Discovery of Novel Biomarkers with Extended Non-Coding RNA Interactor Networks from Genetic and Protein Biomarkers
Abstract
1. Introduction
2. Results
2.1. Proposed Filtering Protocol
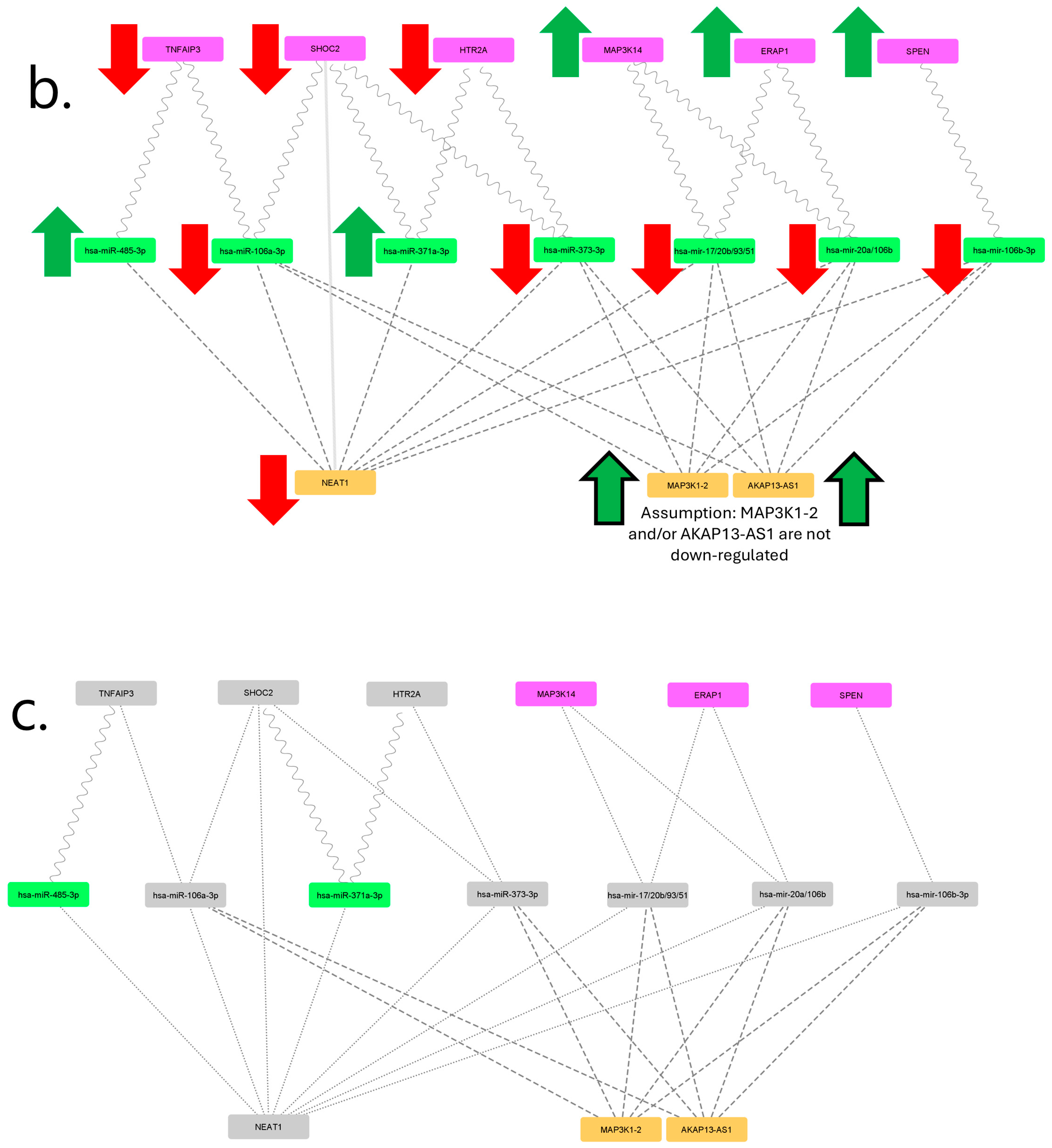
2.2. Filtered mRNA-miRNA–Protein Network
2.3. Gene Ontology Analysis
3. Discussion
4. Materials and Methods
4.1. Interaction Databases
4.2. Biomarker Data
4.3. Gene Ontology
- Biological Process (7 February 2024);
- Cellular Component (7 February 2024);
- Molecular Function (7 February 2024).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, M.A.; Clark, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.; Fiscon, G.; Licursi, V.; Bizzarri, D.; D’Antò, T.; Farina, L.; Paci, P. A paradigm shift in medicine: A comprehensive review of network-based approaches. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194416. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Huntley, R.P.; Sitnikov, D.; Orlic-Milacic, M.; Balakrishnan, R.; D’Eustachio, P.; Gillespie, M.E.; Howe, D.; Kalea, A.Z.; Maegdefessel, L.; Osumi-Sutherland, D.; et al. Guidelines for the functional annotation of microRNAs using the Gene Ontology. RNA 2016, 22, 667–676. [Google Scholar] [CrossRef]
- Lin, X.Y.; Yuen, K.Y.; Chen, H.L.; Shen, M.N.; Huang, Y.; Huang, Q.W.; Liu, Y.; Xu, L.H. Comprehensive Analysis of Potential Biomarkers of Acute Lymphoblastic Leukemia in Children by Using a Competing Endogenous RNA Network. J. Oncol. 2022, 2022, 4563523. [Google Scholar] [CrossRef]
- Mourtzi, N.; Siahanidou, T.; Tsifintaris, M.; Karamichali, E.; Tasiopoulou, A.; Sertedaki, A.; Pesmatzoglou, M.; Kapetanaki, A.; Liosis, G.; Baltatzis, G.; et al. lncRNA NORAD is consistently detected in breastmilk exosomes and its expression is downregulated in mothers of preterm infants. Int. J. Mol. Med. 2021, 48, 216. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, S.; Zou, G.; Ding, X. Paeoniflorin inhibits proliferation and migration of psoriatic keratinocytes via the lncRNA NEAT1/miR-3194-5p/Galectin-7 axis. Anticancer Drugs 2022, 33, e423–e433. [Google Scholar] [CrossRef]
