Unveiling the Role of Endothelial Dysfunction: A Possible Key to Enhancing Catheter Ablation Success in Atrial Fibrillation
Abstract
:1. Introduction
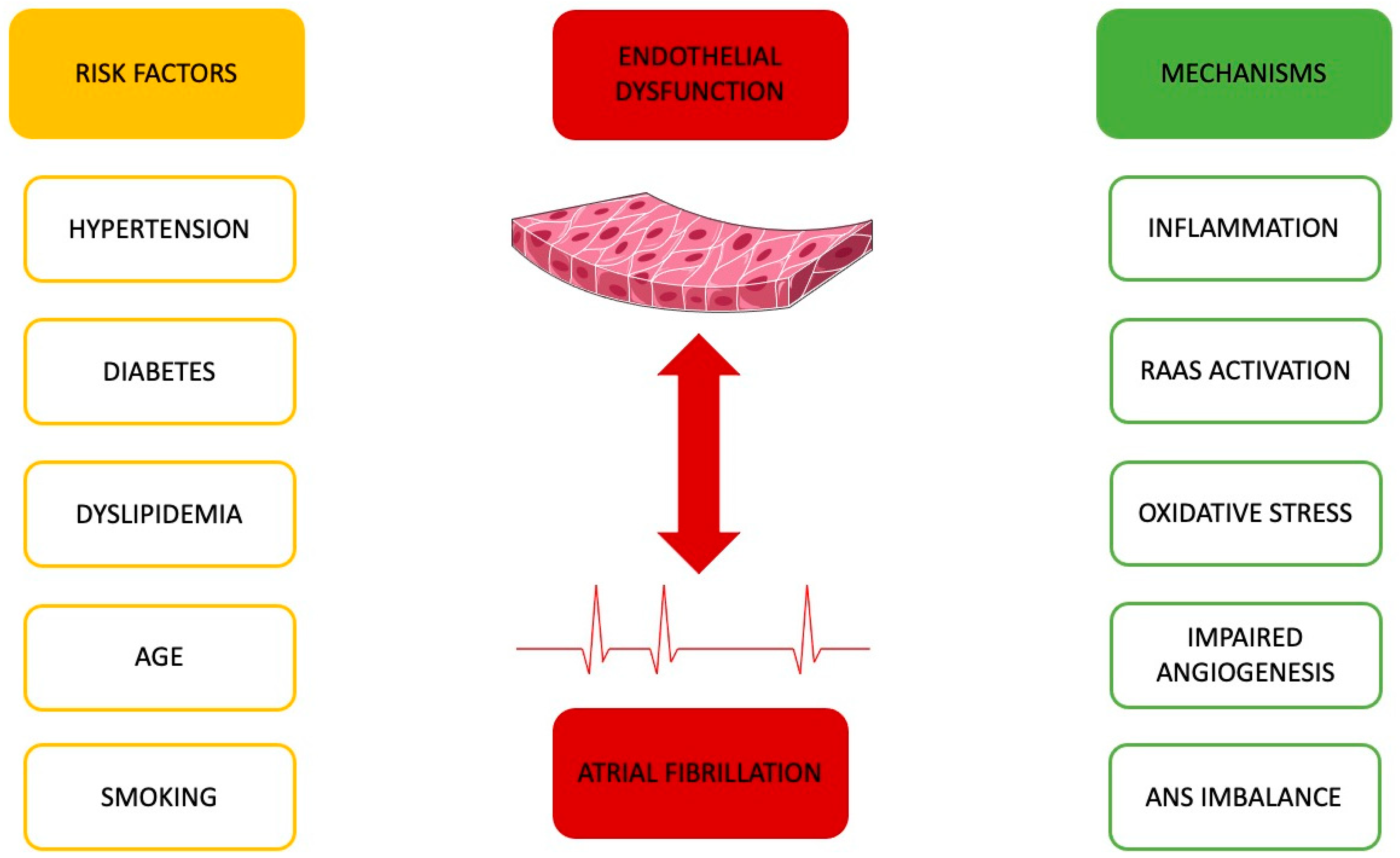
2. Endothelial Dysfunction and Atrial Fibrillation
2.1. Evaluating Indicators of Endothelial Dysfunction and Their Dynamics in the Context of Atrial Fibrillation
2.2. The Impact of Electrical Cardioversion on Endothelial Function
3. Common Ground between Endothelial Function and Atrial Fibrillation
3.1. Hypertension’s Impact
3.2. Ageing and Its Role
3.3. Diabetes and Its Influence
3.4. Lipid Profile’s Significance
3.5. Smoking’s Contribution
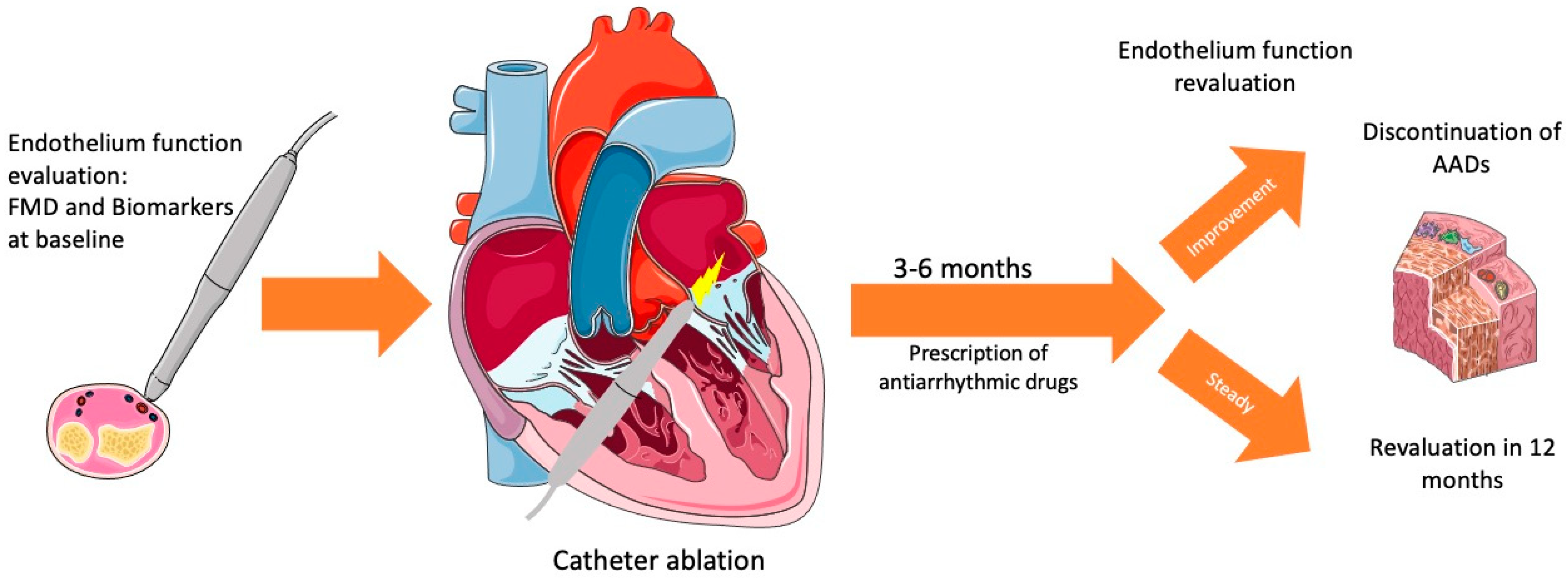
4. Catheter Ablation for Atrial Fibrillation and Endothelial Dysfunction
4.1. The Role of Endothelial Dysfunction in Catheter Ablation
| Studies | Design | Total Cases—Follow Up | Population | Intervention—ED Assessment | Findings |
|---|---|---|---|---|---|
| Okawa et al. [74], 2023 | Prospective Cohort Study | 1040 with AF—Median: 35 months | Japanese (2013–2022) (mean age 67 ± 10) | RF-RHI | Higher 5-year incidence of cardiovascular events in the ED group vs. non-ED group: 98 (11.8%) vs. 13 (6.2%). AF recurrence in ED HR 1.01, 95% CI (0.68–1.5), p = 0.86. |
| Gao et al. [70], 2022 | Case Control | 66 PeAF, 72 PAF, 80 control—6 months | Chinese | RF-ET-1 levels, CTGF | Higher levels of ET-1 and CTGF in PAF and PeAF, compared to control. Higher levels of ET-1 and CTGF in patients with postoperative AF recurrence than those without. Positive correlation of ET-1 and CTGF levels pre- and postoperatively, with PeAF recurrence. |
| Kanazawa et al. [73], 2021 | Retrospective Observational Study | 214 (151 SR, 63 AF)—12 months | Japanese (2013–2016) (mean 61 ± 10) | RF-RH-PAT | LnRHI 3 months after CA (decreased to ≥0.01 compared with that before CA) was an independent marker of suspected AF recurrence (sensitivity, 0.806; specificity, 0.821; area under the curve, 0.792; p < 0.001). Higher probability of AF recurrence when the LnRHI value 3 months after CA was lower than that before CA (log rank test, p < 0.001). |
| Lackermair et al. [71], 2017 | Case Control | 96 AF, 40 Control—3 months | German (61.8 ± 10.9 AF, 60.2 ± 12.58 Control) | RF-ET-1, CGA, MCP-1 | Higher levels of ET-1 in patients with AF, compared to age- and sex-matched healthy volunteers without AF (2.62 pg/mL vs. 1.57 pg/mL; p < 0.001). Lower ET-1 levels prior ablation associated with freedom of AF in the follow-up period of 3 months (2.57 pg/mL vs. 2.96 pg/mL; p = 0.02) (MCP-1 plasma levels increased significantly after ablation independent from AF recurrence; CGA levels increased significantly only in patients without recurrence towards the level of healthy controls). |
| Matsuzawa et al. [75], 2016 | Double-Blind, Placebo-Controlled Trial | 92 (enrolled, 71 follow up)—3 months | American, (January 2008 and December 2009), (mean 57 ± 10) | RF-RH-PAT | Association of Ln_RHI levels with symptomatic AF (hazard ratio [HR] 1.99 [95% CI 0.92–4.51], p = 0.079) and atrial arrhythmia recurrence (HR 1.93 [95% CI 0.99–3.92], p = 0.054); ≤60 years + attenuated endothelial function significantly associated with increased risk of symptomatic AF recurrence (HR 4.01 [95% CI 1.39–14.38], p = 0.009). No significant association in participants aged >60 years. Endothelial dysfunction → higher rates of recurrence of AF (p = 0.010). |
