Role of Gut Microbial Metabolites in Cardiovascular Diseases—Current Insights and the Road Ahead
Abstract
1. Introduction
2. Pathophysiology of Gut Microbial Metabolites in CVDs
2.1. Short-Chain Fatty Acids (SCFAs)
2.2. Bile Acids
2.3. Trimethylamine N-Oxide (TMAO)
2.4. Indole-3-Propionic Acid (IPA)
2.5. Hydrogen Sulfide
2.6. Phenylacetylglutamine (PAGln)
3. Molecular Mechanisms of Gut Microbial Metabolites in Cardiovascular Diseases
3.1. Short-Chain Fatty Acids (SCFAs)
3.2. Trimethylamine-N-Oxide (TMAO)
3.3. Bile Acids (BAs)
3.4. Indole-3-Propionic Acid (IPA)
3.5. Hydrogen Sulfide (H2S)
3.6. Phenylacetylglutamine (PAGln)
4. Therapeutic Perspectives
4.1. Dietary and Lifestyle Modifications
4.2. Fecal Microbial Transplantation (FMT)
4.3. Pharmacological Interventions
4.4. Targeting NLRP3 Inflammasomes
5. Current Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cardiovascular Risk Consortium. Global effect of modifiable risk factors on cardiovascular disease and mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 13 February 2024).
- Dahlöf, B. Cardiovascular disease risk factors: Epidemiology and risk assessment. Am. J. Cardiol. 2010, 105, 3A–9A. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.K.; Rafiq, T. Cardiovascular risk factors and prevention: A perspective from developing countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Katta, N.; Loethen, T.; Lavie, C.J.; Alpert, M.A. Obesity and coronary heart disease: Epidemiology, pathology, and coronary artery imaging. Curr. Probl. Cardiol. 2021, 46, 100655. [Google Scholar] [CrossRef]
- Liang, F.; Wang, Y. Coronary heart disease and atrial fibrillation: A vicious cycle. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H1–H12. [Google Scholar] [CrossRef]
- Dzator, J.S.; Howe, P.R.; Wong, R.H. Profiling cerebrovascular function in migraine: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2021, 41, 919–944. [Google Scholar] [CrossRef]
- Borja, A.J.; Hancin, E.C.; Zhang, V.; Revheim, M.-E.; Alavi, A. Potential of PET/CT in Assessing Dementias with Emphasis on Cerebrovascular Disorders; Springer: Berlin/Heidelberg, Germany, 2020; Volume 47, pp. 2493–2498. [Google Scholar]
- Kim, K.; Anderson, E.M.; Scali, S.T.; Ryan, T.E. Skeletal muscle mitochondrial dysfunction and oxidative stress in peripheral arterial disease: A unifying mechanism and therapeutic target. Antioxidants 2020, 9, 1304. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of peripheral artery disease and polyvascular disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.S.; Nunes, M.C.P.; Aikawa, E. Rheumatic heart valve disease pathophysiology and underlying mechanisms. Front. Cardiovasc. Med. 2021, 7, 612716. [Google Scholar] [CrossRef]
- Marijon, E.; Mocumbi, A.; Narayanan, K.; Jouven, X.; Celermajer, D.S. Persisting burden and challenges of rheumatic heart disease. Eur. Heart J. 2021, 42, 3338–3348. [Google Scholar] [CrossRef]
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2022, 19, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Saef, J.M.; Ghobrial, J. Valvular heart disease in congenital heart disease: A narrative review. Cardiovasc. Diagn. Ther. 2021, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Nordestgaard, B.G. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: A contemporary primary prevention cohort. Lancet 2020, 396, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Ohm, J.; Discacciati, A.; Sundstrom, J.; Hambraeus, K.; Jernberg, T.; Svensson, P. Abdominal obesity and the risk of recurrent atherosclerotic cardiovascular disease after myocardial infarction. Eur. J. Prev. Cardiol. 2020, 27, 1944–1952. [Google Scholar] [CrossRef]
- Chandarana, M.; Curtis, A.; Hoskins, C. The use of nanotechnology in cardiovascular disease. Appl. Nanosci. 2018, 8, 1607–1619. [Google Scholar] [CrossRef]
- Kaptein, F.; Kroft, L.; Hammerschlag, G.; Ninaber, M.; Bauer, M.; Huisman, M.; Klok, F. Pulmonary infarction in acute pulmonary embolism. Thromb. Res. 2021, 202, 162–169. [Google Scholar] [CrossRef]
- Ortega-Paz, L.; Talasaz, A.H.; Sadeghipour, P.; Potpara, T.S.; Aronow, H.D.; Jara-Palomares, L.; Sholzberg, M.; Angiolillo, D.J.; Lip, G.Y.; Bikdeli, B. COVID-19-Associated Pulmonary Embolism: Review of the Pathophysiology, Epidemiology, Prevention, Diagnosis, and Treatment. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2022. [Google Scholar]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.T.; Schwartz, R.J.; Srivastava, D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef]
- Prajnamitra, R.P.; Chen, H.-C.; Lin, C.-J.; Chen, L.-L.; Hsieh, P.C.-H. Nanotechnology approaches in tackling cardiovascular diseases. Molecules 2019, 24, 2017. [Google Scholar] [CrossRef]
- Smith, B.R.; Edelman, E.R. Nanomedicines for cardiovascular disease. Nat. Cardiovasc. Res. 2023, 2, 351–367. [Google Scholar] [CrossRef]
- Soumya, R.S.; Raghu, K.G. Recent advances on nanoparticle-based therapies for cardiovascular diseases. J. Cardiol. 2023, 81, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Tsiamis, E.; Vlachopoulos, C.; Stratos, C.; Toutouzas, K.; Pitsavos, C.; Marakas, S.; Boudoulas, H.; Toutouzas, P. Unfavorable effect of smoking on the elastic properties of the human aorta. Circulation 1997, 95, 31–38. [Google Scholar] [CrossRef]
- Barua, R.S.; Ambrose, J.A.; Eales-Reynolds, L.-J.; DeVoe, M.C.; Zervas, J.G.; Saha, D.C. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 2001, 104, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Lorenzatti, A.J.; Servato, M.L. New evidence on the role of inflammation in CVD risk. Curr. Opin. Cardiol. 2019, 34, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J. Targeting inflammation in CVD: Advances and challenges. Nat. Rev. Cardiol. 2019, 16, 74–75. [Google Scholar] [CrossRef]
- Isordia-Salas, I.; Galván-Plata, M.E.; Leaños-Miranda, A.; Aguilar-Sosa, E.; Anaya-Gómez, F.; Majluf-Cruz, A.; Santiago-Germán, D. Proinflammatory and prothrombotic state in subjects with different glucose tolerance status before cardiovascular disease. J. Diabetes Res. 2014, 2014, 631902. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S. Lipoprotein (a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, H.; Kang, L.; Chu, Q.; Zhao, X.; Li, R. Causal association between basal metabolic rate and risk of cardiovascular diseases: A univariable and multivariable Mendelian randomization study. Sci. Rep. 2023, 13, 12487. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Y.; Zhou, S.; Jin, C.; Xiang, M.; Ma, H. The association between the basal metabolic rate and cardiovascular disease: A two-sample Mendelian randomization study. Eur. J. Clin. Investig. 2024, 54, e14153. [Google Scholar] [CrossRef]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef]
