Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review
Abstract
1. Introduction
2. Synthesis
2.1. Synthesis of Sodalite and Basic Sodalite
2.2. Synthesis of Other Sodalite-Related Materials
3. Crystallography, Crystal Chemistry, and Intra-Cage Chemical Conversions of Sodalite-Type Compounds
3.1. SOD-Type Topology of Zeolites and Related Materials
3.2. Framework Composition, Elasticity and Porosity
3.3. Inorganic and Organic Guest Species in the Sodalite Cages
3.4. Symmetry, Structure Modulations, and Twinning
3.5. Intracage Reactions Involving H, S, C, N, B, Cl, and Mn in Sodalite-Type Compounds
4. Properties and Application of Sodalite-Related Materials
4.1. Ion Exchange; Immobilization of Heavy Metals and Redionuclides
4.2. Hydrogen and Methane Storage
4.3. Gas Sorption; Sodalite-Related Membranes
4.4. Color Centers, Optical Properties, and Luminescence; Sodalite-Based Pigments
4.5. Application of Sodalite-Related Materials for Organic Synthesis
4.6. Electroconductivity
4.7. Magnetic Properties
4.8. Thermal Properties and Thermal Conversions
4.9. Vibrational Spectroscopy of Sodalite-Group Minerals
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Kind of the Framework | Formula | Synthesis Conditions (If Available) | References |
|---|---|---|---|
| [Al–Si–O] | Na8(Al6Si6O24)(HS)2 | Synthesized hydrothermally from kaolin, Na2S·10H2O, and NaOH at 180 °C to 230 °C for 16–24 h. | [26] |
| Na8(Al6Si6O24)(NO3)2 | First, dry gel was prepared by the addition of a solution of aluminum nitrate ethanol solution to a solution of tetraethylorthosilicate. The mixture was held at 70 °C for 17 h, then dried in air at 120 °C for 2 h. The final product was synthesized hydrothermally from the dry gel, at 90 °C for 3 h in the presence of NaOH. | [387] | |
| Na8(Al6Si6O24)(OH)(CO3)0.5·3H2O | Synthesized in the Na2O-SiO2-Al2O3-Na2CO3-H2O system in the presence of 8M NaOH solution, under hydrothermal conditions, at 80 °C. | [372] | |
| Na8(Al6Si6O24)(CO3) | Synthesized in a two-step anion exchange reaction at 700–800 °C in a CO2 atmosphere using basic nitrite sodalite as a starting material. | [372] | |
| Na8(Al6Si6O24)Cl2−xIx | Prepared hydrothermally using zeolite A as a starting material with different proportions of NaCl and NaI, along with appropriate amount of NaOH, at 100 °C for one week. The products obtained were then heated at 300 °C to evaporate adsorbed water. | [388] | |
| Na8(Al6Si6O24)I2 | Prepared hydrothermally from AgI and Zeolite 13 X at 150 °C for 48 h. | [186] | |
| Rb2K6(Al6Si6O24)(OH)2 | Ion exchange of the Na analogue with K and Rb nitrate solutions. | [339] | |
| Na8(Al6Si6O24)(WO4)2 | Synthesized by heating a mixture of zeolite X and WO3 at 660 °C to 800 °C in air for 12 h. | [389] | |
| Na5.12Ca1.92[Al6Si6O24] (MoO4)1.52 | Prepared using a mixture of nepheline gel, anorthite gel, and Na2MoO4·2H2O; first reacted at 1200 °C and 4 kbar during 5–6 h and then at 750 °C and p = 3 kbar during 10 days. | [129] | |
| Na7.68[(Al,Si)12O24] [(Mo0.65W0.35)O4]0.78·H2O | Prepared using a mixture of nepheline gel, Na2MoO4·2H2O, and Na2WO4∙ 2H2O at 750 °C and 3 kbar. | [129] | |
| Na8(Al6Si6O24)(ReO4)2 | Synthesized in equilibrium with Na–Fe-rich aluminosilicate melt and Re metal at 1100 °C and 500 MPa. | [179] | |
| Na8−x(Al6Si6O24)(ReO4, CO3,SO4,MnO4,WO4,Cl)2−y | Synthesized from zeolite 4A in binary solutions containing ReO4 and a corresponding competing anion in the presence of NaOH, at 90 °C for 96 h. | [185] | |
| Na8[Al6Si6O24](ReO4,TcO4)2 | Synthesized using hydrothermal methods by treating zeolite 4A with 8 M NaOH in the presence different amounts of sodium perrhenate and/or sodium pertechnetate at 225 °C for 7 days. | [178] | |
| Na8[Al6Si6O24](ReO4)2 | Synthesized hydrothermally from NaAlO2, Na2SiO3·9H2O, and NaReO4, in the presence of NaOH at 175 °C for 24 h. | [130] | |
| Na8[Al6Si6O24](ReO4,NO3)2 | Synthesized hydrothermally from zeolite A in sodium hydroxide, nitrate, and perrhenate solutions at 90 °C for 24 h. | [181,182] | |
| Na8[Al6Si6O24](SO4)·nH2O | Obtained by precipitation of the desilication product, LTA zeolite, in synthetic Bayer liquor prepared from gibbsite, at 90 °C, in the presence of Na2SO4. | [390] | |
| Ca8(Al12O24)(SO4)2 | Synthesized in a solid-state reaction at 1300 °C using calcite, Al2O3, and gypsum as the starting materials. | [116] | |
| Na7+x[Si5+2xAl7–2xO24](AlF6)x(H2O)4–4x·4H2O (x = 0–1). | Synthesized hydrothermally in the Si–Al–Na–H–O–F system at temperatures of 400–800 °C and H2O pressures of 1–2 kbar. The crystal structure is solved. | [131] | |
| Na7.38[Si6.74Al5.26O24] (AlF6)0.70(H2O)4–4x·4.88H2O | Synthesized hydrothermally from a mixture of nepheline gel and NaF, in the presence of natural sodalite added as a seed, at 650 °C and H2O pressure of 2 kbar. The crystal structure is solved. | [132] | |
| Ag8[Al6Si6O24]I2 | Iodine was captured from the vapour phase using a silver exchanged zrolite and converted to sodalite in hot-isostatic pressing canisters. | [190] | |
| Na8(Al6Si6O24)(NO2)2 | Prepared hydrothermally from kaolin, sodium nitrite, and NaOH at 570 K and 0.1 GPa. | [174] | |
| Na7.7(Al6Si6O24)6(MnO4)1.7 ·0.8H2O | Synthesized hydrothermally from Na2SiO3, NaAlO2, and NaMnO4·H2O in a molar ratio of 1:1:2.6, in the presence of NaOH, at 80 °C, for 48 h. | [156] | |
| Na6Mn2[Al6Si6O24](SO4)2 | Prepared by reacting zeolite A, Na6(Al6Si6O24), with MnSO4 as a compacted mixed-powder monolith at 650 °C under air. | [391] | |
| Na6Zn2[Al6Si6O24](SO4)2 | Prepared in a solid-state reaction of a charge containing appropriate amounts of ZnSO4·H2O and zeolite A at 700 °C for 8 h. | [369] | |
| Li8[Al6Si6O24]Cl2 | No data. | [392] | |
| Li7.4Na0.6[Al6Si6O24](ClO4)2 | Prepared by ion exchange of the sodium perchlorate sodalite (synthesized hydrothermally from the sodium silicate glass, NaClO4 and NaOH at 120 °C under reflux, for 24 h) with LiCl solution at 110 °C for 48 h. | [393] | |
| Na8(Al6Si6O24)Br2 | Synthesized hydrothermally from fly ash and NaBr at 500–550 °C for 2 h. | [394] | |
| (Na,Ag)8[Al6Si6O24]X2 (X = Cl, Br, I) | Aqueous exchanges were used to replace Na+ by Ag+ in hydrothermally synthesized Na8[Al6Si6O24]X2 sodalites (X = Cl, Br, I). | [395,396] | |