| Wang et al. [72], 2012 | Prospective Cohort Study | 103 PAF, 55 PeAF—22-month median follow up | Chinese | RF-big ET-1 | Higher plasma levels of big ET-1 in the recurrence group vs. in the non-recurrence group in all patients (p = 0.001). ET-1 levels were a prognostic predictor of AF recurrence only in patients with paroxysmal AF (p = 0.037). |
| Yang et al. [76], 2011 | Prospective Cohort Study | 138 with AF—3 months | Chinese (June 2007 to October 2009), (49.84 ± 7.47 AF rec, 50.02 ± 6.93 no rec) | RF-ADMA | Higher serum ADMA concentrations before catheter ablation in the recurrence group (0.75 ± 0.24 vs. 0.58 ± 0.14 μmol/L; p < 0.001). Association of recurrences of AF with higher serum ADMA concentration (HR = 4.42; 95% CI, 1.93–10.12; p < 0.001). |
4.2. The Role of Catheter Ablation on Endothelial Dysfunction
4.2.1. Periprocedural and Immediately Postprocedural Role
4.2.2. Long-Term Role
| Studies | Design | Total Cases—Follow Up | Population (Period), (Age) | Intervention—ED Assessment | Findings |
|---|---|---|---|---|---|
| Kanazawa et al. [73], 2021 | Retrospective Observational Study | 214 (151 SR, 63 AF)—12 months | Japanese (2013–2016) (mean 61 ± 10) | RF-RH-PAT | Improvement in LnRHI 6 months after CA (0.61 ± 0.25 versus 0.74 ± 0.22, p < 0.001) in patients without AF recurrence, but not in patients with AF recurrence (0.78 ± 0.25 versus 0.66 ± 0.19, p = 0.055). LnRHI in AF rhythm before CA remained unchanged 6 months after CA. Improvement in LnRHI in AF SR group 3 and 6 months after CA compared with that before (3 months: 0.66 ± 0.24 versus 0.81 ± 0.25, p < 0.001; 6 months: 0.66 ± 0.24 versus 0.76 ± 0.23, p = 0.012). Improvement in LnRHI in patients without AF recurrence after CA to a level similar to those in normal control. |
| Namino et al. [89], 2019 | Prospective Cohort Study | 101–6 months | Japanese (November 2014–August 2015) (mean 61.8 + 8.6) | RG-PAI-1, s-TM, ADMA | Increase after catheter ablation of s-TM and PAI-1 levels at the 6-month follow up compared with baseline in both the restored SR and recurrent AF groups (11.55 [2.92] vs. 13.75 [3.38], p < 0.001; 10.28 [2.78] vs. 11.67 [3.37], p < 0.001) and (25.74 [15.25] vs. 37.79 [19.56], p < 0.001; 26.16 [15.70] vs. 40.74 [22.55], p < 0.001), respectively. No differences in ADMA levels at the 6-month follow up compared with the baseline for either group ((0.625 [0.163] vs. 0.589 [0.101], p = 0.241) and (0.637 [0.143] vs. 0.616 [0.102], p = 0.500)). |
| Wang et al. [91], 2019 | Case Control | 20 PAF, 20 control (+pigs) | Chinese (52.5 ± 9.3 AF, 53 ± 10.8) | RF-miRNA | miR-99b-3p, miR-133a, and miR-99b expression reduced by almost 75%—expression of miR-325, miR-423-5p, and miR-451a reduced by 25% post-ablation. Decreased levels of NO in AF+ groups (both pre-ablation and post-ablation). Implication of miR-155, miR-24, and eNOS on AF pathogenesis (on pigs). |
| Lackermair et al. [71], 2017 | Case Control | 96 AF, 40 Control—3 months | German (61.8 ± 10.9 AF, 60.2 ± 12.58 control) | RF-ET-1 CGA, MCP-1 | Patients without AF recurrence demonstrated a decrease in ET-1 levels three months after ablation getting closer to the level of the healthy volunteers (2.33 pg/mL vs. 2.57 pg/mL; p < 0.01), whereas ET-1 levels in patients with AF recurrence remained unchanged at an elevated level (2.83 pg/mL vs. 2.96 pg/mL; p = 0.09). MCP-1 plasma levels increased after ablation independent from AF recurrence; CGA levels increased significantly only in patients without recurrence towards the level of healthy controls, but not in patients with recurrence. |
