Downregulation of TASK-3 Channel Induces Senescence in Granulosa Cells of Bovine Cystic Ovarian Follicles
Abstract
1. Introduction
2. Results
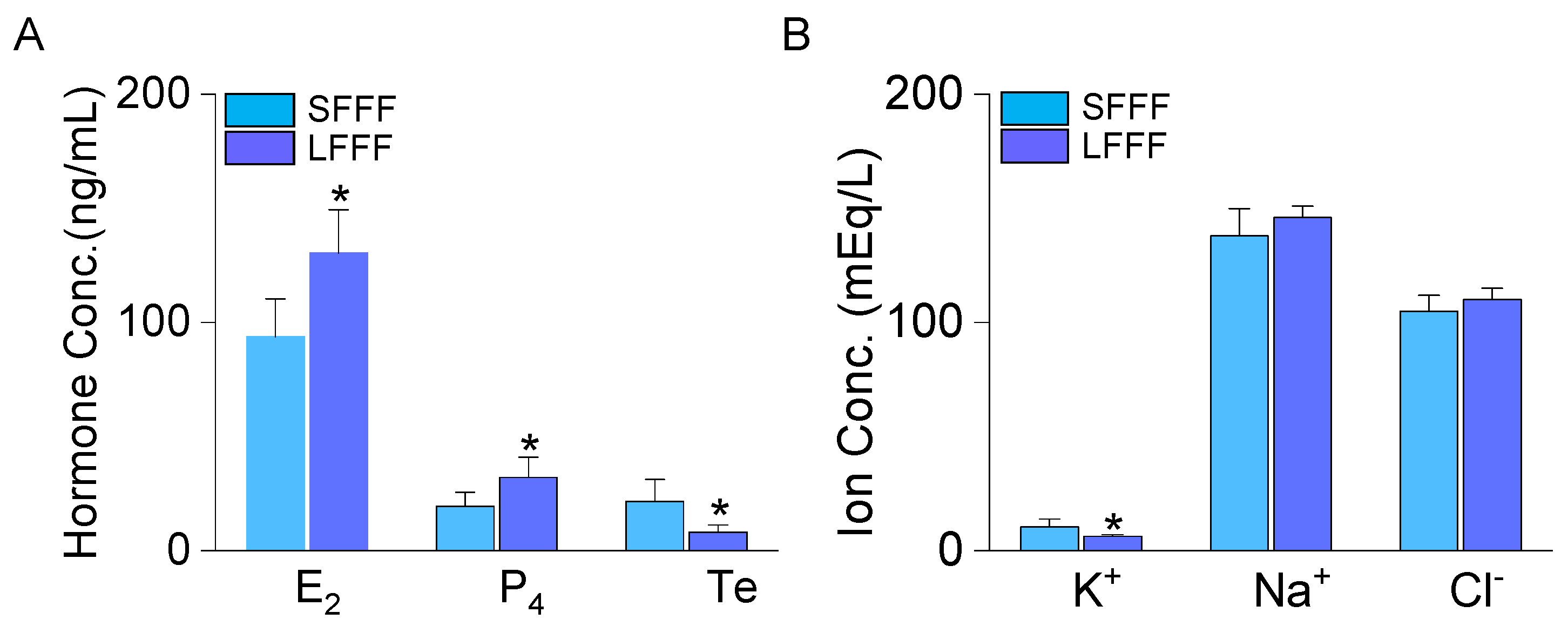
2.1. Low K+ Concentration in Follicular Fluid Obtained from Large-Sized Follicles
2.2. Downregulation of TASK Expression Levels in GCs Obtained from LFs
2.3. Localization of TASK-3 in Ovarian GCs
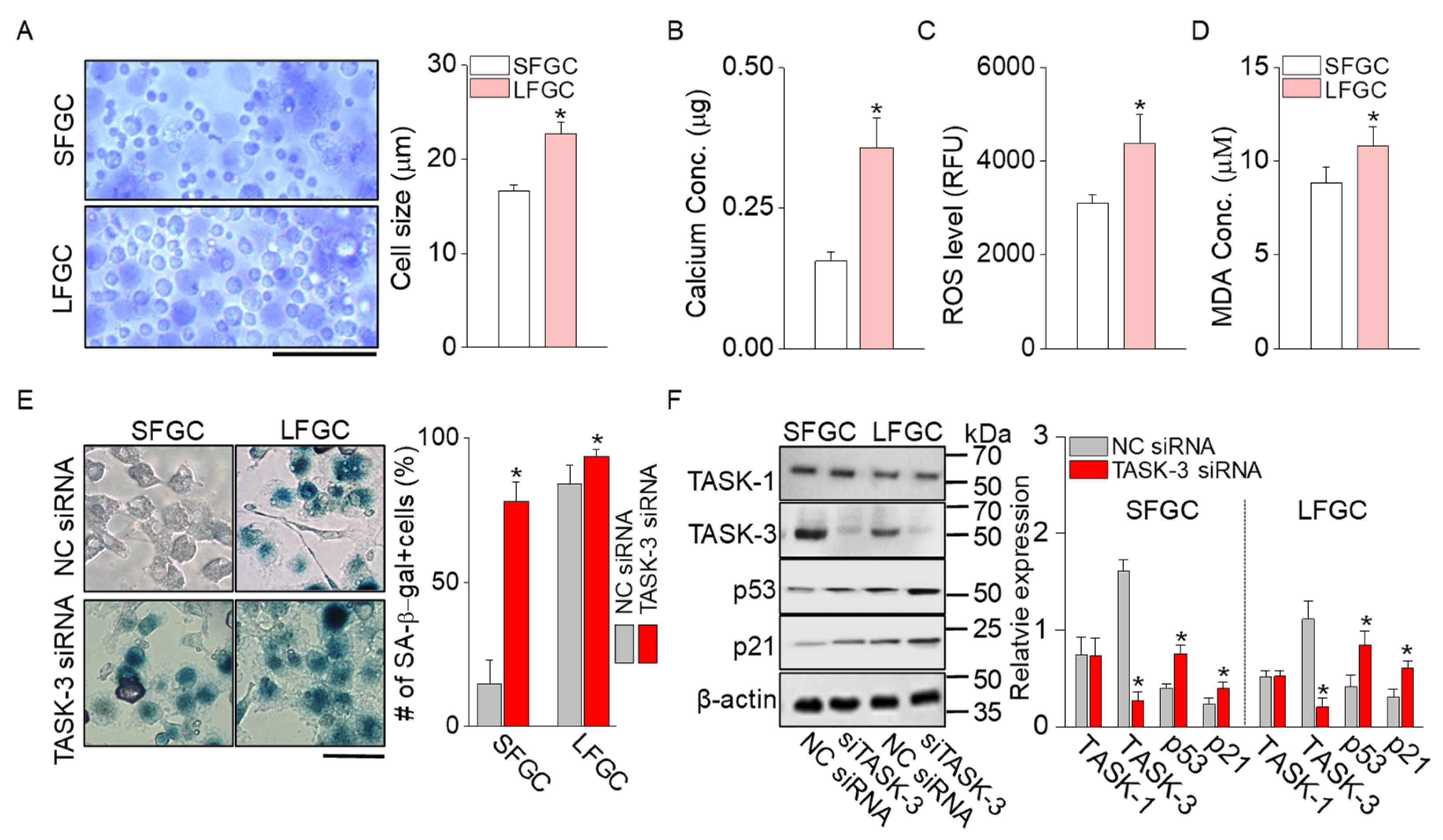
2.4. Senescence Signals Increased in LFGC
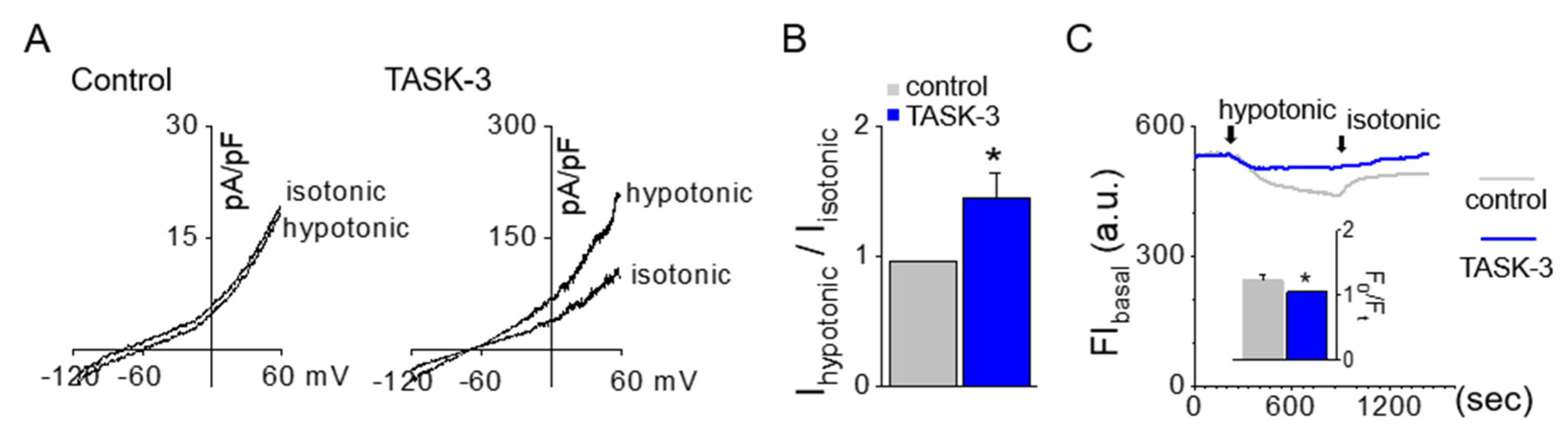
2.5. Hypotonic-Induced Swelling Reduced by TASK-3 Activation
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Isolation of Follicular Fluid and Granulosa Cells
4.3. Measurement of 17β-Estradiol (E2), Progesterone (P4), and Testosterone
4.4. Measurement of Ion Concentrations
4.5. Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR) and Real-Time PCR
4.6. Western Blot Analysis
4.7. Hematoxylin–Eosin (H&E) Staining
4.8. Immunostaining
4.9. Measurement of Free Radical Activity and Calcium and Malondialdehyde (MDA) Concentrations in GCs
4.10. Cellular Senescence Assay
4.11. Recording of Whole-Cell Current
4.12. Measurement of Cell Volume
4.13. Gene Silencing with Small Interfering RNA
4.14. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, A.T. An update on cystic ovarian degeneration in cattle. Reprod. Domest. Anim. 2004, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.A.; Garverick, H.A.; Keisler, D.H.; Xu, Z.Z.; Loos, K.; Youngquist, R.S.; Salfen, B.E. Characterization of ovarian follicular cysts and associated endocrine profiles in dairy cows. Biol. Reprod. 1995, 53, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.H.; Marelli, B.E.; Rey, F.; Amweg, A.N.; Diaz, P.U.; Stangaferro, M.L.; Salvetti, N.R. Molecular aspects of bovine cystic ovarian disease pathogenesis. Reproduction 2015, 149, R251–R264. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, N.R.; Alfaro, N.S.; Velazquez, M.M.; Amweg, A.N.; Matiller, V.; Diaz, P.U.; Ortega, H.H. Alteration in localization of steroid hormone receptors and coregulatory proteins in follicles from cows with induced ovarian follicular cysts. Reproduction 2012, 144, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.M.; Colombero, M.; Amweg, A.N.; Huber, E.; Gareis, N.C.; Salvetti, N.R.; Ortega, H.H.; Rey, F. Involvement of PAPP-A and IGFR1 in Cystic Ovarian Disease in Cattle. Reprod. Domest. Anim. 2015, 50, 659–668. [Google Scholar] [CrossRef]