- Shi, R.; Ma, R.; Jiang, X.; Tang, X.; Gong, Y.; Yu, Z.; Shi, Y. Implications of LncRNAs and CircRNAs in psoriasis: A review. RNA Biol. 2023, 20, 334–347. [Google Scholar] [CrossRef]
- Fierro, C.; Gatti, V.; La Banca, V.; De Domenico, S.; Scalera, S.; Corleone, G.; Fanciulli, M.; De Nicola, F.; Mauriello, A.; Montanaro, M.; et al. The long non-coding RNA NEAT1 is a ΔNp63 target gene modulating epidermal differentiation. Nat. Commun. 2023, 14, 3795. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Zheng, X.; Jin, H. Immune Regulation of TNFAIP3 in Psoriasis through Its Association with Th1 and Th17 Cell Differentiation and p38 Activation. J. Immunol. Res. 2020, 2020, 5980190. [Google Scholar] [CrossRef] [PubMed]
- Sahlol, N.Y.; Mostafa, M.S.; Madkour, L.A.E.; Salama, D.M. Low TNFAIP3 expression in psoriatic skin promotes disease susceptibility and severity. PLoS ONE 2019, 14, e0217352. [Google Scholar] [CrossRef] [PubMed]
- van der Burgt, I. Noonan syndrome. Orphanet J. Rare Dis. 2007, 2, 4. [Google Scholar] [CrossRef]
- Yue, Y.; Zhong, M.; An, X.; Feng, Q.; Lai, Y.; Yu, M.; Zhang, X.; Liao, Z.; Chen, M.; Dong, J.; et al. Serotonin (5-HT) 2A Receptor Involvement in Melanin Synthesis and Transfer via Activating the PKA/CREB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6111. [Google Scholar] [CrossRef]
- Arakawa, A.; Reeves, E.; Vollmer, S.; Arakawa, Y.; He, M.; Galinski, A.; Stöhr, J.; Dornmair, K.; James, E.; Prinz, J.C. ERAP1 Controls the Autoimmune Response against Melanocytes in Psoriasis by Generating the Melanocyte Autoantigen and Regulating Its Amount for HLA-C*06:02 Presentation. J. Immunol. 2021, 207, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Gremel, G.; Ryan, D.; Rafferty, M.; Lanigan, F.; Hegarty, S.; Lavelle, M.; Murphy, I.; Unwin, L.; Joyce, C.; Faller, W.; et al. Functional and prognostic relevance of the homeobox protein MSX2 in malignant melanoma. Br. J. Cancer 2011, 105, 565–574. [Google Scholar] [CrossRef]