| Na8[Si6Al6O24]6·(ClO4)2 | Synthesized by addition of sodium aluminate solution to a concentrated solution of sodium perchlorate and sodium silicate. | [377] | |
| Na8[Si6Al6O24]6·(ClO4)2 | Synthesized by addition of sodium aluminate solution to a concentrated solution of sodium perchlorate and sodium silicate. | [152] | |
| K7.7Na0.3[Al6Si6O24](ClO4)2 | Prepared by ion exchange of the sodium perchlorate sodalite (synthesized hydrothermally from the sodium silicate glass, NaClO4 and NaOH at 120 °C under reflux, for 24 h) with KCl solution at 110 °C for 48 h. | [393] | |
| Na8[Al6Si6O24](XO4)2 (X = Cl, Mn, Re) | Partly prepared hydrothermally from NaAlO2, Na2SiO3 and corresponding Na salts (perchlorate, permanganate, and perrhenate), at 230 °C for perrhenate. | [183,397] | |
| Na8[Al6Si6O24](XO4)2 (X = Cl, Mn) | Synthesized hydrothermally from the sodium silicate glass and NaClO4 or NaMnO4 in the presence of NaOH at 120 °C under reflux, for 24 h. | [393] | |
| Na8−xLix[Al6Si6O24](ClO3)2−y(OH)y | Obtained by aqueous cation exchange of a pure sodium analogue. | [398] | |
| K8−xNax[Al6Si6O24](XO3)2−y(OH)y (X = Cl, Br) | Obtained by aqueous cation exchange of a pure sodium analogue. | [398] | |
| Na8[Al6Si6O24](XO3)2−y(OH)y (X = Cl, Br) | Synthesized by a low-temprtature hydrothermal method. | [398] | |
| Li8−xNax[Al6Si6O24](BrO3)2−y(OH)y | Obtained by aqueous cation exchange of a pure sodium analogue. | [398] | |
| Na8[Si6Al6O24]6·(IO3)2 | Synthesized by hydrothermal treatment from coal fly ash, NaIO3, and NaOH at 100 °C | [197] | |
| Na8[AlxGa6−xSi6O24](NO2)2 0 ≤ x ≤ 6) | Prepared hydrothermally from stoichiometric quantities of Na2SiO3, NaAlO2, NaGaO2, and NaNO2, in the presence of NaOH, at 200 °C for 48 h. | [399] | |
| K8[Al6Si6O24]Cl2 | No data. | [392] | |
| Ag6[Al6Si6O24] | Prepared by ion exchange of hydrous hydroxysodalite, Na8[AlSiO4]6(OH)2·2H2O, with 0.7 M aqueous AgNO3 solution in two 24 h cycles, at 70 °C. | [400] | |
| Tl6[Al6Si6O24] | Prepared by ion exchange of hydrous hydroxysodalite, Na8[AlSiO4]6(OH)2·2H2O, with 1 M aqueous TlNO3 solution at 100 °C. | [400] | |
| (C4H12N)[Al2Si10O24] | Synthesized under hydrothermal conditions in the absence of metal cations. | [401] | |
| Na8[Si6Al6O24]6·(MnO4)2 | Synthesized by addition of sodium aluminate solution to a concentrated solution of sodium permanganate and sodium silicate. | [156] | |
| Na8(Al6Si6O24)(BH4)2 | Synthesized hydrothermally from NaAlO2, Na2SiO3, and NaBH4 at 60 °C for 12 h. | [217] | |
| ~K4Na4(Al6Si6O24)(BH4)2 | Produced by cation exchange between the Na8(Al6Si6O24)(BH4)2 sodalite-type compound and 1 mol/l aqueous solution of KNO3 at 200 °C for 48 h. | [175] | |
| Na8[AlSiO4]6(BH4)2 | Synthesized hydrothermally from kaolinite, NaOH, and NaBH4, at 100 °C, for 24 h. | [219] | |
| Na8[AlSiO4]6(N3)2 | Synthesized hydrothermally from zeolite A and NaN3, at 160 °C, for two weeks. | [162] | |
| Na8[Al6Si6O24]I2 | Prepared hydrothermally from zeolite 4A, kaolinite, and meta-kaolin, as well as colloidal silica and NaAlO2 at 140–180 °C. Decreasing the Al/Si ratio by half increased the crystallization of basic cancrinite. | [402] | |
| [Al–Fe3+–Si–O] | (Na,Fe2+)8−x[(Si,Al,Fe3+)12O24] (OH,Cl)2·nH2O? | Synthesized from Fe- and Al-containing gels, prepared from Fe(NO3)3·9H2O and AlCl3·6H2O in hydrochloric acid solution, and Aldrich type waterglass at 88 °C for 2–2.5 h. | [403] |
| [Al–P–O] | (C3H7NO)2[Al6P6O24]·nH2O | Synthesized hydrothermally. | [153,404,405,406,407] |
| [Mg–Al–P–O] | (C4H12N)2[Mg2Al4P6O24] | Prepared hydrothermally from a gel based on tetramethylammonium hydroxide, MgO, Al2O3, and P2O5. | [408] |
| [Al–O] | Ca8[Al12O24]S2 | Obtained by reduction of the corresponding sulfate material, Ca4[Al96O192]·(SO4)16 via an intra-cage reaction. | [409,410] |
| Ca8[Al12O24]X2 (X = O, Te) | Prepared by solid-state reaction of a stoichiometric mixture of CaO, Al2O3, and CaTe (for X = Te) at 1100 °C for 48. | [411] | |
| Cd8[Al12O24]O2 | Prepared by solid-state reaction of a stoichiometric mixture of CdO and Al2O3 at 1100 °C for 48. | [411] | |
| Ca8[Al12O24](XO4)2 (X = S, Mo, W, Cr) | Synthesized in solid-state reactions. | [154,363,412,413,414,415] | |
| Sr8[Al12O24]X2 (X = S, Te) | Prepared by reduction of the corresponding sulfate and tellurite materials via intra-cage reactions. | [409] | |
| Sr8[Al12O24](TeO3)2 | Prepared using solid-state synthesis from the stoichiometric mixture of oxides at 1200 °C for 48 h. | [416] | |
| Sr8[Al12O24]Te2 | Prepared by the reduction of Sr8[Al12O24](TeO3)2 in a stream of hydrogen at 850 °C for 8 h. | [416] | |
| Sr8[Al12O24](XO4)2 (X = Mo, W, Cr) | Crystals partly grown from the B2O3 flux. | [417,418,419,420] | |
| Cd8[Al12O24]Te2 | Prepared by solid-state reaction of a stoichiometric mixture of CdO, Al2O3 and CdTe at 1100 °C for 48. | [411] | |
| Pb8REE4[Al12O24]O8 (REE = Ho, Lu) | Crystals grown from the melts prepared from corresponding oxides and PbF2 at 850 °C for 7 days. | [421] | |
| [Si–O] | SiO2 | Prepared by thermal treatment of ethylene glycol silica sodalite at 680 °C. | [422] |
| SiO2 | Obtained by heating the AcOH-treated double four-ring lamellar 8TMA(Si8O20)·xH2O precursor (TMA = tetramethylammonium) at 800 °C for one week. | [423] | |
| SiO2 | Silica sodalite formed as a result of the interlayer condensation of the layered silicate RUB-15 during a heat treatment at 800 °C in an inert atmosphere. | [424] | |
| (C2H4(OH)2)2[Si12O24] | Partly synthesized from essentially nonaqueous medium in which ethylene glycol is acting both as a solvent and as a structure-directing agent. | [425,426,427,428] | |
| (C3H6O3)2[Si12O24] | Synthesized from essentially nonaqueous medium in which 1,3,5-trioxane is acting both as a solvent and as a structure-directing agent. | [117,354] | |
| □2−x(C3H6O3)x[Si12O24] | Synthesized from essentially nonaqueous medium in which 1,3,5-trioxane is acting both as a solvent and as a structure-directing agent. | [354] | |
| [Al–Ga–Si–O] | Na6[Al1–yGaySiO4]6(H2O)8 (0 ≤ y ≤ 1) | Prepared from Na6[Al1–yGaySiO4]6(OH·H2O)x(H2O)8–4x sodalites by NaOH/H2O exchange methods carried out in acidic aqueous media at pH = 5.5–6.6. | [429] |
