Novel Synthesis of Zinc Oxide on Cotton Fabric by Cathodic Cage Plasma Deposition for Photocatalytic and Antibacterial Performance
Abstract
1. Introduction
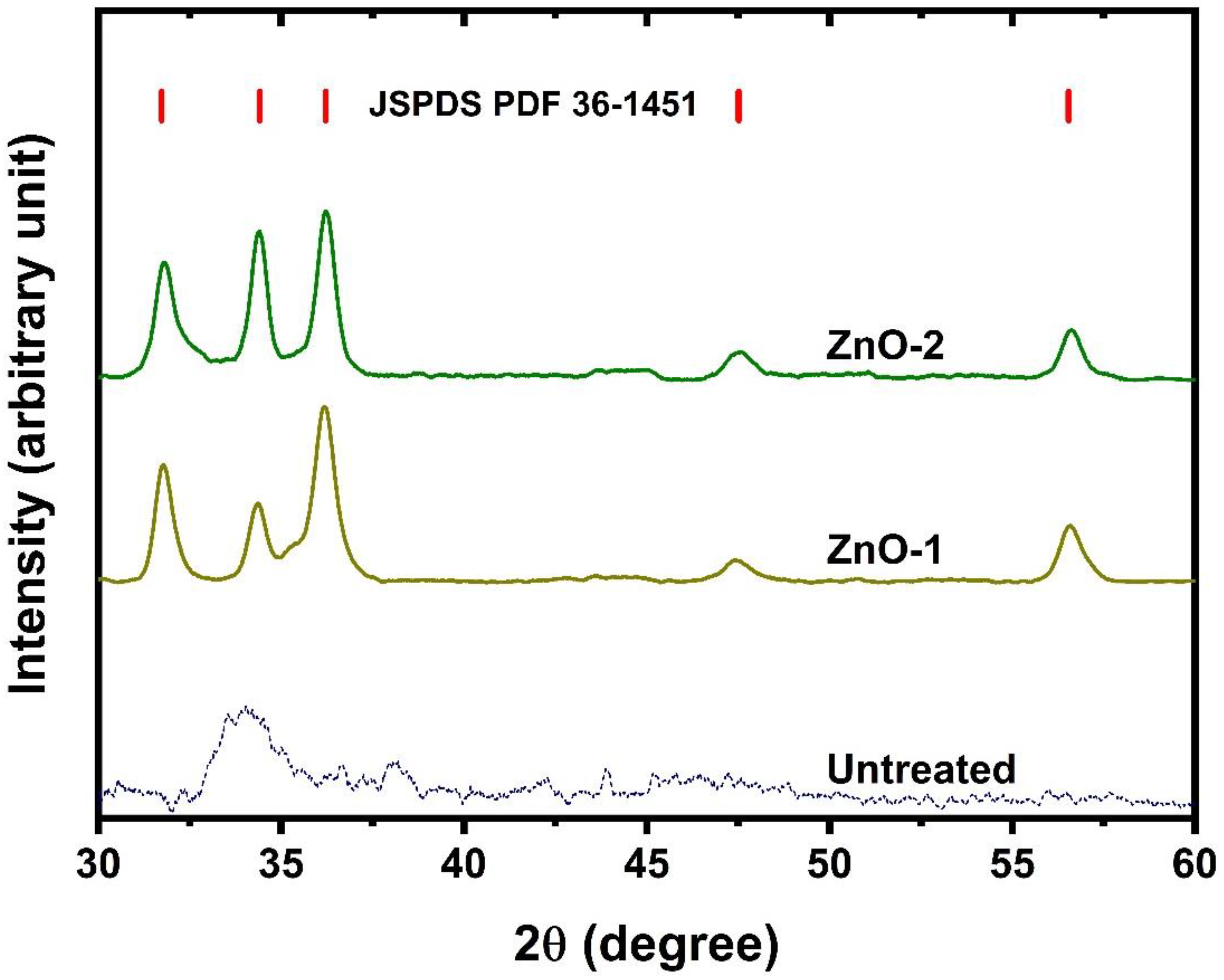
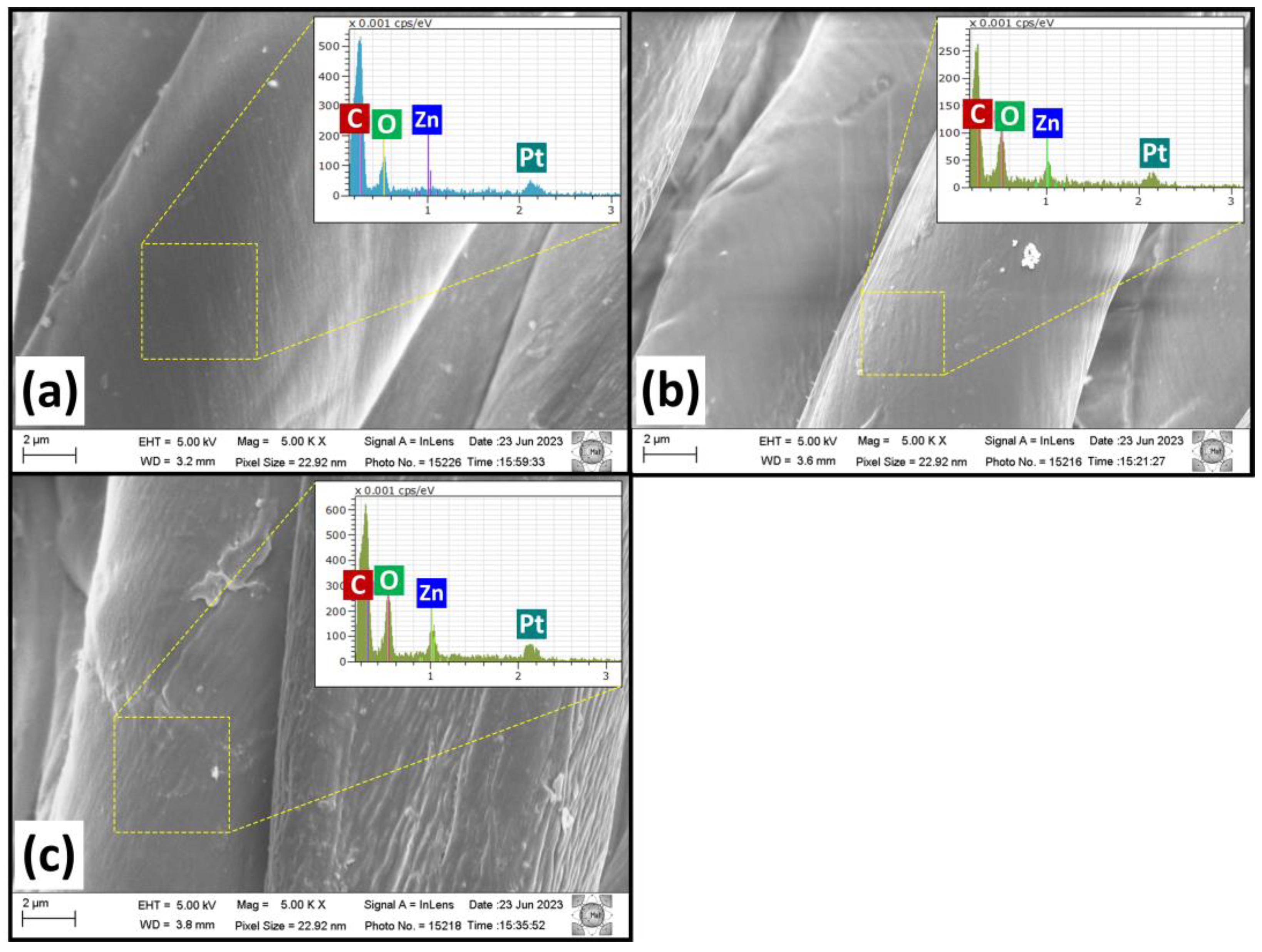
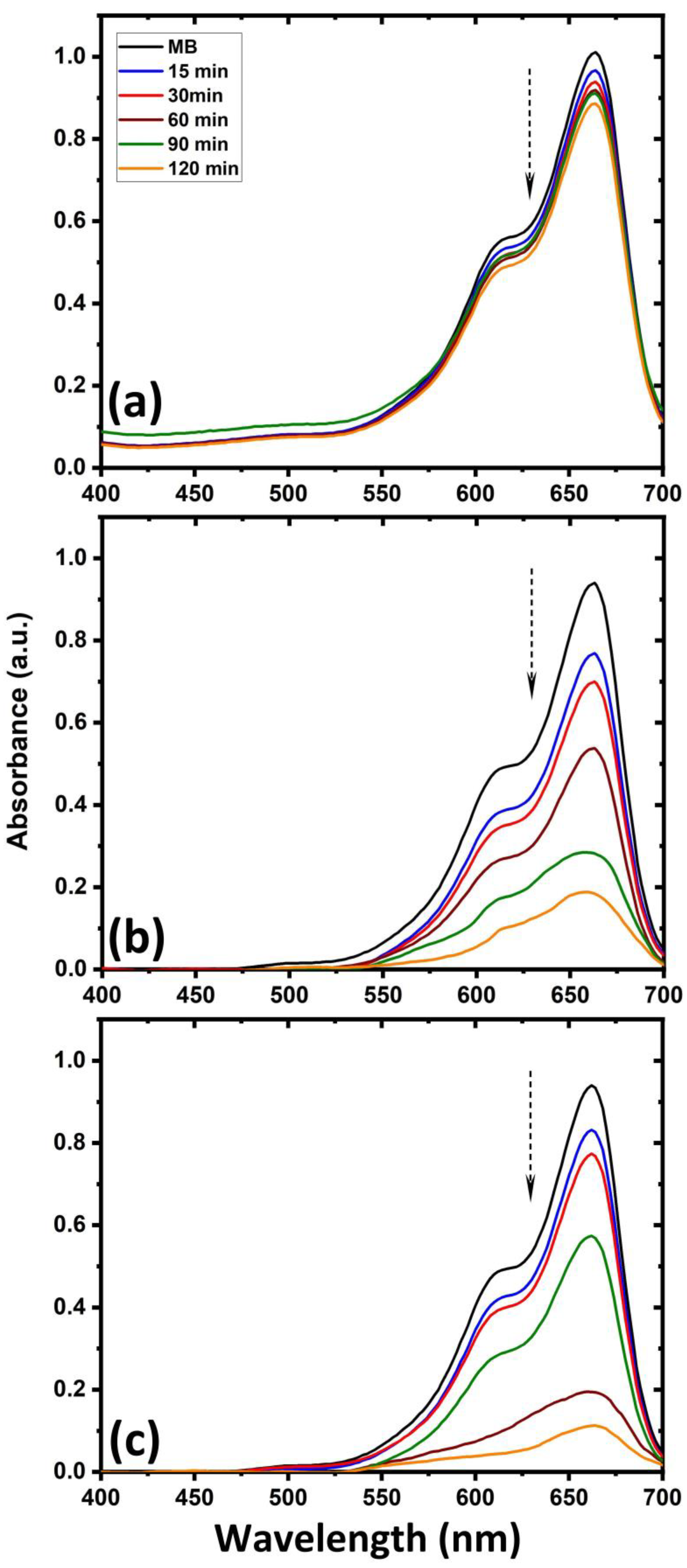
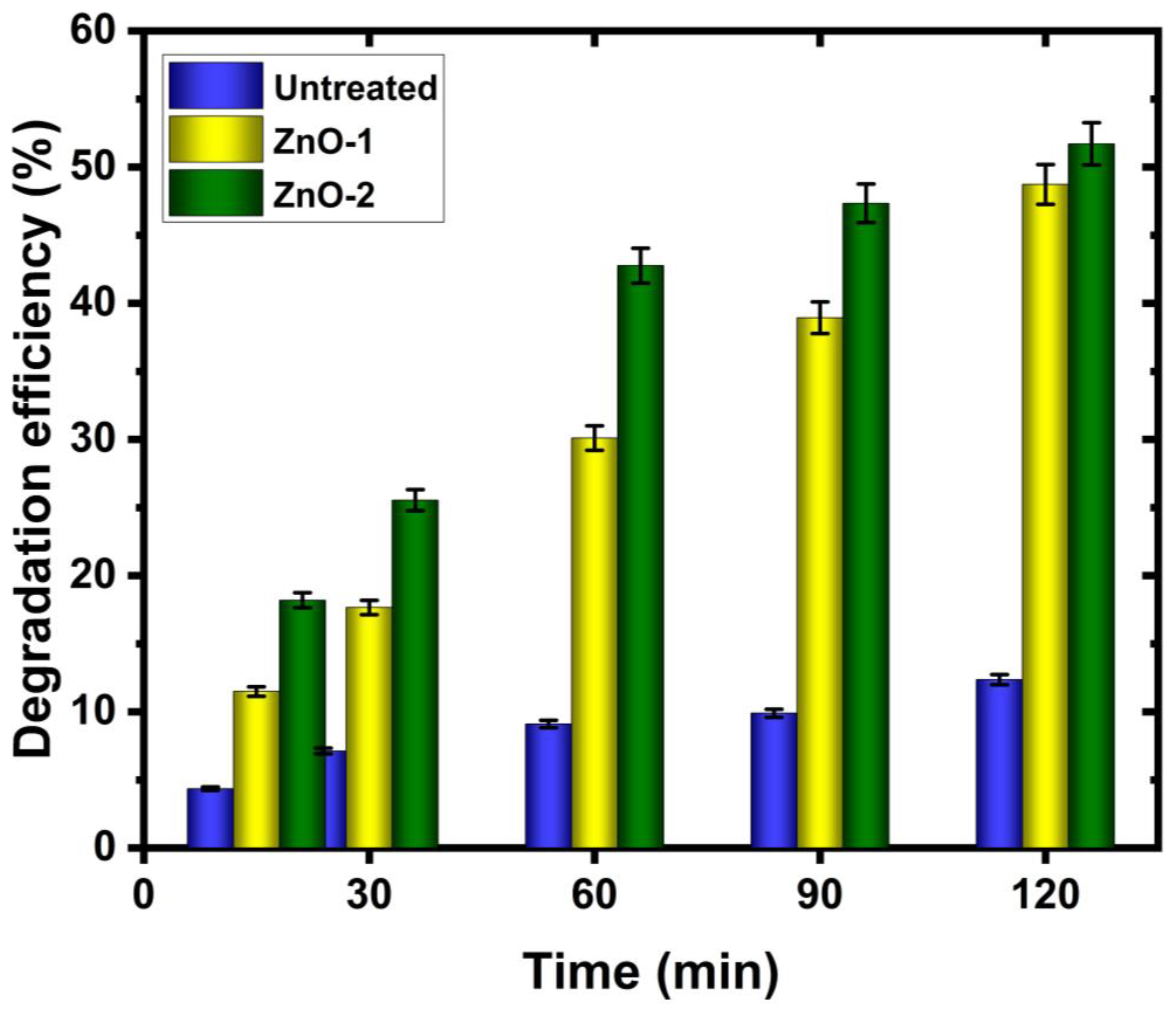
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Characterization Techniques
4. Conclusions
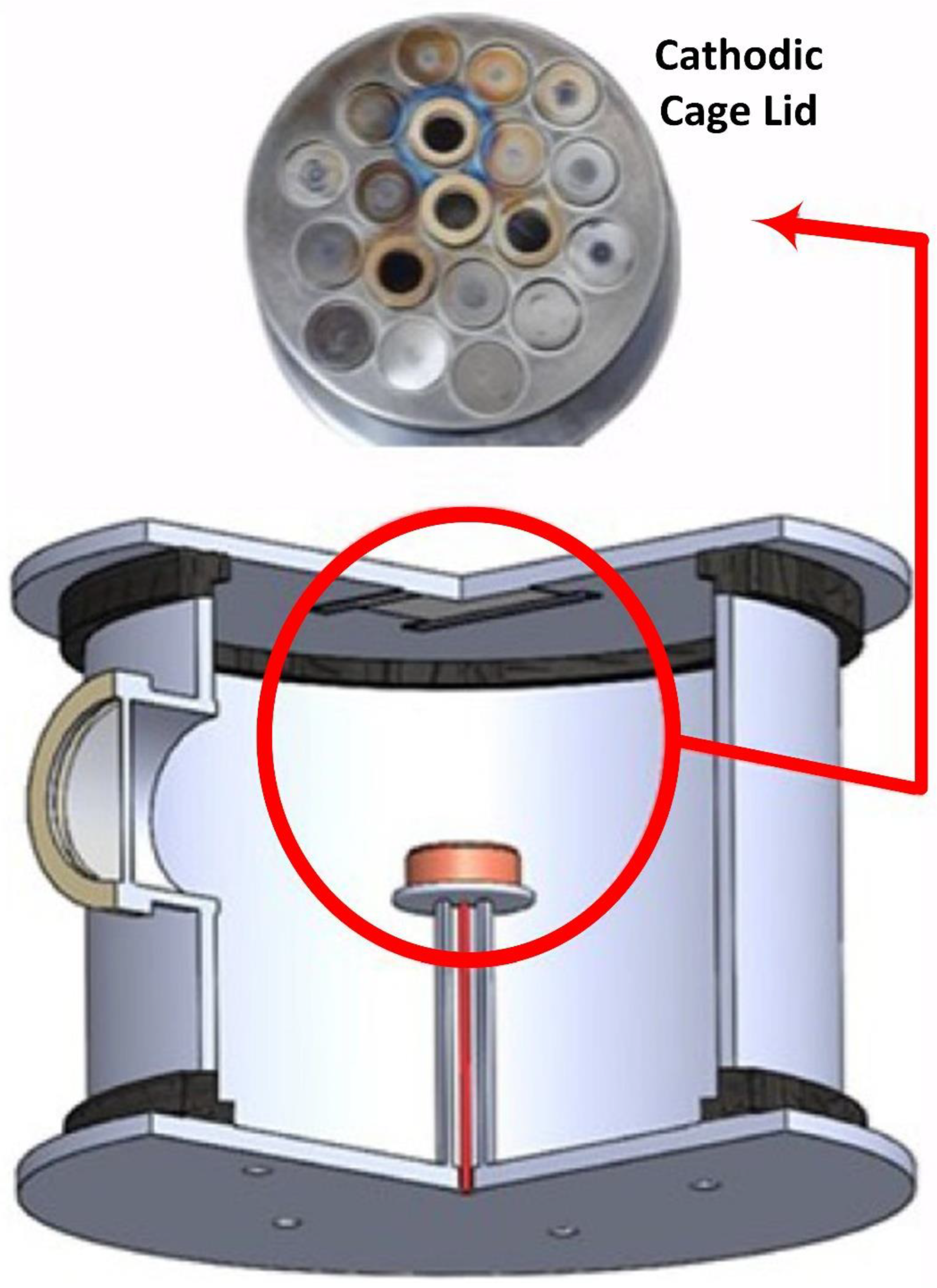
- Instead of conventional techniques, CCPD is an efficient choice for the deposition of ZnO on the cotton fabric, and it can be used for deposition in single-step processes, i.e., separate steps are not required for nanoparticle synthesis and subsequent deposition on fabric.
- The XRD results showed that ZnO is deposited on fabric, which exhibits a hexagonal wurtzite structure.
- The untreated cotton fabric shows that initial dyes still exist in the solution, but for ZnO-deposited cotton fabric, 52% of the initial dyes are degraded after the same immersion time. In particular, the ZnO deposition is efficient when a hydrogen–argon gas mixture is used in the CCPD system.
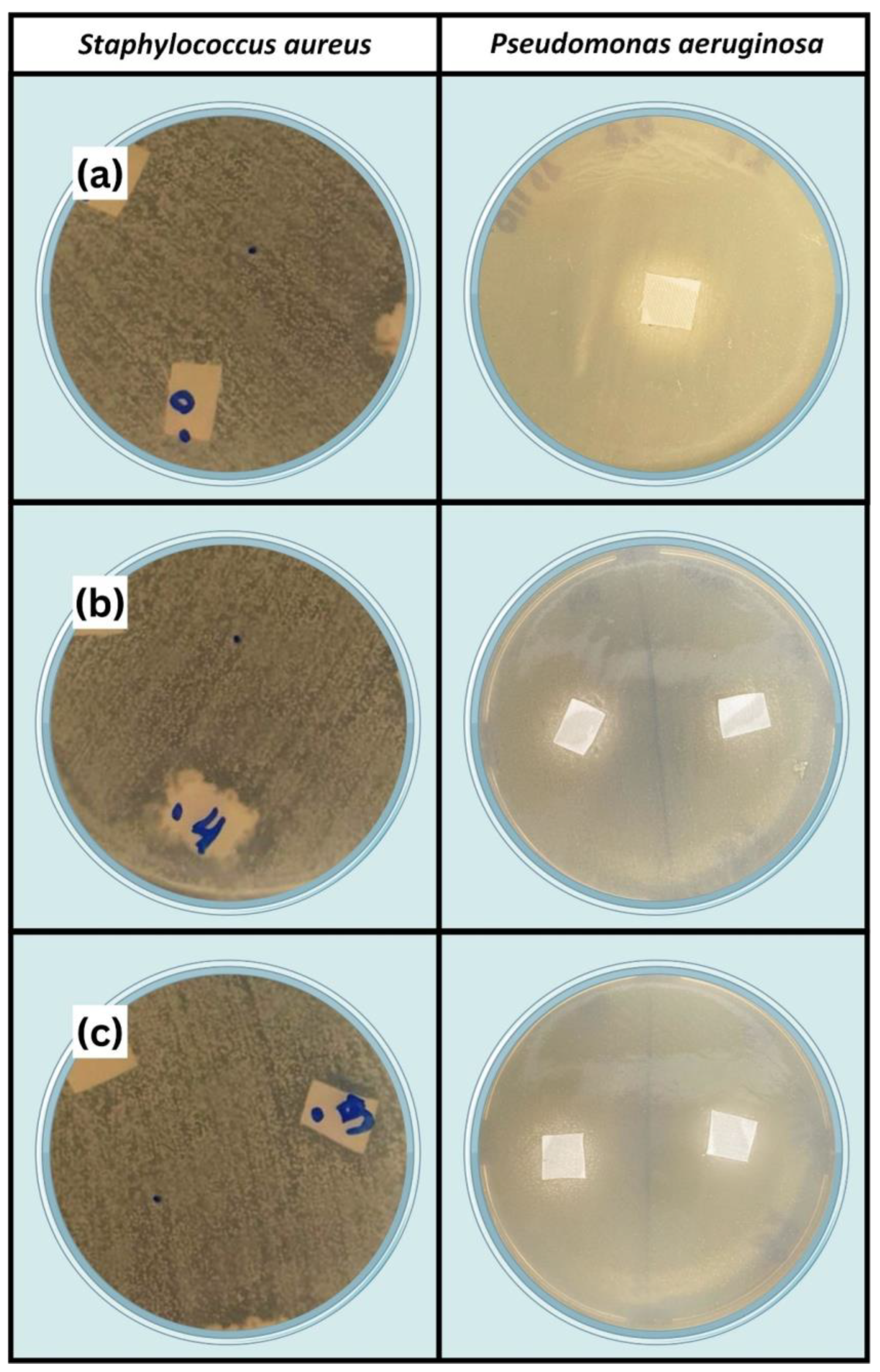
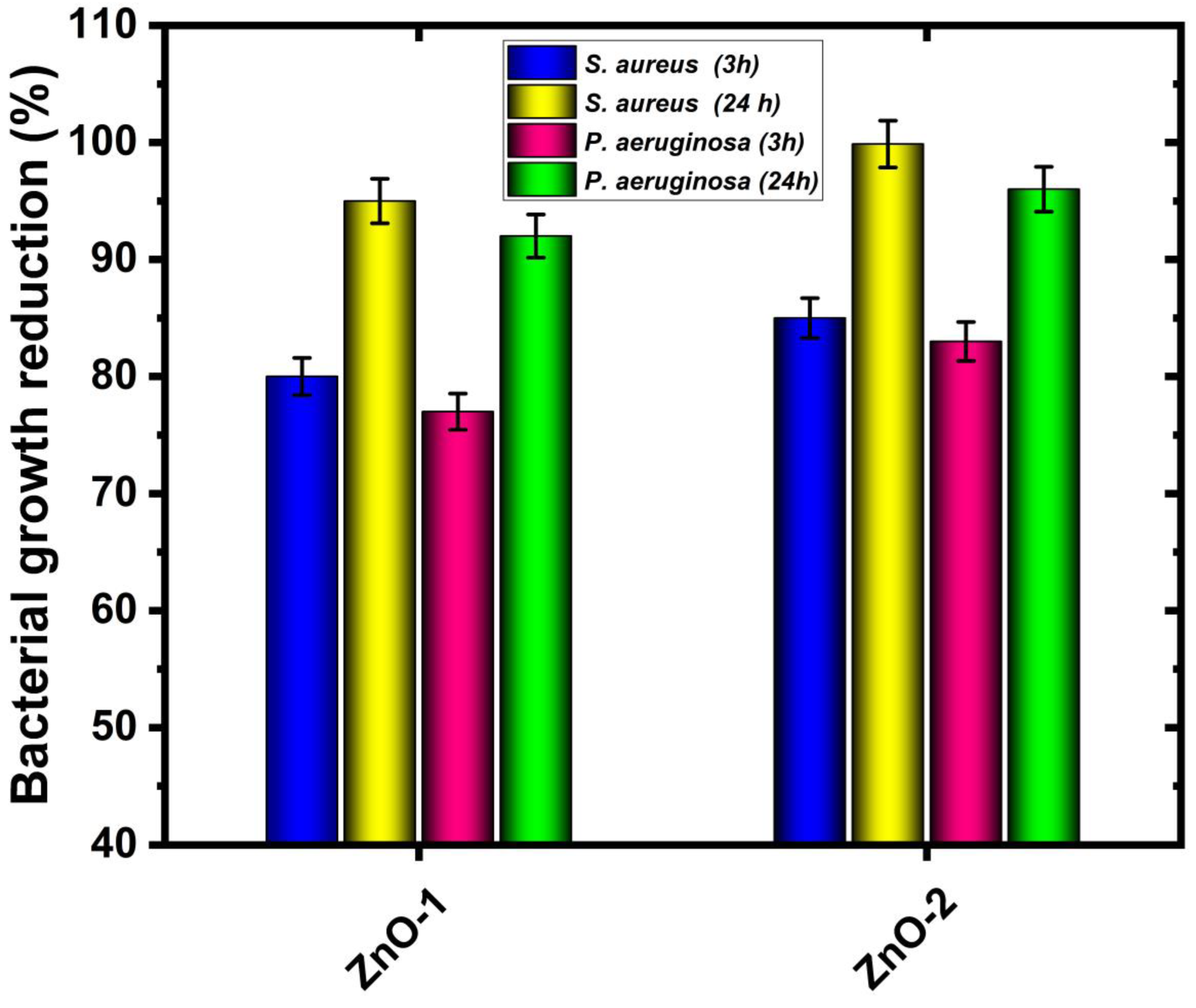
- The antibacterial performance of fabric with ZnO deposition is remarkable, and a reduction in bacterial growth is observed (up to 99% for Staphylococcus aureus and 97% for Pseudomonas aeruginosa bacteria). In the antibacterial test, the ZnO deposition is again more efficient for the hydrogen–argon gas mixture in CCPD operation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evseev, Z.I.; Tarasova, L.A.; Vasilieva, F.D.; Egorova, M.N.; Dmitriev, P.S.; Akhremenko, Y.A.; Smagulova, S.A. Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics. Int. J. Mol. Sci. 2024, 25, 5328. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Karim, Z.; Charnnok, B.; Poonsawat, T.; Posoknistakul, P.; Laosiripojana, N.; Wu, K.C.