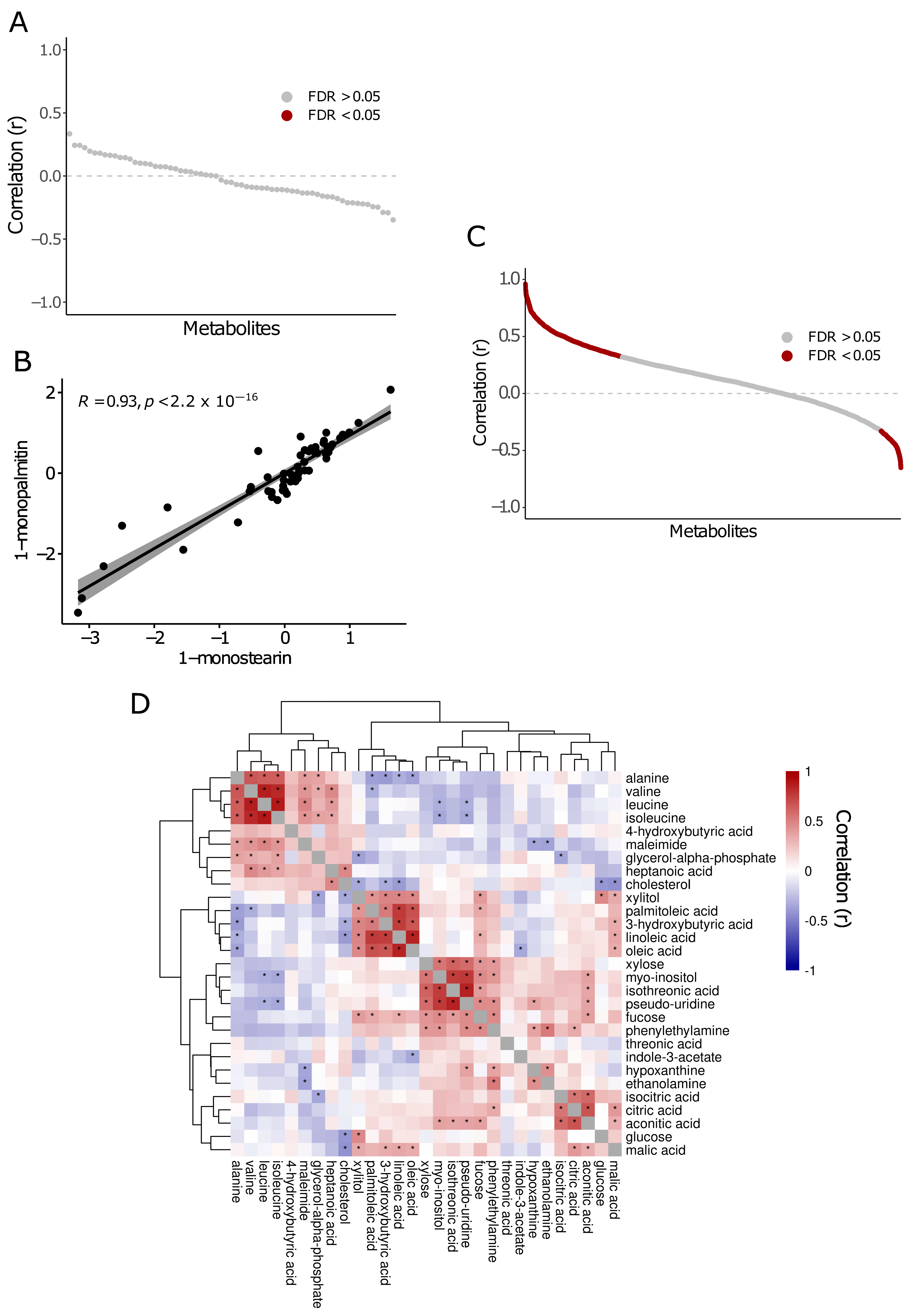
2.3. Metabolite Correlations in Saliva and Plasma
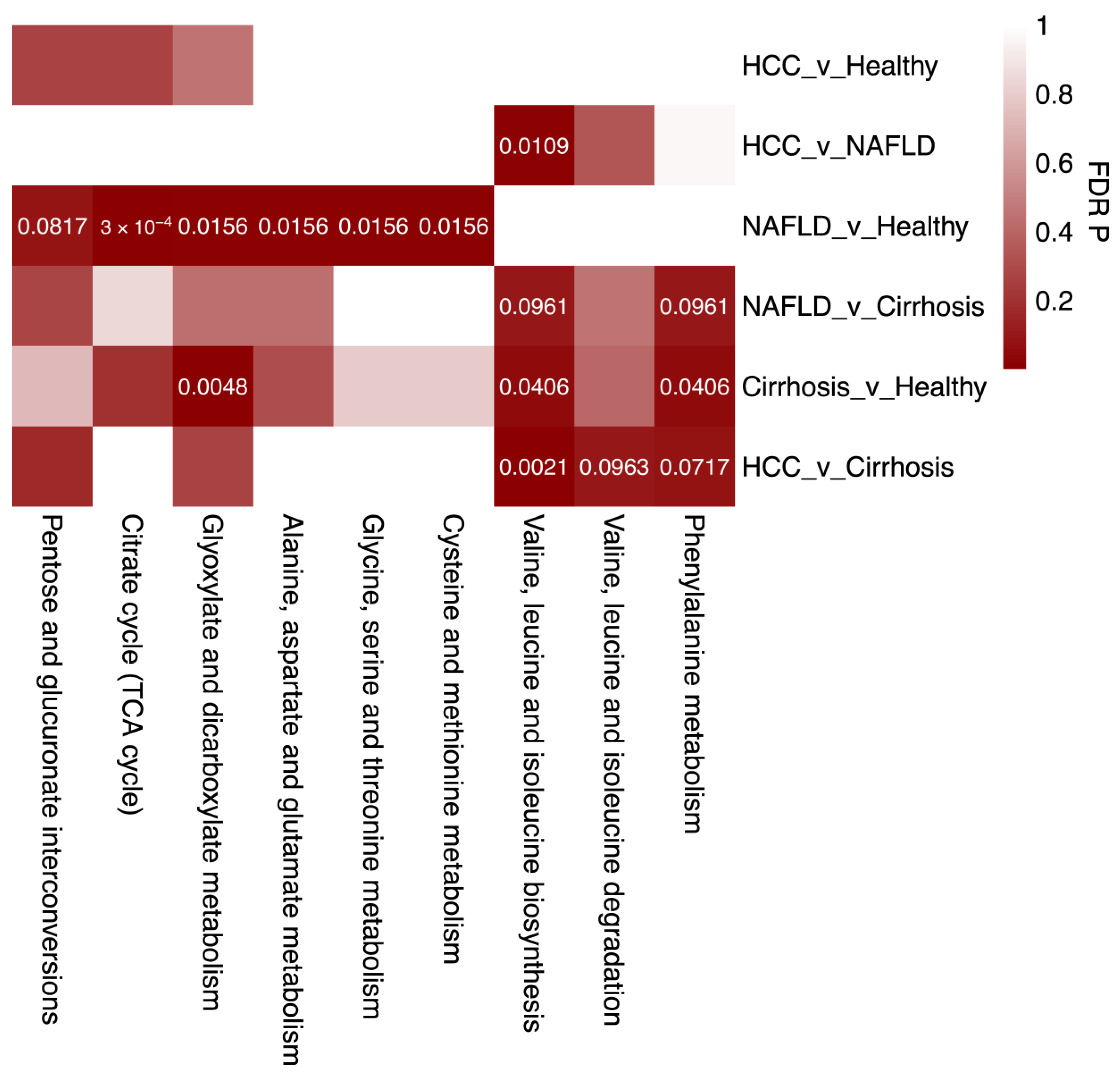
2.4. Enriched Metabolic Pathways Associated with Liver Disease
3. Discussion
3.1. Salivary Metabolites Associated with Liver Disease
3.2. Plasma Metabolites Associated with Liver Disease
4. Materials and Methods
4.1. Subject Recruitment
4.2. Biofluid Collection and Mass Spectrometry
4.3. Metabolomics Data Processing and QC
4.4. Metabolite Associations with Disease Status
4.5. Metabolite Correlations in Biofluids
4.6. Metabolite Pathway Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, J.H.; Jun, D.W. The latest global burden of liver cancer: A past and present threat. Clin. Mol. Hepatol. 2023, 29, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, E.; Paul, S.B.; Kedia, S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J. Med. Res. 2019, 149, 9–17. [Google Scholar] [PubMed]
- Tovo, C.V.; de Mattos, A.Z.; Coral, G.P.; Sartori, G.D.P.; Nogueira, L.V.; Both, G.T.; Villela-Nogueira, C.A.; de Mattos, A.A. Hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis. World J. Gastroenterol. 2023, 29, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.; Cepin, S.; Tesfai, K.; Hofflich, H.; Cadman, K.; Lopez, S.; Madamba, E.; Bettencourt, R.; Richards, L.; Behling, C.; et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J. Hepatol. 2023, 78, 471–478. [Google Scholar] [CrossRef]
- Barb, D.; Repetto, E.M.; Stokes, M.E.; Shankar, S.S.; Cusi, K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity 2021, 29, 1950–1960. [Google Scholar] [CrossRef]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef]
- Daher, D.; Dahan, K.S.E.; Singal, A.G. Non-alcoholic fatty liver disease-related hepatocellular carcinoma. J. Liver Cancer 2023, 23, 127–142. [Google Scholar] [CrossRef]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- Leung, C.; Yeoh, S.W.; Patrick, D.; Ket, S.; Marion, K.; Gow, P.; Angus, P.W. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Mittal, S.; Sada, Y.H.; El-Serag, H.B.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 2015, 13, 594–601.e1. [Google Scholar] [CrossRef]
- Hershberger, C.E.; Raj, R.; Mariam, A.; Aykun, N.; Allende, D.S.; Brown, M.; Aucejo, F.; Rotroff, D.M. Characterization of Salivary and Plasma Metabolites as Biomarkers for HCC: A Pilot Study. Cancers 2023, 15, 4527. [Google Scholar] [CrossRef]