- Marin Lovrić, J.; Filipović, N.; Znaor, L.; Rančić, A.; Petričević, J.; Kunac, N.; Šoljić, V.; Saraga-Babić, M.; Vukojević, K. Expression of Cell Cycle Markers and Proliferation Factors during Human Eye Embryogenesis and Tumorigenesis. Int. J. Mol. Sci. 2022, 23, 9421. [Google Scholar] [CrossRef]
- Alsisi, G.G.; Alsisi, M.H.; Alhowaish, A.K.; Alshammari, W.S. Erythema Dyschromicum Perstans After Adalimumab Treatment. Cureus 2022, 14, e32264. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Molina-Leyva, A.; Garrido-TorresPuchol, V.; Ruiz-Carrascosa, J. Recalcitrant psoriasis responding to new bilogic drug: Ustekinumab. Ann. Saudi Med. 2013, 33, 632–633. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gutiérrez-González, E.; Batalla, A.; de la Mano, D. Multiple lentigines in areas of resolving psoriatic plaques after ustekinumab therapy. Dermatol. Online J. 2014, 20, 22338. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, W.; He, J.; Ke, Y.; Yue, H.; Wang, C.; Zhang, H.; Gu, J.; Hu, W.; Fu, W.; et al. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am. J. Hum. Genet. 2012, 90, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Prasad, H.; Visweswariah, S.S. Impaired Intestinal Sodium Transport in Inflammatory Bowel Disease: From the Passenger to the Driver’s Seat. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 277–292. [Google Scholar] [CrossRef]
- Du, J.; Wei, Y.; Liu, L.; Wang, Y.; Khairova, R.; Blumenthal, R.; Tragon, T.; Hunsberger, J.G.; Machado-Vieira, R.; Drevets, W.; et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc. Natl. Acad. Sci. USA 2010, 107, 11573–11578. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas Ruiz, S.M.; Eldar-Finkelman, H. Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Front. Mol. Neurosci. 2021, 14, 792364. [Google Scholar] [CrossRef]
- Fornai, F.; Longone, P.; Cafaro, L.; Kastsiuchenka, O.; Ferrucci, M.; Manca, M.L.; Lazzeri, G.; Spalloni, A.; Bellio, N.; Lenzi, P.; et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 2052–2057. [Google Scholar] [CrossRef]
