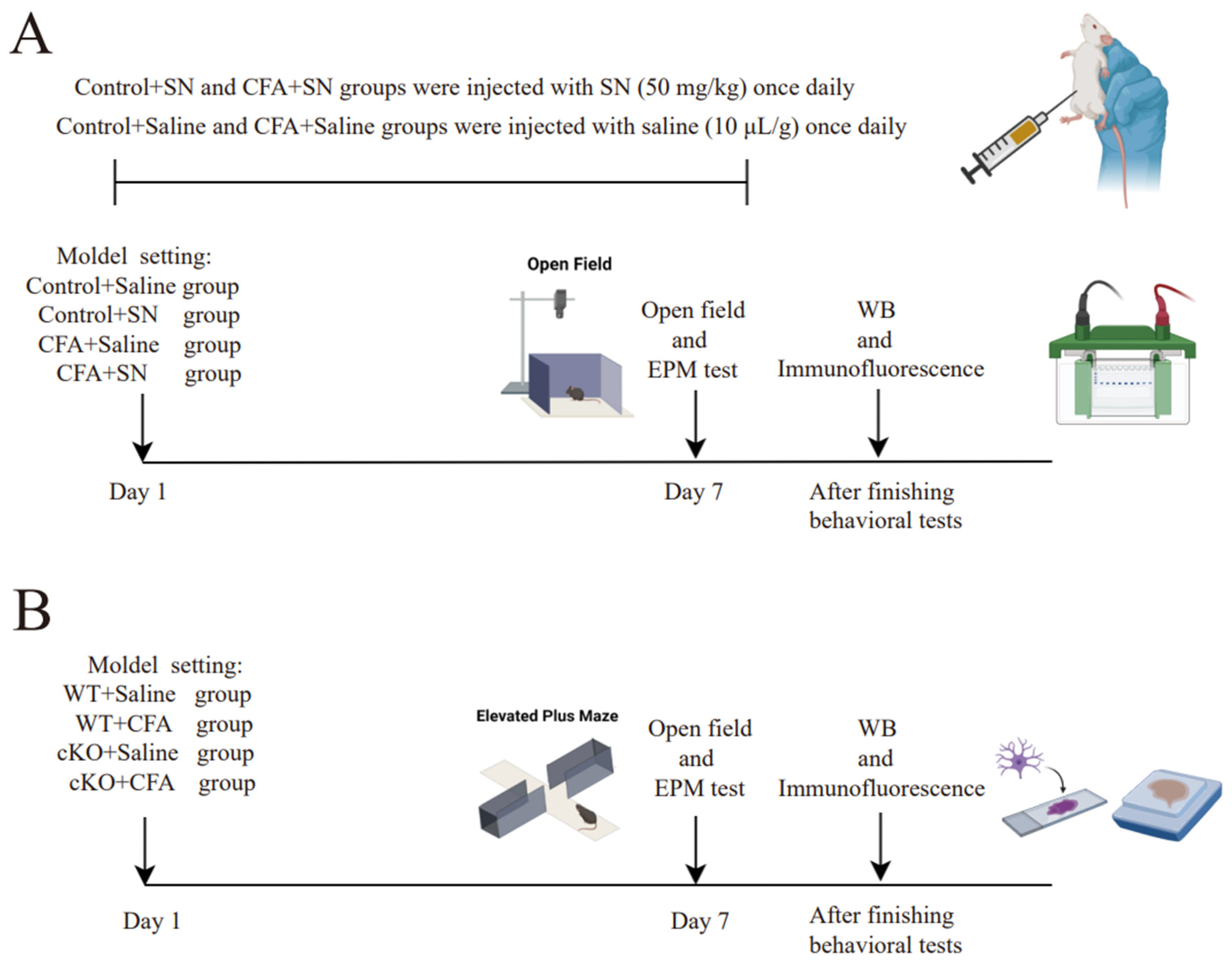
Figure 1.