- Han, W.; Yang, S.; Xiao, H.; Wang, M.; Ye, J.; Cao, L.; Sun, G. Role of adiponectin in cardiovascular diseases related to glucose and lipid metabolism disorders. Int. J. Mol. Sci. 2022, 23, 15627. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, S.; Dreyer, R.P.; Spatz, E.S.; Geda, M.; Lorenze, N.P.; D’Onofrio, G.; Lichtman, J.H.; Spertus, J.A.; Ridker, P.M. Sex differences in inflammatory markers and health status among young adults with acute myocardial infarction: Results from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) study. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003470. [Google Scholar] [CrossRef]
- Bohula, E.A.; Giugliano, R.P.; Cannon, C.P.; Zhou, J.; Murphy, S.A.; White, J.A.; Tershakovec, A.M.; Blazing, M.A.; Braunwald, E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015, 132, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Morrow, D.A.; Rose, L.M.; Rifai, N.; Cannon, C.P.; Braunwald, E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol< 70 mg/dl and C-reactive protein< 2 mg/L: An analysis of the PROVE-IT TIMI-22 trial. J. Am. Coll. Cardiol. 2005, 45, 1644–1648. [Google Scholar]
- Sethi, Y.; Patel, N.; Kaka, N.; Kaiwan, O.; Kar, J.; Moinuddin, A.; Goel, A.; Chopra, H.; Cavalu, S. Precision Medicine and the future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. J. Clin. Med. 2023, 12, 1799. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035s–1045s. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.-C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2020, 11, 13. [Google Scholar] [CrossRef]
- Bai, J.; Hu, Y.; Bruner, D. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef]
- Ojima, M.; Motooka, D.; Shimizu, K.; Gotoh, K.; Shintani, A.; Yoshiya, K.; Nakamura, S.; Ogura, H.; Iida, T.; Shimazu, T. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig. Dis. Sci. 2016, 61, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta-Zuluaga, J.; Escobar, J.S. Considerations for optimizing microbiome analysis using a marker gene. Front. Nutr. 2016, 3, 26. [Google Scholar] [CrossRef]
- Jovel, J.; Nimaga, A.; Jordan, T.; O’keefe, S.; Patterson, J.; Thiesen, A.; Hotte, N.; Bording-Jorgensen, M.; Subedi, S.; Hamilton, J. Metagenomics versus metatranscriptomics of the murine gut microbiome for assessing microbial metabolism during inflammation. Front. Microbiol. 2022, 13, 119. [Google Scholar] [CrossRef]
- Li, X.-Y.; He, C.; Zhu, Y.; Lu, N.-H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Cao, Z.; Li, W.; Li, H.; Lu, C.; Yang, X.; Liu, Y. Gut microbiota as an “invisible organ” that modulates the function of drugs. Biomed. Pharmacother. 2020, 121, 109653. [Google Scholar] [CrossRef]
- García-Ríos, A.; Garcia, A.C.; Perez-Jimenez, F.; Perez-Martinez, P. Gut microbiota: A new protagonist in the risk of cardiovascular disease? Clínica E Investig. En Arterioscler. (Engl. Ed.) 2019, 31, 178–185. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of gut microbiota in neurodegenerative diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the human gut microbiota in metabolic diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: Implications for COVID-19. J. Nutr. Biochem. 2021, 97, 108787. [Google Scholar] [CrossRef]
- Gui, D.-D.; Luo, W.; Yan, B.-J.; Ren, Z.; Tang, Z.-H.; Liu, L.-S.; Zhang, J.-F.; Jiang, Z.-S. Effects of gut microbiota on atherosclerosis through hydrogen sulfide. Eur. J. Pharmacol. 2021, 896, 173916. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Meyer, K.A.; Bennett, B.J. Diet and gut microbial function in metabolic and cardiovascular disease risk. Curr. Diabetes Rep. 2016, 16, 93. [Google Scholar] [CrossRef]
- Zhu, Y.; Shui, X.; Liang, Z.; Huang, Z.; Qi, Y.; He, Y.; Chen, C.; Luo, H.; Lei, W. Gut microbiota metabolites as integral mediators in cardiovascular diseases. Int. J. Mol. Med. 2020, 46, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Feng, W.; Liu, Q.; Zhou, S.; Liu, Q.; Cai, L. The gut microbiota and its interactions with cardiovascular disease. Microb. Biotechnol. 2020, 13, 637–656. [Google Scholar] [CrossRef]
- Tang, W.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; Le Lay, A.; Dumas, M.-E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef]
- Branchereau, M.; Burcelin, R.; Heymes, C. The gut microbiome and heart failure: A better gut for a better heart. Rev. Endocr. Metab. Disord. 2019, 20, 407–414. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Claus, S.P.; Tsang, T.M.; Wang, Y.; Cloarec, O.; Skordi, E.; Martin, F.P.; Rezzi, S.; Ross, A.; Kochhar, S.; Holmes, E. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol. 2008, 4, 219. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.R.; Davenport, E.R.; Gautam, Y.; Bhandari, D.; Tandukar, S.; Ng, K.M.; Fragiadakis, G.K.; Holmes, S.; Gautam, G.P.; Leach, J. Gut microbiome transition across a lifestyle gradient in Himalaya. PLoS Biol. 2018, 16, e2005396. [Google Scholar] [CrossRef]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef]
- Martin, F.-P.J.; Sprenger, N.; Yap, I.K.; Wang, Y.; Bibiloni, R.; Rochat, F.; Rezzi, S.; Cherbut, C.; Kochhar, S.; Lindon, J.C. Panorganismal gut microbiome− host metabolic crosstalk. J. Proteome Res. 2009, 8, 2090–2105. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Juanola, O.; Ferrusquía-Acosta, J.; García-Villalba, R.; Zapater, P.; Magaz, M.; Marín, A.; Olivas, P.; Baiges, A.; Bellot, P.; Turon, F. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB J. 2019, 33, 11595–11605. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.; Branch, W.; Naylor, C.; MacFarlane, G. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O‘Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2014, 64, 1744–1754. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.-L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Karaki, S.-i.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Feingold, K.R.; Moser, A.; Shigenaga, J.K.; Grunfeld, C. Inflammation stimulates niacin receptor (GPR109A/HCA2) expression in adipose tissue and macrophages. J. Lipid Res. 2014, 55, 2501–2508. [Google Scholar] [CrossRef]
- Lukasova, M.; Malaval, C.; Gille, A.; Kero, J.; Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Investig. 2011, 121, 1163–1173. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Biorender. Create Professional Science Figures in Minutes. Available online: https://www.biorender.com/ (accessed on 25 February 2024).
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 2018, 38, 1323–1335. [Google Scholar] [CrossRef]