- Morrison, K.E.; Dhariwal, S.; Hornabrook, R.; Savage, L.; Burn, D.J.; Khoo, T.K.; Kelly, J.; Murphy, C.L.; Al-Chalabi, A.; Dougherty, A.; et al. Lithium in patients with amyotrophic lateral sclerosis (LiCALS): A phase 3 multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2013, 12, 339–345. [Google Scholar] [CrossRef]
- van Eijk, R.P.A.; Jones, A.R.; Sproviero, W.; Shatunov, A.; Shaw, P.J.; Leigh, P.N.; Young, C.A.; Shaw, C.E.; Mora, G.; Mandrioli, J.; et al. Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials. Neurology 2017, 89, 1915–1922. [Google Scholar] [CrossRef]
- Hu, J.H.; Zhang, H.; Wagey, R.; Krieger, C.; Pelech, S.L. Protein kinase and protein phosphatase expression in amyotrophic lateral sclerosis spinal cord. J. Neurochem. 2003, 85, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Leystra-Lantz, C.; Strong, M.J. Upregulation of GSK3beta expression in frontal and temporal cortex in ALS with cognitive impairment (ALSci). Brain Res. 2008, 1196, 131–139. [Google Scholar] [CrossRef]
- Martin, S.A.; Souder, D.C.; Miller, K.N.; Clark, J.P.; Sagar, A.K.; Eliceiri, K.W.; Puglielli, L.; Beasley, T.M.; Anderson, R.M. GSK3β Regulates Brain Energy Metabolism. Cell Rep. 2018, 23, 1922–1931.e1924. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Skelt, L.; Notaro, A.; Highley, J.R.; Fox, A.H.; La Bella, V.; Buchman, V.L.; Shelkovnikova, T.A. ALS-linked FUS mutations confer loss and gain of function in the nucleus by promoting excessive formation of dysfunctional paraspeckles. Acta Neuropathol. Commun. 2019, 7, 7. [Google Scholar] [CrossRef]
- Binothman, N.; Hachim, I.Y.; Lebrun, J.J.; Ali, S. CPSF6 is a Clinically Relevant Breast Cancer Vulnerability Target: Role of CPSF6 in Breast Cancer. EBioMedicine 2017, 21, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Wang, D.; Ping, W.; Sun, W. The roles of CPSF6 in proliferation, apoptosis and tumorigenicity of lung adenocarcinoma. Aging 2022, 14, 9300–9316. [Google Scholar] [CrossRef]
- An, W.; Yu, F. Silencing of CPSF7 inhibits the proliferation, migration, and invasion of lung adenocarcinoma cells by blocking the AKT/mTOR signaling pathway. Open Med. 2022, 17, 1655–1663. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Deng, S.; Zhao, H.; Ye, Y.; Zhang, S.; Huang, X.; Bai, R.; Zhuang, L.; Zhou, Q.; et al. CSTF2 mediated mRNA N. Nat. Commun. 2023, 14, 6334. [Google Scholar] [CrossRef]