| Okawa et al. [93], 2017 | Case Control | 102 PAF, 75 PeAF, 51 control | Japanese (May 2013 to February 2015) (65.9 ± 10.0 PAF, 65.8 ± 10.7 PeAF, 64.9 ± 10.8 control) | RF-RH-PAT | Lowest RHI in the PeAF group (p < 0.001 versus control, p = 0.008 versus PAF groups). Unchanged RHI measurements in the PAF patients prior to the catheter ablation and at 6 and 12 months post-ablation. Increased RHI at the 6-month follow up in the PeAF group (0.53 ± 0.28, p < 0.05), which was maintained at 12 months. |
| Matsuzawa et al. [75], 2016 | Double-Blind, Placebo-Controlled Trial | 92 (enrolled, 71 follow up)—3 months | American, (January 2008 and December 2009), (mean 57 ± 10) | RF-RH-PAT | Unchanged endothelial function after atrial ablation (Ln_RHI from 0.60 ± 0.29 to 0.65 ± 0.25, p = 0.41) after 3 months. Slightly higher endothelial function in the atorvastatin group than in the placebo group (not statistically significant). |
| Lim et al. [90], 2014 | Prospective Cohort Study | 57 AF patients—6 months | Australian (53.8 ± 10.5, SR maintenance, 61.2 ± 9.4, AF recurrence) | RF-ADMA and platelet activation receptors CD62P (P-selectin) and glycoprotein IIb/IIIa (PAC-1) | After catheter ablation and successful maintenance of SR, endothelial dysfunction measured by ADMA levels decreased at 6-month follow up compared with baseline (log ADMA μM/L 0.15 ± 0.02 vs. 0.17 ± 0.04, p = 0.015). No significant improvement in ADMA levels was seen in the group that sustained AF recurrence (log ADMA μM/L 0.16 ± 0.03 vs. 0.16 ± 0.02, p = 0.4). |
| Yoshino et al. [92], 2013 | Case Control | 48 (27 AF, 21 control)—6 month | Japanese (April 2008 to August 2010) (58 ± 12 AF, 56 ± 17 control) | RF-RH-PAT | Higher loge RHI the morning after ABL, compared with that before ABL in day 1-restored SR group (0.53 ± 0.20; 0.73 ± 0.25; p = 0.009), which was maintained after 6 months, and no difference in the day 1-recurred AF group (0.49 ± 0.21; 0.52 ± 0.23; p = 0.787). Similar loge in day 1-recurred AF group for all points (before ABL, day after, and 6 months after). |
| Shin et al. [88], 2011 | Prospective Cohort Study | 61 PAF, 19 PeAF, 80 control—6 months | Korean (53.4 ± 10.4 AF, 54.3 ± 9.3 Control) | RF-FMD + ET-1 | Lower FMD baseline in younger AF patients than control. Greater FMD in AF subjects who remained in SR after a successful CA at 1-month post-CA when compared with FMD baseline, and even more significant increases 6 months post-CA, nearly approaching control levels. Lower FMD baseline in AF recurrence group compared with nonrecurrence group, without increase for 1 month post-CA, even though SR was maintained. |
5. Catheter Ablation and Endothelial Function Treatment
5.1. Treatment of Risk Factors and Lifestyle Interventions
5.2. Antiarrhythmic Drugs after Catheter Ablation
6. Key Points
- Patients undergoing catheter ablation should undergo a preoperative evaluation of endothelial function, as well as follow-up assessments for a period after the procedure. The evaluation of endothelial function, both pre- and postoperative, should encompass a dual approach involving biomarkers and practical methods like FMD and RH-PAT for a thorough assessment. However, it is imperative to note that further studies are required to precisely determine the most effective methods and the optimal time periods for their application.
- Endothelial function should be monitored in patients with atrial fibrillation, as it may significantly influence the treatment decisions for these patients.