Detailed plan and mouse model parameters of the two experiments; (
A) We executed the first experiment according to the detailed mouse model parameters, which are as follows. The two control groups were not injected with CFA (40 μL) but saline (40 μL) in the plantar surface of the hind paw. The control+saline group received daily intra-peritoneal injections of saline (10 μL/g); the control+solanesol group was injected daily with solanesol (50 mg/kg) intra-peritoneally; the CFA+saline group received an intra-plantar CFA (40 μL) injection and daily intra-peritoneal saline injections (10 μL/g); and the CFA+solanesol group received an intra-plantar CFA (40 μL) injection and daily intra-peritoneal solanesol (50 mg/kg) injections. The behavior tests began 1 h after the final injection of solanesol was administered. All mice used in the experiment (
A) were C57BL/6 mice. (
B) We used cKO mice in the second experiment. The detailed mouse model parameters for the experiment (
B) are as follows: mice in the WT+saline and WT+CFA groups were wild-type mice and mice in the cKO+CFA and cKO+saline groups were TIA1
Nestin cKO mice. The WT+saline and cKO+saline groups both received an intra-plantar saline (40 μL) injection, whereas the other two groups received a CFA (40 μL) injection. This figure was created with BioRender.com (partly adapted from ‘Mouse Behavioral Tests: Anxiety & Depression’, by BioRender.com [2024]. Retrieved from
https://app.biorender.com/biorender-templates (accessed on 9 September 2024). Abbreviations: CFA, complete Freund’s adjuvant; SN, solanesol; WB, Western blot; EPM, elevated plus-maze; cKO, TIA1
Nestin knockout; TIA1, T cell-restricted intracellular antigen-1; TIA1
Nestin, TIA1 knockout in Nestin-positive cells; WT, wild-type.
Figure 1.
Detailed plan and mouse model parameters of the two experiments; (
A) We executed the first experiment according to the detailed mouse model parameters, which are as follows. The two control groups were not injected with CFA (40 μL) but saline (40 μL) in the plantar surface of the hind paw. The control+saline group received daily intra-peritoneal injections of saline (10 μL/g); the control+solanesol group was injected daily with solanesol (50 mg/kg) intra-peritoneally; the CFA+saline group received an intra-plantar CFA (40 μL) injection and daily intra-peritoneal saline injections (10 μL/g); and the CFA+solanesol group received an intra-plantar CFA (40 μL) injection and daily intra-peritoneal solanesol (50 mg/kg) injections. The behavior tests began 1 h after the final injection of solanesol was administered. All mice used in the experiment (
A) were C57BL/6 mice. (
B) We used cKO mice in the second experiment. The detailed mouse model parameters for the experiment (
B) are as follows: mice in the WT+saline and WT+CFA groups were wild-type mice and mice in the cKO+CFA and cKO+saline groups were TIA1
Nestin cKO mice. The WT+saline and cKO+saline groups both received an intra-plantar saline (40 μL) injection, whereas the other two groups received a CFA (40 μL) injection. This figure was created with BioRender.com (partly adapted from ‘Mouse Behavioral Tests: Anxiety & Depression’, by BioRender.com [2024]. Retrieved from