- Pan, S.; Liu, R.; Wu, X.; Ma, K.; Luo, W.; Nie, K.; Zhang, C.; Meng, X.; Tong, T.; Chen, X.; et al. LncRNA NEAT1 mediates intestinal inflammation by regulating TNFRSF1B. Ann. Transl. Med. 2021, 9, 773. [Google Scholar] [CrossRef]
- Liu, R.; Tang, A.; Wang, X.; Chen, X.; Zhao, L.; Xiao, Z.; Shen, S. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int. J. Mol. Med. 2018, 42, 2903–2913. [Google Scholar] [CrossRef]
- Ali, M.A.; Shaker, O.G.; Gomaa Ali, E.S.; Ezzat, E.M.; Khalifa, A.A.; Hassan, E.A.; Habib, M.A.; Ahmed, H.M.; Dawood, A.F.A.; Mohamed, E.A. Expression profile of serum LncRNAs. Noncoding RNA Res. 2024, 9, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bhattcharjee, D.; Misra, S.; Saha, A.; Bhattacharyya, N.P.; Ghosh, A. Increase in MEG3, MALAT1, NEAT1 significantly predicts the clinical parameters in patients with rheumatoid arthritis. Per. Med. 2020, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, H.; Teng, X.; Hao, X.; Yan, X.; Tang, Y.; Zhang, W.; Wang, Y.; Zhang, P.; Li, Y.; et al. NPInter v5.0: ncRNA interaction database in a new era. Nucleic Acids Res. 2023, 51, D232–D239. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Coutin, N. biogridr: BioGRID R API. 2015. Available online: https://github.com/npjc/biogridr (accessed on 19 February 2024).
- Jezernik, G.; Mičetić-Turk, D.; Potočnik, U. Molecular Genetic Architecture of Monogenic Pediatric IBD Differs from Complex Pediatric and Adult IBD. J. Pers. Med. 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Jezernik, G.; Gorenjak, M.; Potočnik, U. Gene Ontology Analysis Highlights Biological Processes Influencing Non-Response to Anti-TNF Therapy in Rheumatoid Arthritis. Biomedicines 2022, 10, 1808. [Google Scholar] [CrossRef]
- Krušič, M.; Jezernik, G.; Potočnik, U. Gene Ontology Analysis Highlights Biological Processes Influencing Responsiveness to Biological Therapy in Psoriasis. Pharmaceutics 2023, 15, 2024. [Google Scholar] [CrossRef]