- A comprehensive approach is essential for patients with atrial fibrillation and endothelial dysfunction. This includes managing common risk factors shared by both conditions to effectively treat the patients and minimize potential complications.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Akar, J.G.; Jeske, W.; Wilber, D.J. Acute Onset Human Atrial Fibrillation Is Associated with Local Cardiac Platelet Activation and Endothelial Dysfunction. J. Am. Coll. Cardiol. 2008, 51, 1790–1793. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Kunugita, F.; Ozawa, M.; Satoh, Y.; Yoshizawa, R.; Owada, S.; Sawa, Y.; Morino, Y.; Nakamura, M. Relationship between Impairment of the Vascular Endothelial Function and the CHA2DS2-VASc Score in Patients with Sinus Rhythm and Non-valvular Atrial Fibrillation. Intern. Med. 2018, 57, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Mohammad, F.; Saraf, K.; Morris, G. Endothelial function and atrial fibrillation: A missing piece of the puzzle? J. Car-diovasc. Electrophysiol. 2022, 33, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef] [PubMed]
- Wieczór, A.M.; Wieczór, R.; Kulwas, A.; Rość, D. Asymmetric dimethylarginine and angiogenesis: Biological significance. Int. Angiol. 2018, 37, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Lao, M.C.; Liu, L.J.; Luo, C.F.; Lu, G.H.; Zhai, Y.S.; Chen, X.L.; Gao, X.R. Effect of asymmetrical dimethylarginine for predicting pro-thrombotic risk in atrial fibrillation. Zhonghua Yi Xue Za Zhi 2016, 96, 2059–2063. [Google Scholar]
- De Pablo-Moreno, J.A.; Serrano, L.J.; Revuelta, L.; Sánchez, M.J.; Liras, A. The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V. Int. J. Mol. Sci. 2022, 23, 8283. [Google Scholar] [CrossRef]
- Freestone, B.; Gustafsson, F.; Chong, A.Y.; Corell, P.; Kistorp, C.; Hildebrandt, P.; Lip, G.Y. Influence of Atrial Fibrillation on Plasma Von Willebrand Factor, Soluble E-Selectin, and N-Terminal Pro B-type Natriuretic Peptide Levels in Systolic Heart Failure. Chest 2008, 133, 1203–1208. [Google Scholar] [CrossRef]
- Krieglstein, C.F.; Granger, D. Adhesion molecules and their role in vascular disease. Am. J. Hypertens. 2001, 14 Pt 2, S44–S54. [Google Scholar] [CrossRef]
- Freestone, B.; Chong, A.Y.; Nuttall, S.; Lip, G.Y. Impaired flow mediated dilatation as evidence of endothelial dysfunction in chronic atrial fibrillation: Relationship to plasma von Willebrand factor and soluble E-selectin levels. Thromb. Res. 2008, 122, 85–90. [Google Scholar] [CrossRef]
- Willeit, K.; Pechlaner, R.; Willeit, P.; Skroblin, P.; Paulweber, B.; Schernthaner, C.; Toell, T.; Egger, G.; Weger, S.; Oberhollenzer, M.; et al. Association Between Vascular Cell Adhesion Molecule 1 and Atrial Fibrillation. JAMA Cardiol. 2017, 2, 516–523. [Google Scholar] [CrossRef]
- Ghiadoni, L.; Salvetti, M.; Muiesan, M.L.; Taddei, S. Evaluation of Endothelial Function by Flow Mediated Dilation: Methodological Issues and Clinical Importance. High Blood Press. Cardiovasc. Prev. 2015, 22, 17–22. [Google Scholar] [CrossRef]
- Rosenberry, R.; Nelson, M.D. Reactive hyperemia: A review of methods, mechanisms, and considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef] [PubMed]
- Polovina, M.; Potpara, T.; Giga, V.; Stepanovic, J.; Ostojic, M. Impaired endothelial function in lone atrial fibrillation. Vojn. Pregl. 2013, 70, 908–914. [Google Scholar] [CrossRef]
- Freestone, B.; Chong, A.Y.; Blann, A.D.; Lip, G.Y. The effects of direct current cardioversion for persistent atrial fibrillation on indices of endothelial damage/dysfunction. Thromb. Res. 2006, 118, 479–485. [Google Scholar] [CrossRef]
- Belletti, S.; Lenatti, L.; Bianco, E.; Guazzi, M.D. Effects of cardioversion of atrial fibrillation on endothelial function in hypertension or diabetes. Eur. J. Clin. Investig. 2007, 37, 26–34. [Google Scholar] [CrossRef]
- Skalidis, E.I.; Zacharis, E.A.; Tsetis, D.K.; Pagonidis, K.; Chlouverakis, G.; Yarmenitis, S.; Hamilos, M.; Manios, E.G.; Vardas, P.E. Endothelial Cell Function During Atrial Fibrillation and After Restoration of Sinus Rhythm. Am. J. Cardiol. 2007, 99, 1258–1262. [Google Scholar] [CrossRef]
- Benincasa, G.; Coscioni, E.; Napoli, C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: Novel opportunities for primary prevention. Biochem. Pharmacol. 2022, 202, 115108. [Google Scholar] [CrossRef]
- Zakynthinos, G.E.; Tsolaki, V.; Oikonomou, E.; Vavouranakis, M.; Siasos, G.; Zakynthinos, E. Metabolic Syndrome and Atrial Fi-brillation: Different Entities or Combined Disorders. J. Pers. Med. 2023, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.J.; Soliman, E.Z.; Alonso, A.; Swett, K.; Okin, P.M.; Goff, D.C., Jr.; Heckbert, S.R. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: The Multi-Ethnic Study of Atherosclerosis. Ann. Epidemiology 2015, 25, 71–76.e1. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Coca, A.; Kahan, T.; Boriani, G.; Manolis, A.S.; Olsen, M.H.; Oto, A.; Potpara, T.S.; Steffel, J.; Marín, F.; et al. Hypertension and cardiac arrhythmias: A consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017, 19, 891–911. [Google Scholar] [CrossRef]
- Kockskämper, J.; Pluteanu, F. Left Atrial Myocardium in Arterial Hypertension. Cells 2022, 11, 3157. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.J.; Mackasey, M.; Moghtadaei, M.; Belke, D.D.; Egom, E.E.; Tuomi, J.M.; Rafferty, S.A.; Kirkby, A.W.; Rose, R.A. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J. Mol. Cell. Cardiol. 2018, 124, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.J.; Mackasey, M.; Moghtadaei, M.; Liu, Y.; Kaur, J.; Egom, E.E.; Tuomi, J.M.; Rafferty, S.A.; Kirkby, A.W.; Rose, R.A. NPR-C (Natriuretic Peptide Receptor-C) Modulates the Progression of Angiotensin II–Mediated Atrial Fibrillation and Atrial Remodeling in Mice. Circ. Arrhythmia Electrophysiol. 2019, 12, e006863. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.C.B.; Hornbech, M.S.; Holstein-Rathlou, N.-H. Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus 2011, 1, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [PubMed]