- Hershberger, C.E.; Rodarte, A.I.; Siddiqi, S.; Moro, A.; Acevedo-Moreno, L.A.; Brown, J.M.; Allende, D.S.; Aucejo, F.; Rotroff, D.M. Salivary Metabolites are Promising Non-Invasive Biomarkers of Hepatocellular Carcinoma and Chronic Liver Disease. Liver Cancer Int. 2021, 2, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Mojsak, P.; Buczynska, A.; Suleria, H.A.R.; Kretowski, A.; Ciborowski, M. Altered Metabolome of Lipids and Amino Acids Species: A Source of Early Signature Biomarkers of T2DM. J. Clin. Med. 2020, 9, 2257. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Göring, H.; Cole, S.A.; Comuzzie, A.G. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.T.; Fiehn, O.; Elfert, A.; Helal, M.; Salama, I.; El-Said, H. Sugar Alcohols Have a Key Role in Pathogenesis of Chronic Liver Disease and Hepatocellular Carcinoma in Whole Blood and Liver Tissues. Cancers 2020, 12, 484. [Google Scholar] [CrossRef]
- Osman, D.; Ali, O.; Obada, M.; El-Mezayen, H.; El-Said, H. Chromatographic determination of some biomarkers of liver cirrhosis and hepatocellular carcinoma in Egyptian patients. Biomed. Chromatogr. 2017, 31, e3893. [Google Scholar] [CrossRef]
- Troisi, J.; Pierri, L.; Landolfi, A.; Marciano, F.; Bisogno, A.; Belmonte, F.; Palladino, C.; Guercio Nuzio, S.; Campiglia, P.; Vajro, P. Urinary Metabolomics in Pediatric Obesity and NAFLD Identifies Metabolic Pathways/Metabolites Related to Dietary Habits and Gut-Liver Axis Perturbations. Nutrients 2017, 9, 485. [Google Scholar] [CrossRef]
- Montanari, A.; Simoni, I.; Vallisa, D.; Trifirò, A.; Colla, R.; Abbiati, R.; Borghi, L.; Novarini, A. Free amino acids in plasma and skeletal muscle of patients with liver cirrhosis. Hepatology 1988, 8, 1034–1039. [Google Scholar] [CrossRef]
- Fischer, J.E.; Funovics, J.M.; Aguirre, A.; James, J.H.; Keane, J.M.; Wesdorp, R.I.; Yoshimura, N.; Westman, T. The role of plasma amino acids in hepatic encephalopathy. Surgery 1975, 78, 276–290. [Google Scholar]
