microRNA Temporal-Specific Expression Profiles Reveal longissimus dorsi Muscle Development in Tianzhu White Yak
Abstract
1. Introduction
2. Results
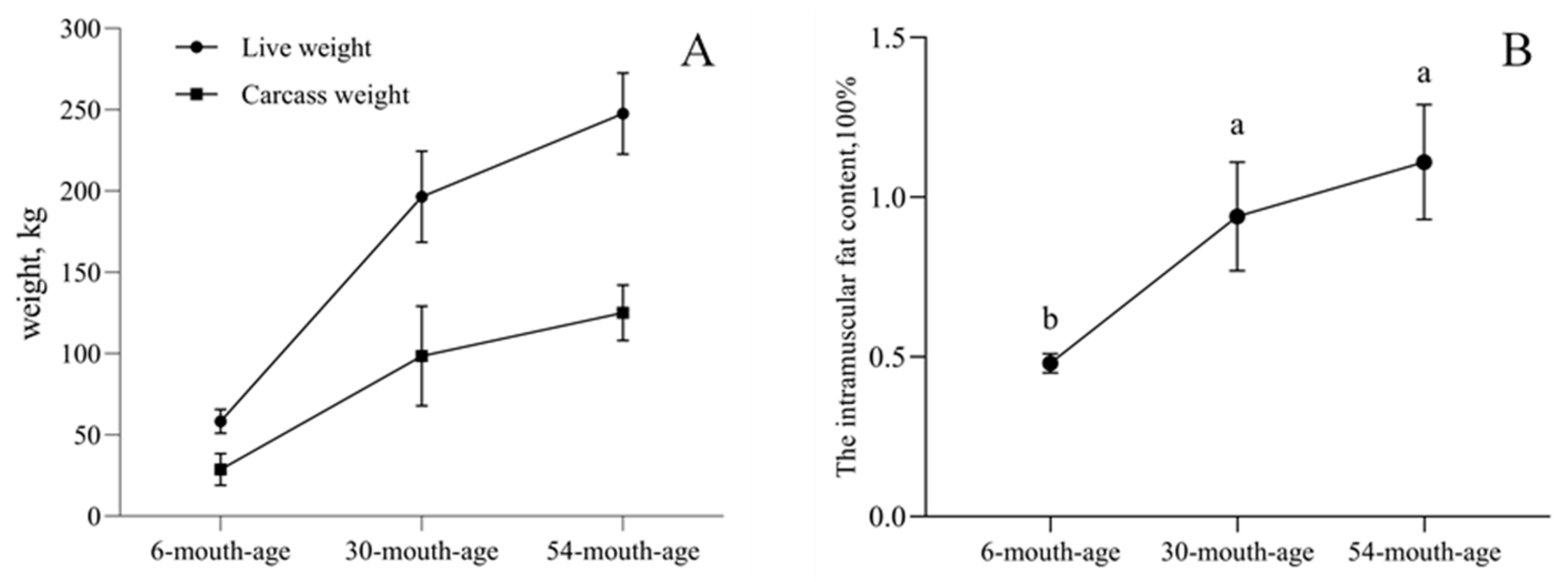
2.1. Intramuscular Fat Contents of the longissimus dorsi Muscle in Yak of Different Ages
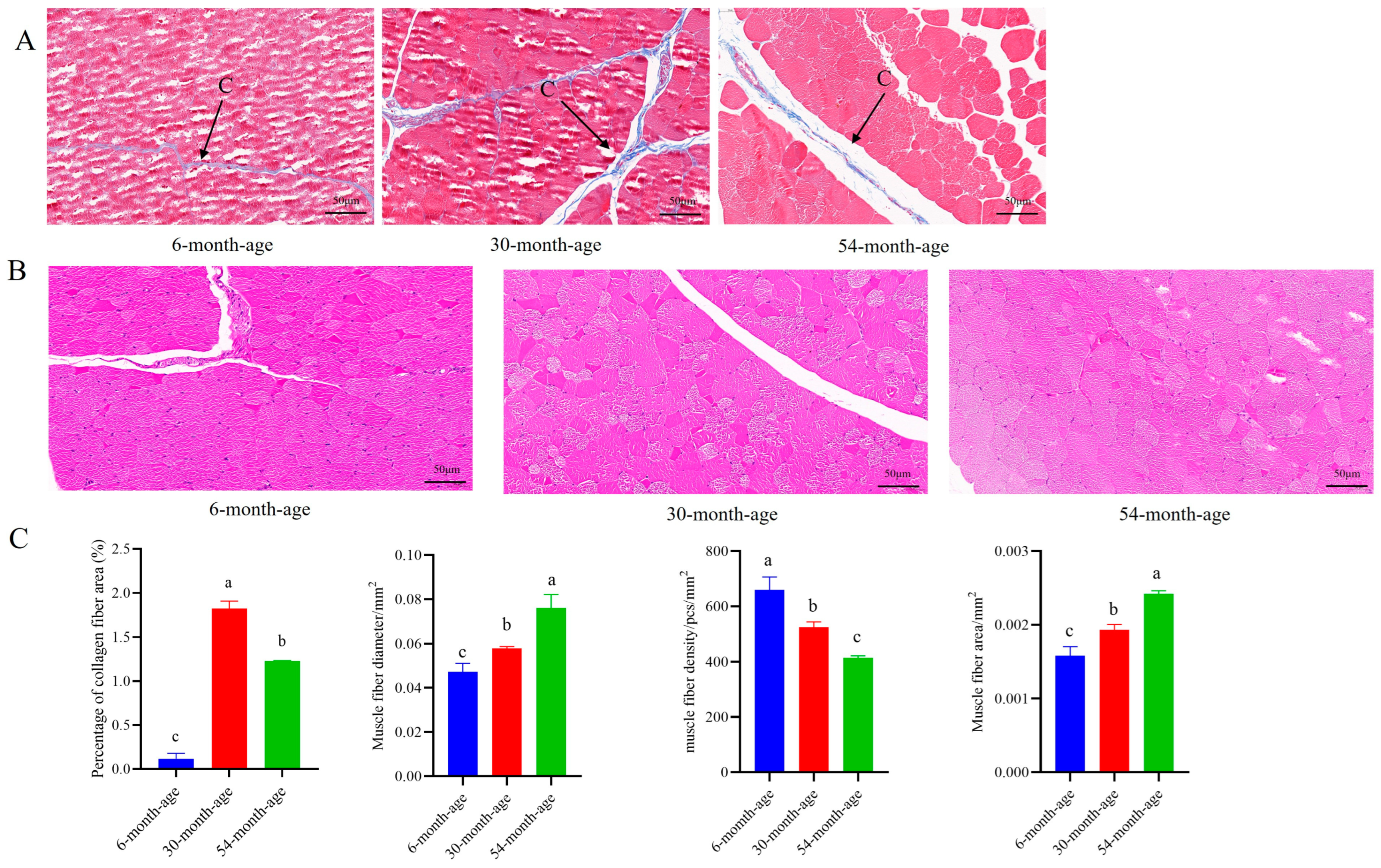
2.2. Characterization of the longissimus dorsi Muscle Tissue at Different Ages
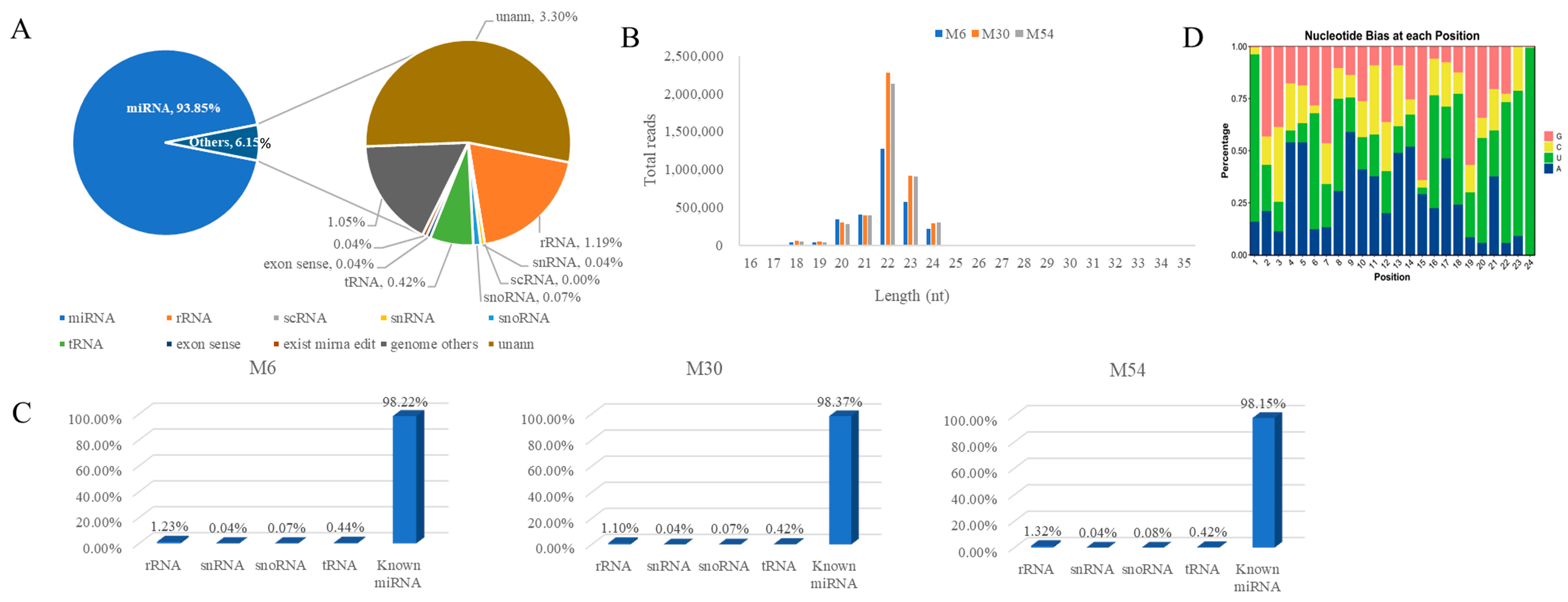
2.3. Small RNA Library Sequencing and Sequence Analysis
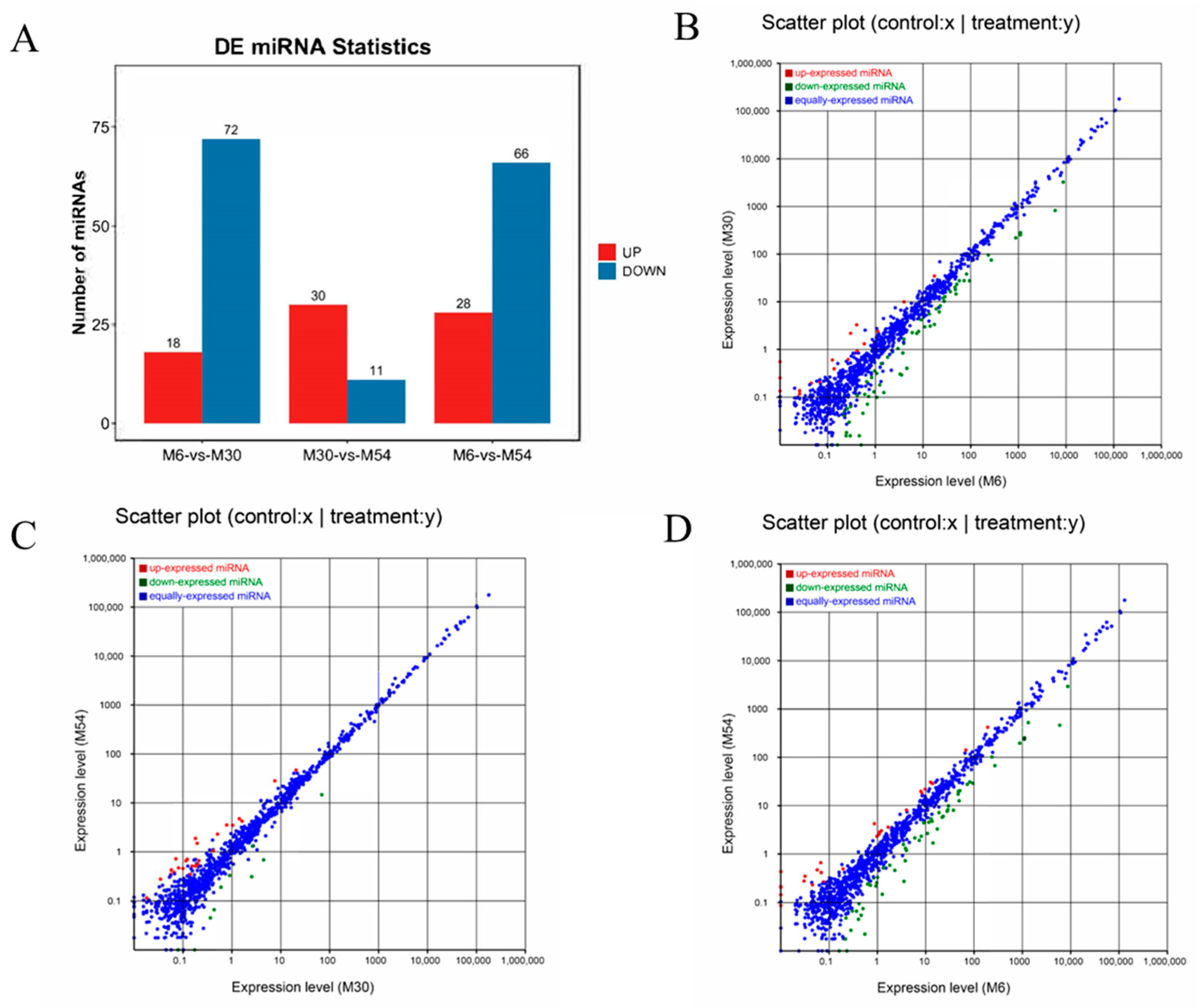
2.4. Analysis of Differentially Expressed miRNAs
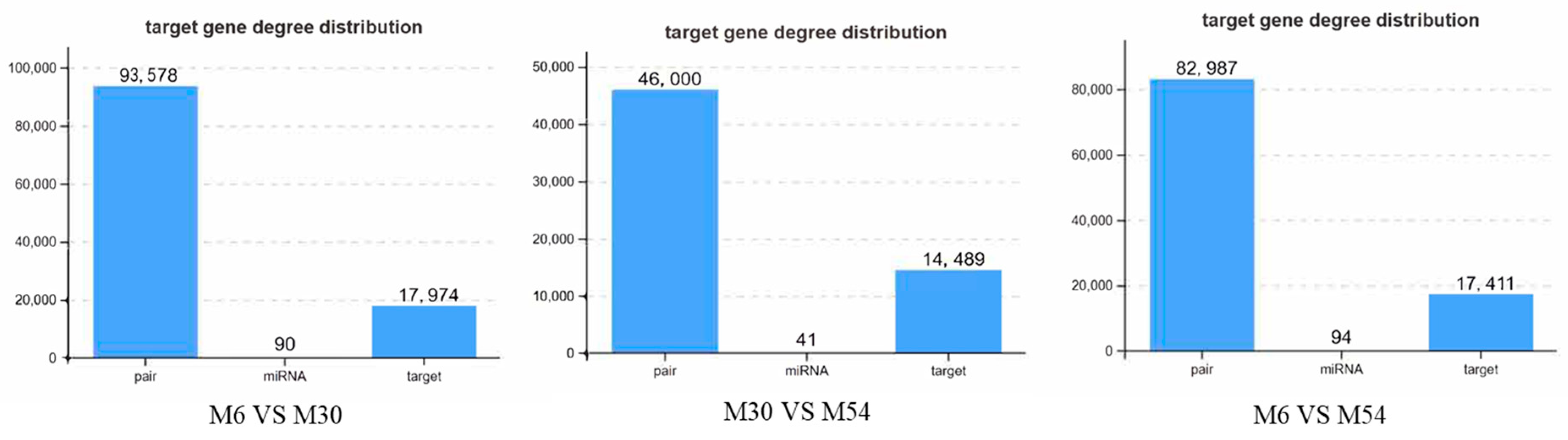
2.5. Target Gene Prediction of DE miRNAs
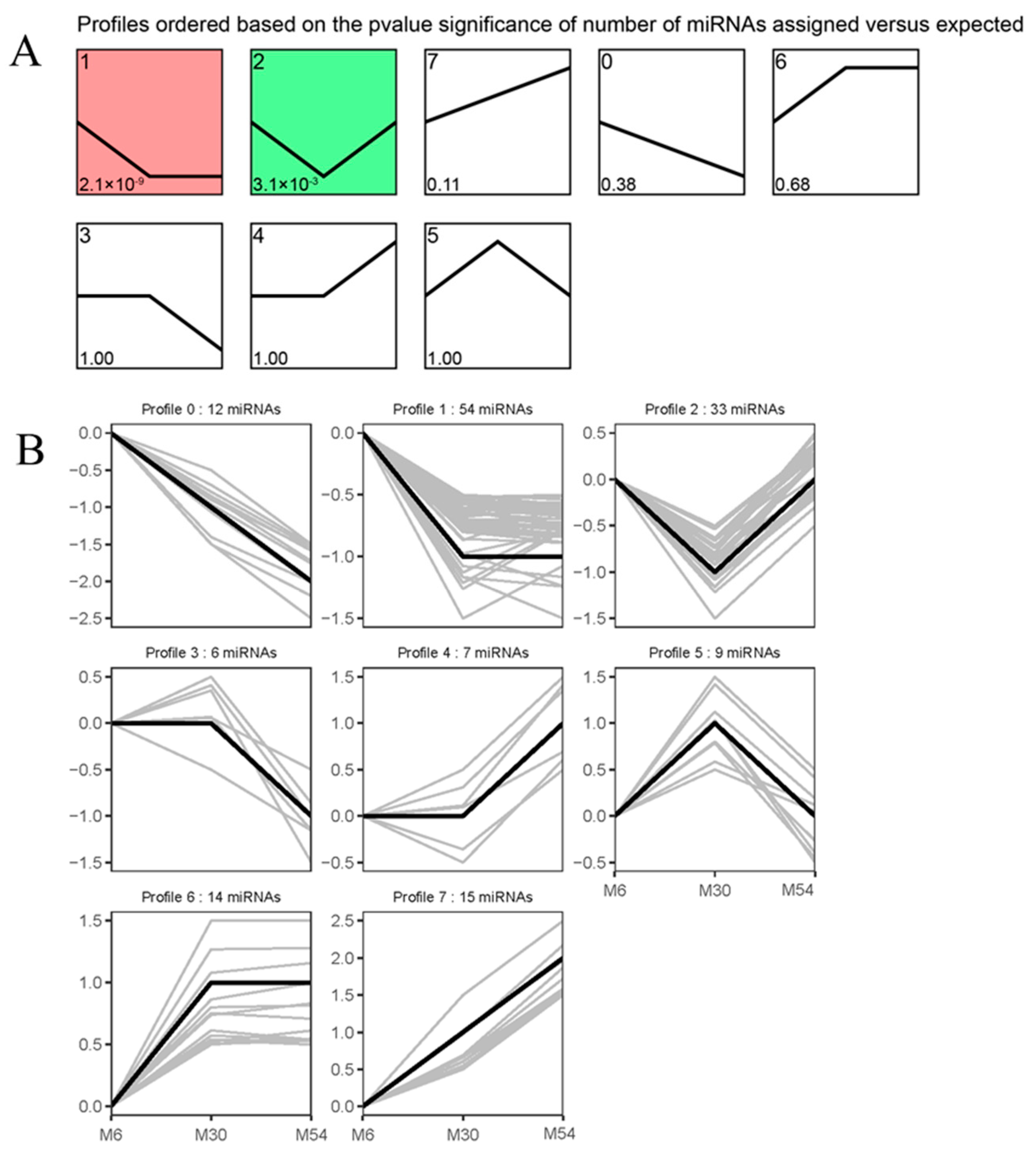
2.6. STEM Analysis of the DE miRNA Expression Profiles
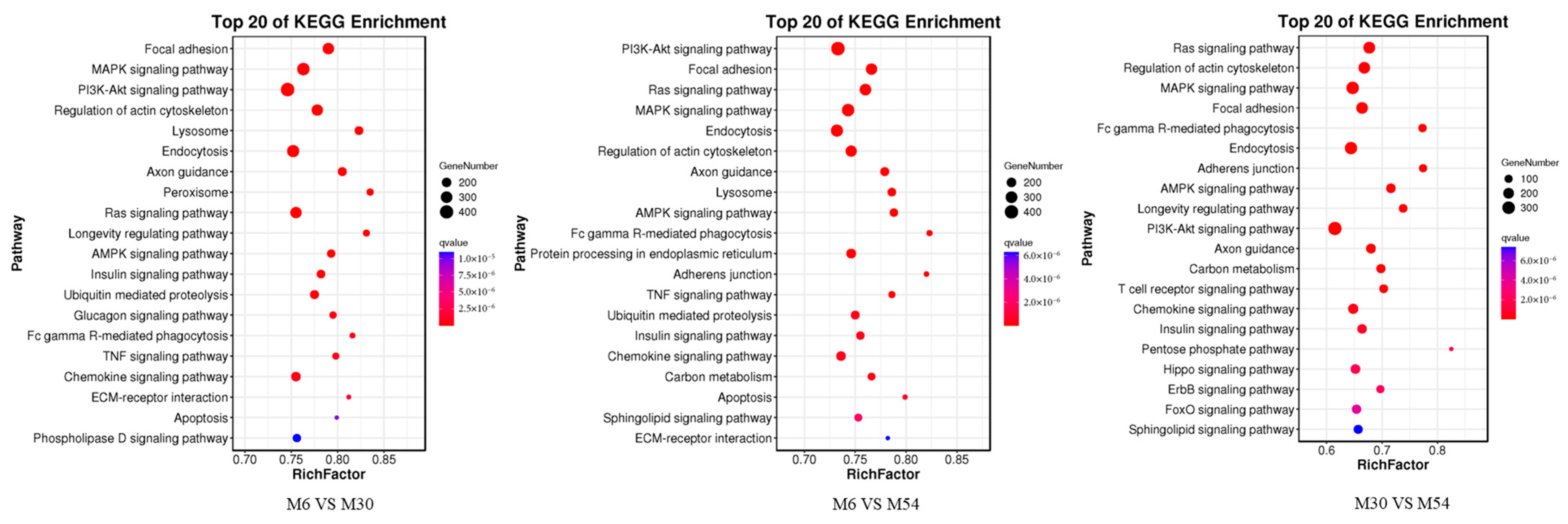
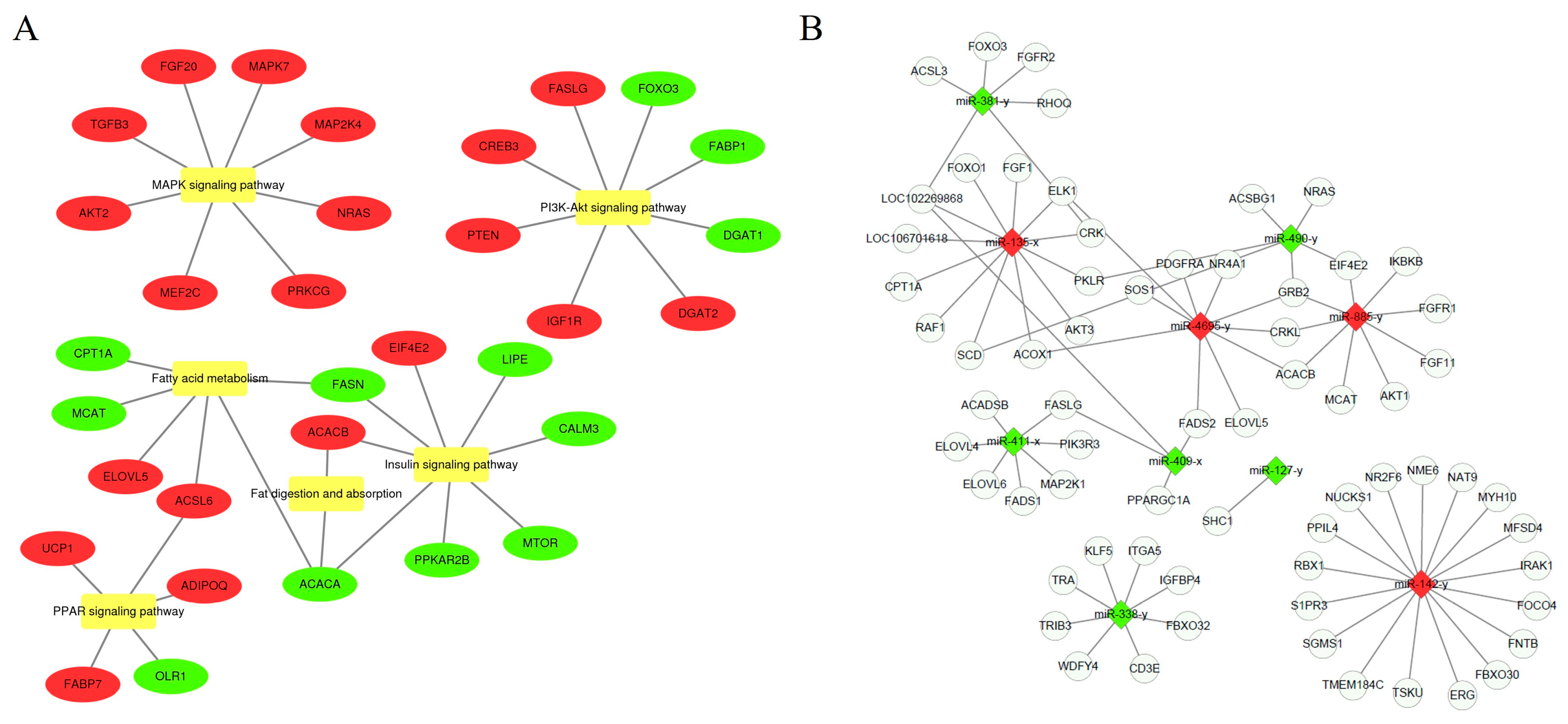
2.7. GO and KEGG Enrichment Analysis of Differentially Expressed miRNAs
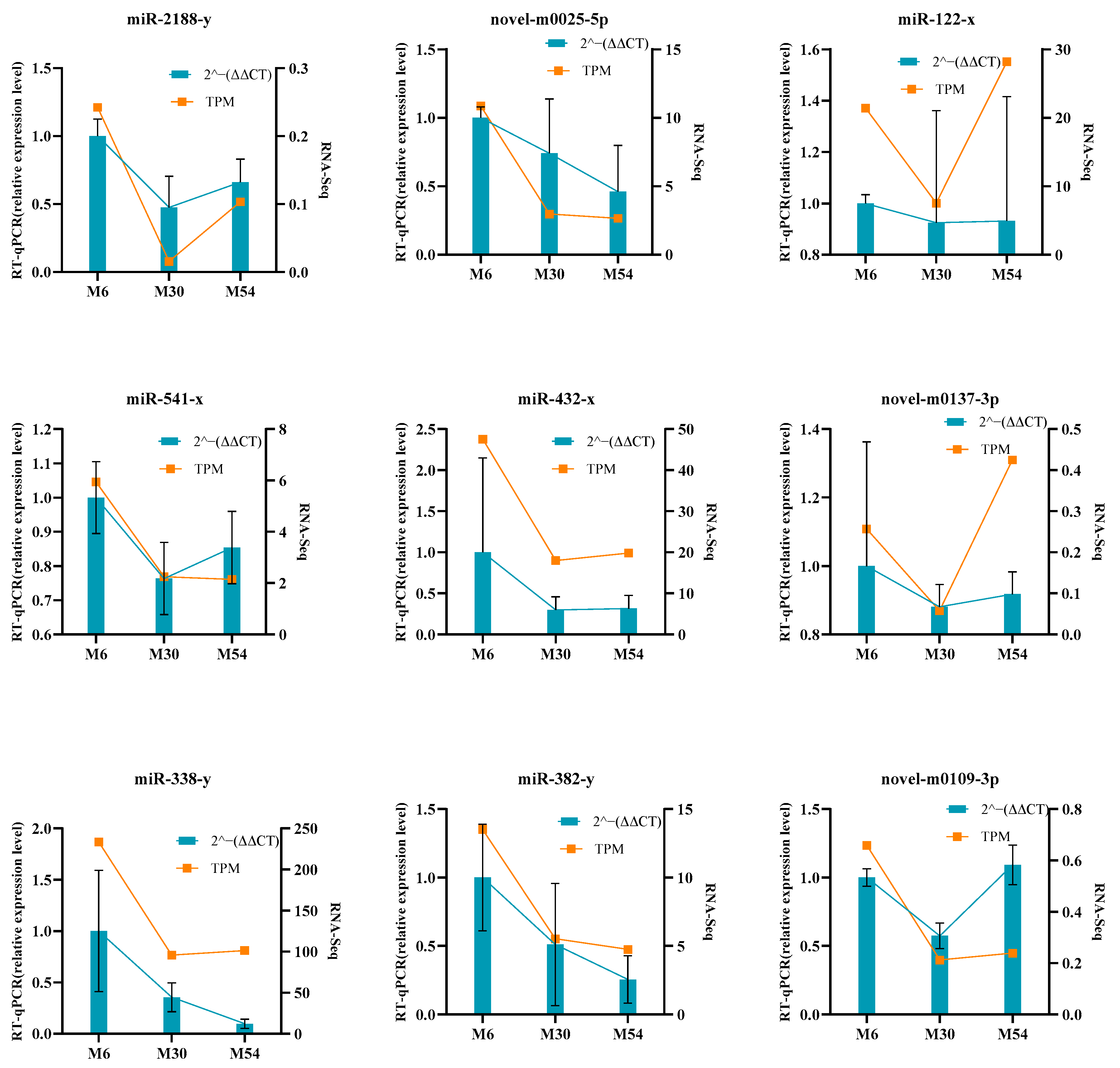
2.8. Validation of Differentially Expressed miRNAs by RT-qPCR
2.9. Construction of the miRNA–mRNA Interaction Network
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Analysis of the Intramuscular Fat Content
4.3. Staining of Muscle Tissue Using Hematoxylin and Eosin and Masson Staining of Collagen Fibers
4.4. RNA Extraction, Library Construction, and Sequencing
4.5. Sequence Analysis and Characterization of miRNAs
4.6. Differential Expression Analysis and Time-Series Expression Profile Clustering
4.7. Prediction of miRNA Targets, GO Enrichment, KEGG Pathway Analysis, and Integrated Analysis of miRNAs and Their Target Genes
4.8. Analysis of miRNA–mRNA Regulatory Networks
4.9. RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Han, L.; Ma, X.; Yu, Q.; Zhao, S. Effect of mitochondrial apoptotic activation through the mitochondrial membrane permeability transition pore on yak meat tenderness during postmortem aging. Food Chem. 2017, 234, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Aykın-Dinçer, E.; Erbaş, M. Quality characteristics of cold-dried beef slices. Meat Sci. 2019, 155, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C. Proteomics discovery of protein biomarkers linked to yak meat tenderness as determined by label-free mass spectrometry. Anim. Sci. J. 2021, 92, e13669. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of functional ingredients in yak milk-derived food on health of Tibetan nomads living under high-altitude stress: A review. Crit. Rev. Food Sci. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Hosaka, T.; Biggs, W.H.; Tieu, D.; Boyer, A.D.; Varki, N.M.; Cavenee, W.K.; Arden, K.C. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 2004, 101, 2975–2980. [Google Scholar] [CrossRef]
- Luo, M.; Wang, H.; Zhang, J.; Yixi, K.; Shu, S.; Fu, C.; Zhong, J.; Peng, W. IMF deposition ceRNA network analysis and functional study of HIF1a in yak. Front. Vet. Sci. 2023, 10, 1272238. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Cossu, G.; Borello, U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999, 18, 6867–6872. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Zhao, Y.; Deng, S.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef]