-W.; Sakdaronnarong, C. Fabrication and Characterization of Functional Biobased Membranes from Postconsumer Cotton Fabrics and Palm Waste for the Removal of Dyes. Int. J. Mol. Sci. 2023, 24, 6030. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Patil, A.H.; Jadhav, S.A.; Magdum, S.S.; Sonawane, K.D.; Patil, P.S. ZnO nanorods-grafted durable antibacterial and hydrophobic cotton fabrics by a new grafting protocol. Inorg. Chem. Commun. 2022, 144, 109947. [Google Scholar] [CrossRef]
- Salam, A.; Siddique, A.; Mubin, M.; Riaz, S. Cotton fabric coated with functionalized Zn-Doped Fe2O3 NPs for a Visible-Light activated durable Antibacterial, UV-Shielding, and organic degradation. Inorg. Chem. Commun. 2024, 162, 112173. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Meyer, S.; Ahmed, O.; Geerts, G.; Madiehe, M.A.; Meyer, M.; Sibuyi, N.R.S. Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 1799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, X.; Zhao, C.; Wang, S.; Tang, X. Recent Advance in Biological Responsive Nanomaterials for Biosensing and Molecular Imaging Application. Int. J. Mol. Sci. 2022, 23, 1923. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Anwar, M.; Kayani, Z.N.; Hassan, A.; Zeeshan, T.; Riaz, S.; Naseem, S. Enhancement in photocatalytic activity and biological properties of Sm doped ZnO nanostructures by the increase in Sm contents. Inorg. Chem. Commun. 2023, 158, 111431. [Google Scholar] [CrossRef]
- Patil, A.H.; Jadhav, S.A.; More, V.B.; Sonawane, K.D.; Vhanbatte, S.H.; Kadole, P.V.; Patil, P.S. A new method for single step sonosynthesis and incorporation of ZnO nanoparticles in cotton fabrics for imparting antimicrobial property. Chem. Pap. 2021, 75, 1247–1257. [Google Scholar] [CrossRef]
- Montazer, M.; Maali Amiri, M. ZnO nano reactor on textiles and polymers: Ex situ and in situ synthesis, application, and characterization. J. Phys. Chem. B 2014, 118, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Kumar, S.; Kathe, A.A.; Varadarajan, P.V.; Prasad, V. Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 2006, 17, 5087. [Google Scholar] [CrossRef]