- Kinny-Köster, B.; Bartels, M.; Becker, S.; Scholz, M.; Thiery, J.; Ceglarek, U.; Kaiser, T. Plasma Amino Acid Concentrations Predict Mortality in Patients with End-Stage Liver Disease. PLoS ONE 2016, 11, e0159205. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. World J. Gastroenterol. 2013, 19, 7620–7629. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Huang, X.; Liao, X.; Zhou, X.; Peng, K.; Wei, Y.; Han, C.; Peng, T.; Wang, J.; Zhu, G. PUS1 May Be a Potential Prognostic Biomarker and Therapeutic Target for Hepatocellular Carcinoma. Pharmgenomics Pers. Med. 2023, 16, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Song, M.; Wang, J.; Zhu, W.; Sun, D.; Liu, H.; Shi, G. Integrative multiomics evaluation reveals the importance of pseudouridine synthases in hepatocellular carcinoma. Front. Genet. 2022, 13, 944681. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Diao, L.T.; Hou, Y.R.; Lv, G.; Tao, S.; Xu, W.Y.; Xie, S.J.; Ren, Y.H.; Xiao, Z.D. Pseudouridine synthase 1 promotes hepatocellular carcinoma through mRNA pseudouridylation to enhance the translation of oncogenic mRNAs. Hepatology 2023. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Garcia-Tsao, G.; Reddy, K.R.; O’Leary, J.G.; Vargas, H.E.; Lai, J.C.; Kamath, P.S.; Tandon, P.; Subramanian, R.M.; Thuluvath, P.; et al. Admission Urinary and Serum Metabolites Predict Renal Outcomes in Hospitalized Patients With Cirrhosis. Hepatology 2021, 74, 2699–2713. [Google Scholar] [CrossRef]
- Amuro, Y.; Nakaoka, H.; Shimomura, S.; Fujikura, M.; Yamamoto, T.; Tamura, S.; Hada, T.; Higashino, K. Serum pseudouridine as a biochemical marker in patients with hepatocellular carcinoma. Clin. Chim. Acta 1988, 178, 151–158. [Google Scholar] [CrossRef]
- Tamura, S.; Fujioka, H.; Nakano, T.; Amuro, Y.; Hada, T.; Nakao, N.; Higashino, K. Urinary pseudouridine as a biochemical marker in the diagnosis and monitoring of primary hepatocellular carcinoma. Am. J. Gastroenterol. 1988, 83, 841–845. [Google Scholar]
- Chen, G.L.; Ye, T.; Chen, H.L.; Zhao, Z.Y.; Tang, W.Q.; Wang, L.S.; Xia, J.L. Xanthine dehydrogenase downregulation promotes TGFβ signaling and cancer stem cell-related gene expression in hepatocellular carcinoma. Oncogenesis 2017, 6, e382. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Z.; Lu, Y.; Liu, Q.; Xu, X.; Xu, J.; Liu, Y.; Yu, H.; Yu, D.; Sun, B. Loss of Xanthine Oxidoreductase Potentiates Propagation of Hepatocellular Carcinoma Stem Cells. Hepatology 2020, 71, 2033–2049. [Google Scholar] [CrossRef]