- Mercatelli, N.; Fittipaldi, S.; De Paola, E.; Dimauro, I.; Paronetto, M.P.; Jackson, M.J.; Caporossi, D. MiR-23-TrxR1 as a novel molecular axis in skeletal muscle differentiation. Sci. Rep. 2017, 7, 7219. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.R.; Legako, J.F.; Dinh, T.T.N.; Garmyn, A.J.; O’Quinn, T.G.; Corbin, C.H.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Assessment of volatile compounds, neutral and polar lipid fatty acids of four beef muscles from USDA Choice and Select graded carcasses and their relationships with consumer palatability scores and intramuscular fat content. Meat Sci. 2016, 116, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, L.; Zhou, H.; Hou, G.; Shi, L.; Li, M.; Huang, X.; Guan, S. Effects of nutritional level of concentrate-based diets on meat quality and expression levels of genes related to meat quality in Hainan black goats. Anim. Sci. J. 2014, 86, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Tang, G.; Liu, S.; Cai, R.; Gao, Y.; Sun, Y.; Yang, G.; Pang, W. Differences between porcine longissimus thoracis and semitendinosus intramuscular fat content and the regulation of their preadipocytes during adipogenic differentiation. Meat Sci. 2018, 147, 116–126. [Google Scholar] [CrossRef]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2002, 107, 823–826. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Valencia-Sanchez, M.A.; Liu, J.; Hannon, G.J.; Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Gene Dev. 2006, 20, 515–524. [Google Scholar] [CrossRef]
- An, X.; Ma, K.; Zhang, Z.; Zhao, T.; Zhang, X.; Tang, B.; Li, Z. miR-17, miR-21, and miR-143 Enhance Adipogenic Differentiation from Porcine Bone Marrow-Derived Mesenchymal Stem Cells. DNA Cell Biol. 2016, 35, 410–416. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Zhang, Y.; Zhang, Y.; Chen, L.; Mo, D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int. J. Biol. Sci. 2012, 8, 1408–1417. [Google Scholar] [CrossRef]
- Clop, A.; Marcq, F.; Takeda, H.; Pirottin, D.; Tordoir, X.; Bibé, B.; Bouix, J.; Caiment, F.; Elsen, J.; Eychenne, F.; et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 2006, 38, 813–818. [Google Scholar] [CrossRef]
- Teleman, A.A.; Maitra, S.; Cohen, S.M. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Gene Dev. 2006, 20, 913. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, X.; Zhang, D.; Li, B.; Gao, N.; Li, X.; Mei, C.; Zan, L. Bta-miR-330 promotes bovine intramuscular pre-adipocytes adipogenesis via targeting SESN3 to activate the Akt-mTOR signaling pathway. Int. J. Biol. Macromol. 2024, 275, 133650. [Google Scholar] [CrossRef]
- Yang, G.; Wu, M.; Liu, X.; Wang, F.; Li, M.; An, X.; Bai, F.; Lei, C.; Dang, R. MiR-24-3p Conservatively Regulates Muscle Cell Proliferation and Apoptosis by Targeting Common Gene CAMK2B in Rat and Cattle. Animals 2022, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tang, K.; Wang, Y.; Zan, L. MiR-27a-5p Increases Steer Fat Deposition Partly by Targeting Calcium-sensing Receptor (CASR). Sci. Rep. 2018, 8, 3012. [Google Scholar] [CrossRef] [PubMed]
- Soulat, J.; Picard, B.; Léger, S.; Monteils, V. Prediction of beef carcass and meat traits from rearing factors in young bulls and cull cows. J. Anim. Sci. 2016, 94, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mishra, B.; Qin, N.; Sun, X.; Zhang, S.; Yang, J.; Xu, R. Differential Transcriptome Analysis of Early Postnatal Developing Longissimus Dorsi Muscle from Two Pig Breeds Characterized in Divergent Myofiber Traits and Fatness. Anim. Biotechnol. 2018, 30, 63–74. [Google Scholar] [CrossRef]
- Choi, Y.M.; Oh, H.K. Carcass Performance, Muscle Fiber, Meat Quality, and Sensory Quality Characteristics of Crossbred Pigs with Different Live Weights. Korean J. Food Sci. Anim. Resour. 2016, 36, 389–396. [Google Scholar] [CrossRef]
- Davoli, R.; Braglia, S. Molecular approaches in pig breeding to improve meat quality. Brief. Funct. Genom. Proteomic 2007, 6, 313–321. [Google Scholar] [CrossRef]
