Helper T Cells are Hyperactive and Contribute to the Dysregulation of Antibody Production in Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Result
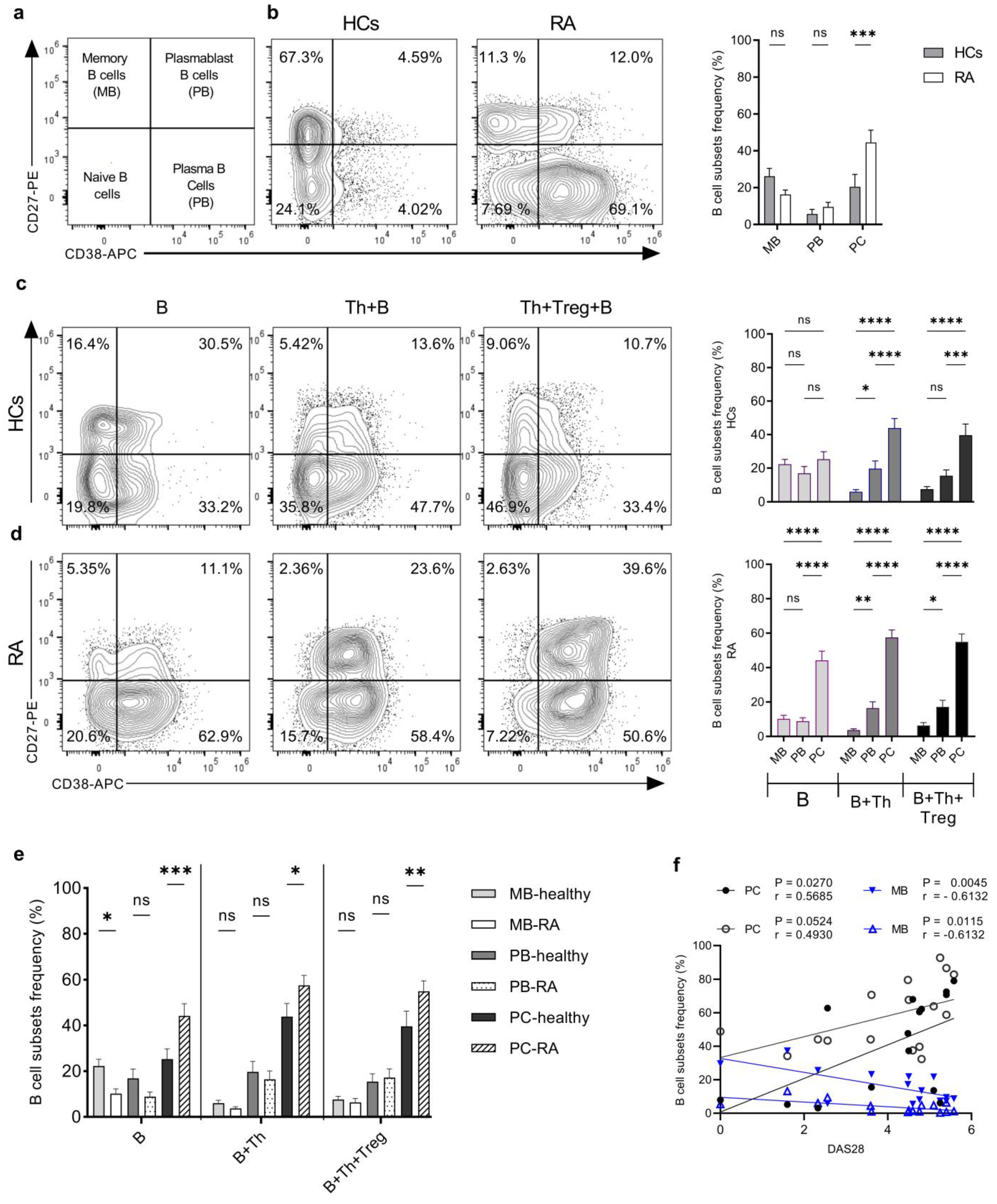
2.1. Characterization and Sorting of the CD4+ T Cell Subsets for Functional Studies
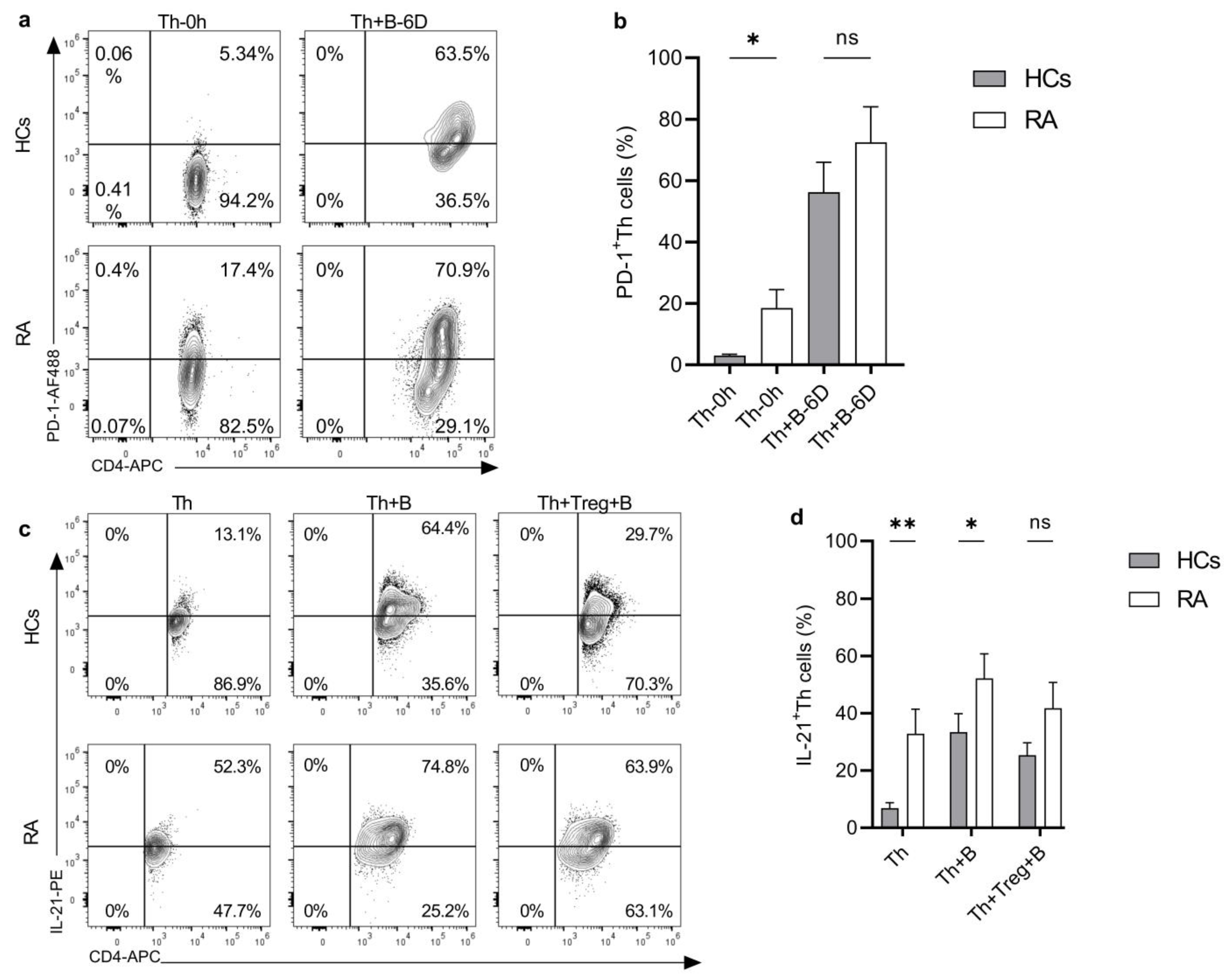
2.2. Helper T Cells from RA Patients Are Hyperactive
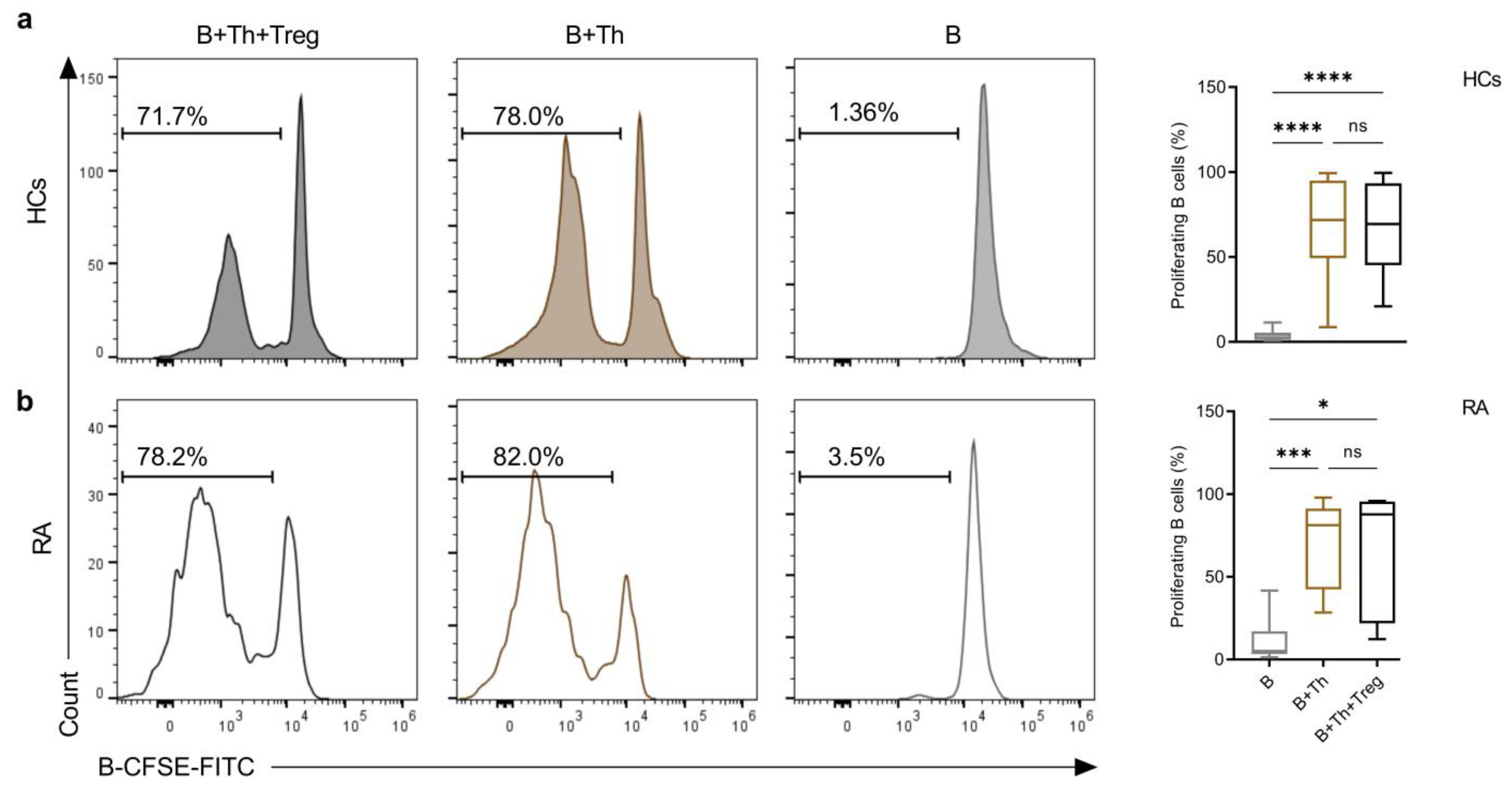
2.3. Th Cells Induce a Similar Rate of B-Cell Proliferation in Co-Cultures from Both Healthy Controls and RA Patients
2.4. Th Cells from RA Patients Induce a Higher Degree of Plasma Cell Differentiation in Th+B Cell Co-Cultures Compared to HCs
2.5. Helper T Cells from RA Patients Induced a Higher IgG Production by Plasma Cells Compared to Healthy Samples
3. Discussion
4. Materials and Methods
4.1. Blood Samples
4.2. Isolation of B and T Cells Using Magnetic Activating Cell Sorting (MACS)
4.3. Isolation of Th Cells and Treg Cells Using Fluorescence-Activated Cell Sorting
4.4. Immunophenotyping Assay
4.5. Proliferation Assay
4.6. ELISA
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7AAD | 7-Aminoactinomycin D |
| ACPA | Anti-citrullinated protein antibody |
| Bcl 6 | B-cell lymphoma 6 |
| BLIMP1 | B-lymphocyte-induced maturation protein 1 |
| CFSE | Carboxyfluorescein diacetate Succinimidyl ester “mixed isomer” |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| FMO | Fluorescent minus one |
| Foxp3 | forkhead box P3 |
| HRP | Horseradish peroxidase |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| ICOS | T-cell inducible co-stimulator |
| MACS | Magnetic activating cells sorting |
| MB | Memory B cells |
| PB | Plasma blast cells |
| PBMCs | Peripheral blood mononuclear cells |
| PC | Plasma B cells |
| PD-1 | Programmed cell death protein 1 |
| PMA | Phorbol 12-myristate 13-acetate |