- Lin, Z.; Xie, Y.Z.; Zhao, M.C.; Hou, P.P.; Tang, J.; Chen, G.L. Xanthine dehydrogenase as a prognostic biomarker related to tumor immunology in hepatocellular carcinoma. Cancer Cell. Int. 2021, 21, 475. [Google Scholar] [CrossRef]
- Linder, N.; Bützow, R.; Lassus, H.; Lundin, M.; Lundin, J. Decreased xanthine oxidoreductase (XOR) is associated with a worse prognosis in patients with serous ovarian carcinoma. Gynecol. Oncol. 2012, 124, 311–318. [Google Scholar] [CrossRef]
- Linder, N.; Martelin, E.; Lundin, M.; Louhimo, J.; Nordling, S.; Haglund, C.; Lundin, J. Xanthine oxidoreductase—Clinical significance in colorectal cancer and in vitro expression of the protein in human colon cancer cells. Eur. J. Cancer 2009, 45, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, K.; Xie, Q.; Wu, Q.; Mack, S.C.; Shi, Y.; Kim, L.J.Y.; Prager, B.C.; Flavahan, W.A.; Liu, X.; et al. Purine synthesis promotes maintenance of brain tumor initiating cells in glioma. Nat. Neurosci. 2017, 20, 661–673. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Bruntz, R.C.; Yang, Y.; Song, H.; Chernyavskaya, Y.; Deng, P.; Zhang, Y.; Shah, P.P.; Beverly, L.J.; Qi, Z.; et al. De novo synthesis of serine and glycine fuels purine nucleotide biosynthesis in human lung cancer tissues. J. Biol. Chem. 2019, 294, 13464–13477. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wan, D.; Zhao, C.; Chen, J.; Zhao, X.; Wang, W.; Lu, X.; Yang, S.; Gu, J.; Xu, G. A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Mol. Biosyst. 2009, 5, 868–876. [Google Scholar] [CrossRef]
- Fitian, A.I.; Nelson, D.R.; Liu, C.; Xu, Y.; Ararat, M.; Cabrera, R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 2014, 34, 1428–1444. [Google Scholar] [CrossRef]
- Gurian, E.; Di Silvestre, A.; Mitri, E.; Pascut, D.; Tiribelli, C.; Giuffrè, M.; Crocè, L.S.; Sergo, V.; Bonifacio, A. Repeated double cross-validation applied to the PCA-LDA classification of SERS spectra: A case study with serum samples from hepatocellular carcinoma patients. Anal. Bioanal. Chem. 2021, 413, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xue, R.; Dong, L.; Liu, T.; Deng, C.; Zeng, H.; Shen, X. Metabolomic profiling of human urine in hepatocellular carcinoma patients using gas chromatography/mass spectrometry. Anal. Chim. Acta 2009, 648, 98–104. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]