- Uğur, Ş.S.; Sarıışık, M.; Aktaş, A.H.; Uçar, M.Ç.; Erden, E. Modifying of cotton fabric surface with nano-ZnO multilayer films by layer-by-layer deposition method. Nanoscale Res. Lett. 2010, 5, 1204–1210. [Google Scholar] [CrossRef]
- Joshi, M.; Bhattacharyya, A.; Agarwal, N.; Parmar, S. Nanostructured coatings for super hydrophobic textiles. Bull. Mater. Sci. 2012, 35, 933–938. [Google Scholar] [CrossRef]
- Patil, A.H.; Jadhav, S.A.; More, V.B.; Sonawane, K.D.; Patil, P.S. Novel one step sonosynthesis and deposition technique to prepare silver nanoparticles coated cotton textile with antibacterial properties. Colloid J. 2019, 81, 720–727. [Google Scholar] [CrossRef]
- Behzadnia, A.; Montazer, M.; Mahmoudi Rad, M. In situ photo sonosynthesis of organic/inorganic nanocomposites on wool fabric introducing multifunctional properties. Photochem. Photobiol. 2016, 92, 76–86. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hassabo, A.G.; Mohamed, A.L.; Shaheen, T.I. Surface modification of SiO2 coated ZnO nanoparticles for multifunctional cotton fabrics. J. Colloid Interface Sci. 2017, 498, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, J.; Yang, P.; Wu, Z.; Xiao, H.; Liu, Y.; Lu, M. Fabrication of ZnO@ Cotton fabric with anti-bacterial and radiation barrier properties using an economical and environmentally friendly method. Cellulose 2020, 27, 2901–2911. [Google Scholar] [CrossRef]
- Tania, I.S.; Ali, M. Effect of the coating of zinc oxide (ZnO) nanoparticles with binder on the functional and mechanical properties of cotton fabric. Mater. Today Proc. 2021, 38, 2607–2611. [Google Scholar] [CrossRef]
- Javed, A.; Azeem, M.; Wiener, J.; Thukkaram, M.; Saskova, J.; Mansoor, T. Ultrasonically assisted in situ deposition of ZnO nano particles on cotton fabrics for multifunctional textiles. Fibers Polym. 2021, 22, 77–86. [Google Scholar] [CrossRef]