- Lingenfelter, B.M.; Dailey, R.A.; Inskeep, E.K.; Vernon, M.W.; Poole, D.H.; Rhinehart, J.D.; Yao, J. Microarray analysis of gene expression in granulosal cells from persistent follicles in cattle. Anim. Reprod. Sci. 2008, 104, 405–413. [Google Scholar] [CrossRef]
- Perks, C.M.; Denning-Kendall, P.A.; Gilmour, R.S.; Wathes, D.C. Localization of messenger ribonucleic acids for insulin-like growth factor I (IGF-I), IGF-II, and the type 1 IGF receptor in the ovine ovary throughout the estrous cycle. Endocrinology 1995, 136, 5266–5273. [Google Scholar] [CrossRef]
- Hastie, P.M.; Haresign, W. Modulating peripheral gonadotrophin levels affects follicular expression of mRNAs encoding insulin-like growth factors and receptors in sheep. Anim. Reprod. Sci. 2008, 109, 110–123. [Google Scholar] [CrossRef]
- Beg, M.A.; Bergfelt, D.R.; Kot, K.; Wiltbank, M.C.; Ginther, O.J. Follicular-fluid factors and granulosa-cell gene expression associated with follicle deviation in cattle. Biol. Reprod. 2001, 64, 432–441. [Google Scholar] [CrossRef]
- Lapp, R.; Rottgen, V.; Viergutz, T.; Weitzel, J.M.; Vernunft, A. Induction of cystic ovarian follicles (COFs) in cattle by using an intrafollicular injection of indomethacin. J. Reprod. Dev. 2020, 66, 181–188. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Yu, D.; Chen, J.; Xing, C.; Li, J.; Li, J.; Cai, Y. The effects of mouse ovarian granulosa cell function and related gene expression by suppressing BMP/Smad signaling pathway. Anim. Cells Syst. 2018, 22, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Dentis, J.L.; Schreiber, N.B.; Burress, A.M.; Spicer, L.J. Effects of angiogenin on granulosa and theca cell function in cattle. Anim. Int. J. Anim. Biosci. 2017, 11, 811–819. [Google Scholar] [CrossRef] [PubMed]
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F.M., Jr. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002, 143, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef]
- Okada, Y.; Maeno, E.; Shimizu, T.; Dezaki, K.; Wang, J.; Morishima, S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 2001, 532, 3–16. [Google Scholar] [CrossRef]
- Kim, J.M.; Song, K.S.; Xu, B.; Wang, T. Role of potassium channels in female reproductive system. Obstet. Gynecol. Sci. 2020, 63, 565–576. [Google Scholar] [CrossRef]