- Li, S.; Wu, Y.; Yu, G.; Xia, Q.; Xu, Y. Angiotensin II Receptor Blockers Improve Peripheral Endothelial Function: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2014, 9, e90217. [Google Scholar] [CrossRef] [PubMed]
- Shahin, Y.; Alam Khan, J.; Samuel, N.; Chetter, I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: A meta-analysis of randomised controlled trials. Atherosclerosis 2011, 216, 7–16. [Google Scholar] [CrossRef]
- Ya, J.; Bayraktutan, U. Vascular Ageing: Mechanisms, Risk Factors, and Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 11538. [Google Scholar] [CrossRef]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace 2018, 20, 1086–1092. [Google Scholar] [CrossRef]
- Caturano, A.; Galiero, R.; Pafundi, P.C. Atrial Fibrillation and Stroke. A Review on the Use of Vitamin K Antagonists and Novel Oral Anticoagulants. Medicina 2019, 55, 617. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yamashita, T.; Sekiguchi, A.; Tsuneda, T.; Sagara, K.; Takamura, M.; Kaneko, S.; Aizawa, T.; Fu, L. AGEs-RAGE System Mediates Atrial Structural Remodeling in the Diabetic Rat. J. Cardiovasc. Electrophysiol. 2008, 19, 415–420. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, S.; Ozturk, S.; Alcelik, A.; Ozlu, M.F.; Erdem, A.; Memioglu, T.; Ozdemir, M.; Yazici, M. Atrial conduction time and atrial mechanical function in patients with impaired fasting glucose. J. Interv. Card. Electrophysiol. 2012, 35, 247–252. [Google Scholar] [CrossRef]
- Kuehl, M.; Stevens, M.J. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 405–416. [Google Scholar] [CrossRef]
- Watanabe, M.; Yokoshiki, H.; Mitsuyama, H.; Mizukami, K.; Ono, T.; Tsutsui, H. Conduction and refractory disorders in the diabetic atrium. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H86–H95. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-F.; Suenari, K.; Chang, S.-L.; Lin, Y.-J.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Tai, C.-T.; Tsao, H.-M.; Li, C.-H.; et al. Atrial Substrate Properties and Outcome of Catheter Ablation in Patients with Paroxysmal Atrial Fibrillation Associated with Diabetes Mellitus or Impaired Fasting Glucose. Am. J. Cardiol. 2010, 106, 1615–1620. [Google Scholar] [CrossRef]
- Demir, K.; Avci, A.; Kaya, Z.; Marakoglu, K.; Ceylan, E.; Yilmaz, A.; Ersecgin, A.; Armutlukuyu, M.; Altunkeser, B.B. Assessment of atrial electromechanical delay and P-wave dispersion in patients with type 2 diabetes mellitus. J. Cardiol. 2016, 67, 378–383. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Matsumoto, S.; Shimabukuro, M.; Fukuda, D.; Soeki, T.; Yamakawa, K.; Masuzaki, H.; Sata, M. Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser(1177)/Thr(497) of endothelial nitric oxide synthase in diabetic mice. Cardiovasc. Diabetol. 2014, 13, 30. [Google Scholar] [CrossRef]
- Paneni, F.; Costantino, S.; Castello, L.; Battista, R.; Capretti, G.; Chiandotto, S.; D’Amario, D.; Scavone, G.; Villano, A.; Rustighi, A.; et al. Targeting prolyl-isomerase Pin1 prevents mi-tochondrial oxidative stress and vascular dysfunction: Insights in patients with diabetes. Eur. Heart J. 2015, 36, 817–828. [Google Scholar] [CrossRef]
- Alam, N.M.; Mills, W.C., IV; Wong, A.A.; Douglas, R.M.; Szeto, H.H.; Prusky, G.T. A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Dis. Model. Mech. 2015, 8, 701–710. [Google Scholar]
- Li, J.; Wang, J.J.; Zhang, S.X. NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. J. Diabetes Res. 2015, 2015, 963289. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Chen, Q.; Jiang, Y.; Lu, X.; Zhao, X. Hydrogen sulfide accelerates wound healing in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 5097–5104. [Google Scholar]
- Bitterli, L.; Afan, S.; Bühler, S.; DiSanto, S.; Zwahlen, M.; Schmidlin, K.; Yang, Z.; Baumgartner, I.; Diehm, N.; Kalka, C. Endothelial progenitor cells as a biological marker of peripheral artery disease. Vasc. Med. 2016, 21, 3–11. [Google Scholar] [CrossRef]
- Rigato, M.; Bittante, C.; Albiero, M.; Avogaro, A.; Fadini, G.P. Circulating Progenitor Cell Count Predicts Microvascular Outcomes in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2015, 100, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Jarajapu, Y.P.; Hazra, S.; Segal, M.; Li Calzi, S.; Jadhao, C.; Qian, K.; Mitter, S.K.; Raizada, M.K.; Boulton, M.E.; Grant, M.B. Vasoreparative dysfunction of CD34+ cells in diabetic in-dividuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS ONE 2014, 9, e93965. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, T.T.; Chen, J.; Qiu, W. Elevated expression levels of serum insulin-like growth factor-1, tumor necrosis factor-alpha and vascular endothelial growth factor 165 might exacerbate type 2 diabetic nephropathy. J. Diabetes Investig. 2017, 8, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Losordo, D.W. Nitric oxide and angiogenesis. Circulation 2002, 105, 2133–2135. [Google Scholar] [CrossRef]
- Movahed, M.-R.; Hashemzadeh, M.; Jamal, M.M. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int. J. Cardiol. 2005, 105, 315–318. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.M.; Shin, D.G.; Kim, J.R.; Cho, K.H. Relation of atrial fibrillation (AF) and change of lipoproteins: Male patients with AF exhibited severe pro-inflammatory and pro-atherogenic properties in lipoproteins. Clin. Biochem. 2014, 47, 869–875. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Oikonomou, E.; Zaromitidou, M.; Stefanadis, C.; Papavassiliou, A.G. Inflammatory markers in hyper-lipidemia: From experimental models to clinical practice. Curr. Pharm. Des. 2011, 17, 4132–4146. [Google Scholar] [CrossRef]