| RANKL | Receptor activator of nuclear factor kappa beta (NF-kβ) ligand |
| RF | Rheumatoid factor |
| SEB | Staphylococcus enterotoxin B |
| Tfh | follicular T helper cells |
| Tph | Peripheral T helper cells |
References
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham III, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- van Delft, M.A.; Huizinga, T.W. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Firestein, G.S.; McInnes, I.B. The Pathogenesis of Rheumatoid Arthritis. Available online: https://www.cell.com/immunity/pdf/S1074-7613(22)00599-4.pdf (accessed on 16 September 2024).
- Scherer, H.U.; van der Woude, D.; Toes, R.E. From risk to chronicity: Evolution of autoreactive B cell and antibody responses in rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Szarka, E.; Aradi, P.; Huber, K.; Pozsgay, J.; Végh, L.; Magyar, A.; Gyulai, G.; Nagy, G.; Rojkovich, B.; Kiss, É.; et al. Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 326. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.-Y.; Tee, S.Z.-Y.; Wong, M.M.-T.; Chow, S.-K.; Peh, S.-C.; Teow, S.-Y. Pathogenic role of immune cells in rheumatoid arthritis: Implications in clinical treatment and biomarker development. Cells 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Luo, P.; Wang, P.; Xu, J.; Hou, W.; Xu, P.; Xu, K.; Liu, L. Immunomodulatory role of T helper cells in rheumatoid arthritis: A comprehensive research review. Bone Jt. Res. 2022, 11, 426–438. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation—Lessons from rheumatoid arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; RSimeonov, D.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef] [PubMed]