- Hur, C.G.; Choe, C.; Kim, G.T.; Cho, S.K.; Park, J.Y.; Hong, S.G.; Han, J.; Kang, D. Expression and localization of two-pore domain K(+) channels in bovine germ cells. Reproduction 2009, 137, 237–244. [Google Scholar] [CrossRef]
- Trimarchi, J.R.; Liu, L.; Smith, P.J.; Keefe, D.L. Apoptosis recruits two-pore domain potassium channels used for homeostatic volume regulation. Am. J. Physiol. Cell Physiol. 2002, 282, C588–C594. [Google Scholar] [CrossRef]
- Bai, X.; Lacey, H.A.; Greenwood, S.L.; Baker, P.N.; Turner, M.A.; Sibley, C.P.; Fyfe, G.K. TASK channel expression in human placenta and cytotrophoblast cells. J. Soc. Gynecol. Investig. 2006, 13, 30–39. [Google Scholar] [CrossRef]
- Hur, C.G.; Kim, E.J.; Cho, S.K.; Cho, Y.W.; Yoon, S.Y.; Tak, H.M.; Kim, C.W.; Choe, C.; Han, J.; Kang, D. K+ efflux through two-pore domain K+ channels is required for mouse embryonic development. Reproduction 2012, 143, 625. [Google Scholar] [CrossRef]
- Niemeyer, M.I.; Cid, L.P.; Barros, L.F.; Sepulveda, F.V. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J. Biol. Chem. 2001, 276, 43166–43174. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Park, H.S.; Kim, E.J.; Cho, Y.W.; Kim, G.T.; Mun, Y.J.; Choi, E.J.; Lee, J.S.; Han, J.; Kang, D. Reduction of breast cancer cell migration via up-regulation of TASK-3 two-pore domain K+ channel. Acta Physiol. 2012, 204, 513–524. [Google Scholar] [CrossRef]
- Zuniga, R.; Valenzuela, C.; Concha, G.; Brown, N.; Zuniga, L. TASK-3 Downregulation Triggers Cellular Senescence and Growth Inhibition in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 1033. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lu, Y.; Du, G.; Liu, J. Advances in the Understanding of Two-Pore Domain TASK Potassium Channels and Their Potential as Therapeutic Targets. Molecules 2022, 27, 8296. [Google Scholar] [CrossRef]
- Restrepo-Angulo, I.; Banuelos, C.; Camacho, J. Ion Channel Regulation by Sex Steroid Hormones and Vitamin D in Cancer: A Potential Opportunity for Cancer Diagnosis and Therapy. Front. Pharmacol. 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T. The modulation of potassium channels by estrogens facilitates neuroprotection. Front. Cell Dev. Biol. 2022, 10, 998009. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kurokawa, J. Involvement of sex hormonal regulation of K(+) channels in electrophysiological and contractile functions of muscle tissues. J. Pharmacol. Sci. 2019, 139, 259–265. [Google Scholar] [CrossRef]