| Saliva | ||||
|---|---|---|---|---|
| Characteristic | Healthy, N = 9 1 | NAFLD, N = 14 1 | Cirrhosis, N = 10 1 | HCC, N = 22 1 |
| Age | 34 (30, 44) | 55 (42, 62) | 64 (62, 66) | 67 (61, 77) |
| Sex | ||||
| Male | 5 (56%) | 4 (29%) | 7 (70%) | 17 (77%) |
| Female | 4 (44%) | 10 (71%) | 3 (30%) | 5 (23%) |
| BMI | 32.6 (28.4, 34.9) | 30.9 (28.9, 35.1) | 28.5 (27.7, 34.9) | 28.2 (26.5, 32.3) |
| Cirrhosis Status | ||||
| Compensated | - | - | 2 (20%) | 7 (32%) |
| Mild | - | - | - | - |
| Decompensated | - | - | 8 (80%) | 7 (32%) |
| Unspecified | - | - | - | 1 (4.5%) |
| None | 9 (100%) | 14 (100%) | - | 7 (32%) |
| Cirrhosis Etiology | ||||
| Alcohol | - | - | 5 (50%) | 1 (4.5%) |
| Alcohol/HBV/HCV | - | - | - | 1 (4.5%) |
| HBV/HCV | - | - | - | 8 (36%) |
| NAFLD/NASH | - | - | 5 (50%) | 2 (9.1%) |
| Other | - | - | - | - |
| Origin Unknown | - | - | - | 3 (14%) |
| None | 9 (100%) | 14 (100%) | - | 7 (32%) |
| Child Pugh | ||||
| A | 9 (100%) | 14 (100%) | 3 (30%) | 18 (82%) |
| B | - | - | 5 (50%) | 4 (18%) |
| C | - | - | 2 (20%) | - |
| Tumor Lesion | ||||
| Multiple | - | - | - | 6 (27%) |
| Single | - | - | - | 16 (73%) |
| Tumor Size | ||||
| <4 | - | - | - | 12 (55%) |
| ≥4 | - | - | - | 10 (45%) |
| Smoker | 2 (22%) | 2 (14%) | 7 (70%) | 13 (59%) |
| Diabetes | ||||
| Type 2 DM | - | 7 (50%) | 5 (50%) | 10 (45%) |
| Prediabetes | - | 2 (14%) | - | - |
| No | 9 (100%) | 5 (36%) | 5 (50%) | 12 (55%) |
| Hypertension | 1 (11%) | 11 (79%) | 8 (80%) | 21 (95%) |
| Coronary Artery Disease | 1 (11%) | 4 (29%) | 4 (40%) | 5 (23%) |
| Hyperlipidemia | 2 (22%) | 10 (71%) | 7 (70%) | 9 (41%) |
| Psychiatric Disorder | 1 (11%) | 4 (29%) | 5 (50%) | 4 (18%) |
| COPD/Asthma/OSA | - | 6 (43%) | 4 (40%) | 7 (32%) |
| Other Cancer | - | 7 (50%) | 1 (10%) | 6 (27%) |
| Thyroid disease | - | 3 (21%) | 2 (20%) | 4 (18%) |
| Other PMH | 3 (33%) | 14 (100%) | 10 (100%) | 19 (86%) |
| Ascites | - | 1 (7.1%) | 7 (70%) | 7 (32%) |
| Encephalopathy | - | - | 7 (70%) | 6 (27%) |
| Mean Hemoglobin (g/dL) | 14.50 (13.50, 14.80) | 12.95 (12.48, 13.98) | 10.95 (8.55, 13.95) | 11.80 (10.23, 13.18) |
| Mean Platelets (k/uL) | 250 (231, 289) | 293 (253, 332) | 99 (84, 118) | 187 (143, 218) |
| Mean AST (U/L) | 20 (18, 25) | 36 (26, 46) | 62 (41, 125) | 119 (84, 232) |