- Glavač, D.; Mladinić, M.; Ban, J.; Mazzone, G.L.; Sámano, C.; Tomljanović, I.; Jezernik, G.; Ravnik-Glavač, M. The Potential Connection between Molecular Changes and Biomarkers Related to ALS and the Development and Regeneration of CNS. Int. J. Mol. Sci. 2022, 23, 11360. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
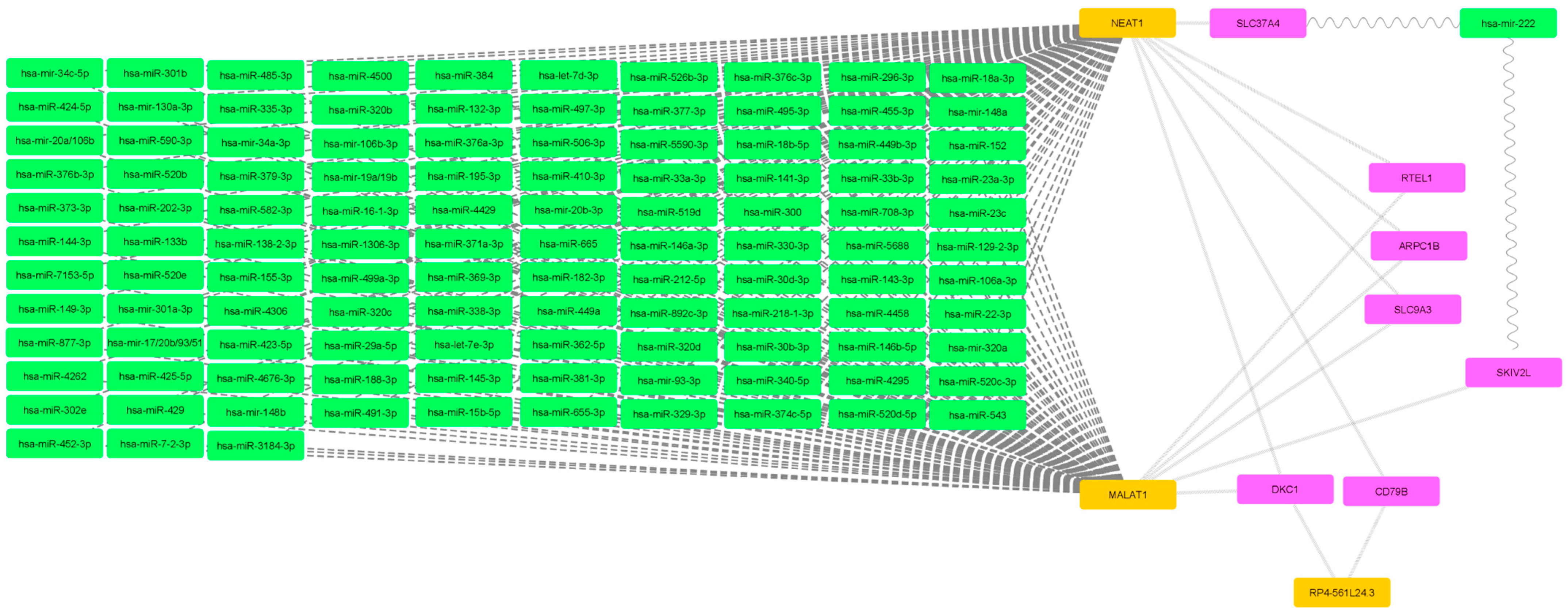

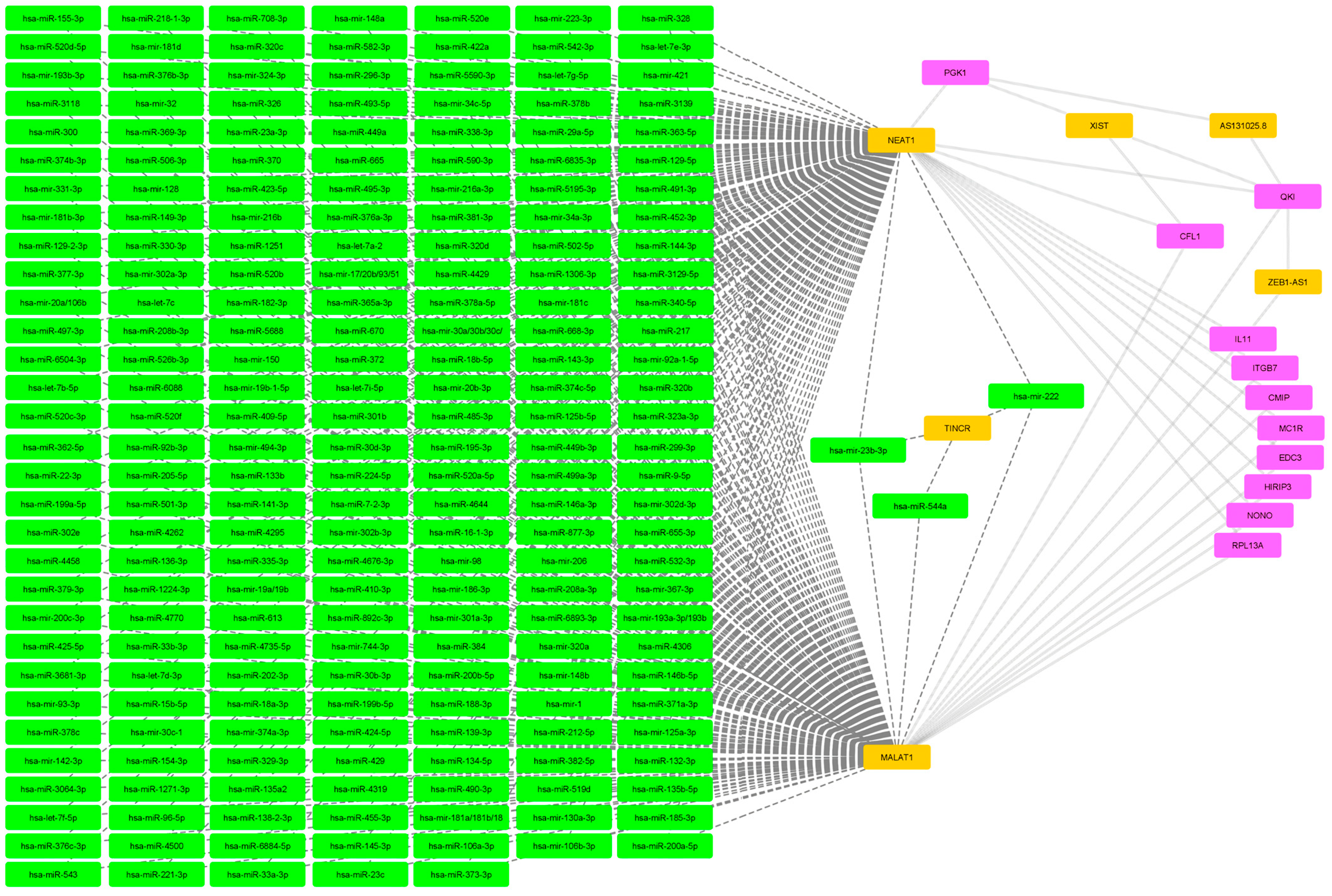
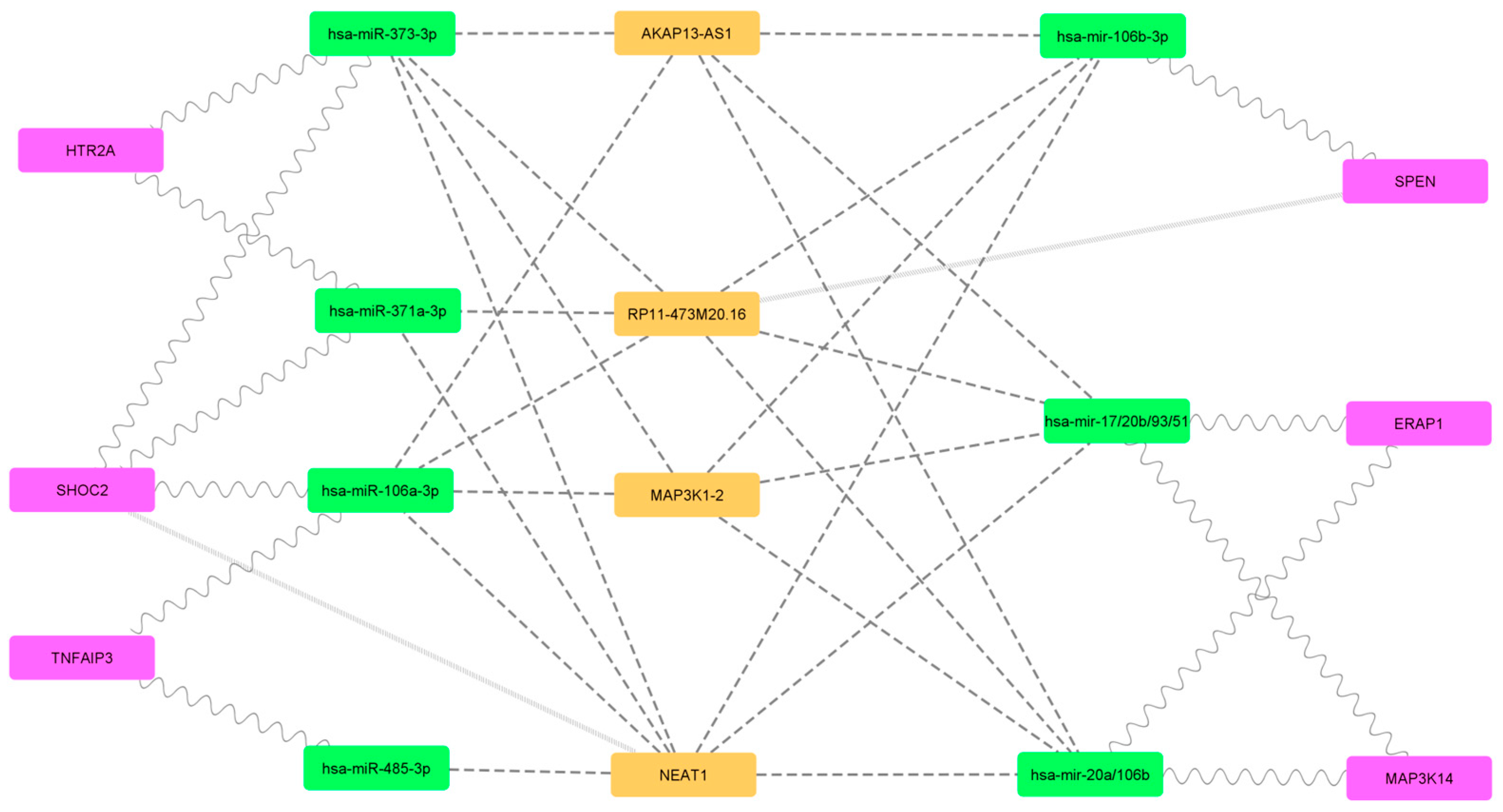



| miRNA | hsa-mir-744-3p, hsa-miR-340-5p, hsa-miR-323a-3p, hsa-miR-208b-3p, hsa-miR-208a-3p, hsa-miR-138-2-3p, hsa-miR-1251, hsa-mir-98, hsa-miR-9-5p, hsa-mir-93-3p, hsa-miR-892c-3p, hsa-miR-877-3p, hsa-miR-7-2-3p, hsa-miR-708-3p, hsa-miR-6893-3p, hsa-miR-6884-5p, hsa-miR-6835-3p, hsa-miR-668-3p, hsa-miR-665, hsa-miR-655-3p, hsa-miR-6504-3p, hsa-miR-6088, hsa-miR-590-3p, hsa-miR-5688, hsa-miR-5590-3p, hsa-miR-543, hsa-miR-542-3p, hsa-miR-526b-3p, hsa-miR-520f, hsa-miR-520e, hsa-miR-520d-5p, hsa-miR-520c-3p, hsa-miR-520b, hsa-miR-520a-5p, hsa-miR-519d, hsa-miR-5195-3p, hsa-miR-506-3p, hsa-miR-502-5p, hsa-miR-501-3p, hsa-miR-495-3p, hsa-mir-494-3p, hsa-miR-493-5p, hsa-miR-491-3p, hsa-miR-485-3p, hsa-miR-4770, hsa-miR-4735-5p, hsa-miR-4676-3p, hsa-miR-455-3p, hsa-miR-452-3p, hsa-miR-4500, hsa-miR-449b-3p, hsa-miR-449a, hsa-miR-4458, hsa-miR-4429, hsa-miR-4319, hsa-miR-4306, hsa-miR-429, hsa-miR-4295, hsa-miR-4262, hsa-miR-425-5p, hsa-miR-424-5p, hsa-miR-423-5p, hsa-miR-422a, hsa-mir-421, hsa-miR-410-3p, hsa-miR-409-5p, hsa-miR-384, hsa-miR-382-5p, hsa-miR-381-3p, hsa-miR-379-3p, hsa-miR-378c, hsa-miR-378b, hsa-miR-378a-5p, hsa-miR-377-3p, hsa-miR-376c-3p, hsa-miR-376b-3p, hsa-miR-376a-3p, hsa-miR-374c-5p, hsa-miR-374b-3p, hsa-mir-374a-3p, hsa-miR-373-3p, hsa-miR-372, hsa-miR-371a-3p, hsa-miR-370, hsa-miR-369-3p, hsa-miR-3681-3p, hsa-mir-367-3p, hsa-miR-365a-3p, hsa-miR-363-5p, hsa-miR-362-5p, hsa-mir-34c-5p, hsa-mir-34a-3p, hsa-miR-33b-3p, hsa-miR-33a-3p, hsa-miR-338-3p, hsa-miR-335-3p, hsa-mir-331-3p, hsa-miR-329-3p, hsa-miR-328, hsa-miR-326, hsa-mir-32, hsa-mir-324-3p, hsa-miR-320d, hsa-miR-320c, hsa-miR-320b, hsa-mir-320a, hsa-miR-3139, hsa-miR-3129-5p, hsa-miR-3118, hsa-miR-30b-3p, hsa-miR-3064-3p, hsa-miR-302e, hsa-mir-302d-3p, hsa-mir-302b-3p, hsa-mir-302a-3p, hsa-miR-301b, hsa-mir-301a-3p, hsa-miR-300, hsa-miR-29a-5p, hsa-miR-299-3p, hsa-miR-296-3p, hsa-miR-23c, hsa-mir-23b-3p, hsa-miR-23a-3p, hsa-miR-224-5p, hsa-miR-22-3p, hsa-mir-223-3p, hsa-mir-222, hsa-miR-221-3p, hsa-miR-218-1-3p, hsa-miR-217, hsa-mir-216b, hsa-mir-216a-3p, hsa-miR-212-5p, hsa-mir-206, hsa-miR-205-5p, hsa-miR-202-3p, hsa-mir-200c-3p, hsa-miR-200b-5p, hsa-miR-200a-5p, hsa-mir-19a/19b, hsa-miR-199b-5p, hsa-miR-199a-5p, hsa-miR-195-3p, hsa-mir-193b-3p, hsa-mir-193a-3p/193b, hsa-miR-18b-5p, hsa-miR-18a-3p, hsa-mir-186-3p, hsa-miR-185-3p, hsa-miR-182-3p, hsa-mir-181d, hsa-mir-181c, hsa-mir-181b-3p, hsa-mir-181a/181b/18, hsa-miR-16-1-3p, hsa-miR-155-3p, hsa-miR-154-3p, hsa-mir-150, hsa-miR-149-3p, hsa-mir-148b, hsa-mir-148a, hsa-miR-146b-5p, hsa-miR-146a-3p, hsa-miR-144-3p, hsa-miR-143-3p, hsa-mir-142-3p, hsa-miR-141-3p, hsa-miR-139-3p, hsa-miR-136-3p, hsa-miR-135b-5p, hsa-miR-135a2, hsa-miR-134-5p, hsa-miR-133b, hsa-miR-132-3p, hsa-mir-130a-3p, hsa-miR-129-2-3p, hsa-miR-129-5p, hsa-mir-128, hsa-miR-125b-5p, hsa-mir-125a-3p, hsa-let-7i-5p, hsa-let-7g-5p, hsa-let-7f-5p, hsa-let-7e-3p, hsa-let-7d-3p, hsa-let-7c, hsa-let-7b-5p, hsa-let-7a-2, hsa-miR-1306-3p, hsa-miR-1271-3p, hsa-miR-1224-3p, hsa-miR-532-3p, hsa-miR-499a-3p, hsa-miR-490-3p, hsa-miR-188-3p, hsa-mir-19b-1-5p, hsa-miR-582-3p, hsa-miR-92b-3p, hsa-miR-497-3p, hsa-mir-20b-3p, hsa-miR-330-3p, hsa-mir-106b-3p, hsa-miR-145-3p, hsa-miR-15b-5p, hsa-mir-1, hsa-miR-30d-3p, hsa-mir-30c-1, hsa-miR-106a-3p, hsa-miR-96-5p, hsa-mir-92a-1-5p, hsa-mir-30a/30b/30c/, hsa-mir-20a/106b, hsa-mir-17/20b/93/51, hsa-miR-4644, hsa-miR-670, hsa-miR-613, hsa-miR-544a |