- Kyeong, K.S.; Hong, S.H.; Kim, Y.C.; Cho, W.; Myung, S.C.; Lee, M.Y.; You, R.Y.; Kim, C.H.; Kwon, S.Y.; Suzuki, H.; et al. Myometrial relaxation of mice via expression of two pore domain acid sensitive K(+) (TASK-2) channels. Korean J. Physiol. Pharmacol. 2016, 20, 547–556. [Google Scholar] [CrossRef]
- Hao, X.; Li, X.; Li, X. 17beta-estradiol downregulated the expression of TASK-1 channels in mouse neuroblastoma N2A cells. J. Membr. Biol. 2014, 247, 273–279. [Google Scholar] [CrossRef]
- Galeska, E.; Wrzecinska, M.; Kowalczyk, A.; Araujo, J.P. Reproductive Consequences of Electrolyte Disturbances in Domestic Animals. Biology 2022, 11, 1006. [Google Scholar] [CrossRef]
- Gumz, M.L.; Rabinowitz, L.; Wingo, C.S. An Integrated View of Potassium Homeostasis. N. Engl. J. Med. 2015, 373, 1787–1788. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Foller, M.; Lang, K.S.; Lang, P.A.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 2005, 205, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Nielsen, B. Comparative physiology of cellular ion and volume regulation. J. Exp. Zool. 1975, 194, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.F.; Litkowski, L.J.; Wilson, T.L.; Guthrie, H.D.; Batta, S.K. Follicular fluid electrolytes and osmolality in cyclic pigs. J. Reprod. Fertil. 1979, 57, 419–422. [Google Scholar] [CrossRef]
- Fatima, S.S.; Rehman, R.; Martins, R.S.; Alam, F.; Ashraf, M. Single nucleotide polymorphisms in Renalase and KCNQ1 genes and female infertility: A cross-sectional study in Pakistan. Andrologia 2019, 51, e13434. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Ren, J.; Liang, X.; Zhang, Q.; Han, Y. Association of Sex with Serum Potassium, Sodium, and Calcium Disorders after Hypertensive Intracerebral Hemorrhage. World Neurosurg. 2020, 141, e367–e373. [Google Scholar] [CrossRef]
- Clarke, H.G.; Hope, S.A.; Byers, S.; Rodgers, R.J. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction 2006, 132, 119–131. [Google Scholar] [CrossRef]