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.; Hennighausen, L. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007, 26, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-X.; Ma, X.-W.; Li, Y.-F.; Lai, N.-L.; Huang, Z.-H.; Fan, K.; Wang, C.-H.; Li, X.-F. The proportion of regulatory T cells in patients with systemic lupus erythematosus: A meta-analysis. J. Immunol. Res. 2018, 2018, 7103219. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol 2021, 12, 626193. [Google Scholar] [CrossRef]
- Yan, S.; Kotschenreuther, K.; Deng, S.; Kofler, D.M. Regulatory T cells in rheumatoid arthritis: Functions, development, regulation, and therapeutic potential. Cell. Mol. Life Sci. 2022, 79, 533. [Google Scholar] [CrossRef]
- Ding, T.; Su, R.; Wu, R.; Xue, H.; Wang, Y.; Su, R.; Gao, C.; Li, X.; Wang, C. Frontiers of Autoantibodies in Autoimmune Disorders: Crosstalk Between Tfh/Tfr and Regulatory B Cells. Front. Immunol. 2021, 12, 641013. [Google Scholar] [CrossRef]
- Gensous, N.; Charrier, M.; Duluc, D.; Contin-Bordes, C.; Truchetet, M.E.; Lazaro, E.; Duffau, P.; Blanco, P.; Richez, C. T Follicular Helper Cells in Autoimmune Disorders. Front. Immunol. 2018, 9, 1637. [Google Scholar] [CrossRef]
- Wei, X.; Niu, X. T follicular helper cells in autoimmune diseases. J. Autoimmun. 2023, 134, 102976. [Google Scholar] [CrossRef]
- Wang, J.; Shan, Y.; Jiang, Z.; Feng, J.; Li, C.; Ma, L.; Jiang, Y. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin. Exp. Immunol. 2013, 174, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H. Peripheral Helper T Cell Responses in Human Diseases. Front. Immunol. 2022, 13, 946786. [Google Scholar] [CrossRef] [PubMed]
- Bocharnikov, A.V.; Keegan, J.; Wacleche, V.S.; Cao, Y.; Fonseka, C.Y.; Wang, G.; Muise, E.S.; Zhang, K.X.; Arazi, A.; Keras, G.; et al. PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhu, C.; Ma, B.; Tian, J.; Baidoo, S.E.; Mao, C.; Wu, W.; Chen, J.; Tong, J.; Yang, M. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012, 2012, 827480. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, Q.; Su, D.; Che, N.; Chen, H.; Geng, L.; Chen, J.; Chen, W.; Li, X.; Sun, L. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R255. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Fraser, J.D.; Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar] [CrossRef]