| [Ga–Si–O] | Na6[GaSiO4]6(H2O)8 | Obtained from Na8[GaSiO4]6I2 by a hydrothermal transformation process in the excess of water, at 200 °C for 24 h. | [429] |
| Na8[Ga6Si6O24]X2 (X = Cl, Br, I) | Prepared under hydrothermal conditions by heating aqueous solutions of NaOH, Ga2O3, Na2SiO3, and NaX for at 160 °C for 100 h. | [352] | |
| Li8[Ga6Si6O24]X2 (X = Cl, Br, I) | Obtained by replacement of Na+ by Li+ in sodalite of composition Na8[GaSiO4]6×2, where X is a halide, in nitrate melt at 240 °C. | [430] | |
| Na6[Ga6Si6O24]·8H2O | Synthesized hydrothermally from corresponding sodium salts. | [364,377,378,379,380] | |
| Na8[Ga6Si6O24]X2 (X = OH, Cl, Br, I) | Synthesized hydrothermally from corresponding sodium salts. | [352,377,431] | |
| Na8[Ga6Si6O24](XO4)2 (X = Cl, Mn) | Synthesized hydrothermally from corresponding sodium salts. | [162,432] | |
| Na8[Ga6Si6O24](XO3)2 (X = Cl, N, C) | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Ga6Si6O24](NO2)2 | Synthesized hydrothermally from corresponding sodium salts. | [353,377,433] | |
| Na8[Ga6Si6O24](BH4)2 | Synthesized under mild hydrothermal conditions from NaAlGeO4 and NaBH4 at 80–120 °C for 12–48 h. Increasing temperatures above 120 °C favor the decomposition of the tetrahydroborate anions before encapsulation within the sodalite framework cavities. | [434] | |
| Na8[Ga6Si6O24](SCN)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Ga6Si6O24](HCO2)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| K4.88Na3.12[Ga6Si6O24](NO2)2 | Obtained by cation exchange of a pure sodium analogue at 100 °C. | [435] | |
| Ag5.98Na2.02[Ga6Si6O24](NO2)2 | Obtained by cation exchange of a pure sodium analogue at 100 °C. | [435] | |
| [Be–Si–O] | Na8[Be3Si9O24]Cl2 | Prepared by a solid-state synthesis method from a sodium silicate glass, Na6Si9O21, stoichiometric quantity of BeO, and a four-fold excess of NaCl at 800 °C for 48 h. | [436] |
| Mn8[Be6Si6O24]X2 (X = S, Se, Te) | Synthesized by solid state reactions of the constituent oxides and MnX at 1000−1100 °C. | [118] | |
| Cd8[Be6Si6O24]X2 (X = S, Se, Te) | Synthesized by hydrothermal reaction of CdO, BeO, SiO2, and X at 750 °C and 2 kbar. | [437] | |
| [Be–Si–O] | Na6[Zn6As6O24]·8H2O | Synthesized hydrothermally from Na2HSO4·7H2O, NaOH, and Zn(NO3)2 at 70 °C for 12 h. | [378,438] |
| [Zn–P–O] | Na6[Zn6P6O24]·Br2? (No data on the chemical composition are provided) | Synthesized from a solution containing Zn(NO3)2, (H3PO4), NaBr, and NaOH at pH 7. | [439] |
| [Ga–As–O] | BaGe8As14 | Synthesized via solid-state reactions. No details provided. | [151] |
| [Zn–P–O] | Na6[Zn6P6O24]·8H2O | Synthesized hydrothermally from ZnO, H3PO4, and NaOH at 50 °C for 12 h. | [438] |
| [Be–P–O] | Li8[Be6P6O24]X2 (X = Cl, Br) | Synthesized at 550 °C and 0.3447 GPa. | [119,440] |
| [Al–Ge–O] | Na8(Al6Ge6O24)X2 (X = Cl, Br, I) | Crystallyzed by solvothermal method at 120 °C, using 50% ethanol + 50% water as a solvent, and NaAlO2, GeO2, NaX, and NaOH as rectants. | [128] |
| Na8(Al6Ge6O24)(ClO3)2 | Synthesized hydrothermally from NaAlO2, GeO2, NaClO3, and NaOH at 120 °C for 120 h. | [158] | |
| Na8(Al6Ge6O24)6(SCN)2 | Synthesized hydrothermally from GeO2 and NaAlO2 in the presence of NaOH, at 150 °C for three days. | [161] | |
| Na8(Al6Ge6O24)Br2 | Synthesized from NaAlO2, GeO2, SiO2, and NaOH under hydrothermal conditions at 320–450°. | [150] | |
| Li8[Al6Ge6O24]X2 (X = Cl, Br, I) | Obtained by replacement of Na+ by Li+ in sodalite of composition Na8[AlGeO4]6×2, where X is a halide, in nitrate melt at 240 °C. | [430] | |
| Na6[Al6Ge6O24]·8H2O | Synthesized hydrothermally from corresponding sodium salts. | [377,378] | |
| Na6+x[Al6Ge6O24](OH)x·3H2O | Synthesized by reacting Al2O3, GeO2, and NaOH solution under mild hydrothermal conditions. | [441] | |
| Na8[Al6Ge6O24]X2 (X = OH, Cl, Br, I) | Synthesized hydrothermally from sodium aluminate, GeO2 and corresponding sodium salts. | [120,377,442,443] | |
| Na8[Al6Ge6O24](S2/S3)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Al6Ge6O24](Se2)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Al6Ge6O24](XO4)2 (X = Cl, S, Mn) | Synthesized hydrothermally from sodium aluminate, GeO2, and corresponding sodium salts. | [377,444] | |
| Na8[Al6Ge6O24](NO2)2 | Synthesized hydrothermally in the system Na2O–GeO2–Al2O3–NaNO2–H2O at 200 °C. | [445] | |
| Na8[Al6Ge6O24](XO3)2 (X = Cl, Br, N, C, Se) | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Al6Ge6O24](SCN)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Al6Ge6O24](HCO2)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Al6Ge6O24](H3C2O2)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| K8−xNax[Al6Ge6O24](ClO4)2 | Obtained by cation exchange of corresponding pure Na analogue. | [444] | |
| [Zn–Si–O] | (C4H12N)2−xNax[Zn1.6Si10.4O24] | Obtained from a silicate gel with included Zn2+. | [446] |
| [Ga–Co–Si–O] | (C4H10N2)2[Ga2Co4P6O24] | Synthesized hydrothermally from C4H10N2, Ga2O3, and Co phosphate. | [447,448] |
| [Be–Ge–O] | Mn8[Be6Ge6O24]X2 (X = S, Se) | Synthesized by solid state reactions of the constituent oxides and MnX at 1000−1100 °C in sealed quartz ampoules. | [118] |
| [Al–Co–P–O] | (C4H10N2)2[Al2Co4P6O24] | Synthesized either hydrothermally from C4H10N2, Al2O3, and Co phosphate or by an ionothermal method (an ionic liquid as both solvent and structure-directing agent has been used). | [447,449] |
| [Zn–Ga–P–O] | (C4H12N)2[Zn2Ga4P6O24] | Synthesized hydrothermally from C4H12N, Ga2O3, and Zn phosphate. | [448] |
| [Zn–Ga–As–O] | (C4H12N)2[Zn2Ga4As6O24] | Synthesized hydrothermally from C4H12N, Ga2O3, and Zn arsenate. | [448,450] |
| (C4H10N2)2[Zn4Ga2As6O24] | Synthesized hydrothermally from C4H10N2, Ga2O3, and Zn arsenate. | [450] | |
| [Zn–Al–As–O] | (C4H12N)2[Zn2Al4As6O24] | Obtained by successive adding of As2O5 + C4H12N, Al nitrate, Zn arsenate, and HNO3 to a mixed water–ethylene glycol solvent to pH of 5.25 and heating at 150 °C for 4 days. | [450] |
| [Ga–Ge–O] | Na8−x(Ga6Ge6O24) [B(OH)4]2−x(H2O)x (?) | Synthesized hydrothermally, using NaGaGeO4 and Na[B(OH)4] as the starting materials, at 120 °C for 24 h. | [451] |