| Interacting lncRNA | AC009133.12, EPB41L4A-AS1, GAS5, H1FX-AS1, JPX, LENG8-AS1, LINC00324, OIP5-AS1, PCBP1-AS1, RP11-473I1.9, SLC25A5-AS1, SMAD5-AS1, SNHG1, SNHG10, SNHG11, SNHG3, SNHG5, SNHG6, SNHG7, SNHG9, XIST, SNHG16, SNHG15, DHRS4-AS1, ZFAS1, HOXB-AS1, ZNF503-AS2, MAPKAPK5-AS1, LINC00662, NDUFB2-AS1, ADPGK-AS1, SNHG17, IQCH-AS1, PROSER2-AS1, HOXA-AS2, LINC00240, LINC00910, LIMD1-AS1, TMPO-AS1, AC092159.2, LINC00511, STARD4-AS1, CIRBP-AS1, TINCR, CRNDE, SNHG11, SNORD109A |
| Interacting mRNA or proteins | DDX3X, DNMT1, CDK6, CPSF7, CPSF6, CSTF2, RBM6, UPF1 |
| Name | Type | Biomarker Type (Source) | Number of Markers | Citation |
|---|---|---|---|---|
| Pediatric IBD and IBD-like genes | Genetics | DNA (PBMC) | 84 | [46] |
| Adult complex IBD loci | Genetics | DNA (PBMC) | 164 | [46] |
| Rheumatoid arthritis | Pharmacogenomics | DNA and RNA (PBMC) Proteins (PBMC, blood serum) | 390 | [47] |
| Psoriasis | Pharmacogenomics | DNA (PBMC) | 64 | [48] |
| Amyotrophic lateral sclerosis | mRNA-miRNA-lncRNA network | RNA (spinal cord tissue) | 38 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jezernik, G.; Glavač, D.; Skok, P.; Krušič, M.; Potočnik, U.; Gorenjak, M. Discovery of Novel Biomarkers with Extended Non-Coding RNA Interactor Networks from Genetic and Protein Biomarkers. Int. J. Mol. Sci. 2024, 25, 10210. https://doi.org/10.3390/ijms251810210
Jezernik G, Glavač D, Skok P, Krušič M, Potočnik U, Gorenjak M. Discovery of Novel Biomarkers with Extended Non-Coding RNA Interactor Networks from Genetic and Protein Biomarkers. International Journal of Molecular Sciences. 2024; 25(18):10210. https://doi.org/10.3390/ijms251810210
Chicago/Turabian StyleJezernik, Gregor, Damjan Glavač, Pavel Skok, Martina Krušič, Uroš Potočnik, and Mario Gorenjak. 2024. "Discovery of Novel Biomarkers with Extended Non-Coding RNA Interactor Networks from Genetic and Protein Biomarkers" International Journal of Molecular Sciences 25, no. 18: 10210. https://doi.org/10.3390/ijms251810210
APA StyleJezernik, G., Glavač, D., Skok, P., Krušič, M., Potočnik, U., & Gorenjak, M. (2024). Discovery of Novel Biomarkers with Extended Non-Coding RNA Interactor Networks from Genetic and Protein Biomarkers. International Journal of Molecular Sciences, 25(18), 10210. https://doi.org/10.3390/ijms251810210