- Takaeko, Y.; Kajikawa, M.; Kishimoto, S.; Yamaji, T.; Harada, T.; Han, Y.; Kihara, Y.; Hida, E.; Chayama, K.; Goto, C.; et al. Low Levels of Low-Density Lipoprotein Cholesterol and Endothelial Function in Subjects without Lipid-Lowering Therapy. J. Clin. Med. 2020, 9, 3796. [Google Scholar] [CrossRef]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Crinelli, R.; Nasoni, M.G.; Benedetti, S.; Palma, F.; Fraternale, A.; Iuliano, L. LDL receptors, caveolae and cholesterol in endothelial dysfunction: oxLDLs accomplices or victims? Br. J. Pharmacol. 2021, 178, 3104–3114. [Google Scholar] [CrossRef]
- Toma, L.; Stancu, C.S.; Sima, A.V. Endothelial Dysfunction in Diabetes Is Aggravated by Glycated Lipoproteins; Novel Molecular Therapies. Biomedicines 2020, 9, 18. [Google Scholar] [CrossRef]
- Soga, J.; Noma, K.; Hata, T.; Hidaka, T.; Fujii, Y.; Idei, N.; Fujimura, N.; Mikami, S.; Maruhashi, T.; Kihara, Y.; et al. Rho-associated kinase activity, endothelial function, and cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.K.; Devaraj, S.; Yuhanna, I.; Shaul, P.; Jialal, I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 2002, 106, 1439–1441. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708. [Google Scholar] [CrossRef]
- Riwanto, M.; Landmesser, U. High density lipoproteins and endothelial functions: Mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 2013, 54, 3227–3243. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Besler, C.; Rohrer, L.; Meyer, M.; Heinrich, K.; Bahlmann, F.H.; Mueller, M.; Horváth, T.; Doerries, C.; Heinemann, M.; et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010, 121, 110–122. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Duval, S.; Soliman, E.Z.; Ambrose, M.; Eberly, L.E.; Alonso, A. Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011, 8, 1160–1166. [Google Scholar] [CrossRef]
- Golbidi, S.; Edvinsson, L.; Laher, I. Smoking and Endothelial Dysfunction. Curr. Vasc. Pharmacol. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Pehrson, S.M.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; Hansen, P.S. Long-term efficacy of catheter ablation as first-line therapy for paroxysmal atrial fibrillation: 5-year outcome in a randomised clinical trial. Heart 2017, 103, 368–376. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; et al. Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; Natale, A.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. JAMA 2014, 311, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Kuniss, M.; Pavlovic, N.; Velagic, V.; Hermida, J.S.; Healey, S.; Arena, G.; Badenco, N.; Meyer, C.; Chen, J.; Iacopino, S.; et al. Cryoballoon ablation vs. antiarrhythmic drugs: First-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021, 23, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Geng, J.; Ding, Y.; Yao, Z.; Meng, J.; Wang, C.; Zhang, H.; Kang, P.; Tang, B. Serum levels of endothelin-1 and connective tissue growth factor are elevated in patients with atrial fibrillation and correlated with relapse following radiofrequency ablation. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Lackermair, K.; Clauss, S.; Voigt, T.; Klier, I.; Summo, C.; Hildebrand, B.; Nickel, T.; Estner, H.L.; Kääb, S.; Wakili, R.; et al. Alteration of Endothelin 1, MCP-1 and Chromogranin A in patients with atrial fibrillation undergoing pulmonary vein isolation. PLoS ONE 2017, 12, e0184337. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Fang, P.; Lei, S.; Li, X.; Hou, Y.; Zhang, S. Big endothelin-1 as a predictor of atrial fibrillation recurrence after primary ablation only in patients with paroxysmal atrial fibrillation. Herz 2012, 37, 919–925. [Google Scholar] [CrossRef]
- Kanazawa, H.; Kaikita, K.; Ito, M.; Kawahara, Y.; Hoshiyama, T.; Kanemaru, Y.; Kiyama, T.; Iwashita, S.; Tabata, N.; Yamanaga, K.; et al. Improvement of Vascular Endothelial Function Reflects Nonrecurrence After Catheter Ablation for Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e021551. [Google Scholar] [CrossRef]
- Okawa, K.; Sogo, M.; Morimoto, T.; Tsushima, R.; Sudo, Y.; Saito, E.; Ozaki, M.; Takahashi, M. Relationship Between Endothelial Dysfunction and the Outcomes After Atrial Fibrillation Ablation. J. Am. Heart Assoc. 2023, 12, e028482. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Suleiman, M.; Guddeti, R.R.; Kwon, T.; Monahan, K.H.; Lerman, L.O.; Friedman, P.A.; Lerman, A. Age-Dependent Predictive Value of Endothelial Dysfunction for Arrhythmia Recurrence Following Pulmonary Vein Isolation. J. Am. Heart Assoc. 2016, 5, e003183. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiufen, Q.; Shuqin, S.; Yang, Y.; Ying, S.; Yanwei, Y.; Wei, F.; Dechun, Y. Asymmetric dimethylarginine concentration and recurrence of atrial tachyarrythmias after catheter ablation in patients with persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2011, 32, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Demaria, R.G.; Pagé, P.; Leung, T.K.; Dubuc, M.; Malo, O.; Carrier, M.; Perrault, L.P. Surgical radiofrequency ablation induces coronary endothelial dysfunction in porcine coronary arteries. Eur. J. Cardio-Thoracic Surg. 2003, 23, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Bulava, A.; Slavík, L.; Fiala, M.; Heinc, P.; Škvařilova, M.; Lukl, J.; Krčová, V.; Indrák, K. Endothelial Damage and Activation of the Hemostatic System During Radiofrequency Catheter Isolation of Pulmonary Veins. J. Interv. Card. Electrophysiol. 