- Verbič, A.; Šala, M.; Jerman, I.; Gorjanc, M. Novel green in situ synthesis of ZnO nanoparticles on cotton using pomegranate peel extract. Materials 2021, 14, 4472. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Ferri, A.; Ferraris, M.; Balagna, C. Antimicrobial functionalization of cotton fabric with silver nanoclusters/silica composite coating via RF co-sputtering technique. Cellulose 2017, 24, 2331–2345. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.X.; Dong, H.; Bell, T. Study on the active screen plasma nitriding and its nitriding mechanism. Surf. Coat. Technol. 2006, 201, 2320–2325. [Google Scholar] [CrossRef]
- Gallo, S.C.; Dong, H. On the fundamental mechanisms of active screen plasma nitriding. Vacuum 2009, 84, 321–325. [Google Scholar] [CrossRef]
- Saeed, A.; Khan, A.W.; Jan, F.; Abrar, M.; Khalid, M.; Zakaullah, M. Validity of “sputtering and re-condensation” model in active screen cage plasma nitriding process. Appl. Surf. Sci. 2013, 273, 173–178. [Google Scholar] [CrossRef]
- Naeem, M.; Raja, F.Q.; Nolêto, B.J.S.; Serra, P.L.C.; Costa, T.H.C.; Díaz-Guillén, J.C.; Hdz-García, H.M.; Iqbal, J.; Sousa, R.R.M. Surface modification of AISI-420 steel by cathodic cage plasma niobium nitride deposition. Mater. Sci. Technol. 2024, 40. [Google Scholar] [CrossRef]
- Zaka-ul-Islam, M.; Naeem, M.; Shafiq, M.; Al-Rajab, A.J.; Zakaullah, M. Active screen cage pulsed dc discharge for implanting copper in polytetrafluoroethylene (PTFE). Mater. Res. Express 2017, 4, 075304. [Google Scholar] [CrossRef]
- Naeem, M.; Shafiq, M.; Zaka-ul-Islam, M.; Ashiq, A.; Díaz-Guillén, J.C.; Shahzad, M.; Zakaullah, M. Enhanced surface properties of plain carbon steel using plasma nitriding with austenitic steel cathodic cage. Mater. Des. 2016, 108, 745–753. [Google Scholar] [CrossRef]
- Naeem, M.; Shafiq, M.; Zaka-ul-Islam, M.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Ullah, N.; Zakaullah, M. Improved surface properties of AISI-304 by novel duplex cathodic cage plasma nitriding. Mater. Lett. 2017, 189, 213–216. [Google Scholar] [CrossRef]