- Wahlström, A.; Kovatcheva-Datchary, P.; Ståhlman, M.; Khan, M.-T.; Bäckhed, F.; Marschall, H.-U. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J. Lipid Res. 2017, 58, 412–419. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Xie, G.; Raufman, J.-P.; Hogan, S.; Griffin, T.L.; Packard, C.A.; Chatfield, D.A.; Hagey, L.R.; Steinbach, J.H.; Hofmann, A.F. Human cecal bile acids: Concentration and spectrum. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G256–G263. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Van Best, N.; Rolle-Kampczyk, U.; Schaap, F.; Basic, M.; Olde Damink, S.; Bleich, A.; Savelkoul, P.; Von Bergen, M.; Penders, J.; Hornef, M. Bile acids drive the newborn’s gut microbiota maturation. Nat. Commun. 2020, 11, 3692. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic resistance and regulation of the gram-negative bacterial outer membrane barrier by host innate immune molecules. mBio 2016, 7, e01541-16. [Google Scholar] [CrossRef]
- Li, Y.; Tang, R.; Leung, P.S.; Gershwin, M.E.; Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017, 16, 885–896. [Google Scholar] [CrossRef]
- Theriot, C.M.; Bowman, A.A.; Young, V.B. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 2016, 1, e00045-15. [Google Scholar] [CrossRef]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 2009, 183, 6251–6261. [Google Scholar] [CrossRef]
- Mencarelli, A.; Renga, B.; Palladino, G.; Ricci, P.; Distrutti, E.; Barbanti, M.; Baldelli, F.; Fiorucci, S. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur. J. Pharmacol. 2011, 668, 317–324. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Bandsma, R.H.; Ouwens, M.; van der Sluijs, F.H.; Havinga, R.; Boer, T.; Reijngoud, D.-J.; Romijn, J.A.; Kuipers, F. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 2003, 52, 1081–1089. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef]
- Bernstein, H.; Bernstein, C.; Payne, C.; Dvorakova, K.; Garewal, H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res./Rev. Mutat. Res. 2005, 589, 47–65. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Qiao, D.; Gaitonde, S.V.; Qi, W.; Martinez, J.D. Deoxycholic acid suppresses p53 by stimulating proteasome-mediated p53 protein degradation. Carcinogenesis 2001, 22, 957–964. [Google Scholar] [CrossRef]
- Payne, C.M.; Bernstein, C.; Dvorak, K.; Bernstein, H. Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis. Clin. Exp. Gastroenterol. 2008, 1, 19–47. [Google Scholar] [CrossRef]
- Washo-Stultz, D.; Crowley-Weber, C.L.; Dvorakova, K.; Bernstein, C.; Bernstein, H.; Kunke, K.; Waltmire, C.N.; Garewal, H.; Payne, C.M. Role of mitochondrial complexes I and II, reactive oxygen species and arachidonic acid metabolism in deoxycholate-induced apoptosis. Cancer Lett. 2002, 177, 129–144. [Google Scholar] [CrossRef]
- Tang, W.W.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Katz, D.L.; Gnanaraj, J.; Treu, J.A.; Ma, Y.; Kavak, Y.; Njike, V.Y. Effects of egg ingestion on endothelial function in adults with coronary artery disease: A randomized, controlled, crossover trial. Am. Heart J. 2015, 169, 162–169. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Wang, Z.; Levison, B.S.; Hazen, J.E.; Donahue, L.; Li, X.-M.; Hazen, S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014, 455, 35–40. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Gao, C.; Catucci, G.; Castrignanò, S.; Gilardi, G.; Sadeghi, S.J. Inactivation mechanism of N61S mutant of human FMO3 towards trimethylamine. Sci. Rep. 2017, 7, 14668. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E., III; Polhemus, D.J.; Gupta, N.; Goodchild, T.T.; Tang, W.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef]
- Zhang, W.; Miikeda, A.; Zuckerman, J.; Jia, X.; Charugundla, S.; Zhou, Z.; Kaczor-Urbanowicz, K.E.; Magyar, C.; Guo, F.; Wang, Z. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci. Rep. 2021, 11, 518. [Google Scholar] [CrossRef]
- Gupta, N.; Buffa, J.A.; Roberts, A.B.; Sangwan, N.; Skye, S.M.; Li, L.; Ho, K.J.; Varga, J.; DiDonato, J.A.; Tang, W.W. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1239–1255. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef]
- Mohammadi, A.; Najar, A.G.; Yaghoobi, M.M.; Jahani, Y.; Vahabzadeh, Z. Trimethylamine-N-oxide treatment induces changes in the ATP-binding cassette transporter A1 and scavenger receptor A1 in murine macrophage J774A. 1 cells. Inflammation 2016, 39, 393–404. [Google Scholar] [CrossRef]
- Mondal, J.A. Effect of trimethylamine N-oxide on interfacial electrostatics at phospholipid monolayer–water interfaces and its relevance to cardiovascular disease. J. Phys. Chem. Lett. 2016, 7, 1704–1708. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef]
- Danaceau, J.P.; Anderson, G.M.; McMahon, W.M.; Crouch, D.J. A liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. J. Anal. Toxicol. 2003, 27, 440–444. [Google Scholar] [CrossRef]
- Negatu, D.A.; Gengenbacher, M.; Dartois, V.; Dick, T. Indole propionic acid, an unusual antibiotic produced by the gut microbiota, with anti-inflammatory and antioxidant properties. Front. Microbiol. 2020, 11, 575586. [Google Scholar] [CrossRef]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as essential mediators in the gut-brain axis. Their Role Alzheimer’s disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef]
- Melhem, N.J.; Taleb, S. Tryptophan: From diet to cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J. Agric. Food Chem. 2020, 69, 1487–1495. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Ding, W.; Ruan, Z.; Zhang, L. Enhanced intestinal barriers by puerarin in combination with tryptophan. J. Agric. Food Chem. 2021, 69, 15575–15584. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Ramachandran, S.; Ganapathy, V. Cell-surface and nuclear receptors in the colon as targets for bacterial metabolites and its relevance to colon health. Nutrients 2017, 9, 856. [Google Scholar] [CrossRef]
- Stasiak, M.; Zasada, K.; Lewinski, A.; Karbownik-Lewinska, M. Melatonin restores the basal level of lipid peroxidation in rat tissues exposed to potassium bromate in vitro. Neuroendocr. Lett 2010, 31, 363–369. [Google Scholar]
- Karbownik, M.; Stasiak, M.; Zygmunt, A.; Zasada, K.; Lewiński, A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem. Funct. Cell. Biochem. Its Modul. By Act. Agents Or Dis. 2006, 24, 483–489. [Google Scholar] [CrossRef]