2004, 10, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Dinov, B.; Blann, A.D.; Rolf, S.; Arya, A.; Schmidl, J.; Husser, D.; Hindricks, G.; Bollmann, A.; Lip, G.Y.H. Effects of Radiofrequency Catheter Ablation of Atrial Fibrillation on Soluble P-Selectin, Von Willebrand Factor and IL-6 in the Peripheral and Cardiac Circulation. PLoS ONE 2014, 9, e111760. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liu, X.; Zheng, M.; Zhao, R.; Liu, X.; Yin, X.; Liu, X.; Tian, Y.; Shi, L.; Sun, K.; et al. Effect of remote ischemic preconditioning on left atrial remodeling and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Pacing Clin. Electrophysiol. 2018, 41, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.E.; Choi, C.U.; Na, J.O.; Choi, J.-I.; Kim, S.H.; Kim, J.W.; Kim, E.J.; Han, S.W.; Park, S.W.; Rha, S.-W.; et al. Effects of Iatrogenic Myocardial Injury on Coronary Microvascular Function in Patients Undergoing Radiofrequency Catheter Ablation of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2013, 6, 318–326. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, G.; Xu, G.; Weng, S.; Lu, X. Effect of radiofrequency catheter ablation on endothelial function and oxidative stress. Acta Cardiol. 2006, 61, 339–342. [Google Scholar] [CrossRef]
- Antolič, B.; Pernat, A.; Cvijić, M.; Žižek, D.; Jan, M.; Šinkovec, M. Radiofrequency catheter ablation versus balloon cryoablation of atrial fibrillation: Markers of myocardial damage, inflammation, and thrombogenesis. Wien. Klin. Wochenschr. 2016, 128, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Malmborg, H.; Christersson, C.; Lonnerholm, S.; Blomstrom-Lundqvist, C. Comparison of effects on coagulation and inflam-matory markers using a duty-cycled bipolar and unipolar radiofrequency pulmonary vein ablation catheter vs. a cryoballoon catheter for pulmonary vein isolation. Europace 2013, 15, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Hisazaki, K.; Hasegawa, K.; Kaseno, K.; Miyazaki, S.; Amaya, N.; Shiomi, Y.; Tama, N.; Ikeda, H.; Fukuoka, Y.; Morishita, T.; et al. Endothelial damage and thromboembolic risk after pulmonary vein isolation using the latest ablation technologies: A comparison of the second-generation cryoballoon vs. contact force-sensing radiofrequency ablation. Heart Vessel. 2019, 34, 509–516. [Google Scholar] [CrossRef]
- Hajas, O.; Bagoly, Z.; Tóth, N.K.; Urbancsek, R.; Kiss, A.; Kovács, K.B.; Sarkady, F.; Nagy, A.; Oláh, A.V.; Nagy, L.; et al. Intracardiac Fibrinolysis and Endothelium Activation Related to Atrial Fibrillation Ablation with Different Techniques. Cardiol. Res. 2020, 2020, 1570483. [Google Scholar] [CrossRef]
- Osmancik, P.; Bacova, B.; Hozman, M.; Pistkova, J.; Kunstatova, V.; Sochorova, V.; Waldauf, P.; Hassouna, S.; Karch, J.; Vesela, J.; et al. Myocardial Damage, Inflammation, Coagulation, and Platelet Activity During Catheter Ablation Using Radiofrequency and Pulsed-Field Energy. JACC Clin. Electrophysiol. 2023. [Google Scholar] [CrossRef]
- Shin, S.Y.; NA, J.O.; Lim, H.E.; Choi, C.U.; Choi, J.I.; Kim, S.H.; Kim, E.J.; Park, S.W.; Rha, S.; Park, C.G.; et al. Improved Endothelial Function in Patients with Atrial Fibrillation through Maintenance of Sinus Rhythm by Successful Catheter Ablation. J. Cardiovasc. Electrophysiol. 2011, 22, 376–382. [Google Scholar] [CrossRef]
- Namino, F.; Yamakuchi, M.; Iriki, Y.; Okui, H.; Ichiki, H.; Maenosono, R.; Oketani, N.; Masamoto, I.; Miyata, M.; Horiuchi, M.; et al. Dynamics of Soluble Thrombomodulin and Circulating miRNAs in Patients with Atrial Fibrillation Undergoing Radiofrequency Catheter Ablation. Clin. Appl. Thromb. 2019, 25, 1076029619851570. [Google Scholar] [CrossRef]
- Lim, H.S.; Willoughby, S.R.; Schultz, C.; Chakrabarty, A.; Alasady, M.; Lau, D.H.; Roberts-Thomson, K.C.; Worthley, M.I.; Young, G.D.; Sanders, P. Successful catheter ablation decreases platelet activation and improves endothelial function in patients with atrial fibrillation. Heart Rhythm. 2014, 11, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, L.; Ding, W.; Cai, S.; Zhao, Q. Ablation alleviates atrial fibrillation by regulating the signaling pathways of en-dothelial nitric oxide synthase/nitric oxide via miR-155-5p and miR-24-3p. J. Cell Biochem. 2019, 120, 4451–4462. [Google Scholar] [CrossRef]
- Yoshino, S.; Yoshikawa, A.; Hamasaki, S.; Ishida, S.; Oketani, N.; Saihara, K.; Okui, H.; Kuwahata, S.; Fujita, S.; Ichiki, H.; et al. Atrial fibrillation-induced endothelial dysfunction improves after restoration of sinus rhythm. Int. J. Cardiol. 2013, 168, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Okawa, K.; Miyoshi, T.; Tsukuda, S.; Hara, S.; Matsuo, N.; Nishibe, N.; Sogo, M.; Okada, T.; Nosaka, K.; Sakane, K.; et al. Differences in endothelial dysfunction induced by paroxysmal and persistent atrial fibrillation: Insights from restoration of sinus rhythm by catheter ablation. Int. J. Cardiol. 2017, 244, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Baranchuk, A.; Crystal, E.; Morillo, C.A.; Garfinkle, M.; Yusuf, S.; Connolly, S.J. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: A meta-analysis. J. Am. Coll. Cardiol. 2005, 45, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Aagaard, P.; Kanj, M.; Jaber, W.; Elshazly, M.; Hoosien, M.; Baranowski, B.; Hussein, A.; Saliba, W.; Wazni, O. Association Between Pre-Ablation Glycemic Control and Outcomes Among Patients with Diabetes Undergoing Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2019, 5, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-H.; Liu, N.; Bai, R.; Yao, Y.; Li, S.-N.; Yu, R.-H.; Sang, C.-H.; Tang, R.