- Naeem, M.; Torres, A.V.R.; Serra, P.L.C.; Monção, R.M.; Junior, C.A.A.; Rossino, L.S.; Costa, T.H.C.; Costa, C.; Iqbal, J.; Sousa, R.R.M. Combined plasma treatment of AISI-1045 steel by hastelloy deposition and plasma nitriding. J. Build. Eng. 2022, 47, 103882. [Google Scholar] [CrossRef]
- Nishimoto, A.; Nakazawa, K. Active screen plasma nitriding of titanium alloy using titanium double screen. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2017; pp. 11–17. [Google Scholar]
- Naeem, M.; Monção, R.M.; Díaz-Guillén, J.C.; Hdz-García, H.M.; Costa, T.H.C.; Safeen, K.; Iqbal, J.; Khan, K.H.; Sousa, R.R.M. Improved mechanical and wear properties of AISI-420 steel by cathodic cage plasma vanadium nitride deposition. Phys. Scr. 2023, 98, 115602. [Google Scholar]
- Naeem, M.; Fortaleza, V.C.; Serra, P.L.C.; Lima, C.L.; Costa, T.H.C.; Sousa, R.R.M.; Díaz-Guillén, J.C.; Iqbal, J. Enhanced Wear Resistance of AISI-316 Steel by Low-Temperature Molybdenum Cathodic Cage Plasma Deposition. J. Mater. Eng. Perform. 2021, 30, 8947–8955. [Google Scholar] [CrossRef]
- Fernades, F.; Filho, E.R.; Souza, I.; Nascimento, I.; Sousa, R.; Almeida, E.; Feitor, M.; Costa, T.; Naeem, M.; Iqbal, J. Novel synthesis of copper oxide on fabric samples by cathodic cage plasma deposition. Polym. Adv. Technol. 2020, 31, 520–526. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Loconsole, D.; Sallustio, A.; Picca, R.A.; Felici, R.; Chironna, M.; Cioffi, N. On the Efficacy of ZnO Nanostructures against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3040. [Google Scholar] [CrossRef]
- Sathish, T.; Chandramohan, D.; Dinesh Kumar, S.; Rajkumar, S.; Vijayan, V. A facile synthesis of Ag/ZnO nanocomposites prepared via novel green mediated route for catalytic activity. Appl. Phys. A 2021, 127, 692. [Google Scholar] [CrossRef]