https://app.biorender.com/biorender-templates (accessed on 9 September 2024). Abbreviations: CFA, complete Freund’s adjuvant; SN, solanesol; WB, Western blot; EPM, elevated plus-maze; cKO, TIA1
Nestin knockout; TIA1, T cell-restricted intracellular antigen-1; TIA1
Nestin, TIA1 knockout in Nestin-positive cells; WT, wild-type.
![Ijms 25 10165 g001]()
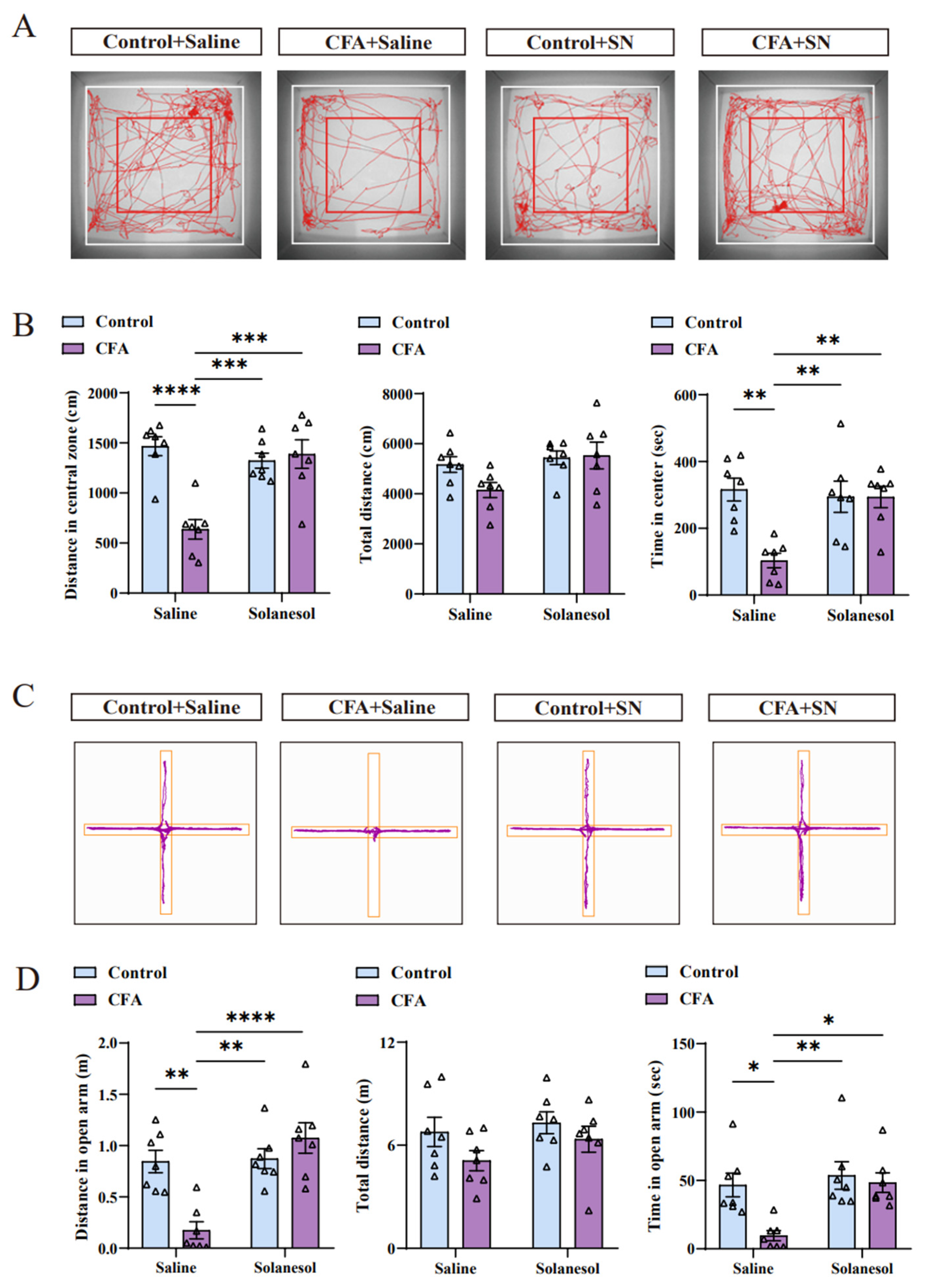
Figure 2.
Solanesol ameliorated the anxiety-like behaviors induced by CFA injection. (A,B) Solanesol treatment significantly increased the number of foot tracks in the red square box (central zone) and inside the white square box (total zone), as indicated in the tracking map (A). Based on the detailed data (B), the distance traveled in the central zone was significantly decreased after CFA injection, which was ameliorated by solanesol, whereas the total distances did not differ significantly. Moreover, times in the central zone were similar in the context of distance. Solanesol injection had no influence on the behaviors of normal control mice. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; ** p < 0.01, *** p < 0.001, **** p < 0.0001). (C,D) Cross-track plot of the elevated plus-maze test (C) shows that the movements of mice in the open arm in the solanesol group were similar to those in the control group. Based on the specific data (D), the time and distance in the open arm both increased after solanesol treatment, which was significantly decreased after CFA injection. As in the open-field test, solanesol did not change the behaviors of control mice in the elevated plus-maze test. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; * p < 0.05, ** p < 0.01, **** p < 0.0001). Abbreviations: ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; and SN, solanesol.
Figure 2.
Solanesol ameliorated the anxiety-like behaviors induced by CFA injection. (A,B) Solanesol treatment significantly increased the number of foot tracks in the red square box (central zone) and inside the white square box (total zone), as indicated in the tracking map (A). Based on the detailed data (B), the distance traveled in the central zone was significantly decreased after CFA injection, which was ameliorated by solanesol, whereas the total distances did not differ significantly. Moreover, times in the central zone were similar in the context of distance. Solanesol injection had no influence on the behaviors of normal control mice. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; ** p < 0.01, *** p < 0.001, **** p < 0.0001). (C,D) Cross-track plot of the elevated plus-maze test (C) shows that the movements of mice in the open arm in the solanesol group were similar to those in the control group. Based on the specific data (D), the time and distance in the open arm both increased after solanesol treatment, which was significantly decreased after CFA injection. As in the open-field test, solanesol did not change the behaviors of control mice in the elevated plus-maze test. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; * p < 0.05, ** p < 0.01, **** p < 0.0001). Abbreviations: ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; and SN, solanesol.