| (Am)2[Ga2Ge10O24] (Am = ammonium cation) | Synthesized hydrothermally from GeO2, Ga(NO3)3·nH2O, and corresponding amine at 180 °C for 8 days. | [452] | |
| Na6[Ga6Ge6O24]·xH2O | Prepared from sodium germanate and sodium gallate in aqueous sodium tetramethylammonium hydroxide solution, in the presence of Na2SO4, at 100 °C. | [377,453] | |
| Na8[Ga6Ge6O24](OH)2·6H2O | Prepared from sodium germanate and sodium gallate in aqueous sodium tetramethylammonium hydroxide solution, in the presence of Na2SO4, at 100–150 °C. | [453] | |
| Na8[Ga6Ge6O24]X2 (X = Cl, Br, I) | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Ga6Ge6O24](ClO4)2 | Synthesized hydrothermally from corresponding sodium salts. | [377] | |
| Na8[Ga6Ge6O24](NO2)2 | Synthesized starting with the formation of gallogermanate basic-hydro-sodalite under hydrothermal conditions, at 100 °C, followed by hydrothermal treatment in concentrated NaNO2 solution. | [454] | |
| Na8[Ga6Ge6O24](BH4)2 | Separate suspensions of NaGaGeO4 and NaBH4 in sodium hydroxide solutions were combined before the hydrothermal treatment in the presence of NaOH at 150 °C for 4 h. | [370] | |
| [Al–Be–Si–O] | Na8[Al2Be2Si8O24]Cl2 | Prepared by a solid-state synthesis method from a sodium silicate glass, Na6Si8O19, stoichiometric quantities of BeO and Al2O3, and a four-fold excess of NaCl at 800 °C for 48 h. | [436] |
| Na8[Al4BeSi7O24]X2 (X = Cl, Br) | Prepared by a solid-state synthesis method from a sodium silicate glass, Na6Si7O17, stoichiometric quantities of BeO and Al2O3, and a four-fold excess of NaCl or NaBr at 800 °C for 48 h. | [436] | |
| [Be–As–O] | Li8[Be6As6O24]Cl2 | No data. | [119] |
| [B–O] | (Zn,Ga)8(B12O24)(Se,P)2 | Prepared by a solid-state synthesis method from a mixture of ZnB4O7, ZnO, ZnSe, and GaP at 900–950 °C for 12–36 h. | [121] |
| Co[B12O24]S2 | Synthesized from a stoichiometric mixture of B2O3, CoO, and CoS at 920 °C. | [455] | |
| Zn[B12O24]S2 | Synthesized from a stoichiometric mixture of B2O3, ZnO, and ZnS at 920 °C. | [455] | |
| (Zn,Mn)4[B6O12]O | Synthesized via solid-state reaction from ZnO, H3BO3 and MnCO3, first at 500 °C for 5 h in air atmosphere, then at 700 and 900 °C for 7 h in a reduced carbon atmosphere. | [304] | |
| [B–Ge–O] | Cs2[B2Ge10O24] | Obtained by the solvothermal reaction of H3BO3, GeO2, CsCl, HF, pyridine, and H2O at 200 °C for 7 days. | [456] |
| Organic and metal–organic | [(CH3)2NH2]4[In6(BTC)12]2[(M3OH)4(H2O)36][(In2MO)4(BTC)4(H2O)12]·(solvent)x (M = Mg, Mn, Co, Ni, and Cd). | Synthesized by a single-step low-temperature method of simultaneous formation of sodalite-type frameworks and covalent attachment of transition- and non-transition-metal clusters in a solution containing 1,3,5-benzenetricarboxylate (BTC). | [457,458] |
| Zn(im)2 (zinc imidazolate) | Synthesized mechanochemically at room temperature. | [459,460] | |
| Zn(im)2·nC60 (im = imidazolate, n = 0.15–1) | Fullerene C60 was entrapped in the cages of the sodalite-type imidazolate framework by mechanochemical processing. | [460] | |
| Zn(im)2 (zinc imidazilate) | Synthesized via solvent-assisted linker exchange of ZIF-8 (Zn(Me-im)2). | [461] | |
| Zn(5-mtz)(2-eim) | Obtained by heating a mixture of Zn(CH3CO2)2·2H2O, 5-methyltetrazole (5-mtz) and 2-ethylimidazole (2-eim) in dimethylformamide and ethanol to 120 °C. | [329,330,331] | |
| Zn(Me-im)2 | Synthesized from methyl imidazol (Me-im) and a Zn salt at room temperature under aqueous conditions for 10 min. | [462] | |
| Co(Me-im)2 | Synthesized from methyl imidazol (Me-im) and a Co salt at room temperature under aqueous conditions for 10 min. | [462] | |
| [(Cu4Cl)3(H0.5BTT)8(H2O)12]·3MeOH·9DMF | Prepared from CuCl2, 5,5′-(1,4-phenylene) bis(1H-tetrazole) (H3BTT), methanol, and dimethyl formamide (DMF). | [316] | |
| Cu3[(Cu4Cl)3(TPB-3tz)8]2·11CuCl2·8H2O·120DMF | Obtained in the reactions of 2,4,6-tri-p-(tetrazol-5-yl)-phenyl-s-triazine (H3TPB-3tz) with CuCl2in the presence of dimethyl formamide (DMF). | [205] | |
| Mn3[(Mn4Cl)3(TPT-3tz)8]2 ·25H2O·15CH3OH·95DMF | Obtained in the reactions of 2,4,6-tri-p-(tetrazol-5-yl)-phenyl-s-triazine (H3TPB-3tz) with MnCl2 in the presence of dimethyl formamide (DMF). | [205] | |
| Cu3[(Cu4Cl)3(TPT-3tz)8]2·x(solvent) | Obtained in the reactions of 2,4,6-tri-p-(tetrazol-5-yl)- phenyl-s-triazine (H3TPB-3tz) with CuCl2 in different solvents. | [205] | |
| ML2 (M = PdII or CuII, L = 2-hydroxypyrimidine or 4-hydroxypyrimidine) | Prepared by a solid–liquid sorption method. | [233] | |
| (Et2NH2)3[(Cu4Cl)3(TTCA)8] ·26DEF | Synthesized in the solvothermal reaction of triphenylene-2,6,10-tricarboxylic acid (H3TTCA)9 with CuCl2·2H2O in N,N-diethylformamide (DEF) at 110 °C for 48 h. | [214] | |
| Li3[(Cu4Cl)3(TTCA)8]·26DEF | Ion exchange of (Et2NH2)3[(Cu4Cl)3(TTCA)8]·26DEF with Li+. | [214] | |
| Fe3[(Fe4Cl)3(BTT)8]2·22DMF ·32DMSO·11H2O | Synthesized in the reaction between FeCl2 and H3BTT·2HCl (BTT3− = 1,3,5-benzenetristetrazolate) in a mixture of dimethylformamide (DMF) and dimethylsulfoxide (DMSO). | [215] | |
| [Zn(HL)]·DMA | Solvothermally synthesized based on an N-rich aromatic ligand L = 4,5-di(1H-tetrazol-5-yl)-2H-1,2,3-triazole in dimethyl acetate (DMA) | [230] |
References
- Peterson, R.C. The structure of hackmanite, a variety of sodalite, from Mont St-Hilaire, Quebec. Can. Mineral. 1983, 21, 549–552. [Google Scholar]
- Hassan, I.; Grundy, H.D. The crystal structures of sodalite-group minerals. Acta Crystallogr. Sect. B Struct. Sci. 1984, 40, 6–13. [Google Scholar] [CrossRef]
- Löhn, J.; Schulz, H. Strukturverfeinerung am gestörten Haüyn, (Na5K1Ca2)Al6Si6O24(SO4)1.5. Neues Jahrb. Mineral. Abh. 1968, 109, 201–210. (In German) [Google Scholar]
- Hassan, I.; Grundy, H.D. The crystal structure of haüyne at 273 and 153 K. Can. Mineral. 1991, 29, 123–130. [Google Scholar]
- Hassan, I.; Peterson, R.C.; Grundy, H.D. The structure of lazurite, ideally Na6Ca2(Al6Si6O24)S2, a member of the sodalite group. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1985, 41, 827–832. [Google Scholar] [CrossRef]
- IMA, List of Mineral Species. 2020. Available online: http://cnmnc.main.jp/ (accessed on 21 August 2024).