- Khosravian, S.; Montazer, M.; Malek, R.M.A.; Harifi, T. In situ synthesis of nano ZnO on starch sized cotton introducing nano photo active fabric optimized with response surface methodology. Carbohydr. Polym. 2015, 132, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, R.; Haq, M.O.; Rahman, M.A. Influence of metal doping on topographic, microstructural, optical and thermal properties of zinc oxide nanoparticles. Appl. Phys. A 2024, 130, 473. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.K.; Jeevanandam, J.; Muthalagu, M.; Danquah, M.K. Surface cationization of cellulose to enhance durable antibacterial finish in phytosynthesized silver nanoparticle treated cotton fabric. Cellulose 2021, 28, 5895–5910. [Google Scholar] [CrossRef]
- Roy, T.; Basak, N.; Mainak, S.; Das, S.; Ali, S.I.; Islam, E. Burkholderia sp. EIKU21 mediated synthesis of biogenic ZnO nanoparticle–based pigment for development of antibacterial cotton fabric through nanocoating. Biomass Convers. Biorefinery 2024, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent advances in zinc oxide nanostructures with antimicrobial activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Kupińska, K.; Michalik, M.; Krajenta, J.; Bielicka, M.; Markiewicz, K.H.; Kalska-Szostko, B.; Wilczewska, A.Z. An in-situ fabrication method of ZnO and Other Zn (II) compounds containing polypropylene composites. Int. J. Mol. Sci. 2023, 24, 2357. [Google Scholar] [CrossRef] [PubMed]
- Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Functional properties of gelatin/polyvinyl alcohol films containing black cumin cake extract and zinc oxide nanoparticles produced via casting technique. Int. J. Mol. Sci. 2022, 23, 2734. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, T.; Ogorek, M.; Skuza, Z.; Prusak, R. Mechanism of ion nitriding of 316L austenitic steel by active screen method in a hydrogen-nitrogen atmosphere. Int. J. Adv. Manuf. Technol. 2020, 109, 1357–1368. [Google Scholar] [CrossRef]