![Ijms 25 10165 g002]()
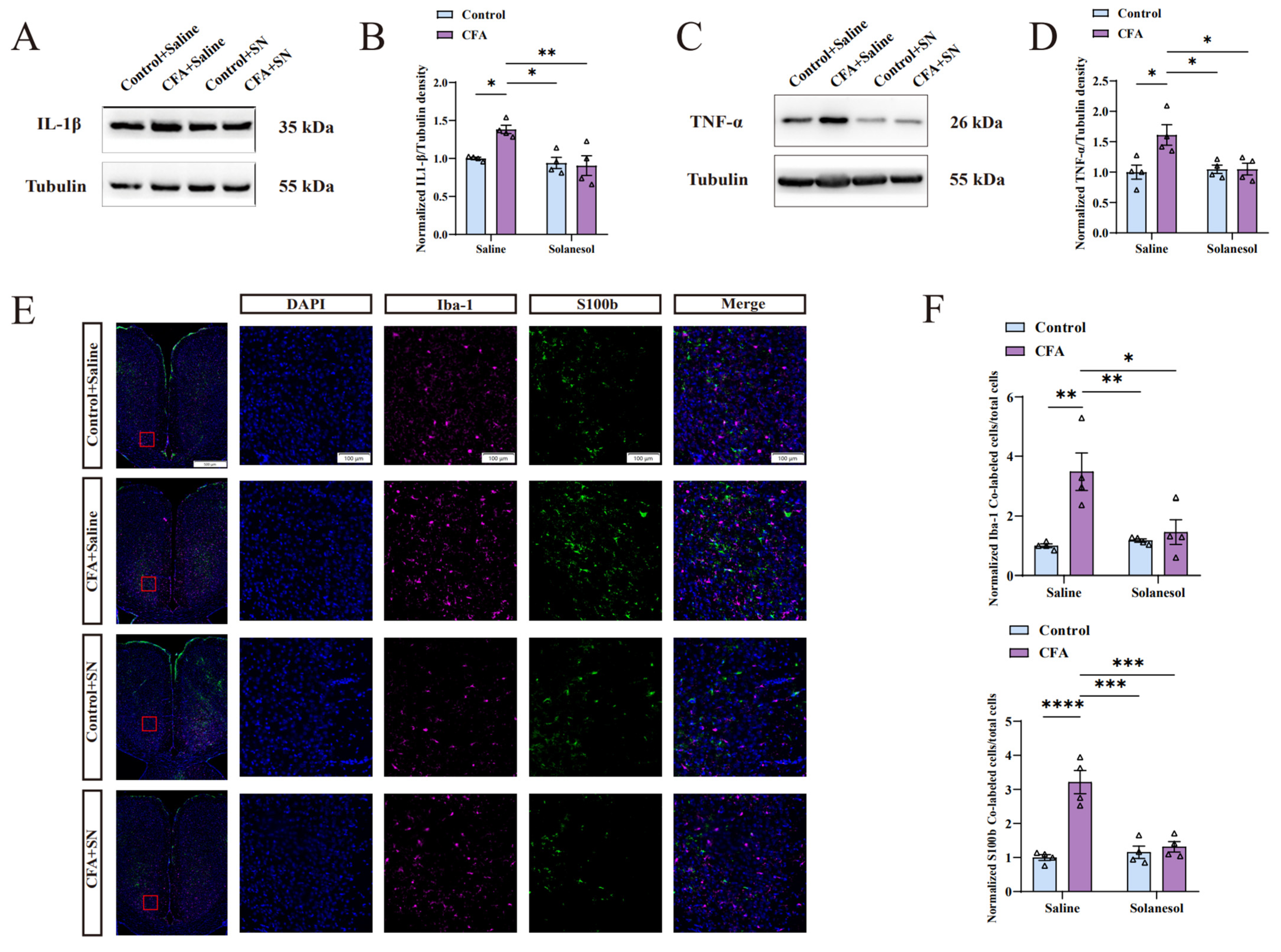
Figure 3.
Solanesol mitigated the neuro-inflammation induced by CFA in the ACC. (A) Representative Western blot analysis results for IL-1β. (B) Normalized density ratio of IL-1β to β-tubulin, which shows that solanesol reduced the CFA-induced increase in IL-1β levels. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Solanesol did not affect IL-1β levels of control mice without CFA injection. Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01). (C) Representative Western blot analysis results for TNF-α. (D) Normalized density ratio of TNF-α to β-tubulin, which shows that solanesol decreases TNF-α levels after CFA injection. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Solanesol also had no influence on the TNF-α levels of control mice. Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05). (E) Sections were immune-stained for the microglial marker Iba-1 (red) and the astrocytic marker S100b (green), and nuclei were stained with DAPI (blue). Scale bar for the 16 smaller figures on the right = 100 μm; scale bar for the remaining four larger figures on the left = 500 μm. Red boxes in the larger figures represent the locations of the 16 smaller figures in the ACC. (F) The ratio of cells with Iba-1 and DAPI co-localization and cells with S100b and DAPI co-localization to the total number of cells indicates the degree of microglial and astrocytic activation. Solanesol clearly reduced microglial and astrocytic activation in the ACC of CFA-injected mice, although it had no influence on normal microglial and astrocytic activation in control mice, indicating that solanesol ameliorates anxiety-like behaviors by inhibiting microglial and astrocytic activation. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001). Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; IL, interleukin; TNF-α, tumor necrosis factor α; SN, solanesol; DAPI, 4′,6-diamidino-2-phenylindole; Iba-1, ionized calcium-binding adapter molecule 1; S100b, S100 calcium-binding protein B; kDa, kilodalton.