- Sapozhnikov, A.N.; Tauson, V.L.; Lipko, S.V.; Shendrik, R.Y.; Levitskii, V.I.; Suvorova, L.F.; Chukanov, N.V.; Vigasina, M.F. On the crystal chemistry of sulfur-rich lazurite, ideally Na7Ca(Al6Si6O24)(SO4)(S3)–·nH2O. Am. Mineral. 2020, 106, 226–234. [Google Scholar] [CrossRef]
- Hassan, I.; Grundy, H.D. The structure of nosean, ideally Na8[Al6Si6O24]SO4∙H2O. Can. Mineral. 1989, 27, 165–172. [Google Scholar]
- Sahl, K. Refinement of the crystal structure of bicchulite, Ca2[Al2SiO6](OH)2. Z. Krist. Cryst. Mater. 1980, 152, 13–22. [Google Scholar] [CrossRef]
- Uchida, E.; Iiyama, J.T. On kamaishilite, Ca2Al2SiO6(OH)2, a new mineral dimorphous (tetragonal) with bicchulite from the Kamaishi mine, Japan. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1981, 57, 239–243. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Xu, H.; Xu, H.; Jacobs, R.; Morgan, D. Valleyite: A new magnetic mineral with the sodalite-type structure. Am. Mineral. 2019, 104, 1238–1245. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Kaneva, E.V.; Cherepanov, D.I.; Suvorova, L.F.; Levitsky, V.I.; Ivanova, L.A.; Reznitsky, L.Z. Vladimirivanovite, Na6Ca2[Al6Si6O24](SO4,S3,S2,Cl)2·H2O, a new mineral of sodalite group. Geol. Ore Depos. 2012, 54, 557–564. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Rybakov, V.B.; Pautov, L.A. Crystal structure of a new natural tetramethyammonium aluminosilicate [N(CH3)4][Si2(Si0.5Al0.5)O6]2. Proc. USSR Acad. Sci. 1991, 317, 884–887. (In Russian) [Google Scholar]
- Pautov, L.A.; Karpenko, V.Y.; Sokolova, E.V.; Ignatenko, K.I. Tsaregorodtsevite N(CH3)4[Si2(Si0.5Al0.5)O6]2—A new mineral. Zap. Vses. Miner. Obsh. 1993, 121, 128–135. (In Russian) [Google Scholar]
- Hassan, I.; Grundy, H.D. The crystal structure and thermal expansion of tugtupite, Na8[Al2Be2Si8O24]Cl2. Can. Mineral. 1991, 29, 385–390. [Google Scholar]
- Hassan, I.; Grundy, H.D. The crystal structures of helvite group minerals, (Mn,Fe,Zn)8(Be6Si6O24)S2. Am. Mineral. 1985, 70, 186–192. [Google Scholar]
- Chukanov, N.V.; Zubkova, N.V.; Pekov, I.V.; Shendrik, R.Y.; Varlamov, D.A.; Vigasina, M.F.; Belakovskiy, D.I.; Britvin, S.N.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Sapozhnikovite, Na8(Al6Si6O24)(HS)2, a new sodalite-group mineral from the Lovozero alkaline massif, Kola Peninsula. Mineral. Mag. 2022, 86, 49–59. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Bolotina, N.B.; Chukanov, N.V.; Shendrik, R.Y.; Kaneva, E.V.; Vigasina, M.F.; Ivanova, L.A.; Tauson, V.L.; Lipko, S.V. Slyudyankaite, Na28Ca4(Si24Al24O96)(SO4)6(S6)1/3(CO2)·2H2O, a new sodalite group mineral from the Malo-Bystrinskoe lazurite deposit, Baikal Lake area, Russia. Am. Mineral. 2023, 108, 1805–1817. [Google Scholar] [CrossRef]
- Dunn, P.E. Genthelvite and the helvine group. Mineral. Mag. 1976, 40, 627–636. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Chukanov, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Tauson, V.L.; Lipko, S.V.; Belakovskiy, D.I.; Levitskii, V.I.; Suvorova, L.G.; Ivanova, L.A. Lazurite: Validation as a mineral species with the formula Na7Ca(Al6Si6O24)(SO4)S3•–·H2O and new data. Geol. Ore Depos. 2022, 64, 470–475. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Schäfer, C.; Pekov, I.V.; Shendrik, R.Y.; Vigasina, M.F.; Belakovskiy, D.I.; Britvin, S.N.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Bolotinaite, (Na6K−)(Al6Si6O24)F·4H2O, a new sodalite-group mineral from the Eifel paleovolcanic region, Germany. Mineral. Mag. 2022, 86, 920–928. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K. Structural chemistry, IR spectroscopy, properties, and genesis of natural and synthetic microporous cancrinite- and sodalite-related materials: A review. Microporous Mesoporous Mater. 2021, 323, 111098. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Vigasina, M.F.; Zubkova, N.V.; Pekov, I.V.; Schäfer, C.; Kasatkin, A.V.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Extra-framework content in sodalite-group minerals: Complexity and new aspects of its study using infrared and Raman spectroscopy. Minerals 2020, 10, 363. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Pekov, I.V.; Sapozhnikov, A.N.; Shcherbakov, V.D.; Varlamov, D.A. Crystal chemistry, isomorphism, and thermal conversions of extra-framework components in sodalite-group minerals. Minerals 2022, 12, 887. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Vigasina, M.F.; Shendrik, R.Y.; Varlamov, D.A.; Pekov, I.V.; Zubkova, N.V. Nature and isomorphism of extra-framework components in cancrinite- and sodalite-related minerals: New data. Minerals 2022, 12, 729. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Shchipalkina, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Tauson, V.L.; Lipko, S.V.; Varlamov, D.A.; Shcherbakov, V.D.; Sapozhnikov, A.N.; Kasatkin, A.V.; et al. Isomorphism and mutual transformations of S-bearing components in feldspathoids with microporous structures. Minerals 2022, 12, 1456. [Google Scholar] [CrossRef]
- NChukanov, V.; Sapozhnikov, A.N.; Shendrik, R.Y.; Vigasina, M.F.; Steudel, R. Spectroscopic and crystal-chemical features of sodalite-group minerals from gem lazurite deposits. Minerals 2020, 10, 1042. [Google Scholar] [CrossRef]
- NBolotina, B.; Sapozhnikov, A.N.; Chukanov, N.V.; Vigasina, M.F. Structure modulations and symmetry of lazurite-related sodalite-group minerals. Crystals 2023, 13, 768. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Heflik, W.; Pieczka, A.; Sikorska, M.; Dąbrowa, Ł. The transformation of nepheline and albite into sodalite in pegmatitic mariupolite of the Oktiabrski Massif (SE Ukraine). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 837–845. [Google Scholar] [CrossRef]
- Schneider, J.B.; Jenkins, D.M. Stability of sodalite relative to nepheline in NaCl–H2O brines at 750 °C: Implications for hydrothermal formation of sodalite. Can. Mineral. 2020, 58, 3–18. [Google Scholar] [CrossRef]
- Hassan, I.; Grundy, H.D. Structure of basic sodalite, Na8Al6Si6O24(OH)2·2H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1983, 39, 3–5. [Google Scholar] [CrossRef]
- Felsche, J.; Luger, S.; Baerlocher, C. Crystal structures of the hydro-sodalite Na6[AlSiO4]6·8H2O and of the anhydrous sodalite Na6[AlSiO4]6. Zeolites 1986, 6, 367–372. [Google Scholar] [CrossRef]
- Fazal, T. High Temperature Studies of Sodalites. Master’s Thesis, University of Birmingham, Birmingham, UK, 2011. [Google Scholar]
- Felsche, J.; Luger, S. Phases and thermal decomposition characteristics of hydro-sodalites Na6+x[AISiO4]6(OH)x·nH2O. Thermochim. Acta 1987, 118, 35–55. [Google Scholar] [CrossRef]
- Sari, M.E.F.; Suprapto, S.; Prasetyoko, D. Direct synthesis of sodalite from kaolin: The influence of alkalinity. Indones. J. Chem. 2018, 18, 607–613. [Google Scholar] [CrossRef]
- Khalifah, S.N.; Cahyawati, M.; Cahyani, D.K.D.; Arifah, A.; Prasetyo, A. Synthesis of sodalite from indonesian kaolin with conventional and alkali fusion method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012006. [Google Scholar] [CrossRef]
- Reyes, C.A.R.; Williams, C.; Mauricio, O.; Alarcón, C. Nucleation and growth process of sodalite and cancrinite from kaolinite-rich clay under low-temperature hydrothermal conditions. Mater. Res. 2013, 16, 424–438. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Yang, X.; Wang, C.; Luo, X. Synthesis of pure sodalite with wool ball morphology from alkali fusion kaolin. Mater. Lett. 2015, 161, 157–159. [Google Scholar] [CrossRef]