- Karbownik, M.; Stasiak, M.; Zasada, K.; Zygmunt, A.; Lewinski, A. Comparison of potential protective effects of melatonin, indole-3-propionic acid, and propylthiouracil against lipid peroxidation caused by potassium bromate in the thyroid gland. J. Cell. Biochem. 2005, 95, 131–138. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, C.; Buac, D.; Fan, Y.; Zhang, X.; Zuo, J.; Zhang, P.; Zhang, N.; Dong, L.; Dou, Q.P. Organic cadmium complexes as proteasome inhibitors and apoptosis inducers in human breast cancer cells. J. Inorg. Biochem. 2013, 123, 1–10. [Google Scholar] [CrossRef]
- Xiao, H.-w.; Cui, M.; Li, Y.; Dong, J.-l.; Zhang, S.-q.; Zhu, C.-c.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.-C. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.; Matsubara, E.; Vidal, R.; Pacheco-Quinto, J.; Poeggeler, B.; Zagorski, M.; Sambamurti, K. Melatonin treatment enhances Aβ lymphatic clearance in a transgenic mouse model of amyloidosis. Curr. Alzheimer Res. 2018, 15, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Bendheim, P.E.; Poeggeler, B.; Neria, E.; Ziv, V.; Pappolla, M.A.; Chain, D.G. Development of indole-3-propionic acid (OXIGON™) for alzheimer’s disease. J. Mol. Neurosci. 2002, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, A.; Poisson, L.; Nemutlu, E.; Datta, I.; Denic, A.; Dzeja, P.; Rodriguez, M.; Rattan, R.; Giri, S. Profile of circulatory metabolites in a relapsing-remitting animal model of multiple sclerosis using global metabolomics. J. Clin. Cell. Immunol. 2013, 4, 1–25. [Google Scholar]
- Gaetani, L.; Boscaro, F.; Pieraccini, G.; Calabresi, P.; Romani, L.; Di Filippo, M.; Zelante, T. Host and microbial tryptophan metabolic profiling in multiple sclerosis. Front. Immunol. 2020, 11, 157. [Google Scholar] [CrossRef]
- Rabus, R.; Venceslau, S.S.; Woehlbrand, L.; Voordouw, G.; Wall, J.D.; Pereira, I.A. A post-genomic view of the ecophysiology, catabolism and biotechnological relevance of sulphate-reducing prokaryotes. Adv. Microb. Physiol. 2015, 66, 55–321. [Google Scholar]
- Rey, F.E.; Gonzalez, M.D.; Cheng, J.; Wu, M.; Ahern, P.P.; Gordon, J.I. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci. USA 2013, 110, 13582–13587. [Google Scholar] [CrossRef]
- Gibson, G.; Macfarlane, G.; Cummings, J. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J. Appl. Bacteriol. 1988, 65, 103–111. [Google Scholar] [CrossRef]
- Awano, N.; Wada, M.; Mori, H.; Nakamori, S.; Takagi, H. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 2005, 71, 4149–4152. [Google Scholar] [CrossRef]
- Blachier, F.; Davila, A.-M.; Mimoun, S.; Benetti, P.-H.; Atanasiu, C.; Andriamihaja, M.; Benamouzig, R.; Bouillaud, F.; Tomé, D. Luminal sulfide and large intestine mucosa: Friend or foe? Amino Acids 2010, 39, 335–347. [Google Scholar] [CrossRef]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Russo, G.; Caliendo, G.; Santagada, V.; Cirino, G.; Wallace, J.L. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1, 2] dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J. Pharmacol. Exp. Ther. 2006, 319, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R.; McKnight, G.W.; Dicay, M.S.; Coffin, C.S.; Ferraz, J.G.; Wallace, J.L. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig. Liver Dis. 2010, 42, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 2009, 137, 569–578.e561. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Distrutti, E.; Rizzo, G.; Mencarelli, A.; Orlandi, S.; Zanardo, R.; Renga, B.; Di Sante, M.; Morelli, A. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 2005, 129, 1210–1224. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, R.; Zhou, X.; Ji, F.; Zhang, B. Hydrogen sulfide improves colonic barrier integrity in DSS-induced inflammation in Caco-2 cells and mice. Int. Immunopharmacol. 2016, 39, 121–127. [Google Scholar] [CrossRef]
- Figliuolo, V.R.; Dos Santos, L.M.; Abalo, A.; Nanini, H.; Santos, A.; Brittes, N.M.; Bernardazzi, C.; de Souza, H.S.P.; Vieira, L.Q.; Coutinho-Silva, R. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 2017, 189, 29–38. [Google Scholar] [CrossRef]
- Lohninger, L.; Tomasova, L.; Praschberger, M.; Hintersteininger, M.; Erker, T.; Gmeiner, B.M.; Laggner, H. Hydrogen sulphide induces HIF-1α and Nrf2 in THP-1 macrophages. Biochimie 2015, 112, 187–195. [Google Scholar] [CrossRef]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y. Hydrogen sulfide promotes Tet1-and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, P.; Yang, Y.; Ge, H.; Chen, W.; Qu, J.; Shi, J.; Cui, G.; Liu, X.; Feng, H. Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J. Neuroinflamm. 2017, 14, 163. [Google Scholar] [CrossRef]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 2020, 180, 862–877.e822. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Sun, Z.; Xie, S.; Hu, Z.; Lan, Q.; Sun, Y.; Yuan, L.; Zhai, C. Intestinal flora derived metabolites affect the occurrence and development of cardiovascular disease. J. Multidiscip. Healthc. 2022, 15, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin. Chim. Acta 2020, 507, 236–241. [Google Scholar] [CrossRef]
- Huynh, K. Novel gut microbiota-derived metabolite promotes platelet thrombosis via adrenergic receptor signalling. Nat. Rev. Cardiol. 2020, 17, 265. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, X.; Feng, X.; Wei, M.; Luo, Y.; Zhao, T.; Xiao, B.; Xia, J. Phenylacetylglutamine, a novel biomarker in acute ischemic stroke. Front. Cardiovasc. Med. 2021, 8, 798765. [Google Scholar] [CrossRef]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable atherosclerotic plaque: Is there a molecular signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The implication of the gut microbiome in heart failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Fang, C.; Zuo, K.; Fu, Y.; Li, J.; Wang, H.; Xu, L.; Yang, X. Dysbiosis of gut microbiota and metabolite phenylacetylglutamine in coronary artery disease patients with stent stenosis. Front. Cardiovasc. Med. 2022, 9, 832092. [Google Scholar] [CrossRef] [PubMed]