-B.; Long, D.-Y.; Du, X.; et al. HbA1c levels as predictors of ablation outcome in type 2 diabetes mellitus and paroxysmal atrial fibrillation. Herz 2015, 40 (Suppl. S2), S130–S136. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Kartas, A.; Moysidis, D.V.; Tsagkaris, C.; Papadakos, S.P.; Bekiaridou, A.; Samaras, A.; Karagiannidis, E.; Papadakis, M.; Giannakoulas, G. Glycemic control and atrial fibrilla-tion: An intricate relationship, yet under investigation. Cardiovasc. Diabetol. 2022, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, W.; Pan, Q.; Guo, L. Effects of SGLT-2 Inhibitors on Vascular Endothelial Function and Arterial Stiffness in Subjects with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 826604. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Exercise training and endothelial function in patients with type 2 diabetes: A meta-analysis. Cardiovasc. Diabetol. 2018, 17, 64. [Google Scholar] [CrossRef]
- Fauchier, L.; Pierre, B.; de Labriolle, A.; Grimard, C.; Zannad, N.; Babuty, D. Antiarrhythmic effect of statin therapy and atrial fi-brillation a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2008, 51, 828–835. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Korantzopoulos, P.; Liu, E.; Li, G. Statin use and development of atrial fibrillation: A systematic review and me-ta-analysis of randomized clinical trials and observational studies. Int. J. Cardiol. 2008, 126, 160–170. [Google Scholar] [CrossRef]
- Peña, J.M.; MacFadyen, J.; Glynn, R.J.; Ridker, P.M. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: An exploratory analysis of the JUPITER trial. Eur. Heart J. 2012, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, Y.; Zhao, Y.; Xiao, H. The effect of statins on the recurrence rate of atrial fibrillation after catheter ablation: A meta-analysis. Pacing Clin. Electrophysiol. 2018, 41, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Reriani, M.K.; Dunlay, S.M.; Gupta, B.; West, C.P.; Rihal, C.S.; Lerman, L.; Lerman, A. Effects of statins on coronary and peripheral endothelial function in humans: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2011, 18, 704–716. [Google Scholar] [CrossRef]
- Williams, I.L.; Chowienczyk, P.J.; Wheatcroft, S.B.; Patel, A.G.; Sherwood, R.; Momin, A.; Shah, A.M.; Kearney, M.T. Endothelial Function and Weight Loss in Obese Humans. Obes. Surg. 2005, 15, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Middeldorp, M.E.; Lau, D.H.; Mehta, A.B.; Mahajan, R.; Twomey, D.; Alasady, M.; Hanley, L.; Antic, N.A.; McEvoy, R.D.; et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: The ARREST-AF cohort study. J. Am. Coll. Cardiol. 2014, 64, 2222–2231. [Google Scholar] [CrossRef]
- Pathak, R.K.; Evans, M.; Middeldorp, M.E.; Mahajan, R.; Mehta, A.B.; Meredith, M.; Twomey, D.; Wong, C.X.; Hendriks, J.M.; Abhayaratna, W.P.; et al. Cost-Effectiveness and Clinical Effectiveness of the Risk Factor Management Clinic in Atrial Fibrillation: The CENT Study. JACC Clin. Electrophysiol. 2017, 3, 436–447. [Google Scholar] [CrossRef]
- Kaitani, K.; Inoue, K.; Kobori, A.; Nakazawa, Y.; Ozawa, T.; Kurotobi, T.; Morishima, I.; Miura, F.; Watanabe, T.; Masuda, M.; et al. Efficacy of Antiarrhythmic Drugs Short-Term Use After Catheter Ablation for Atrial Fibrillation (EAST-AF) trial. Eur. Heart J. 2016, 37, 610–618. [Google Scholar] [CrossRef]
- Leong-Sit, P.; Roux, J.-F.; Zado, E.; Callans, D.J.; Garcia, F.; Lin, D.; Marchlinski, F.E.; Bala, R.; Dixit, S.; Riley, M.; et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circ. Arrhythmia Electrophysiol. 2009, 120, 1036–1040. [Google Scholar] [CrossRef]
- Duytschaever, M.; Demolder, A.; Phlips, T.; Sarkozy, A.; El Haddad, M.; Taghji, P.; Knecht, S.; Tavernier, R.; Vandekerckhove, Y.; De Potter, T. PulmOnary vein isolation with vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): Results from a multicentre randomized trial. Eur. Heart J. 2018, 39, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Taddei, S. How to evaluate microvascular organ damage in hypertension: Assessment of endothelial function. High Blood Press Cardiovasc Prev. 2011, 18, 163–167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakynthinos, G.E.; Tsolaki, V.; Oikonomou, E.; Pantelidis, P.; Gialamas, I.; Kalogeras, K.; Zakynthinos, E.; Vavuranakis, M.; Siasos, G. Unveiling the Role of Endothelial Dysfunction: A Possible Key to Enhancing Catheter Ablation Success in Atrial Fibrillation. Int. J. Mol. Sci. 2024, 25, 2317. https://doi.org/10.3390/ijms25042317
Zakynthinos GE, Tsolaki V, Oikonomou E, Pantelidis P, Gialamas I, Kalogeras K, Zakynthinos E, Vavuranakis M, Siasos G. Unveiling the Role of Endothelial Dysfunction: A Possible Key to Enhancing Catheter Ablation Success in Atrial Fibrillation. International Journal of Molecular Sciences. 2024; 25(4):2317. https://doi.org/10.3390/ijms25042317
Chicago/Turabian StyleZakynthinos, George E., Vasiliki Tsolaki, Evangelos Oikonomou, Panteleimon Pantelidis, Ioannis Gialamas, Konstantinos Kalogeras, Epaminondas Zakynthinos, Manolis Vavuranakis, and Gerasimos Siasos. 2024. "Unveiling the Role of Endothelial Dysfunction: A Possible Key to Enhancing Catheter Ablation Success in Atrial Fibrillation" International Journal of Molecular Sciences 25, no. 4: 2317. https://doi.org/10.3390/ijms25042317
APA StyleZakynthinos, G. E., Tsolaki, V., Oikonomou, E., Pantelidis, P., Gialamas, I., Kalogeras, K., Zakynthinos, E., Vavuranakis, M., & Siasos, G. (2024). Unveiling the Role of Endothelial Dysfunction: A Possible Key to Enhancing Catheter Ablation Success in Atrial Fibrillation. International Journal of Molecular Sciences, 25(4), 2317. https://doi.org/10.3390/ijms25042317