Figure 3.
Solanesol mitigated the neuro-inflammation induced by CFA in the ACC. (A) Representative Western blot analysis results for IL-1β. (B) Normalized density ratio of IL-1β to β-tubulin, which shows that solanesol reduced the CFA-induced increase in IL-1β levels. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Solanesol did not affect IL-1β levels of control mice without CFA injection. Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01). (C) Representative Western blot analysis results for TNF-α. (D) Normalized density ratio of TNF-α to β-tubulin, which shows that solanesol decreases TNF-α levels after CFA injection. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Solanesol also had no influence on the TNF-α levels of control mice. Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05). (E) Sections were immune-stained for the microglial marker Iba-1 (red) and the astrocytic marker S100b (green), and nuclei were stained with DAPI (blue). Scale bar for the 16 smaller figures on the right = 100 μm; scale bar for the remaining four larger figures on the left = 500 μm. Red boxes in the larger figures represent the locations of the 16 smaller figures in the ACC. (F) The ratio of cells with Iba-1 and DAPI co-localization and cells with S100b and DAPI co-localization to the total number of cells indicates the degree of microglial and astrocytic activation. Solanesol clearly reduced microglial and astrocytic activation in the ACC of CFA-injected mice, although it had no influence on normal microglial and astrocytic activation in control mice, indicating that solanesol ameliorates anxiety-like behaviors by inhibiting microglial and astrocytic activation. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001). Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; IL, interleukin; TNF-α, tumor necrosis factor α; SN, solanesol; DAPI, 4′,6-diamidino-2-phenylindole; Iba-1, ionized calcium-binding adapter molecule 1; S100b, S100 calcium-binding protein B; kDa, kilodalton.
![Ijms 25 10165 g003]()
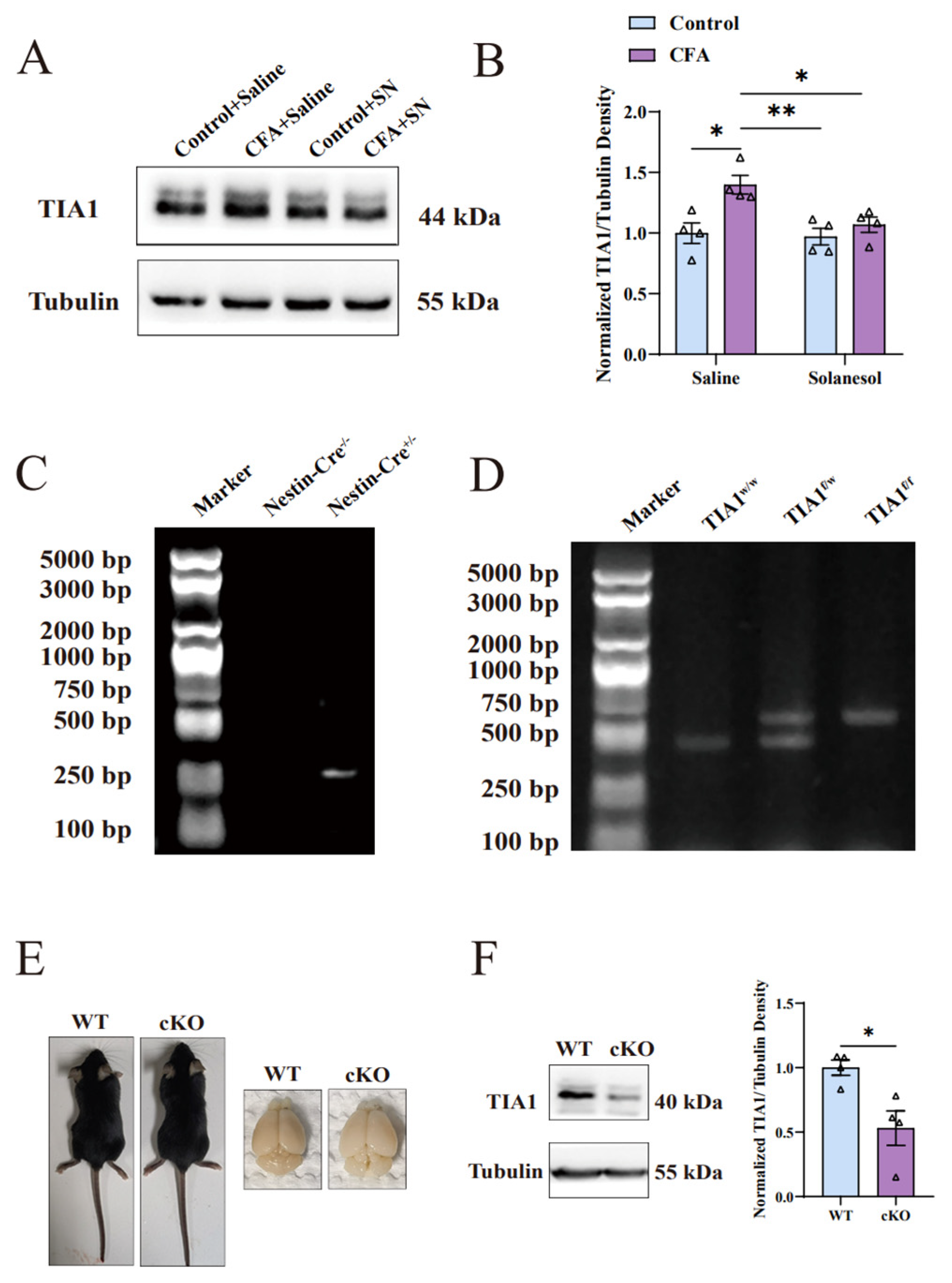
Figure 4.