- Polsinelli, V.B.; Marteau, L.; Shah, S.J. The role of splanchnic congestion and the intestinal microenvironment in the pathogenesis of advanced heart failure. Curr. Opin. Support. Palliat. Care 2019, 13, 24. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Z.; Ferrari, M.W.; Liu, Y.; Li, C.; Zhang, T.; Lyu, G. The correlation between gut microbiota and serum metabolomic in elderly patients with chronic heart failure. Mediat. Inflamm. 2021, 2021, 5587428. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Navab-Moghadam, F.; Sedighi, M.; Khamseh, M.E.; Alaei-Shahmiri, F.; Talebi, M.; Razavi, S.; Amirmozafari, N. The association of type II diabetes with gut microbiota composition. Microb. Pathog. 2017, 110, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mamic, P.; Chaikijurajai, T.; Tang, W.W. Gut microbiome-A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J. Mol. Cell. Cardiol. 2021, 152, 105–117. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, T.; Hu, H.; Zhang, W.; Hua, X. Association study of gut flora in coronary heart disease through high-throughput sequencing. BioMed Res. Int. 2017, 2017, 3796359. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Gallo, A.; Macerola, N.; Favuzzi, A.M.; Nicolazzi, M.A.; Gasbarrini, A.; Montalto, M. The gut in heart failure: Current knowledge and novel frontiers. Med. Princ. Pract. 2022, 31, 203–214. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Gerdes, V.; Gueimonde, M.; Pajunen, L.; Nieuwdorp, M.; Laitinen, K. How strong is the evidence that gut microbiota composition can be influenced by lifestyle interventions in a cardio-protective way? Atherosclerosis 2020, 311, 124–142. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.; Van Der Velden, S.; Ríos-Morales, M.; Van Faassen, M.J.; Loreti, M.G.; De Bruin, A. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Emoto, T.; Yamashita, T.; Kobayashi, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: Gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessel. 2017, 32, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Emoto, T.; Yamashita, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; Mizoguchi, T. Analysis of gut microbiota in coronary artery disease patients: A possible link between gut microbiota and coronary artery disease. J. Atheroscler. Thromb. 2016, 23, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hua, Y.; Ren, J. Short-chain fatty acid propionate alleviates Akt2 knockout-induced myocardial contractile dysfunction. J. Diabetes Res. 2012, 2012, 851717. [Google Scholar] [CrossRef]
- Gelis, L.; Jovancevic, N.; Veitinger, S.; Mandal, B.; Arndt, H.-D.; Neuhaus, E.M.; Hatt, H. Functional characterization of the odorant receptor 51E2 in human melanocytes. J. Biol. Chem. 2016, 291, 17772–17786. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Bumbalo, R.; Bautze, V.; Strotmann, J.; Breer, H. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res. 2015, 361, 697–710. [Google Scholar] [CrossRef]
- Pluznick, J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol.-Ren. Physiol. 2013, 305, F439–F444. [Google Scholar] [CrossRef]
- Zhu, Y.; Jameson, E.; Crosatti, M.; Schäfer, H.; Rajakumar, K.; Bugg, T.D.; Chen, Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 4268–4273. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481-14. [Google Scholar] [CrossRef]
- Dong, Z.; Liang, Z.; Guo, M.; Hu, S.; Shen, Z.; Hai, X. The association between plasma levels of trimethylamine N-oxide and the risk of coronary heart disease in Chinese patients with or without type 2 diabetes mellitus. Dis. Markers 2018, 2018, 1578320. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Yang, X.; Wang, H.; Saaoud, F.; Sun, Y.; Fong, D. The microbial metabolite trimethylamine N-oxide links vascular dysfunctions and the autoimmune disease rheumatoid arthritis. Nutrients 2019, 11, 1821. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Boini, K.M.; Hussain, T.; Li, P.-L.; Koka, S.S. Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell. Physiol. Biochem. 2018, 44, 152–162. [Google Scholar] [CrossRef]
- Singh, G.B.; Zhang, Y.; Boini, K.M.; Koka, S. High mobility group box 1 mediates TMAO-induced endothelial dysfunction. Int. J. Mol. Sci. 2019, 20, 3570. [Google Scholar] [CrossRef]
- Ke, Y.; Li, D.; Zhao, M.; Liu, C.; Liu, J.; Zeng, A.; Shi, X.; Cheng, S.; Pan, B.; Zheng, L. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic. Biol. Med. 2018, 116, 88–100. [Google Scholar] [CrossRef]
- González-Loyola, A.; Petrova, T.V. Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 2021, 169, 63–78. [Google Scholar] [CrossRef]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021, 43, 377–394. [Google Scholar] [CrossRef]
- Chen, M.l.; Zhu, X.h.; Ran, L.; Lang, H.d.; Yi, L.; Mi, M.t. Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Boutagy, N.E.; Neilson, A.P.; Osterberg, K.L.; Smithson, A.T.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 2015, 23, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: Inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: New insights into atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Durpès, M.-C.; Morin, C.; Paquin-Veillet, J.; Beland, R.; Paré, M.; Guimond, M.-O.; Rekhter, M.; King, G.L.; Geraldes, P. PKC-β activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovasc. Res. 2015, 106, 303–313. [Google Scholar] [CrossRef]
- Liu, H.; Jia, K.; Ren, Z.; Sun, J.; Pan, L.-L. PRMT5 critically mediates TMAO-induced inflammatory response in vascular smooth muscle cells. Cell Death Dis. 2022, 13, 299. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Rimm, E.B.; Hu, F.B.; Albert, C.M.; Rexrode, K.M.; Manson, J.E.; Qi, L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am. J. Clin. Nutr. 2016, 104, 173–180. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam cells in atherosclerosis: Novel insights into its origins, consequences, and molecular mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, J.; Shan, J.; Hong, Y.; Zhu, W.; Nie, Z.; Zhang, Y.; Ji, N.; Luo, X.; Zhang, T. Gut-flora-dependent metabolite trimethylamine-N-oxide promotes atherosclerosis-associated inflammation responses by indirect ROS stimulation and signaling involving AMPK and SIRT1. Nutrients 2022, 14, 3338. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 2022, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.V.; Okros, M.; Shivel, M.; Armwald, B.; Bridges, C.; Fu, Y.; Martin, C.; Schilmiller, A.L.; Miller, W.M.; Ziegler, K.M. Bile salt hydrolase acyltransferase activity expands bile acid diversity. Nature 2024, 626, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, J.; Jiang, L.; Geng, T.; Tian, S.; Liao, Y.; Yang, K.; Zheng, Y.; He, M.; Tang, H. Gut microbiota-derived secondary bile acids, bile acids receptor polymorphisms, and risk of cardiovascular disease in individuals with newly diagnosed type 2 diabetes: A cohort study. Am. J. Clin. Nutr. 2023, 119, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Matthan, N.R. Nutrition and gastrointestinal microbiota, microbial-derived secondary bile acids, and cardiovascular disease. Curr. Atheroscler. Rep. 2020, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef]
- Li, W.; Shu, S.; Cheng, L.; Hao, X.; Wang, L.; Wu, Y.; Yuan, Z.; Zhou, J. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis 2020, 292, 193–200. [Google Scholar] [CrossRef]
- Fiorucci, S.; Zampella, A.; Cirino, G.; Bucci, M.; Distrutti, E. Decoding the vasoregulatory activities of bile acid-activated receptors in systemic and portal circulation: Role of gaseous mediators. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H21–H32. [Google Scholar] [CrossRef]