- Maia, A.Á.B.; Neves, R.F.; Angélica, R.S.; Pöllmann, H. Synthesis of sodalite from Brazilian kaolin wastes. Clay Miner. 2015, 50, 663–675. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Q.; Liu, Y. Ionothermal synthesis of sodalite from metakaolin. Adv. Mater. Res. 2012, 487, 789–792. [Google Scholar] [CrossRef]
- Wahyuni, T.; Prasetyoko, D.; Suprapto, S.; Qoniah, I.; Bahruji, H.; Dawam, A.; Triwahyono, S.; Jalil, A.A. Direct synthesis of sodalite from indonesian kaolin for adsorption of Pb2+ solution, kinetics, and isotherm approach. Bull. Chem. React. Eng. Catal. 2019, 14, 502–512. [Google Scholar] [CrossRef]
- Esaifan, M.; Warr, L.N.; Grathoff, G.; Meyer, T.; Schafmeister, M.-T.; Kruth, A.; Testrich, H. Synthesis of hydroxy-sodalite/cancrinite zeolites from calcite-bearing kaolin for the removal of heavy metal ions in aqueous media. Minerals 2019, 9, 484. [Google Scholar] [CrossRef]
- Yanga, J.; Li, T.; Bao, X.; Yue, Y.; Liua, H. Mesoporogen-free synthesis of hierarchical sodalite as a solid basecatalyst from sub-molten salt-activated aluminosilicate. Particuology 2020, 48, 48–54. [Google Scholar] [CrossRef]
- Passos, F.A.C.M.; Castro, D.C.; Ferreira, K.K.; Simoes, K.M.A.; Bertolino, L.C.; Barbato, C.N.; Garrido, F.M.S.; Felix, A.A.S.; Silva, F. Synthesis and Characterization of Sodalite and Cancrinite from Kaolin; Ikhmayies, S., Li, B., Carpenter, J.S., Li, J., Hwang, J.Y., Monteiro, S.N., Firrao, D., Zhang, M., Peng, Z., Escobedo Diaz, J.P., et al., Eds.; Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2017; pp. 279–288. [Google Scholar] [CrossRef]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Zong, Y.-B.; Zhao, C.-Y.; Chen, W.-H.; Liu, Z.-B.; Cang, D.-Q. Preparation of hydro-sodalite from fly ash using a hydrothermal method with a submolten salt system and study of the phase transition process. Int. J. Miner. Metall. Mater. 2020, 27, 55–62. [Google Scholar] [CrossRef]
- Shabani, J.M.; Babajide, O.; Oyekola, O.; Petrik, L. Synthesis of hydroxy sodalite from coal fly ash for biodiesel production from waste-derived maggot oil. Catalysts 2019, 9, 1052. [Google Scholar] [CrossRef]
- Luo, J.; Zhangb, H.; Yang, J. Hydrothermal synthesis of sodalite on alkali-activated coal fly ash for removal of lead ions. Procedia Environ. Sci. 2016, 31, 605–614. [Google Scholar] [CrossRef]
- Oh, J.E.; Moon, J.; Mancio, M.; Clark, S.M.; Monteiro, P.J.M. Bulk modulus of basic sodalite, Na8[AlSiO4]6(OH)2·2H2O, a possible zeolitic precursor in coal-fly-ash-based geopolymers. Cem. Concr. Res. 2011, 41, 107–112. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Gitari, W.M.; Balfour, G.; Hums, E. Optimization of hydrothermal synthesis of pure phase zeolite Na-P1 from South African coal fly ashes. J. Environ. Sci. Health A 2012, 47, 337–350. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Wang, J.; Yu, G.; Liu, J.; Du, Y.; Li, Y.; Li, H. Controlled synthesis of Zeolite adsorbent from lowgrade diatomite: A case study of self-assembled sodalite microspheres. J. Environ. Sci. 2020, 91, 92–104. [Google Scholar] [CrossRef]
- Kamyab, S.M.; Williams, C.D.; Badiei, A. Synthesis of sodalite from sepiolite by alkali fusion method and its application to remove Fe3+, Cr3+, and Cd2+ from aqueous solutions. Environ. Eng. Sci. 2020, 37, 689–701. [Google Scholar] [CrossRef]
- Xie, L.; Wang, P.; Li, Z.; Peng, H.; Li, X.; Zhou, Y. Preparation and performance study on sodalite/magnetite from tourmaline. Mater. Sci. Eng. 2018, 381, 012109. [Google Scholar] [CrossRef]
- Esaifan, M.; Hourani, M.; Khoury, H.; Rahier, H.; Wastiels, J. Synthesis of hydroxysodalite zeolite by alkali-activation of basalt powder rich in calc-plagioclase. Adv. Powder Technol. 2017, 28, 473–480. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Feng, L.; Duanmu, C.; Jin, Y.; Hu, T.; Wu, J. Controllable synthesis of sodalite submicron crystals and microspheres from palygorskite clay using a two-step approach. Powder Technol. 2012, 217, 298–303. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. R. Soc. Chem. Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Kim, J.-C.; Choi, M.; Kim, D.-S.; Song, H.J.; Kim, D.-W. Windshield-waste-driven synthesis of hydroxy sodalite. J. Ceram. Soc. Jpn. 2015, 123, 2022–2026. [Google Scholar] [CrossRef]
- Naskar, M.K.; Kundu, D.; Chatterjee, M. Coral-like hydroxy sodalite particles from rice husk ash as silica source. Mater. Lett. 2011, 65, 3408–3410. [Google Scholar] [CrossRef]
- Choy, H.; Lee, S.-R.; Han, Y.-S.; Park, M.; Park, G.-S. Solid–solid transformation mechanism for nanocrystalline sodalite from pillared clay. Chem. Commun. 2003, 15, 1922–1923. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, faujasite and A-type zeolite from 2:1dioctahedral and 2:1:1 trioctahedral clay minerals. A singular review of synthesis methods through laboratory trials at a low incubation temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Mundus, C.; Müller-Warmuth, W.; Buhl, J.-C. Crystallization of a basic sodalite under hydrothermal conditions studied by MAS-NMR, XRD and DTA/DTG. Eur. J. Mineral. 1996, 8, 231–240. [Google Scholar] [CrossRef]
- Fan, W.; Morozumi, K.; Kimura, R.; Yokoi, T.; Okubo, T. Synthesis of nanometer-sized sodalite without adding organic additives. Langmuir 2008, 24, 6952–6958. [Google Scholar] [CrossRef]
- You, S.; Zhang, Y.; Chen, F.; Cao, S.; Zhang, Y. Transformation of NaCaHSiO4 to sodalite and katoite in sodium aluminate solution. Hydrometallurgy 2014, 141, 43–48. [Google Scholar] [CrossRef]
- Naskar, M.K.; Kundu, D.; Chatterjee, M.I. An aqueous-based synthesis of flower-like hydroxy sodalite particles in the presence of cetyltrimethylammonium bromide. Am. Ceram. Soc. 2011, 94, 1643–1646. [Google Scholar] [CrossRef]
- Lapari, S.S.; Ramli, Z.; Triwahyono, S. Effect of different templates on the synthesis of mesoporous sodalite. J. Chem. 2015, 2015, 272613. [Google Scholar] [CrossRef]
- Cui, L.; Han, R.; Yang, L.; Wu, Y.; Pei, R.; Li, F. Synthesis and characterization of mesoporous sodalite and investigation of the effects of inorganic salts on its structure and properties. Microporous Mesoporous Mater. 2020, 306, 110385. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, J.; Zhang, X.; Kong, C.; Chen, H.; Liu, D.; Tsapatsis, M.; Hill, M.R.; Hill, A.J.; Wang, H. Role of ethanol in sodalite crystallization in an ethanol–Na2O–Al2O3–SiO2–H2O system. CrystEngComm 2011, 13, 4714–4722. [Google Scholar] [CrossRef]
- Naskar, M.K.; Kundu, D.; Chatterjee, M. Effect of process parameters on surfactant-based synthesis of hydroxyl sodalite particles. Mater. Lett. 2011, 65, 436–438. [Google Scholar] [CrossRef]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. The mechanism of the sodalite-to-cancrinite phase transformation in synthetic spent Bayer liquor. Microporous Mesoporous Mater. 1999, 31, 287–302. [Google Scholar] [CrossRef]
- Xu, B.; Smith, P.; Wingate, C.; De Silva, L. The effect of calcium and temperature on the transformation of sodalite to cancrinite in Bayer digestion. Hydrometallurgy 2010, 105, 75–81. [Google Scholar] [CrossRef]
- Buhl, J.-C. Enhanced methods of crystallization: The crossover synthesis from gel to melt flow—A case study on sodalites. Microporous Mesoporous Mater. 2016, 236, 13–20. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, R.; Zhang, Z.; Qiu, S. Solventless green synthesis of sodalite zeolite using diatomite as silica source by a microwave heating technique. Inorg. Chem. Commun. 2016, 70, 168–171. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, R.; Li, A.; Huang, W.; Zhang, Z.; Qiu, S. Solvent-free synthesis of nanosized hierarchical sodalite zeolite with a multi-hollow polycrystalline structure. CrystEngComm 2016, 18, 6779–6783. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Pasculli, A.; Di Sabatino, B. Synthesis and characterization of sodalite using natural kaolinite: An analytical and mathematical to simulate the loss in weight of chlorine during the synthesis process. Fresenius Environ. Bull. 2010, 19, 1109–1117. [Google Scholar]