Solanesol decreased the upregulation of TIA1 induced by CFA in the ACC. (A,B) Representative Western blot analysis results for TIA1 (A) and density ratio of TIA1 to β-tubulin (B) indicated that solanesol significantly decreased TIA1 expression, which was elevated after CFA injection to approximately the normal level; however, it did not influence the normal TIA1 expression in the control group. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01). (C,D) Comparison of mice with and without the Nestin-Cre gene after polymerase chain reaction (C). Comparison of mice with and without TIA1 floxed after polymerase chain reaction (D). TIA1f/f represents the homozygote of TIA1-floxed mice. TIA1f/w represents the heterozygote of TIA1-floxed mice. TIA1w/w represents TIA1 wild-type mice. (E) Body and brain size comparisons between cKO and wild-type mice. (F) Representative Western blot analysis results for TIA1 in the ACC showed that its expression decreased after the conditional knockout compared with the wild type. Statistical analysis was performed using an unpaired t-test. Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05). Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; TIA1, T cell-restricted intracellular antigen-1; SN, solanesol; cKO, TIA1Nestin knockout; WT, wild-type; kDa, kilodalton; bp, base pair.
Figure 4.
Solanesol decreased the upregulation of TIA1 induced by CFA in the ACC. (A,B) Representative Western blot analysis results for TIA1 (A) and density ratio of TIA1 to β-tubulin (B) indicated that solanesol significantly decreased TIA1 expression, which was elevated after CFA injection to approximately the normal level; however, it did not influence the normal TIA1 expression in the control group. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01). (C,D) Comparison of mice with and without the Nestin-Cre gene after polymerase chain reaction (C). Comparison of mice with and without TIA1 floxed after polymerase chain reaction (D). TIA1f/f represents the homozygote of TIA1-floxed mice. TIA1f/w represents the heterozygote of TIA1-floxed mice. TIA1w/w represents TIA1 wild-type mice. (E) Body and brain size comparisons between cKO and wild-type mice. (F) Representative Western blot analysis results for TIA1 in the ACC showed that its expression decreased after the conditional knockout compared with the wild type. Statistical analysis was performed using an unpaired t-test. Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05). Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; TIA1, T cell-restricted intracellular antigen-1; SN, solanesol; cKO, TIA1Nestin knockout; WT, wild-type; kDa, kilodalton; bp, base pair.