- Sauerbruch, T.; Hennenberg, M.; Trebicka, J.; Beuers, U. Bile acids, liver cirrhosis, and extrahepatic vascular dysfunction. Front. Physiol. 2021, 12, 718783. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zou, Y.; Han, X.; Bae, J.-W.; Jeon, C.O. Gut microbiome-mediated mechanisms for reducing cholesterol levels: Implications for ameliorating cardiovascular disease. Trends Microbiol. 2023, 31, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; King, N.; Chen, K.Y.; Breslow, J.L. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice [S]. J. Lipid Res. 2009, 50, 2004–2013. [Google Scholar] [CrossRef]
- Sui, Y.; Xu, J.; Rios-Pilier, J.; Zhou, C. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J. Lipid Res. 2011, 52, 1652–1659. [Google Scholar] [CrossRef]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [PubMed]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef]
- Ning, B.; Chen, Y.; Waqar, A.B.; Yan, H.; Shiomi, M.; Zhang, J.; Chen, Y.E.; Wang, Y.; Itabe, H.; Liang, J. Hypertension enhances advanced atherosclerosis and induces cardiac death in Watanabe heritable hyperlipidemic rabbits. Am. J. Pathol. 2018, 188, 2936–2947. [Google Scholar] [CrossRef]
- Huc, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut 422 bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and 423 central mechanisms in rats. Pharmacol Res 2018, 130, 424. [Google Scholar] [CrossRef]
- Gesper, M.; Nonnast, A.B.; Kumowski, N.; Stoehr, R.; Schuett, K.; Marx, N.; Kappel, B.A. Gut-derived metabolite indole-3-propionic acid modulates mitochondrial function in cardiomyocytes and alters cardiac function. Front. Med. 2021, 8, 648259. [Google Scholar] [CrossRef]
- Konopelski, P.; Chabowski, D.; Aleksandrowicz, M.; Kozniewska, E.; Podsadni, P.; Szczepanska, A.; Ufnal, M. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, increases blood pressure via cardiac and vascular mechanisms in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 321, R969–R981. [Google Scholar] [CrossRef]
- Venu, V.K.P.; Saifeddine, M.; Mihara, K.; Tsai, Y.-C.; Nieves, K.; Alston, L.; Mani, S.; McCoy, K.D.; Hollenberg, M.D.; Hirota, S.A. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E350–E361. [Google Scholar] [CrossRef]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B. Plasma microbiome-modulated indole-and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J. Vasc. Surg. 2018, 68, 1552–1562.e1557. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Chen, X.; Yu, C.; Deng, Y.; Zhang, Y.; Chen, S.; Chen, X.; Chen, K.; Yang, Y.; Ling, W. Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ. Res. 2022, 131, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Cuchel, M.; Rader, D.J. Macrophage reverse cholesterol transport: Key to the regression of atherosclerosis? Circulation 2006, 113, 2548–2555. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.-C.; Phillips, M.C.; Rader, D.J. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; la Bastide-van Gemert, S.; Wang, N. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation 2018, 138, 898–912. [Google Scholar] [CrossRef]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q. Cholesterol and lipoprotein metabolism and atherosclerosis: Recent advances in reverse cholesterol transport. Ann. Hepatol. 2018, 16, 27–42. [Google Scholar] [CrossRef]
- Mani, S.; Untereiner, A.; Wu, L.; Wang, R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid. Redox Signal. 2014, 20, 805–817. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-H.; Ren, Z.; Qu, S.-L.; Liu, M.-H.; Liu, L.-S.; Jiang, Z.-S. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol. Cell. Biol. 2013, 33, 1104–1113. [Google Scholar] [CrossRef]
- Zhao, Z.-Z.; Wang, Z.; Li, G.-H.; Wang, R.; Tan, J.-M.; Cao, X.; Suo, R.; Jiang, Z.-S. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp. Biol. Med. 2011, 236, 169–176. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Wang, R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006, 20, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, C.; Zhang, A.; Fan, Y.; Gu, T.; Wu, D.; Sparatore, A.; Wang, C. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur. J. Pharmacol. 2012, 697, 106–116. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 173–179. [Google Scholar] [CrossRef]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017, 15, 711–723. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef]
- Kiss, L.; Deitch, E.A.; Szabó, C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci. 2008, 83, 589–594. [Google Scholar] [CrossRef]
- Wang, J.; Gareri, C.; Rockman, H.A. G-protein–coupled receptors in heart disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef]
- Iaccarino, G.; Ciccarelli, M.; Sorriento, D.; Galasso, G.; Campanile, A.; Santulli, G.; Cipolletta, E.; Cerullo, V.; Cimini, V.; Altobelli, G.G. Ischemic neoangiogenesis enhanced by β2-adrenergic receptor overexpression: A novel role for the endothelial adrenergic system. Circ. Res. 2005, 97, 1182–1189. [Google Scholar] [CrossRef]
- Jansen, V.L.; Gerdes, V.E.; Middeldorp, S.; van Mens, T.E. Gut microbiota and their metabolites in cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101492. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Nemet, I.; Prasad Saha, P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A. Gut microbiota-generated phenylacetylglutamine and heart failure. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Phenylacetylglutamine causes a pathologic inflammation state and enhances atherosclerosis through the b2-adrenergic receptor cAMP PKA NF-kappaB pathway in diabetes. Eur. Heart J. 2023, 44, ehad655.3289. [Google Scholar] [CrossRef]
- Xu, H.; Yang, N.; Wang, B.Y.; Zhou, L.; Xu, L.L.; Chen, Y.; Wang, D.J.; Ge, W.H. Phenylacetyl glutamine (PAGln) enhances cardiomyocyte death after myocardial infarction through β1 adrenergic receptor. Environ. Toxicol. 2024, 39, 1682–1699. [Google Scholar] [CrossRef]
- Adithya, K.; Rajeev, R.; Selvin, J.; Seghal Kiran, G. Dietary influence on the dynamics of the human gut microbiome: Prospective implications in interventional therapies. ACS Food Sci. Technol. 2021, 1, 717–736. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; de Vos, W.M. Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Adamberg, K.; Kolk, K.; Jaagura, M.; Vilu, R.; Adamberg, S. The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: An isothermal microcalorimetry study. Benef. Microbes 2018, 9, 21–34. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef]