- Li, L.Q. Physicochemical Characterization of Meat Quality of Qinchuan Cattle and Its Positive Regulation. Doctoral Dissertation, Northwest Agriculture and Forestry University, Xianyang, China, 2010. [Google Scholar]
- .Gagaoua, M.; Bonnet, M.; Picard, B. Protein Array-Based Approach to Evaluate Biomarkers of Beef Tenderness and Marbling in Cows: Understanding of the Underlying Mechanisms and Prediction. Foods 2020, 9, 1180. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhu, L.; Han, M.; Gao, S.; Luo, X. Effect of packaging atmospheres on storage quality characteristics of heavily marbled beef longissimus steaks. Meat Sci. 2016, 117, 50–56. [Google Scholar] [CrossRef]
- Jiang, C.; Shi, P.; Li, S.; Dong, R.; Tian, J.; Wei, J.; Luo, S. Gene expression profiling of skeletal muscle of nursing piglets. Int. J. Biol. Sci. 2010, 6, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S.; Zhu, L.G.; McKeith, F.K. Marbling effects on quality characteristics of pork loin chops: Consumer purchase intent, visual and sensory characteristics. Meat Sci. 2001, 59, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Salazar, E.G.; Almeraya, E.V.; López-Perez, T.V.; Patiño, N.; Salmeron, J.; Velázquez-Cruz, R. MicroRNA-548-3p overexpression inhibits proliferation, migration and invasion in osteoblast-like cells by targeting STAT1 and MAFB. J. Biochem. 2020, 168, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y.; Kim, Y. Roles of GPRC5 family proteins: Focusing on GPRC5B and lipid-mediated signalling. J. Biochem. 2020, 167, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Ikura, M.; Ikura, T. Epigenetic interplays between DNA demethylation and histone methylation for protecting oncogenesis. J. Biochem. 2019, 165, 297–299. [Google Scholar] [CrossRef]
- Shi, C.; Huang, F.; Gu, X.; Zhang, M.; Wen, J.; Wang, X.; You, L.; Cui, X.; Ji, C.; Guo, X. Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity. Oncotarget 2016, 7, 40830–40845. [Google Scholar] [CrossRef]
- Wang, X.; Yan, P.; Feng, S.; Luo, Y.; Liang, J.; Zhao, L.; Liu, H.; Tang, Q.; Long, K.; Jin, L.; et al. Identification and expression pattern analysis of miRNAs in pectoral muscle during pigeon (Columba livia) development. PeerJ 2021, 9, e11438. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, Y.; Li, Y.; Li, G.; Chen, Y.; Li, Z.; Sun, G.; Li, H.; Kang, X.; Yan, F. Characterization of miRNA transcriptome profiles related to breast muscle development and intramuscular fat deposition in chickens. J. Cell Biochem. 2018, 119, 7063–7079. [Google Scholar] [CrossRef]
- Shen, J.; Hao, Z.; Luo, Y.; Zhen, H.; Liu, Y.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Zhao, Z.; et al. Deep Small RNA Sequencing Reveals Important miRNAs Related to Muscle Development and Intramuscular Fat Deposition in Longissimus dorsi Muscle From Different Goat Breeds. Front. Vet. Sci. 2022, 9, 911166. [Google Scholar] [CrossRef]
- Koopmans, P.J.; Ismaeel, A.; Goljanek-Whysall, K.; Murach, K.A. The roles of miRNAs in adult skeletal muscle satellite cells. Free Radic. Biol. Med. 2023, 209, 228–238. [Google Scholar] [CrossRef]
- Yun, Y.; Wu, R.; He, X.; Qin, X.; Chen, L.; Sha, L.; Yun, X.; Nishiumi, T.; Borjigin, G. Integrated Transcriptome Analysis of miRNAs and mRNAs in the Skeletal Muscle of Wuranke Sheep. Genes 2023, 14, 2034. [Google Scholar] [CrossRef] [PubMed]