![Ijms 25 10165 g004]()
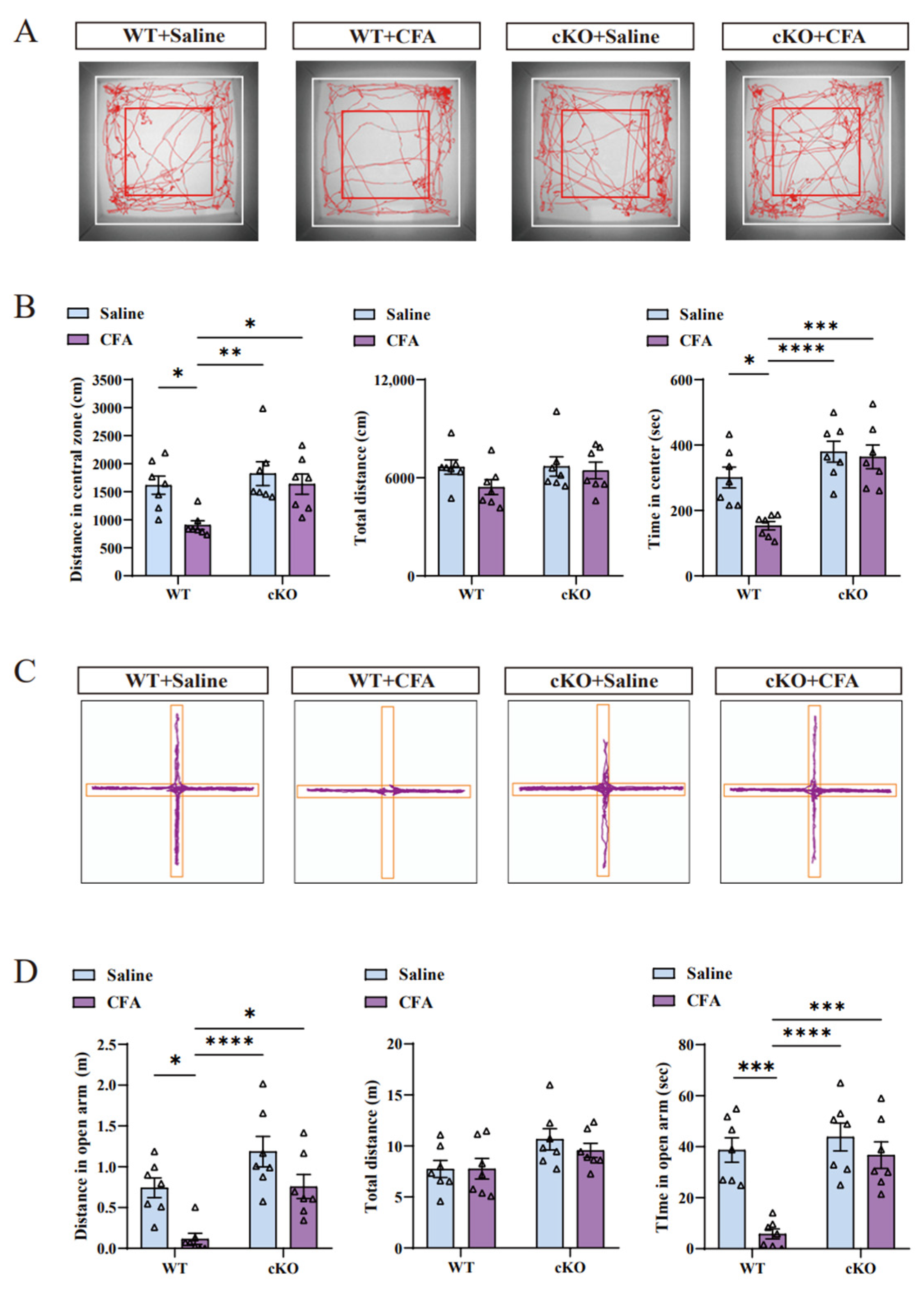
Figure 5.
Conditional knockout of TIA1 improved the anxiety-like behaviors induced by CFA injection. (A,B) Based on the tracking map of the open field test (A), cKO mice did not show decreased levels of exploration in the central area after CFA injection. The time and distance in the central zone (B) clearly indicate that cKO mice after CFA treatment did not exhibit anxiety-like behaviors compared with control mice after CFA treatment. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001). (C,D) Tracking plot of the elevated plus-maze (C) shows that cKO mice did not have decreased motivation with respect to discovering the open arm after CFA injection. The time and distance in the open arm (D) prove that cKO mice were not anxious after CFA injection. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; * p < 0.05, *** p < 0.001, and **** p < 0.0001). Abbreviations: ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; cKO, TIA1Nestin knockout; TIA1Nestin, TIA1 knockout in Nestin-positive cells; WT, wild-type.
Figure 5.
Conditional knockout of TIA1 improved the anxiety-like behaviors induced by CFA injection. (A,B) Based on the tracking map of the open field test (A), cKO mice did not show decreased levels of exploration in the central area after CFA injection. The time and distance in the central zone (B) clearly indicate that cKO mice after CFA treatment did not exhibit anxiety-like behaviors compared with control mice after CFA treatment. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001). (C,D) Tracking plot of the elevated plus-maze (C) shows that cKO mice did not have decreased motivation with respect to discovering the open arm after CFA injection. The time and distance in the open arm (D) prove that cKO mice were not anxious after CFA injection. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 7/group; * p < 0.05, *** p < 0.001, and **** p < 0.0001). Abbreviations: ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; cKO, TIA1Nestin knockout; TIA1Nestin, TIA1 knockout in Nestin-positive cells; WT, wild-type.