- Luo, Q.; Ye, J.; Zeng, L.; Luo, Z.; Deng, Z.; Li, X.; Guo, Y.; Huang, Z.; Li, J. Elevated expression of PD-1 on T cells correlates with disease activity in rheumatoid arthritis. Mol. Med. Rep. 2018, 17, 3297–3305. [Google Scholar] [CrossRef]
- Fortea-Gordo, P.; Nuno, L.; Villalba, A.; Peiteado, D.; Monjo, I.; Sanchez-Mateos, P.; Puig-Kroger, A.; Balsa, A.; Miranda-Carus, M.E. Two populations of circulating PD-1hiCD4 T cells with distinct B cell helping capacity are elevated in early rheumatoid arthritis. Rheumatology 2019, 58, 1662–1673. [Google Scholar] [CrossRef]
- McHeyzer-Williams, M.; McHeyzer-Williams, L.; Panus, J.; Pogue-Caley, R.; Bikah, G.; Driver, D.; Eisenbraun, M. Helper T-cell-regulated B-cell immunity. Microbes Infect. 2003, 5, 205–212. [Google Scholar] [CrossRef]
- Horii, M.; Matsushita, T. Regulatory B cells and T cell regulation in cancer. J. Mol. Biol. 2021, 433, 166685. [Google Scholar] [CrossRef]
- Pierce, S.K. Understanding B cell activation: From single molecule tracking, through Tolls, to stalking memory in malaria. Immunol. Res. 2009, 43, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Garcia-Ibanez, L.; Toellner, K.M. Regulation of germinal center B-cell differentiation. Immunol. Rev. 2016, 270, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Hamel, K.M.; Liarski, V.M.; Clark, M.R. Germinal center B-cells. Autoimmunity 2012, 45, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015, 36, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H.; Ueno, H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell. Mol. Immunol. 2021, 18, 523–527. [Google Scholar] [CrossRef]