- Martignani, E.; Miretti, S.; Vincenti, L.; Baratta, M. Correlation between estrogen plasma level and miRNAs in muscle of Piedmontese cattle. Domest. Anim. Endocrin 2018, 67, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Mai, M.; Jin, L.; Tian, S.; Liu, R.; Huang, W.; Tang, Q.; Ma, J.; Jiang, A.; Wang, X.; Hu, Y.; et al. Deciphering the microRNA transcriptome of skeletal muscle during porcine development. PeerJ 2016, 4, e1504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Gao, X.; Ren, H.; Gao, S. Identification and functional analysis of miRNAs in skeletal muscle of juvenile and adult largemouth bass, Micropterus salmoides. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100985. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Zhao, R.; Wu, J.; Li, Z. Transcriptome analysis of mRNAs, lncRNAs, and miRNAs in the skeletal muscle of Tibetan chickens at different developmental stages. Front. Physiol. 2023, 14, 1225349. [Google Scholar] [CrossRef]
- Gizak, A.; Duda, P.; Pielka, E.; McCubrey, J.A.; Rakus, D. GSK3 and miRNA in neural tissue: From brain development to neurodegenerative diseases. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118696. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, Q.; Wu, F.; Wang, M.; Chen, L.; Hu, L.; Zhao, J.; Loor, J.J.; Zhang, J. Arginine Alters miRNA Expression Involved in Development and Proliferation of Rat Mammary Tissue. Animals 2021, 11, 535. [Google Scholar] [CrossRef]
- Youssef, E.M.; Elfiky, A.M.; BanglySoliman, N.; Abu-Shahba, N.; Elhefnawi, M.M. Expression profiling and analysis of some miRNAs in subcutaneous white adipose tissue during development of obesity. Genes. Nutr. 2020, 15, 8. [Google Scholar] [CrossRef]
- Lerman, D.A.; Diaz, M.; Peault, B. P1850Changes in coexpression of pericytes and endogenous cardiac progenitor cells from heart development to disease state. Eur. Heart J. 2018, 39, P1850. [Google Scholar] [CrossRef]
- Wu, C.; Hsieh, K.; Yeh, S.; Lu, Y.; Chen, L.; Ku, M.S.B.; Li, W. Simultaneous detection of miRNA and mRNA at the single-cell level in plant tissues. Plant Biotechnol. J. 2022, 21, 136–149. [Google Scholar] [CrossRef]
- Granados-López, A.J.; Ruiz-Carrillo, J.L.; Servín-González, L.S.; Martínez-Rodríguez, J.L.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; López, J.A. Use of Mature miRNA Strand Selection in miRNAs Families in Cervical Cancer Development. Int. J. Mol. Sci. 2017, 18, 407. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, C.; Tilahun Bizuayehu, T.; Kopp, M.; Fernandes, J.M.O.; Babiak, I. Dynamics of miRNA transcriptome during gonadal development of zebrafish. Sci. Rep. 2017, 7, 43850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, T. Modulation of microRNAs in Tooth Root and Periodontal Tissue Development. Curr. Stem Cell Res. T 2017, 13, 118–124. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, X.; Zhao, B.; Liu, G.; Tian, Y.; Zhang, G.; Wei, C.; Mao, J.; Tian, K. Integrated analysis of miRNAs and mRNA profiling reveals the potential roles of miRNAs in sheep hair follicle development. BMC Genom. 2022, 23, 722. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.A.; Zou, K.; Li, Z.; Ying, S. Mechanism and functions of identified miRNAs in poultry skeletal muscle development. Ann. Anim. Sci. 2019, 19, 887–904. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Xu, Z.; Gao, J.; Mishra, S.K.; Zhu, Q.; Zhao, X.; Wang, Y.; Yin, H.; Fan, X.; et al. MiRNA Profiling in Pectoral Muscle Throughout Pre- to Post-Natal Stages of Chicken Development. Front. Genet. 2020, 11, 570. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, K.; Zhang, L.; Li, P.; He, M.; Zhang, X.; Ye, H.; Zhang, Q.; Wei, Q.; Zhang, G. High-throughput sequencing reveals crucial miRNAs in skeletal muscle development of Bian chicken. Brit. Poult. Sci. 2021, 62, 658–665. [Google Scholar] [CrossRef]