![Ijms 25 10165 g005]()
Figure 6.
Neuro-inflammation in the ACC of cKO mice did not occur after CFA injection. (A) Markers (Iba-1, S100b, and DAPI) used in the second experiment were the same as in the first experiment, as well as the scale bar and significance of the red boxes. (B) Microglia and astrocytes in the ACCs of cKO mice that show increased activation after CFA injection were restricted, indicating that the TIA1 knockout in neural stem cells maintained microglial and astrocytic activation at a normal level, similar to that in cKO mice and WT mice without CFA injections, and this confirmed that solanesol plays a role through TIA1 regulation. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; *** p < 0.001, **** p < 0.0001). (C) Representative Western blot analysis results for IL-1β and TNF-α. (D) The normalised density ratio of IL-1β to tubulin shows that the knockout of TIA1 resulted in decreased IL-1β expression after CFA injection. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01, *** p < 0.001). (E) Normalized density ratio of TNF-α to β-tubulin shows that the TNF-α levels of cKO mice were maintained at a normal level after CFA injection. Combined with IL-1β density results (D), these findings suggest that solanesol ameliorates increased IL-1β and TNF-α levels via TIA1 regulation. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05 and ** p < 0.01). Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; cKO, TIA1Nestin knockout; DAPI, 4′,6-diamidino-2-phenylindole; Iba-1, ionized calcium-binding adapter molecule 1; S100b, S100 calcium binding protein B; TIA1, T cell-restricted intracellular antigen-1; TIA1Nestin, TIA1 knockout in Nestin-positive cells; IL, interleukin; TNF-α, tumor necrosis factor α; WT, wild-type; kDa, kilodalton.
Figure 6.
Neuro-inflammation in the ACC of cKO mice did not occur after CFA injection. (A) Markers (Iba-1, S100b, and DAPI) used in the second experiment were the same as in the first experiment, as well as the scale bar and significance of the red boxes. (B) Microglia and astrocytes in the ACCs of cKO mice that show increased activation after CFA injection were restricted, indicating that the TIA1 knockout in neural stem cells maintained microglial and astrocytic activation at a normal level, similar to that in cKO mice and WT mice without CFA injections, and this confirmed that solanesol plays a role through TIA1 regulation. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; *** p < 0.001, **** p < 0.0001). (C) Representative Western blot analysis results for IL-1β and TNF-α. (D) The normalised density ratio of IL-1β to tubulin shows that the knockout of TIA1 resulted in decreased IL-1β expression after CFA injection. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05, ** p < 0.01, *** p < 0.001). (E) Normalized density ratio of TNF-α to β-tubulin shows that the TNF-α levels of cKO mice were maintained at a normal level after CFA injection. Combined with IL-1β density results (D), these findings suggest that solanesol ameliorates increased IL-1β and TNF-α levels via TIA1 regulation. Statistical analysis was performed using two-way ANOVA (followed by Bonferroni’s multiple comparisons test). Values are expressed as mean ± S.E.M. (n = 4/group; * p < 0.05 and ** p < 0.01). Abbreviations: ACC, anterior cingulate cortex; ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; cKO, TIA1Nestin knockout; DAPI, 4′,6-diamidino-2-phenylindole; Iba-1, ionized calcium-binding adapter molecule 1; S100b, S100 calcium binding protein B; TIA1, T cell-restricted intracellular antigen-1; TIA1Nestin, TIA1 knockout in Nestin-positive cells; IL, interleukin; TNF-α, tumor necrosis factor α; WT, wild-type; kDa, kilodalton.
![Ijms 25 10165 g006]()
Figure 7.
The key mechanisms of solanesol in the alleviation of anxiety. Solanesol can ameliorate anxiety-like behaviors induced by neuroinflammation by inhibiting glial activation and decreasing TIA1 expression, thereby reducing pro-inflammatory cytokines. This figure was created using BioRender.com. Abbreviations: cKO, TIA1Nestin knockout; TIA1, T cell-restricted intracellular antigen-1; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor α.
Figure 7.
The key mechanisms of solanesol in the alleviation of anxiety. Solanesol can ameliorate anxiety-like behaviors induced by neuroinflammation by inhibiting glial activation and decreasing TIA1 expression, thereby reducing pro-inflammatory cytokines. This figure was created using BioRender.com. Abbreviations: cKO, TIA1Nestin knockout; TIA1, T cell-restricted intracellular antigen-1; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor α.