- Goñi, I.; Hernández-Galiot, A. Intake of nutrient and non-nutrient dietary antioxidants. contribution of macromolecular antioxidant polyphenols in an elderly Mediterranean population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef]
- Colman, R.J.; Rubin, D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis 2014, 8, 1569–1581. [Google Scholar] [CrossRef]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-Term Follow-Up of Colonoscopic Fecal Microbiota Transplant for RecurrentClostridium difficileInfection. Off. J. Am. Coll. Gastroenterol. ACG 2012, 107, 1079–1087. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Khan, M.Y.; Dirweesh, A.; Khurshid, T.; Siddiqui, W.J. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; O’Donovan, A.N.; Caplice, N.M.; Ross, R.P.; Stanton, C. Exploring the gut microbiota and cardiovascular disease. Metabolites 2021, 11, 493. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e917. [Google Scholar] [CrossRef]
- Hu, X.-F.; Zhang, W.-Y.; Wen, Q.; Chen, W.-J.; Wang, Z.-M.; Chen, J.; Zhu, F.; Liu, K.; Cheng, L.-X.; Yang, J. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol. Res. 2019, 139, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Robles-Vera, I.; De la Visitacion, N.; Romero, M.; Yang, T.; Sánchez, M.; Gómez-Guzmán, M.; Jiménez, R.; Raizada, M.K.; Duarte, J. Critical role of the interaction gut microbiota–sympathetic nervous system in the regulation of blood pressure. Front. Physiol. 2019, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.; Kemper, E.M. Effect of vegan fecal microbiota transplantation on carnitine-and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J. Am. Heart Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.T.; Parajuli, N.; Sung, M.M.; Bairwa, S.C.; Levasseur, J.; Soltys, C.-L.M.; Wishart, D.S.; Madsen, K.; Schertzer, J.D.; Dyck, J.R. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E511–E519. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.L.; Mukherjee, J.; Jenkins-Jones, S.; Holden, S.; Currie, C. Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: A retrospective, observational study. Diabetes Obes. Metab. 2014, 16, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef]
- Montandon, S.A.; Jornayvaz, F.R. Effects of antidiabetic drugs on gut microbiota composition. Genes 2017, 8, 250. [Google Scholar] [CrossRef]
- Napolitano, A.; Miller, S.; Nicholls, A.W.; Baker, D.; Van Horn, S.; Thomas, E.; Rajpal, D.; Spivak, A.; Brown, J.R.; Nunez, D.J. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS ONE 2014, 9, e100778. [Google Scholar] [CrossRef] [PubMed]
- Van Stee, M.F.; de Graaf, A.A.; Groen, A.K. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc. Diabetol. 2018, 17, 94. [Google Scholar] [CrossRef]
- Brønden, A.; Albér, A.; Rohde, U.; Rehfeld, J.F.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Single-dose metformin enhances bile acid–induced glucagon-like peptide-1 secretion in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 4153–4162. [Google Scholar] [CrossRef]
- De La Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Ning, F.; Tuomilehto, J.; Pyörälä, K.; Onat, A.; Söderberg, S.; Qiao, Q.; Group, D.S. Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care 2010, 33, 2211–2216. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ. Res. 2018, 122, 1439–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Li, M.; Yu, M.; Ping, F.; Zheng, J.; Wang, T.; Wang, X. Vildagliptin increases butyrate-producing bacteria in the gut of diabetic rats. PLoS ONE 2017, 12, e0184735. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Rajamäki, K.; Lappalainen, J.; Öörni, K.; Välimäki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Yu, Z.-L.; Chen, J.-Y.; Li, X.-Y.; Wang, Z.-P.; Wu, M.; Liu, L.-T. The crosstalk between NLRP3 inflammasome and gut microbiome in atherosclerosis. Pharmacol. Res. 2022, 181, 106289. [Google Scholar] [CrossRef]
- Li, X.; Geng, J.; Zhao, J.; Ni, Q.; Zhao, C.; Zheng, Y.; Chen, X.; Wang, L. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front. Physiol. 2019, 10, 866. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, H.-Y.; Zhao, Y.-C.; Zheng, X.-R.; Wang, F.; Zhao, G.-R. NLRP3 inflammasome in atherosclerosis: Putting out the fire of inflammation. Inflammation 2023, 46, 35–46. [Google Scholar] [CrossRef]
- Han, L.; Li, M.; Liu, X. Effects of long-term atorvastatin treatment on cardiac aging. Exp. Ther. Med. 2013, 6, 721–726. [Google Scholar] [CrossRef]
- Gong, X.; Ma, Y.; Ruan, Y.; Fu, G.; Wu, S. Long-term atorvastatin improves age-related endothelial dysfunction by ameliorating oxidative stress and normalizing eNOS/iNOS imbalance in rat aorta. Exp. Gerontol. 2014, 52, 9–17. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Lin, L.; Cai, X.; Huang, W.; Huang, Z. Atorvastatin suppresses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB signaling in PMA-stimulated THP-1 monocytes. Biomed. Pharmacother. 2016, 82, 167–172. [Google Scholar] [CrossRef]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, Y.; Zhang, M.; An, F. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc. Drugs Ther. 2014, 28, 33–43. [Google Scholar] [CrossRef]
- Lv, Z.-H.; Phuong, T.A.; Jin, S.-J.; Li, X.-X.; Xu, M. Protection by simvastatin on hyperglycemia-induced endothelial dysfunction through inhibiting NLRP3 inflammasomes. Oncotarget 2017, 8, 91291. [Google Scholar] [CrossRef]
- Abderrazak, A.; Couchie, D.; Mahmood, D.F.D.; Elhage, R.; Vindis, C.; Laffargue, M.; Matéo, V.; Büchele, B.; Ayala, M.R.; El Gaafary, M. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2. Ki mice fed a high-fat diet. Circulation 2015, 131, 1061–1070. [Google Scholar] [CrossRef]
- Luo, F.; Das, A.; Chen, J.; Wu, P.; Li, X.; Fang, Z. Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc. Diabetol. 2019, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Ferrell, W.; Greer, I.A.; Petrie, J.R.; Cobbe, S.M.; Sattar, N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 2006, 48, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Preiss, D.; Lloyd, S.M.; Ford, I.; McMurray, J.J.; Holman, R.R.; Welsh, P.; Fisher, M.; Packard, C.J.; Sattar, N. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 116–124. [Google Scholar] [CrossRef]
- Holman, R.R.; Coleman, R.L.; Chan, J.C.; Chiasson, J.-L.; Feng, H.; Ge, J.; Gerstein, H.C.; Gray, R.; Huo, Y.; Lang, Z. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 877–886. [Google Scholar] [CrossRef]