- Guo, D.; Wei, Y.; Li, X.; Bai, Y.; Liu, Z.; Li, J.; Chen, Z.; Shi, B.; Zhang, X.; Zhao, Z.; et al. Comprehensive Analysis of miRNA and mRNA Expression Profiles during Muscle Development of the Longissimus Dorsi Muscle in Gannan Yaks and Jeryaks. Genes 2023, 14, 2220. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Huang, W.; Miao, X. Identification and characterization of differentially expressed miRNAs in subcutaneous adipose between Wagyu and Holstein cattle. Sci. Rep. 2017, 7, 44026. [Google Scholar] [CrossRef]
- Duckett, S.K.; Greene, M.A. Identification of microRNA Transcriptome Involved in Bovine Intramuscular Fat Deposition. Front. Vet. Sci. 2022, 9, 883295. [Google Scholar] [CrossRef]
- Zhang, J.S.; Xu, H.Y.; Fang, J.C.; Yin, B.Z.; Wang, B.B.; Pang, Z.; Xia, G.J. Integrated microRNA–mRNA analysis reveals the roles of microRNAs in the muscle fat metabolism of Yanbian cattle. Anim. Genet. 2021, 52, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, B.; Shang, P.; Fu, Y.; Nie, R.; Chamba, Y.; Zhang, H. Comparative Transcriptomic Profiles of Differentiated Adipocytes Provide Insights into Adipogenesis Mechanisms of Subcutaneous and Intramuscular Fat Tissues in Pigs. Cells 2022, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, J.; Zhu, H.; Fan, G.; Zhou, G. Expression profiles of miRNAs in giant cell tumor of bone showed miR-187-5p and miR-1323 can regulate biological functions through inhibiting FRS2. Cancer Med. 2020, 9, 3163–3173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, L.; Li, Z.; Wang, W.; Hao, C. miR-6835-3p regulates the function of pancreatic islet cells by modulating the expression of AdipoR1. Int. J. Mol. Med. 2018, 42, 1317–1326. [Google Scholar] [CrossRef]
- Feng, H.; Liu, T.; Yousuf, S.; Zhang, X.; Huang, W.; Li, A.; Xie, L.; Miao, X. Identification and analysis of lncRNA, miRNA and mRNA related to subcutaneous and intramuscular fat in Laiwu pigs. Front. Endocrinol. 2023, 13, 1081460. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wang, Y.; Wang, H.; Zhang, S.; Hong, J.; Guo, H.; Chen, D.; Yang, Y.; Zan, L. Transcriptome analysis of mRNA and microRNAs in intramuscular fat tissues of castrated and intact male Chinese Qinchuan cattle. PLoS ONE 2017, 12, e0185961. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and mechanisms of microRNA-mediated gene regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef]
- Harquail, J.; LeBlanc, N.; Ouellette, R.J.; Robichaud, G.A. miRNAs 484 and 210 regulate Pax-5 expression and function in breast cancer cells. Carcinogenesis 2019, 40, 1010–1020. [Google Scholar] [CrossRef]
- Du, J.; Xu, Y.; Zhang, P.; Zhao, X.; Gan, M.; Li, Q.; Ma, J.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, J.; Zhang, M.; Zhang, S.; Lei, C.; Chen, H.; Guo, W.; Lan, X. Role of bta-miR-204 in the regulation of adipocyte proliferation, differentiation, and apoptosis. J. Cell Physiol. 2019, 234, 11037–11046. [Google Scholar] [CrossRef]
- Khan, R.; Raza, S.H.A.; Junjvlieke, Z.; Wang, X.; Wang, H.; Cheng, G.; Mei, C.; Elsaeid Elnour, I.; Zan, L. Bta-miR-149-5p inhibits proliferation and differentiation of bovine adipocytes through targeting CRTCs at both transcriptional and posttranscriptional levels. J. Cell Physiol. 2020, 235, 5796–5810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, X.; Dong, M.; Tan, J.; Zhang, J.; Zhuang, L.; Liu, S.; Xin, Y. MiR-30b-5p regulates the lipid metabolism by targeting PPARGC1A in Huh-7 cell line. Lipids Health Dis. 2020, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Andreote, A.P.D.; Rosario, M.F.; Ledur, M.C.; Jorge, E.C.; Sonstegard, T.S.; Matukumalli, L.; Coutinho, L.L. Identification and characterization of microRNAs expressed in chicken skeletal muscle. Genet. Mol. Res. 2014, 13, 1465–1479. [Google Scholar] [CrossRef] [PubMed]