- Sun, X.L.; Ding, J.H.; Fan, Y.; Zhang, J.; Gao, L.; Hu, G. Aquaporin 4 regulates the effects of ovarian hormones on monoamine neurotransmission. Biochem. Biophys. Res. Commun. 2007, 353, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-W.; Choi, E.-J.; Kim, E.-J.; Siregar, A.S.; Han, J.; Kang, D. Aquaporin 4 expression is downregulated in large bovine ovarian follicles. J. Anim. Reprod. Biotechnol. (KSARB) 2020, 35, 315–322. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Tesarik, J.; Galan-Lazaro, M.; Mendoza-Tesarik, R. Ovarian Aging: Molecular Mechanisms and Medical Management. Int. J. Mol. Sci. 2021, 22, 1371. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.; Gonczi, M.; Dienes, B.; Szoor, A.; Fodor, J.; Nagy, Z.; Toth, A.; Fodor, T.; Bai, P.; Szucs, G.; et al. Silencing the KCNK9 potassium channel (TASK-3) gene disturbs mitochondrial function, causes mitochondrial depolarization, and induces apoptosis of human melanoma cells. Arch. Dermatol. Res. 2014, 306, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Jarmuszkiewicz, W.; Kunz, W.S. Mitochondrial potassium channels. IUBMB Life 2009, 61, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; McHedlishvili, D.; McIntire, W.E.; Guagliardo, N.A.; Erisir, A.; Coburn, C.A.; Santarelli, V.P.; Bayliss, D.A.; Barrett, P.Q. Functional TASK-3-Like Channels in Mitochondria of Aldosterone-Producing Zona Glomerulosa Cells. Hypertension 2017, 70, 347–356. [Google Scholar] [CrossRef]
- Bachmann, M.; Rossa, A.; Antoniazzi, G.; Biasutto, L.; Carrer, A.; Campagnaro, M.; Leanza, L.; Gonczi, M.; Csernoch, L.; Paradisi, C.; et al. Synthesis and cellular effects of a mitochondria-targeted inhibitor of the two-pore potassium channel TASK-3. Pharmacol. Res. 2021, 164, 105326. [Google Scholar] [CrossRef]
- Szewczyk, A. Understanding mitochondrial potassium channels: 33 years after discovery. Acta Biochim. Pol. 2024, 71, 13126. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sakemura, R.; Kumagai, A.; Sumikawa, E.; Fujii, M.; Ayusawa, D. Nuclear swelling occurs during premature senescence mediated by MAP kinases in normal human fibroblasts. Biosci. Biotechnol. Biochem. 2008, 72, 1122–1125. [Google Scholar] [CrossRef][Green Version]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca(2+) in aging and age-related diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef]