- Pascull, A.; Novembre, D. Phenomenological–mathematical approach in simulating the loss in weight of chlorine during sodalite synthesis. Comput. Geosci. 2012, 42, 110–117. [Google Scholar] [CrossRef]
- Song, Q.; Shen, J.; Yang, Y.; Wang, J.; Yang, Y.; Sun, J.; Jiang, B.; Liao, Z. Effect of temperature on the synthesis of sodalite by crystal transition process. Microporous Mesoporous Mater. 2020, 292, 109755. [Google Scholar] [CrossRef]
- Hayashi, T.; Shiga, H.; Sadakata, M.; Okuboa, T. Hydrothermal growth of millimeter-sized aluminosilicate sodalite single crystals in noble metal capsules. J. Mater. Res. 1998, 13, 891–895. [Google Scholar] [CrossRef]
- Klunk, M.; Dasgupta, S.; Das, M.; Cunha, M.G.; Wander, P.R. Synthesis of sodalite zeolite and adsorption study of crystal violet dye. ECS J. Solid State Sci. Technol. 2019, 8, N144–N150. [Google Scholar] [CrossRef]
- Ding, L.; Yang, H.; Rahimi, P.; Omotoso, O.; Friesen, W.; Fairbridge, C.; Shi, Y.; Ng, S. Solid transformation of zeolite NaA to sodalite. Microporous Mesoporous Mater. 2010, 130, 303–308. [Google Scholar] [CrossRef]
- Greer, H.; Wheatley, P.S.; Ashbrook, S.E.; Morris, R.E.; Zhou, W. Early stage reversed crystal growth of zeolite A and its phase transformation to sodalite. J. Am. Chem. Soc. 2009, 131, 17986–17992. [Google Scholar] [CrossRef]
- Depmeier, W. The sodalite family—A simple but versatile framework structure. Rev. Mineral. Geochem. 2005, 57, 203–240. [Google Scholar] [CrossRef]
- Baur, W.H.; Fischer, R.X. ZeoBase, a Databank for Zeolitetype Crystal Structures; Northwestern University, Evanston and Universitat Bremen: Evanston, IL, USA, 2008. [Google Scholar]
- Fischer, R.X.; Baur, W.H. Symmetry relationships of sodalite (SOD)-type crystal structures. Z. Krist. 2009, 224, 185–197. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 6149. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Khay, I.; Chaplais, G.; Nouali, H.; Ortiz, G.; Marichal, C.; Patarin, J. Assessment of the energetic performances of various ZIFs with SOD or RHO topology using high pressure water intrusion–extrusion experiments. Dalton Trans. 2016, 45, 4392–4400. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhu, Y.; Fu, F.; Wang, L.L.; Wang, J.; Du, H. Theoretical prediction of the mechanical properties of zeolitic imidazolate frameworks (ZIFs). RSC Adv. 2017, 7, 41499–41503. [Google Scholar] [CrossRef]
- Andres-Garcia, E.; López-Cabrelles, J.; Oar-Arteta, L.; Roldan-Martinez, B.; Cano-Padilla, M.; Gascon, J.; Espallargas, G.M.; Kapteijn, F. Cation influence in adsorptive propane/propylene separation in ZIF-8 (SOD) topology. Chem. Eng. J. 2019, 371, 848–856. [Google Scholar] [CrossRef]
- Bose, R.; Ethiraj, J.; Sridhar, P.; Varghese, J.J.; Kaisare, N.S.; Selvam, P. Adsorption of hydrogen and carbon dioxide in zeolitic imidazolate framework structure with SOD topology: Experimental and modelling studies. Adsorption 2020, 26, 1027–1038. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Yang, D.-A.; Ahn, W.-S. Carbon dioxide adsorption over zeolite-like metal organic frameworks (ZMOFs) having a sod topology: Structure and ion-exchange effect. Chem. Eng. J. 2011, 168, 1134–1139. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wang, F.; Zhang, J. Zeolitic Tetrazolate–Imidazolate Frameworks with SOD Topology for Room Temperature Fixation of CO 2 to Cyclic Carbonates. Cryst. Growth Des. 2020, 20, 2866–2870. [Google Scholar] [CrossRef]
- Huh, S.; Kwon, T.-H.; Park, N.; Kim, S.-J.; Kim, Y. Nanoporous In-MOF with multiple one-dimensional pores. Chem. Commun. 2009, 33, 4953–4955. [Google Scholar] [CrossRef]
- Sun, L.; Xing, H.; Liang, Z.; Yu, J.; Xu, R. A 4 + 4 strategy for synthesis of zeolitic metal–organic frameworks: An indium-MOF with SOD topology as a light-harvesting antenna. Chem. Commun. 2013, 49, 11155. [Google Scholar] [CrossRef]
- Alsadun, N.; Mouchaham, G.; Guillerm, V.; Czaban-Jóźwiak, J.; Shkurenko, A.; Jiang, H.; Bhatt, P.M.; Parvatkar, P.; Eddaoudi, M. Introducing a Cantellation Strategy for the Design of Mesoporous Zeolite-like Metal–Organic Frameworks: Zr-sod-ZMOFs as a Case Study. J. Am. Chem. Soc. 2020, 142, 20547–20553. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeoung, S.; Chung, Y.G.; Moon, H.R. Elucidation of flexible metal-organic frameworks: Research progresses and recent developments. Coord. Chem. Rev. 2019, 389, 161–188. [Google Scholar] [CrossRef]
- Gao, M.; Huang, R.-K.; Zheng, B.; Wang, P.; Shi, Q.; Zhang, W.-X.; Dong, J. Large breathing effect in ZIF-65(Zn) with expansion and contraction of the SOD cage. Nat. Commun. 2022, 13, 4569. [Google Scholar] [CrossRef] [PubMed]
- Trojan, I.A.; Semenok, D.V.; Ivanova, A.G.; Kvashnin, A.G.; Zhou, D.; Sadakov, A.V.; Sobolevsky, O.A.; Pudalov, V.M.; Lyubutin, I.S.; Oganov, A.R. High-temperature superconductivity in hydrides. Usp. Fiz. Nauk. 2022, 192, 799–813. [Google Scholar] [CrossRef]
- Semenok, D.V.; Kvashnin, A.G.; Ivanova, A.G.; Svitlyk, V.; Fominski, V.Y.; Sadakov, A.V.; Sobolevskiy, O.A.; Pudalov, V.M.; Troyan, I.A.; Oganov, A.R. Superconductivity at 161 K in thorium hydride ThH10: Synthesis and properties. Mater. Today 2020, 33, 36–44. [Google Scholar] [CrossRef]
- Semenok, D.V.; Kruglov, I.A.; Savkin, I.A.; Kvashnin, A.G.; Oganov, A.R. On Distribution of Superconductivity in Metal Hydrides. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100808. [Google Scholar] [CrossRef]
- Liu, H.; Naumov, I.I.; Hoffmann, R.; Ashcroft, N.W.; Hemley, R.J. Potential high- T c superconducting lanthanum and yttrium hydrides at high pressure. Proc. Natl. Acad. Sci. USA 2017, 114, 6990–6995. [Google Scholar] [CrossRef]
- Heil, C.; di Cataldo, S.; Bachelet, G.B.; Boeri, L. Superconductivity in sodalite-like yttrium hydride clathrates. Phys. Rev. B 2019, 99, 220502. [Google Scholar] [CrossRef]
- Semenok, D.V.; Troyan, I.A.; Ivanova, A.G.; Kvashnin, A.G.; Kruglov, I.A.; Hanfland, M.; Sadakov, A.V.; Sobolevskiy, O.A.; Pervakov, K.S.; Lyubutin, I.S.; et al. Superconductivity at 253 K in lanthanum–yttrium ternary hydrides. Mater. Today 2021, 48, 18–28. [Google Scholar] [CrossRef]
- Du, M.; Song, H.; Zhang, Z.; Duan, D.; Cui, T. Room-Temperature Superconductivity in Yb/Lu Substituted Clathrate Hexahydrides under Moderate Pressure. Research 2022, 2022, 9784309. [Google Scholar] [CrossRef]
- Tsuppayakorn-aek, P.; Phaisangittisakul, N.; Ahuja, R.; Bovornratanaraks, T. High-temperature superconductor of sodalite-like clathrate hafnium hexahydride. Sci. Rep. 2021, 11, 16403. [Google Scholar] [CrossRef]
- Ma, L.; Wang, K.; Xie, Y.; Yang, X.; Wang, Y.; Zhou, M.; Liu, H.; Yu, X.; Zhao, Y.; Wang, H.; et al. High-temperature superconducting phase in clathrate calcium hydride CaH6 up to 215 K at a pressure of 172 GPa. Phys. Rev. Lett. 2022, 128, 167001. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, A.J.; Fässler, T.F.; Linnolahti, M.; Pakkanen, T.A. Structural Principles of Semiconducting Group 14 Clathrate Frameworks. Inorg. Chem. 2011, 50, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Borstad, G.M.; Liu, H.; Guńka, P.A.; Guerette, M.; Dolyniuk, J.-A.; Meng, Y.; Greenberg, E.; Prakapenka, V.B.; Chaloux, B.L.; et al. Carbon-boron clathrates as a new class of sp 3 -bonded framework materials. Sci. Adv. 2020, 6, eaay8361. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xie, T.; Zhang, L.; Wang, P.; Fang, Z. Si2Ge: A New VII-Type Clathrate with Ultralow Thermal Conductivity and High Thermoelectric Property. Sci. Rep. 2020, 10, 3068. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.D. Does porous mean soft? On the elastic behaviour and structural evolution of zeolites under pressure. Z. Krist. Cryst. Mater. 2008, 223, 160–170. [Google Scholar] [CrossRef]