- Sengar, G.S.; Deb, R.; Singh, U.; Raja, T.V.; Kant, R.; Sajjanar, B.; Alex, R.; Alyethodi, R.R.; Kumar, A.; Kumar, S.; et al. Differential expression of microRNAs associated with thermal stress in Frieswal (Bos taurus x Bos indicus) crossbred dairy cattle. Cell Stress. Chaperones 2017, 23, 155–170. [Google Scholar] [CrossRef]
- Lang, L.; Xu, B.; Li, S.; Guo, W.; Yuan, J.; Zang, S.; Chen, Y.; Yang, H.; Lian, S. Rno-miR-425-5p targets the DLST and SLC16A1 genes to reduce liver damage caused by excessive energy mobilization under cold stress. J. Anim. Physiol. An. N. 2019, 103, 1251–1262. [Google Scholar] [CrossRef]
- Muroya, S.; Shibata, M.; Hayashi, M.; Oe, M.; Ojima, K. Differences in Circulating microRNAs between Grazing and Grain-Fed Wagyu Cattle Are Associated with Altered Expression of Intramuscular microRNA, the Potential Target PTEN, and Lipogenic Genes. PLoS ONE 2016, 11, e0162496. [Google Scholar] [CrossRef]
- Bianchi, M.; Renzini, A.; Adamo, S.; Moresi, V. Coordinated Actions of MicroRNAs with other Epigenetic Factors Regulate Skeletal Muscle Development and Adaptation. Int. J. Mol. Sci. 2017, 18, 840. [Google Scholar] [CrossRef]
- Morenikeji, O.B.; Hawkes, M.E.; Hudson, A.O.; Thomas, B.N. Computational Network Analysis Identifies Evolutionarily Conserved miRNA Gene Interactions Potentially Regulating Immune Response in Bovine Trypanosomosis. Front. Microbiol. 2019, 10, 2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; He, J.; Chen, D. From Nutrient to MicroRNA: A Novel Insight into Cell Signaling Involved in Skeletal Muscle Development and Disease. Int. J. Biol. Sci. 2016, 12, 1247–1261. [Google Scholar] [CrossRef]
- Baik, M.; Kang, H.J.; Park, S.J.; Na, S.W.; Piao, M.; Kim, S.Y.; Fassah, D.M.; Moon, Y.S. TRIENNIAL GROWTH AND DEVELOPMENT SYMPOSIUM: Molecular mechanisms related to bovine intramuscular fat deposition in the longissimus muscle. J. Anim. Sci. 2017, 95, 2284–2303. [Google Scholar]
- Wang, H.; Zhong, J.; Zhang, C.; Chai, Z.; Cao, H.; Wang, J.; Zhu, J.; Wang, J.; Ji, Q. The whole-transcriptome landscape of muscle and adipose tissues reveals the ceRNA regulation network related to intramuscular fat deposition in yak. BMC Genom. 2020, 21, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Wang, X.; Zhang, D.; Zhang, H.; Wu, Q.; He, Y.; Wang, J.; Zhang, L.; Xia, H.; et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia 2013, 56, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qi, R.; Wang, J.; Huang, W.; Wu, Y.; Huang, X.; Yang, F.; Huang, J. Differential expression profile of miRNAs in porcine muscle and adipose tissue during development. Gene 2017, 618, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Valášková, S.; Gažová, A.; Vrbová, P.; Koller, T.; Šalingova, B.; Adamičková, A.; Chomaničová, N.; Hulajová, N.; Payer, J.; Kyselovič, J. The Severity of Muscle Performance Deterioration in Sarcopenia Correlates with Circulating Muscle Tissue-Specific miRNAs. Physiol. Res. 2021, 70, S91–S98. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, J. MicroRNAs in skeletal myogenesis. Cell Cycle 2011, 10, 441–448. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 101964. [Google Scholar] [CrossRef]
- Ma, L.; Mondal, A.K.; Murea, M.; Sharma, N.K.; Tönjes, A.; Langberg, K.A.; Das, S.K.; Franks, P.W.; Kovacs, P.; Antinozzi, P.A.; et al. The effect of ACACB cis-variants on gene expression and metabolic traits. PLoS ONE 2011, 6, e23860. [Google Scholar] [CrossRef]