- Belrose, J.C.; Xie, Y.F.; Gierszewski, L.J.; MacDonald, J.F.; Jackson, M.F. Loss of glutathione homeostasis associated with neuronal senescence facilitates TRPM2 channel activation in cultured hippocampal pyramidal neurons. Mol. Brain 2012, 5, 11. [Google Scholar] [CrossRef]
- Pei, L.; Wiser, O.; Slavin, A.; Mu, D.; Powers, S.; Jan, L.Y.; Hoey, T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc. Natl. Acad. Sci. USA 2003, 100, 7803–7807. [Google Scholar] [CrossRef]
- Choe, C.; Son, D.S.; Choi, G.C.; Song, S.H.; Choe, C.Y.; Choi, S.H.; Kim, H.J.; Cho, S.R.; Hur, C.G.; Kang, D. Survey on the Incidence of Reproductive Disorders in Hanwoo. Korean J. Emb. Trans. 2006, 21, 331–338. [Google Scholar]
- Perl, K.; Ushakov, K.; Pozniak, Y.; Yizhar-Barnea, O.; Bhonker, Y.; Shivatzki, S.; Geiger, T.; Avraham, K.B.; Shamir, R. Reduced changes in protein compared to mRNA levels across non-proliferating tissues. BMC Genom. 2017, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Choe, C.; Cho, Y.W.; Kim, C.W.; Son, D.S.; Han, J.; Kang, D. Identification of differentially expressed genes in bovine follicular cystic ovaries. Korean J. Physiol. Pharmacol. 2010, 14, 265–272. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.-J.; Shin, E.-J.; Kim, C.-W.; Lee, D.; Hong, S.-G.; Han, J.; Kang, D. TRPV1 Is Associated with Testicular Apoptosis in Mice. J. Anim. Reprod. Biotechnol. 2019, 34, 7. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Woo, M.S.; Cao, D.L.; Kim, E.J.; Jeong, Y.Y.; Koh, E.H.; Cho, K.M.; Kang, S.S.; Kang, D. Fermented and Aged Ginseng Sprouts (Panax ginseng) and Their Main Component, Compound K, Alleviate Asthma Parameters in a Mouse Model of Allergic Asthma through Suppression of Inflammation, Apoptosis, ER Stress, and Ferroptosis. Antioxidants 2022, 11, 2052. [Google Scholar] [CrossRef]
- Nyiramana, M.M.; Cho, S.B.; Kim, E.J.; Kim, M.J.; Ryu, J.H.; Nam, H.J.; Kim, N.G.; Park, S.H.; Choi, Y.J.; Kang, S.S.; et al. Sea Hare Hydrolysate-Induced Reduction of Human Non-Small Cell Lung Cancer Cell Growth through Regulation of Macrophage Polarization and Non-Apoptotic Regulated Cell Death Pathways. Cancers 2020, 12, 726. [Google Scholar] [CrossRef]
- Niemeyer, M.I.; Cid, L.P.; Sepulveda, F.V. K+ conductance activated during regulatory volume decrease. The channels in Ehrlich cells and their possible molecular counterpart. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 565–575. [Google Scholar] [CrossRef]