- Lucas, C.; Perdriger, A.; Amé, P. Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment. Semin. Arthritis Rheum. 2020, 50, 867–872. [Google Scholar] [CrossRef]
- Bautista-Caro, M.B.; Arroyo-Villa, I.; Castillo-Gallego, C.; de Miguel, E.; Peiteado, D.; Plasencia-Rodríguez, C.; Villalba, A.; Sánchez-Mateos, P.; Puig-Kröger, A.; Martín-Mola, E.; et al. Decreased frequencies of circulating follicular helper T cell counterparts and plasmablasts in ankylosing spondylitis patients Naïve for TNF blockers. PLoS ONE 2014, 9, e107086. [Google Scholar] [CrossRef]
- Yamada, H. The Search for the Pathogenic T Cells in the Joint of Rheumatoid Arthritis: Which T-Cell Subset Drives Autoimmune Inflammation? Int. J. Mol. Sci. 2023, 24, 6930. [Google Scholar] [CrossRef]
- Yoshitomi, H. Peripheral helper T cells, mavericks of peripheral immune responses. Int. Immunol. 2024, 36, 9–16. [Google Scholar] [CrossRef]
- Pincus, S.H.; Clegg, D.O.; Ward, J.R. Characterization of T cells bearing HLA-DR antigens in rheumatoid arthritis. Arthritis Rheum. 1985, 28, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kunkl, M.; Amormino, C.; Caristi, S.; Tedeschi, V.; Fiorillo, M.T.; Levy, R.; Popugailo, A.; Kaempfer, R.; Tuosto, L. Binding of Staphylococcal Enterotoxin B (SEB) to B7 Receptors Triggers TCR- and CD28-Mediated Inflammatory Signals in the Absence of MHC Class II Molecules. Front. Immunol. 2021, 12, 723689. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Schmitt, N.; Bentebibel, S.E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B Cells in Rheumatoid Arthritis: Pathogenic Mechanisms and Treatment Prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar] [CrossRef]
- Koga, T.; Kawakami, A.; Tsokos, G.C. Current insights and future prospects for the pathogenesis and treatment for rheumatoid arthritis. Clin. Immunol. 2021, 225, 108680. [Google Scholar] [CrossRef]