| Mean ALT (U/L) | 24 (16, 29) | 46 (29, 53) | 27 (24, 134) | 125 (68, 291) |
| Mean ALP (U/L) | 63 (54, 72) | 97 (82, 195) | 124 (79, 179) | 114 (77, 144) |
| Mean Bilirubin, Total (mg/dL) | 0.40 (0.30, 0.50) | 0.50 (0.43, 0.58) | 1.30 (1.10, 1.40) | 1.00 (0.63, 1.48) |
| Mean Albumin (g/dL) | 4.80 (4.30, 4.80) | 4.40 (4.15, 4.50) | 3.55 (3.03, 3.70) | 3.50 (3.25, 3.78) |
| Mean PT-INR | 1.00 (1.00, 1.10) | 1.00 (1.00, 1.00) | 1.20 (1.13, 1.20) | 1.20 (1.10, 1.30) |
| Mean Glucose (mg/dL) | 89 (87, 91) | 97 (88, 110) | 138 (122, 164) | 122 (112, 140) |
| Mean Creatinine (mg/dL) | 0.82 (0.81, 0.90) | 0.82 (0.73, 0.93) | 1.36 (0.71, 1.86) | 0.92 (0.77, 1.29) |
| Plasma | ||||
|---|---|---|---|---|
| Characteristic | Healthy, N = 9 1 | NAFLD, N = 14 1 | Cirrhosis, N = 10 1 | HCC, N = 34 1 |
| Age | 41 (30, 46) | 55 (42, 62) | 64 (62, 66) | 68 (63, 74) |
| Sex | ||||
| Male | 4 (44%) | 4 (29%) | 7 (70%) | 26 (76%) |
| Female | 5 (56%) | 10 (71%) | 3 (30%) | 8 (24%) |
| BMI | 32.8 (30.2, 34.9) | 30.9 (28.9, 35.1) | 28.5 (27.7, 34.9) | 29.7 (26.5, 32.4) |
| Cirrhosis Status | ||||
| Compensated | - | - | 2 (20%) | 8 (24%) |
| Mild | - | - | - | 1 (2.9%) |
| Decompensated | - | - | 8 (80%) | 8 (24%) |
| Unspecified | - | - | - | 1 (2.9%) |
| None | 9 (100%) | 14 (100%) | - | 16 (47%) |
| Cirrhosis Etiology | ||||
| Alcohol | - | - | 5 (50%) | 2 (5.9%) |
| Alcohol/HBV/HCV | - | - | - | 2 (5.9%) |
| HBV/HCV | - | - | - | 7 (21%) |
| NAFLD/NASH | - | - | 5 (50%) | 2 (5.9%) |
| Other | - | - | - | 2 (5.9%) |
| Origin Unknown | - | - | - | 3 (8.8%) |
| None | 9 (100%) | 14 (100%) | - | 16 (47%) |
| Child Pugh | ||||
| A | 9 (100%) | 14 (100%) | 3 (30%) | 27 (79%) |
| B | - | - | 5 (50%) | 7 (21%) |
| C | - | - | 2 (20%) | - |
| Tumor Lesion | ||||
| Multiple | - | - | - | 13 (38%) |
| Single | - | - | - | 21 (62%) |
| Tumor Size | ||||
| <4 | - | - | - | 14 (41%) |
| ≥4 | - | - | - | 20 (59%) |
| Smoker | 2 (22%) | 2 (14%) | 7 (70%) | 23 (68%) |
| Diabetes | ||||
| Type 2 DM | - | 7 (50%) | 5 (50%) | 16 (47%) |
| Prediabetes | - | 2 (14%) | - | - |
| No | 9 (100%) | 5 (36%) | 5 (50%) | 18 (53%) |
| Hypertension | 1 (11%) | 11 (79%) | 8 (80%) | 27 (79%) |
| Coronary Artery Disease | 1 (11%) | 4 (29%) | 4 (40%) | 7 (21%) |
| Hyperlipidemia | 2 (22%) | 10 (71%) | 7 (70%) | 12 (35%) |