- Way, A.L. Isolation and culture of bovine oviductal epithelial cells for use in the anatomy and physiology laboratory and undergraduate research. Adv. Physiol. Educ. 2006, 30, 237–241. [Google Scholar] [CrossRef]


| Gene Name (Channel Name) | Species | GenBank Accession Number | Primer Sequences (5′–3′) | Application | Expected Size (bp) |
|---|---|---|---|---|---|
| Kcnk3 (TASK-1) | Bovine | XM_597401 | F: CAGGCCTACTACTACTGCT R: GGCCCGTGAGGATGTAGA | qRT-PCR | 133 |
| F: ACACCTTCGTGAAGTACCTG R: GGATGTAGACGAAGCTGAAG | RT-PCR | 287 | |||
| Kcnk9 (TASK-3) | Bovine | XM_588194 | F: CTACTACTGCTTCATCACGTTG R: CCCACCAGGATATACATAAAGCTA | qRT-PCR | 123 |
| F: CTACGTGGCCTTTAGCTTTA R: GTCGGTAAAGCTGTGTAACC | RT-PCR | 433 | |||
| GAPDH | Bovine | NM_001034034 | F: ATGGTCTACATGTTCCAG R: AAGATGGTGATGGCCTTT | qRT-PCR | 104 |
| F: CAGCGACACTCACTCTTCTAC R: GGAAGTCAGGAGATTCTCAGT | RT-PCR | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-W.; Kim, E.-J.; Woo, M.S.; Cao, D.L.; Cirunduzi, A.C.; Ryu, J.H.; Kong, I.-K.; Lee, D.K.; Hong, S.-G.; Han, J.; et al. Downregulation of TASK-3 Channel Induces Senescence in Granulosa Cells of Bovine Cystic Ovarian Follicles. Int. J. Mol. Sci. 2024, 25, 10199. https://doi.org/10.3390/ijms251810199
Kim C-W, Kim E-J, Woo MS, Cao DL, Cirunduzi AC, Ryu JH, Kong I-K, Lee DK, Hong S-G, Han J, et al. Downregulation of TASK-3 Channel Induces Senescence in Granulosa Cells of Bovine Cystic Ovarian Follicles. International Journal of Molecular Sciences. 2024; 25(18):10199. https://doi.org/10.3390/ijms251810199
Chicago/Turabian StyleKim, Chang-Woon, Eun-Jin Kim, Min Seok Woo, Dang Long Cao, Asifiwe Clarisse Cirunduzi, Ji Hyeon Ryu, Il-Keun Kong, Dong Kun Lee, Seong-Geun Hong, Jaehee Han, and et al. 2024. "Downregulation of TASK-3 Channel Induces Senescence in Granulosa Cells of Bovine Cystic Ovarian Follicles" International Journal of Molecular Sciences 25, no. 18: 10199. https://doi.org/10.3390/ijms251810199
APA StyleKim, C.-W., Kim, E.-J., Woo, M. S., Cao, D. L., Cirunduzi, A. C., Ryu, J. H., Kong, I.-K., Lee, D. K., Hong, S.-G., Han, J., & Kang, D. (2024). Downregulation of TASK-3 Channel Induces Senescence in Granulosa Cells of Bovine Cystic Ovarian Follicles. International Journal of Molecular Sciences, 25(18), 10199. https://doi.org/10.3390/ijms251810199