- Naeem, M.; Khattak, Z.I.; Zaka-ul-Islam, M.; Shabir, S.; Khan, A.W.; Zakaullah, M. Investigation of plasma parameters in an active screen cage-pulsed dc plasma used for plasma nitriding. Radiat. Eff. Defects Solids 2014, 169, 893–905. [Google Scholar] [CrossRef]
- Budtz-Jørgensen, C.V.; Kringhøj, P.; Bøttiger, J. The critical role of hydrogen for physical sputtering with Ar–H2 glow discharges. Surf. Coat. Technol. 1999, 116, 938–943. [Google Scholar] [CrossRef]
- Junior, W.N.; Naeem, M.; Costa, T.H.C.; Díaz-Guillén, J.C.; Díaz-Guillén, M.R.; Iqbal, J.; Jelani, M.; Sousa, R.R.M. Surface modification of AISI-304 steel by ZnO synthesis using cathodic cage plasma deposition. Mater. Res. Express 2021, 8, 096403. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Maity, J. Synthesis, characterization and photocatalytic properties of ZnO nanoparticles and cotton fabric modified with ZnO nanoparticles via in-situ hydrothermal coating technique: Dual response. Mater. Technol. 2018, 33, 884–891. [Google Scholar] [CrossRef]
- Li, L.; Fan, T.; Hu, R.; Liu, Y.; Lu, M. Surface micro-dissolution process for embedding carbon nanotubes on cotton fabric as a conductive textile. Cellulose 2017, 24, 1121–1128. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Li, A.-D.; Li, X.-Y.; Zhai, H.-F.; Zhang, W.-Q.; Gong, Y.-P.; Li, H.; Wu, D. Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J. Solid State Chem. 2010, 183, 1359–1364. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rosan, S.A.; Albiss, B.A. Photocatalytic degradation of methylene blue using polymeric membranes based on cellulose acetate impregnated with ZnO nanostructures. Polymers 2021, 13, 3451. [Google Scholar] [CrossRef] [PubMed]
- Albiss, B.; Abu-Dalo, M. Photocatalytic degradation of methylene blue using zinc oxide nanorods grown on activated carbon fibers. Sustainability 2021, 13, 4729. [Google Scholar] [CrossRef]
- Huang, N.; Shu, J.; Wang, Z.; Chen, M.; Ren, C.; Zhang, W. One-step pyrolytic synthesis of ZnO nanorods with enhanced photocatalytic activity and high photostability under visible light and UV light irradiation. J. Alloys Compd. 2015, 648, 919–929. [Google Scholar] [CrossRef]