- Muroya, S.; Taniguchi, M.; Shibata, M.; Oe, M.; Ojima, K.; Nakajima, I.; Chikuni, K. Profiling of differentially expressed microRNA and the bioinformatic target gene analyses in bovine fast- and slow-type muscles by massively parallel sequencing. J. Anim. Sci. 2013, 91, 90–103. [Google Scholar] [CrossRef]
- Ylioja, C.M.; Rolf, M.M.; Mamedova, L.K.; Bradford, B.J. Associations between body condition score at parturition and microRNA profile in colostrum of dairy cows as evaluated by paired mapping programs. J. Dairy. Sci. 2019, 102, 11609–11621. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Somwar, R.; Koterski, S.; Sweeney, G.; Sciotti, R.; Djuric, S.; Berg, C.; Trevillyan, J.; Scherer, P.E.; Rondinone, C.M.; Klip, A. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J. Biol. Chem. 2002, 277, 50386–50395. [Google Scholar] [CrossRef] [PubMed]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Seo, H.G.; Son, S.; Choi, H.; Kim, B.G.; Kweon, T.H.; Kim, S.; Pai, J.; Shin, I.; Yang, W.H.; et al. O-GlcNAcylation of Mef2c regulates myoblast differentiation. Biochem. Bioph Res. Commun. 2020, 529, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N.; Perry, M.; Schulz, R.A. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev. Biol. 1995, 172, 2–14. [Google Scholar] [CrossRef]
- Ornatsky, O.I.; Andreucci, J.J.; McDermott, J.C. A dominant-negative form of transcription factor MEF2 inhibits myogenesis. J. Biol. Chem. 1997, 272, 33271–33278. [Google Scholar] [CrossRef]
- Materna, S.C.; Sinha, T.; Barnes, R.M.; Lammerts Van Bueren, K.; Black, B.L. Cardiovascular development and survival require Mef2c function in the myocardial but not the endothelial lineage. Dev. Biol. 2018, 445, 170–177. [Google Scholar] [CrossRef]
- Di Giorgio, E.; Hancock, W.W.; Brancolini, C. MEF2 and the tumorigenic process, hic sunt leones. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 261–273. [Google Scholar] [CrossRef]
- Liu, N.; Nelson, B.R.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 4109–4114. [Google Scholar] [CrossRef]
- Cao, X.; Zhan, Z.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Chen, H. Variants and haplotypes within MEF2C gene influence stature of chinese native cattle including body dimensions and weight. Livest. Sci. 2016, 185, 106–109. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E.; Flisikowski, K.; Wicińska, K. Nucleotide sequence and variations of the bovine myocyte enhancer factor 2C (MEF2C) gene promoter in Bos taurus cattle. Mol. Biol. Rep. 2011, 38, 1269–1276. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.; Cao, J.; Sun, Y.; Zhou, H.; Zhu, Q.; Dai, D.; Zhan, S.; Guo, J.; Zhong, T.; Wang, L.; et al. METTL3 Promotes the Differentiation of Goat Skeletal Muscle Satellite Cells by Regulating MEF2C mRNA Stability in a m6A-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 14115. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, B.; Dai, S.; Tang, Z.; Zhao, W.; Zhan, S.; Wang, L.; Zhong, T.; Guo, J.; Zhang, H. The novel mutations in the MEF2C gene associate with growth of Nanjiang Yellow goats. Gene Rep. 2019, 14, 100–109. [Google Scholar] [CrossRef]
- Ren, H.; Xiao, W.; Qin, X.; Cai, G.; Chen, H.; Hua, Z.; Cheng, C.; Li, X.; Hua, W.; Xiao, H.; et al. Myostatin regulates fatty acid desaturation and fat deposition through MEF2C/miR222/SCD5 cascade in pigs. Commun. Biol. 2020, 3, 612. [Google Scholar] [CrossRef] [PubMed]