| RA (n = 25) | Reference Value (RV) | |
|---|---|---|
| Age (y; mean [range]) | 60.4 (24–85) | - |
| M/F (N) | 1/24 | - |
| Disease duration (y; mean [range]) | 11.07 (2–27) | - |
| Treatments: | ||
| Methotrexate | 12 | - |
| Methotrexate + folic acid | 1 | - |
| Methotrexate + Medrol | 6 | - |
| Hydroxychloroquin + Medrol | 1 | - |
| Methotrexate + Medrol + tofacitinib | 1 | - |
| Cimzia | 1 | - |
| Somatostatin Analogues + Cimzia | 1 | - |
| Leflunomide | 2 | - |
| CRP (mg/L; mean [range]) | 11.07 (0.58–46) | <8 mg/L |
| DAS28 [ESR-based] (mean [range]) | 4.19 (1.6–5.58) | |
| aCCP (IU/mL) | <20 EU/mL | |
| aCCP− | n = 5 | |
| aCCP+ (mean [range]) | 1623.16 (70.15–3200) | |
| RF(IU/mL) | <20 IU/mL | |
| RF− | n = 2 | |
| RF+ (mean [range]) | 186.1 (12–650) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, M.; Gyebrovszki, B.; Bőgér, D.; Csomor, R.; Mészáros, A.; Fodor, A.; Rojkovich, B.; Sármay, G. Helper T Cells are Hyperactive and Contribute to the Dysregulation of Antibody Production in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 10190. https://doi.org/10.3390/ijms251810190
Talib M, Gyebrovszki B, Bőgér D, Csomor R, Mészáros A, Fodor A, Rojkovich B, Sármay G. Helper T Cells are Hyperactive and Contribute to the Dysregulation of Antibody Production in Patients with Rheumatoid Arthritis. International Journal of Molecular Sciences. 2024; 25(18):10190. https://doi.org/10.3390/ijms251810190
Chicago/Turabian StyleTalib, Mustafa, Balázs Gyebrovszki, Dorottya Bőgér, Réka Csomor, Anna Mészáros, Anna Fodor, Bernadette Rojkovich, and Gabriella Sármay. 2024. "Helper T Cells are Hyperactive and Contribute to the Dysregulation of Antibody Production in Patients with Rheumatoid Arthritis" International Journal of Molecular Sciences 25, no. 18: 10190. https://doi.org/10.3390/ijms251810190