| Psychiatric Disorder | 2 (22%) | 4 (29%) | 5 (50%) | 12 (35%) |
| COPD/Asthma/OSA | - | 6 (43%) | 4 (40%) | 12 (35%) |
| Other Cancer | - | 7 (50%) | 1 (10%) | 12 (35%) |
| Thyroid disease | - | 3 (21%) | 2 (20%) | 3 (8.8%) |
| Other PMH | 4 (44%) | 14 (100%) | 10 (100%) | 33 (97%) |
| Ascites | - | 1 (7.1%) | 7 (70%) | 10 (29%) |
| Encephalopathy | - | - | 7 (70%) | 10 (29%) |
| Mean Hemoglobin (g/dL) | 13.90 (13.50, 14.60) | 12.95 (12.48, 13.98) | 10.95 (8.55, 13.95) | 12.05 (10.73, 13.08) |
| Mean Platelets (k/uL) | 269 (231, 301) | 293 (253, 332) | 99 (84, 118) | 197 (172, 231) |
| Mean AST (U/L) | 20 (18, 24) | 36 (26, 46) | 62 (41, 125) | 135 (96, 209) |
| Mean ALT (U/L) | 20 (16, 29) | 46 (29, 53) | 27 (24, 134) | 154 (82, 202) |
| Mean ALP (U/L) | 63 (54, 72) | 97 (82, 195) | 124 (79, 179) | 100 (69, 130) |
| Mean Bilirubin, Total (mg/dL) | 0.40 (0.30, 0.50) | 0.50 (0.43, 0.58) | 1.30 (1.10, 1.40) | 0.80 (0.63, 1.20) |
| Mean Albumin (g/dL) | 4.60 (4.20, 4.80) | 4.40 (4.15, 4.50) | 3.55 (3.03, 3.70) | 3.60 (3.20, 3.80) |
| Mean PT-INR | 1.00 (1.00, 1.10) | 1.00 (1.00, 1.00) | 1.20 (1.13, 1.20) | 1.10 (1.10, 1.20) |
| Mean Glucose (mg/dL) | 89 (88, 96) | 97 (88, 110) | 138 (122, 164) | 123 (108, 140) |
| Mean Creatinine (mg/dL) | 0.82 (0.69, 0.84) | 0.82 (0.73, 0.93) | 1.36 (0.71, 1.86) | 0.94 (0.69, 1.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, N.J.; Hershberger, C.E.; Kerosky, M.; Wehrle, C.J.; Raj, R.; Aykun, N.; Allende, D.S.; Aucejo, F.N.; Rotroff, D.M. Biomarker Discovery in Liver Disease Using Untargeted Metabolomics in Plasma and Saliva. Int. J. Mol. Sci. 2024, 25, 10144. https://doi.org/10.3390/ijms251810144
Daniels NJ, Hershberger CE, Kerosky M, Wehrle CJ, Raj R, Aykun N, Allende DS, Aucejo FN, Rotroff DM. Biomarker Discovery in Liver Disease Using Untargeted Metabolomics in Plasma and Saliva. International Journal of Molecular Sciences. 2024; 25(18):10144. https://doi.org/10.3390/ijms251810144
Chicago/Turabian StyleDaniels, Noah J., Courtney E. Hershberger, Matthew Kerosky, Chase J. Wehrle, Roma Raj, Nihal Aykun, Daniela S. Allende, Federico N. Aucejo, and Daniel M. Rotroff. 2024. "Biomarker Discovery in Liver Disease Using Untargeted Metabolomics in Plasma and Saliva" International Journal of Molecular Sciences 25, no. 18: 10144. https://doi.org/10.3390/ijms251810144