- Gerbens, F.; Verburg, F.J.; Van Moerkerk, H.T.; Engel, B.; Buist, W.; Veerkamp, J.H.; Te, P.M. Associations of heart and adipocyte fatty acid-binding protein gene expression with intramuscular fat content in pigs. J. Anim. Sci. 2001, 79, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, G.; Guo, Z.; Yu, Q.; Han, L.; Han, M.; Zhu, Y. Study on the apoptosis mediated by apoptosis-inducing-factor and influencing factors of bovine muscle during postmortem aging. Food Chem. 2018, 266, 359–367. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, D.; Bai, Y.; Liu, Z.; Li, J.; Chen, Z.; Shi, B.; Zhao, Z.; Hu, J.; Han, X.; et al. Transcriptome Analysis of mRNA and lncRNA Related to Muscle Growth and Development in Gannan Yak and Jeryak. Int. J. Mol. Sci. 2023, 24, 16991. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Betel, D.; Koppal, A.; Agius, P.; Sander, C.; Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010, 11, R90. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]

| Sample | Average Clean_Reads | Average High_Quality | Average 3′Adapter_Null | Average Insert_Null | Average 5′Adapter_Contaminants | Average polyA | Average Clean_ Tags |
|---|---|---|---|---|---|---|---|
| M6 | 13,786,039 (100%) | 13,669,782 (99.1560%) | 12,780 (0.0911%) | 53,070 (0.3819%) | 6541 (0.0467%) | 173 (0.0012%) | 13,227,222 (96.1951%) |
| M30 | 16,023,867 (100%) | 15,874,203 (99.1314%) | 10,477 (0.0579%) | 51,679 (0.3151%) | 5659 (0.0420%) | 209 (0.0012%) | 15,627,938 (96.7044%) |
| M54 | 16,575,445 (100%) | 13,640,341 (98.9557%) | 14,968 (0.0780%) | 85,117 (0.4558%) | 9939 (0.0580%) | 284 (0.0015%) | 16,518,724 (96.4666%) |
| Sample | Average Total_Abundance | Average Match_Abundance | Average Other_Abundance |
|---|---|---|---|
| M6 | 13,517,788 | 8,987,399 (75.75%) | 3,451,495 (23.93%) |
| M30 | 15,500,216 | 12,281,680 (79.21%) | 3,467,715 (20.79%) |
| M54 | 15,990,108 | 11,910,843 (78.73%) | 3,335,252 (21.27%) |
| Reagent | Usage Amount |
|---|---|
| 2 × SuperReal premix Plus | 10 μL |
| Upstream primer | 0.4 μL |
| Downstream primer | 0.4 μL |
| cDNA templates | 2 μL |
| 50 × ROX Reference Dye1 | 0.4 μL |
| RNase-Free ddH2O | 0.8 μL |
| miRNA/Gene | Forward Primer | Reverse Primer |
|---|---|---|
| miR-2188-y | GCTGTGTGAGGTCGGACCTATC | |
| novel-m0025-5p | AGGACTCCATTTGTTTTGATGA | / |
| miR-122-x | TGGAGTGTGACAATGGTGTTT | / |
| miR-541-x | AAAGGATTCTGCTGTCGGTCCCACT | / |
| miR-432-x | TCTTGGAGTAGGTCATTGGGTGT | / |
| novel-m0137-3p | CCGAGCCTGACAGATCACACAC | / |
| miR-338-y | TCCAGCATCAGTGATTTTGTT | / |
| miR-382-y | AATCATTCACGGACAACACTTT | / |
| novel-m0109-3p | AGCCCGAGGGTCCCAAGCTGAGC | / |
| U6 | ACGGACAGGATTGACAGATT | TCGCTCCACCAACTAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Zhu, C.; Wang, X.; Qi, Y.; Hu, J.; Liu, X.; Wang, J.; Hao, Z.; Zhao, Z.; Zhang, X. microRNA Temporal-Specific Expression Profiles Reveal longissimus dorsi Muscle Development in Tianzhu White Yak. Int. J. Mol. Sci. 2024, 25, 10151. https://doi.org/10.3390/ijms251810151
Shi B, Zhu C, Wang X, Qi Y, Hu J, Liu X, Wang J, Hao Z, Zhao Z, Zhang X. microRNA Temporal-Specific Expression Profiles Reveal longissimus dorsi Muscle Development in Tianzhu White Yak. International Journal of Molecular Sciences. 2024; 25(18):10151. https://doi.org/10.3390/ijms251810151
Chicago/Turabian StyleShi, Bingang, Chune Zhu, Xiangyan Wang, Youpeng Qi, Jiang Hu, Xiu Liu, Jiqing Wang, Zhiyun Hao, Zhidong Zhao, and Xiaolan Zhang. 2024. "microRNA Temporal-Specific Expression Profiles Reveal longissimus dorsi Muscle Development in Tianzhu White Yak" International Journal of Molecular Sciences 25, no. 18: 10151. https://doi.org/10.3390/ijms251810151
APA StyleShi, B., Zhu, C., Wang, X., Qi, Y., Hu, J., Liu, X., Wang, J., Hao, Z., Zhao, Z., & Zhang, X. (2024). microRNA Temporal-Specific Expression Profiles Reveal longissimus dorsi Muscle Development in Tianzhu White Yak. International Journal of Molecular Sciences, 25(18), 10151. https://doi.org/10.3390/ijms251810151