- Lahnwong, S.; Chattipakorn, S.C.; Chattipakorn, N. Potential mechanisms responsible for cardioprotective effects of sodium–glucose co-transporter 2 inhibitors. Cardiovasc. Diabetol. 2018, 17, 101. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; An, J.; Lee, S.; Choi, D.; Kong, H.; Song, Y.; Park, I.H.; Lee, C.-K.; Kim, K. Downregulation of IL-18 expression in the gut by metformin-induced gut microbiota modulation. Immune Netw. 2019, 19, e28. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Tang, W.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Gluck, M.; Koon, S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment ofclostridium difficileinfection despite asymptomatic donors and lack of sick contacts. Off. J. Am. Coll. Gastroenterol. ACG 2013, 108, 1367. [Google Scholar] [CrossRef]
- De Leon, L.M.; Watson, J.B.; Kelly, C.R. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2013, 11, 1036–1038. [Google Scholar] [CrossRef]
- Ndeh, D.; Gilbert, H.J. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol. Rev. 2018, 42, 146–164. [Google Scholar] [CrossRef]
- Hickey, C.A.; Kuhn, K.A.; Donermeyer, D.L.; Porter, N.T.; Jin, C.; Cameron, E.A.; Jung, H.; Kaiko, G.E.; Wegorzewska, M.; Malvin, N.P. Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe 2015, 17, 672–680. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, Å.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Russell, B.J.; Brown, S.D.; Siguenza, N.; Mai, I.; Saran, A.R.; Lingaraju, A.; Maissy, E.S.; Machado, A.C.D.; Pinto, A.F.; Sanchez, C. Intestinal transgene delivery with native E. coli chassis allows persistent physiological changes. Cell 2022, 185, 3263–3277.e3215. [Google Scholar]
- Praveschotinunt, P.; Duraj-Thatte, A.M.; Gelfat, I.; Bahl, F.; Chou, D.B.; Joshi, N.S. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 2019, 10, 5580. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Caparrós-Martin, J.A.; Gray, N.; Lodge, S.; Wist, J.; Lee, S.; O’Gara, F.; Dwivedi, G.; Ward, N.C. Gut microbiota and metabolomics profiles in patients with chronic stable angina and acute coronary syndrome. Physiol. Genom. 2024, 56, 48–64. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Caparrós-Martin, J.A.; Gray, N.; Lodge, S.; Wist, J.; Lee, S.; O’Gara, F.; Shah, A.; Ward, N.C.; Dwivedi, G. Insights into the associations between the gut microbiome, its metabolites, and heart failure. Am. J. Physiol.-Heart Circ. Physiol. 2023, 325, H1325–H1336. [Google Scholar] [CrossRef]
- Wymore Brand, M.; Wannemuehler, M.J.; Phillips, G.J.; Proctor, A.; Overstreet, A.-M.; Jergens, A.E.; Orcutt, R.P.; Fox, J.G. The altered Schaedler flora: Continued applications of a defined murine microbial community. ILAR J. 2015, 56, 169–178. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814.e805. [Google Scholar] [CrossRef]
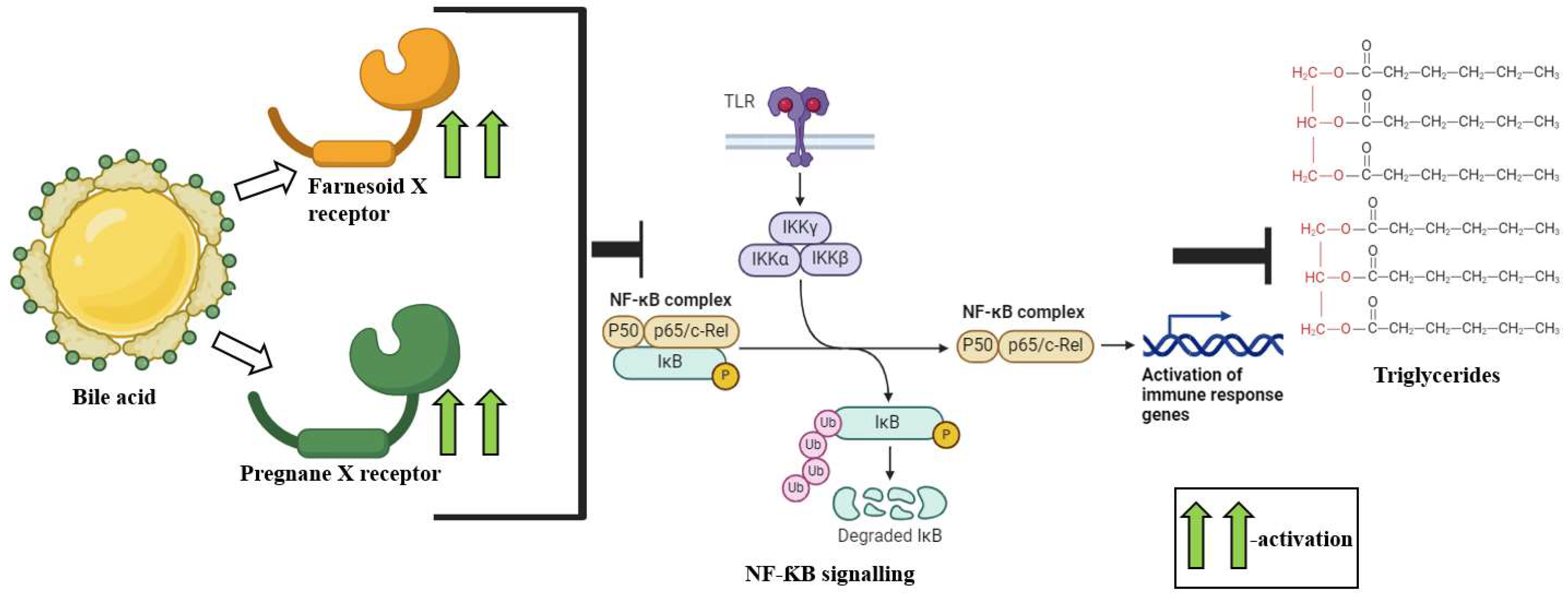
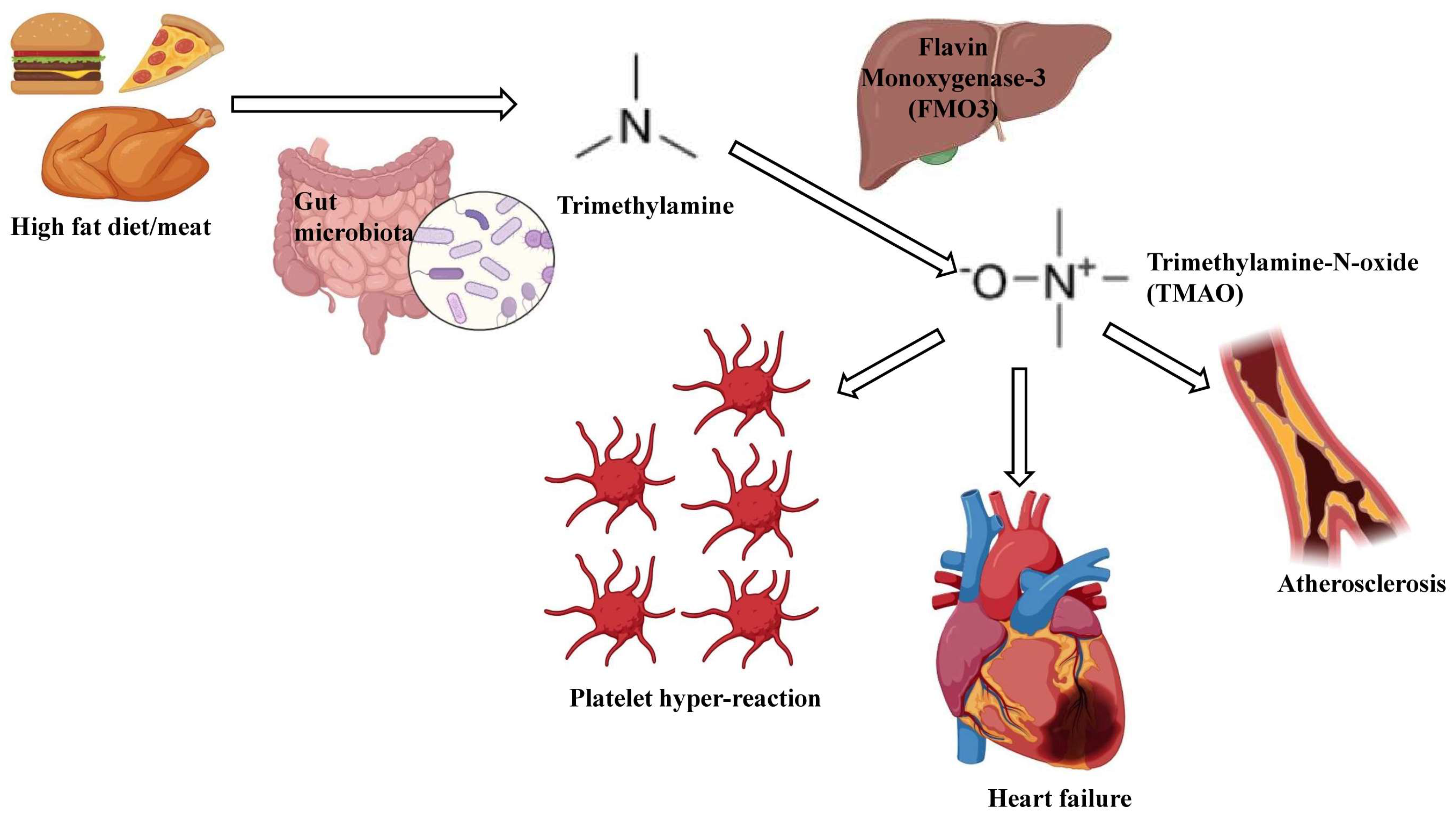
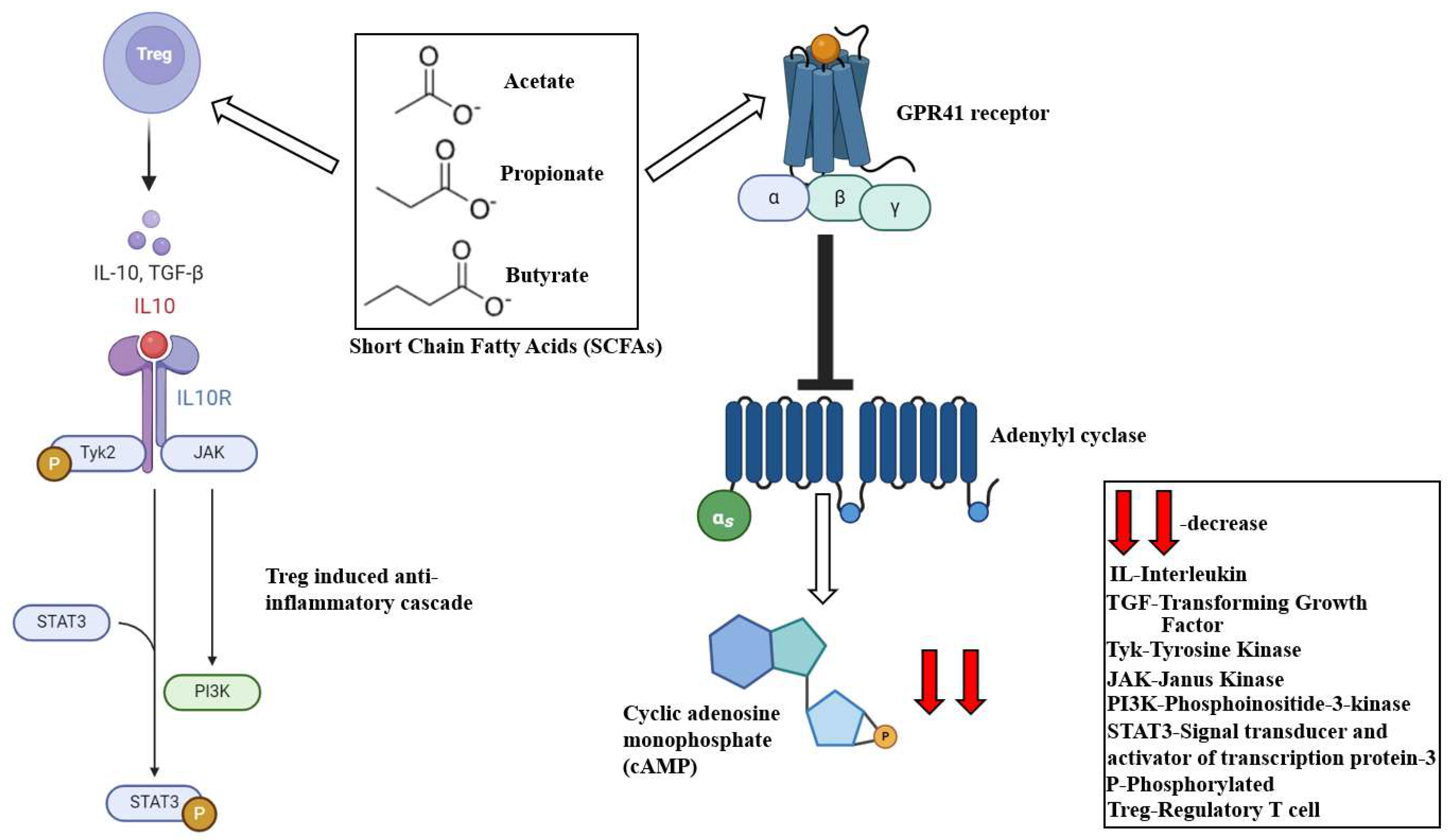
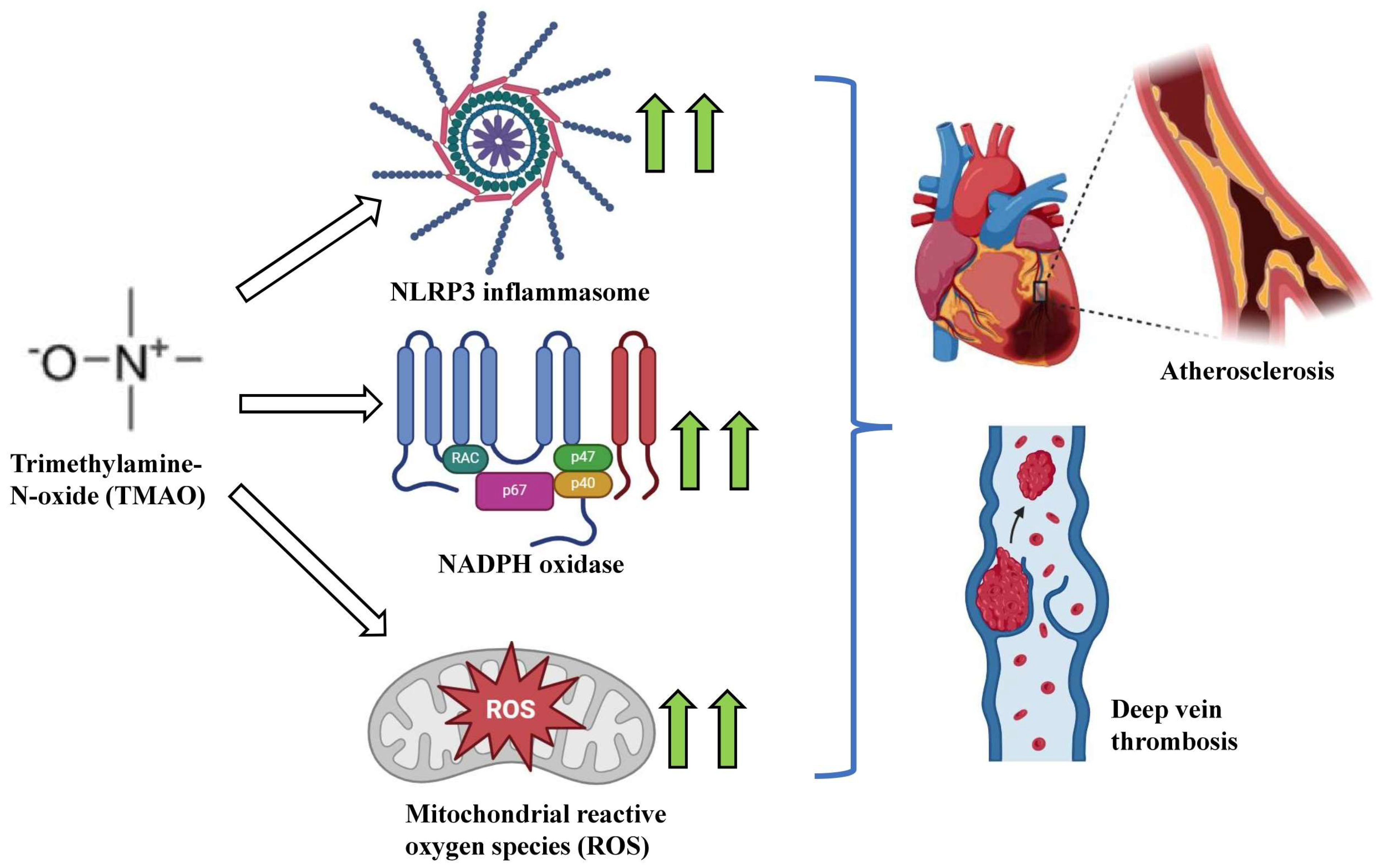
| Metabolite | Source | Mechanism of Action | Impact on CVDs | References |
|---|---|---|---|---|
| Short-Chain Fatty acids (SCFAs) | Gut microbial fermentation of dietary macromolecules | Block cholesterol generation and their hepatic localization, promote Treg-induced anti-inflammatory signaling and reduce the local infiltration of immune cells, promote the expression of protein phosphatases, eNOS, PTEN and GSK-3β | Downregulate cardiac hypertrophy, fibrosis and associated vascular dysfunction, inhibit onset of coronary diseases, reverses cardiomyocyte contraction defects | [185,193,196,198] |
| Trimethylamine-N-oxide (TMAO) | Gut-microbiota-driven breakdown of high-fat dietary food components via microbial TMA lyases | Enhances oxidative stress and ROS production via NLRP3 inflammasome activation, downregulates SIRT1 levels and promotes oxidative stress via the p53/p21/retinoblastoma (Rb) signaling, upregulates vascular oxidative stress via an upsurge in NADPH oxidase activity | Promotes monocyte adhesion and deteriorates endothelium self-repair capacity, upregulates foam cell formation, augments macrophage LDL aggregation and is cumulatively predisposed towards atherosclerosis | [214,216,217,218,219,220,225] |
| Bile acids (BAs) | Amphipathic molecules generated in the liver from cholesterol metabolism | Activate FXR, TGR5, modulation of Ca2+ inflow in ventricular cardiomyocytes, induce PXR activation, downregulating ApoA-IV, Cyp39a1 and augments CD36 | Influence vascular tone and overall cardiovascular function, a reduction in secondary BA and a rise in primary BAs promotes CAD and hypercholesterolemia, increases total cholesterol, VLDL, and LDL, and decreases HDL that lead to atherosclerosis | [229,230,232,234,235] |
| Indole-3-propionic acid (IPA) | Gut-microbiota-driven tryptophan breakdown | Enhances ABCA1 and macrophage-associated reverse cholesterol transport | Induces myocardial contractility, the vasoconstriction of endothelium-denuded mesenteric arteries | [242,243,244,245] |
| Hydrogen sulfide (H2S) | Sulfate-reducing gut microbiota | Induces the downregulation of macrophage CX3CR1 expression, promotes PPAR-γ and downregulates NF-κB/ICAM-1 | Exhibits vascular protective functions, regulates vascular tone, reduces leukocyte adhesion, mitochondrial ROS stress, decreases vascular proliferation and LDL oxidation. | [255,256,257,259,260] |
| Phenylacetylglutamine (PAGln) | Phenylalanine metabolism, primarily driven via bacterial PAGln synthase | Interacts and activates α2A, α2B and β2- adrenergic GPCRs | Associated with sarcomere functional defects, influences cardiac and platelet functions that eventually culminate to atherosclerosis | [175,262,263,264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datta, S.; Pasham, S.; Inavolu, S.; Boini, K.M.; Koka, S. Role of Gut Microbial Metabolites in Cardiovascular Diseases—Current Insights and the Road Ahead. Int. J. Mol. Sci. 2024, 25, 10208. https://doi.org/10.3390/ijms251810208
Datta S, Pasham S, Inavolu S, Boini KM, Koka S. Role of Gut Microbial Metabolites in Cardiovascular Diseases—Current Insights and the Road Ahead. International Journal of Molecular Sciences. 2024; 25(18):10208. https://doi.org/10.3390/ijms251810208
Chicago/Turabian StyleDatta, Sayantap, Sindhura Pasham, Sriram Inavolu, Krishna M. Boini, and Saisudha Koka. 2024. "Role of Gut Microbial Metabolites in Cardiovascular Diseases—Current Insights and the Road Ahead" International Journal of Molecular Sciences 25, no. 18: 10208. https://doi.org/10.3390/ijms251810208
APA StyleDatta, S., Pasham, S., Inavolu, S., Boini, K. M., & Koka, S. (2024). Role of Gut Microbial Metabolites in Cardiovascular Diseases—Current Insights and the Road Ahead. International Journal of Molecular Sciences, 25(18), 10208. https://doi.org/10.3390/ijms251810208