- Yamamoto, O.; Sawai, J.; Sasamoto, T. Change in antibacterial characteristics with doping amount of ZnO in MgO–ZnO solid solution. Int. J. Inorg. Mater. 2000, 2, 451–454. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.S.M.d.; Naeem, M.; da Silva, A.L.; De Medeiros Aires, M.; de Sousa, R.R.M.; de Carvalho Costa, T.H.; Rocha, H.A.O.; De Melo, M.C.N.; Feitor, M.C. Novel Synthesis of Zinc Oxide on Cotton Fabric by Cathodic Cage Plasma Deposition for Photocatalytic and Antibacterial Performance. Int. J. Mol. Sci. 2024, 25, 10192. https://doi.org/10.3390/ijms251810192
Santos RSMd, Naeem M, da Silva AL, De Medeiros Aires M, de Sousa RRM, de Carvalho Costa TH, Rocha HAO, De Melo MCN, Feitor MC. Novel Synthesis of Zinc Oxide on Cotton Fabric by Cathodic Cage Plasma Deposition for Photocatalytic and Antibacterial Performance. International Journal of Molecular Sciences. 2024; 25(18):10192. https://doi.org/10.3390/ijms251810192
Chicago/Turabian StyleSantos, Rayane Saory Medeiros dos, Muhammad Naeem, Anderson Lucas da Silva, Michelle De Medeiros Aires, Rômulo R. Magalhães de Sousa, Thércio Henrique de Carvalho Costa, Hugo Alexandre Oliveira Rocha, Maria Celeste Nunes De Melo, and Michelle Cequeira Feitor. 2024. "Novel Synthesis of Zinc Oxide on Cotton Fabric by Cathodic Cage Plasma Deposition for Photocatalytic and Antibacterial Performance" International Journal of Molecular Sciences 25, no. 18: 10192. https://doi.org/10.3390/ijms251810192
APA StyleSantos, R. S. M. d., Naeem, M., da Silva, A. L., De Medeiros Aires, M., de Sousa, R. R. M., de Carvalho Costa, T. H., Rocha, H. A. O., De Melo, M. C. N., & Feitor, M. C. (2024). Novel Synthesis of Zinc Oxide on Cotton Fabric by Cathodic Cage Plasma Deposition for Photocatalytic and Antibacterial Performance. International Journal of Molecular Sciences, 25(18), 10192. https://doi.org/10.3390/ijms251810192