- Bau, W.H.; Fischer, R.X. The flexibility of the T–X–T hinges between the coordination tetrahedra in various zeolitic frameworks: An empirical structural study. Mineral. Petrol. 2023, 117, 165–179. [Google Scholar]
- Li, Z.; Nevitt, M.V.; Ghose, S. Elastic constants of sodalite Na4Al3Si3O12Cl. Appl. Phys. Lett. 1989, 55, 1730–1731. [Google Scholar] [CrossRef]
- Ulian, G.; Valdrè, G. Structural and Elastic Behaviour of Sodalite Na8(Al6Si6O24)Cl2 at High-Pressure by First-Principle Simulations. Minerals 2022, 12, 1323. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sharp, Z.D. Compressibility of sodalite and scapolite. Am. Mineral. 1988, 73, 1120–1122. [Google Scholar]
- Elhachemi, K.; Khellafi, H.; Bendouba, M.; Djebli, A. Quantification of Young's modulus of kaolin, sodalite and nanocomposite based polycaprolactone/sodalite using atomic force microscopy. Mater. Res. Express 2024, 11, 075008. [Google Scholar] [CrossRef]
- Hargis, C.W.; Moon, J.; Lothenbach, B.; Winnefeld, F.; Wenk, H.-R.; Monteiro, P.J.M. Calcium sulfoaluminate sodalite (Ca4Al6O12SO4) crystal structure evaluation and bulk modulus determination. J. Am. Ceram. Soc. 2014, 97, 892–898. [Google Scholar] [CrossRef]
- Fütterer, K.; Depmeier, W.; Altorfer, F.; Behrens, P.; Felsche, J. Compression mechanism in trioxane silica sodalite, [Si12O24]·2C3H6O3. Z. Krist. Cryst. Mater. 1994, 209, 517–523. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T.; Rainford, B.D.; Adroja, D.T. Synthesis, structure, optical properties, and magnetism of the manganese chalcogenide ceryllosilicate and beryllogermanate sodalites. Inorg. Chem. 1997, 36, 5278–5283. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Gier, T.E.; Stucky, G.D. Two lithium chloroberyllo(phosphate/arsenate) sodalites: Li4Cl(BePO4)3 and Li4Cl(BeAsO4)3. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 471–473. [Google Scholar] [CrossRef]
- Johnson, G.M.; Weller, M.T. Synthesis and characterisation of gallium and germanium containing sodalites. Stud. Surf. Sci. Catal. 1997, 105, 269–275. [Google Scholar] [CrossRef]
- Moran, K.L.; Gier, T.E.; Harrison, W.T.A.; Stucky, G.D.; Eckert, H.; Eichele, K.; Wasylishen, R.E. Synthesis and characterization of mixed ZnSe/GaP semiconductor species included in the sodalite structure. J. Am. Chem. Soc. 1993, 115, 10553–10558. [Google Scholar] [CrossRef]
- Moon, D.J.; Lim, W.T. Minireview of pentatomic cations in sodalite cavities. J. Porous Mater. 2020, 27, 563–564. [Google Scholar] [CrossRef]
- Kim, S.H.; Ha, S.G.; Heo, N.H.; Seff, K. A crystallographic study of the decomposition of NO in fully indium exchanged zeolite Y. In+, In3+, and In3+–NO3– complexes with facially coordinating nitrate ions are in supercages. Distorted cubic In4O44+ clusters fill sodalite cavities. J. Phys. Chem. C 2011, 115, 20248–20257. [Google Scholar] [CrossRef]
- Frank, S.M.; Barber, T.L.; Lambregts, M.J. Powder diffraction of sodalite in a multiphase ceramic used to immobilize radioactive waste. Powder Diffr. 2005, 20, 212–214. [Google Scholar] [CrossRef]
- Riley, B.J.; Peterson, J.A.; Chong, S.; Vienna, J.D. Influence of ion site occupancies on the unit cell parameters, specific volumes, and densities of M8(AlSiO4)6X2 sodalites where M = Li, Na, K, Rb, and Ag and X = Cl, Br, and I. Phys. Chem. Miner. 2021, 48, 3. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Taylor, D.; Henderson, C.M.B. A computer model for the cubic sodalite structure. Phys. Chem. Miner. 1978, 2, 325–336. [Google Scholar] [CrossRef]
- Borhade, A.V.; Dholi, A.G.; Tope, D.P.; Wakchaure, S.G. Solvithermal synthesis and crystal structure of aluminogermanate halide sodalites using organic solvent. Indian J. Pure Appl. Phys. 2012, 50, 576–582. [Google Scholar]
- Shchipalkina, N.V.; Zubkova, N.N.; Kotelnikov, A.R.; Koshlyakova, N.N.; Pekov, I.V.; Ksenofontov, D.A.; Britvin, S.N. Crystal chemistry and Raman spectroscopy of two synthetic sodalite-type aluminosilicates with [MoO4]2− and [WO4]2− groups. Phys. Chem. Miner. 2021, 48, 19. [Google Scholar] [CrossRef]
- Mattigod, S.V.; McGrail, B.P.; McCready, D.E.; Wang, L.-Q.; Parker, K.E.; Young, J.S. Synthesis and structure of perrhenate sodalite. Microporous Mesoporous Mater. 2006, 91, 139–144. [Google Scholar] [CrossRef]
- Gramenitskii, E.N.; Kotel’nikov, A.R.; Shchekina, T.I.; Yakubovich, O.V.; Devyatova, V.N.; Zubkov, E.S.; Suk, N.I.; Vigasina, M.F.; Kotel’nikova, Z.A. Composition, structure, and conditions of formation of fluorine-bearing sodalite: Experimental evidence. Geochem. Int. 2018, 56, 521–534. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Kotel’nikov, A.R.; Shchekina, T.I.; Gramenitskiy, E.N.; Zubkov, E.S. New representative in the dodalite dtructure type with extraframework anions [AlF6]3−. Crystallogr. Rep. 2011, 56, 190–197. [Google Scholar] [CrossRef]
- Van Peteghem, J.K.; Burley, B.J. Studies on solid solution between sodalite, nosean and haüyne. Can. Mineral. 1963, 3, 808–816. [Google Scholar]
- Ito, T.; Nakajima, Y.; Morimoto, N.; Sadanaga, R. On the polysynthetic structure of haüyne. Acta Crystallogr. 1966, 21, A55. [Google Scholar]
- Taylor, D. The sodalite group of minerals, Contrib. Mineral. Petrol. 1967, 16, 172–188. [Google Scholar] [CrossRef]
- Schulz, H. Struktur- and Überstrukturuntersuchungen an Nosean-Einkristallen. Z. Kristallogr. 1970, 131, 114–138. (In German) [Google Scholar] [CrossRef]
- Tsuchiya, N.; Takéuchi, Y. Fine texture of hauyne having modulated structure. Z. Kristallogr. 1985, 173, 273–281. [Google Scholar] [CrossRef]
- Hassan, I.; Buseck, P.R. Cluster ordering and antiphase domain boundaries in haüyne. Can. Mineral. 1989, 27, 173–180. [Google Scholar]
- Xu, H.; Veblen, D.R. Transmission electron microscopy study of optically anisotropic and isotropic haüyne. Am. Mineral. 1995, 80, 87–93. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N. Modulated structure of lazurite from deposits in southwestern Pamir. Sov. Phys. Crystallogr. 1992, 37, 470–472. [Google Scholar]
- Sapozhnikov, N.; Ivanov, V.G.; Levitsky, V.I.; Piskunova, L.F. Structural-mineralogical peculiarities of lazurite from the south-western Pamir. Zap. Vses. Mineral. Obsh. 1993, 122, 108–115. (In Russian) [Google Scholar]
- Evsyunin, V.G.; Sapozhnikov, A.N.; Rastsvetaeva, R.K.; Kashaev, A.A. Modulated structure of orthorhombic lazurite. Crystallogr. Rep. 1998, 43, 999–1002. [Google Scholar]
- Rastsvetaeva, R.K.; Bolotina, N.B.; Sapozhnikov, A.N.; Kashaev, A.A.; Schoenleber, A.; Chapuis, G. Average structure of cubic lazurite with a three-dimensional incommensurate modulation. Crystallogr. Rep. 2002, 47, 404–407. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Rastsvetaeva, R.K.; Sapozhnikov, A.N.; Kashaev, A.A.; Shönleber, A.; Chapuis, G. Incommensurately modulated structure of isotropic lazurite as a product of twinning of two-dimensionally modulated domains. Crystallogr. Rep. 2003, 48, 721–727. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Rastsvetaeva, R.K.; Chapuis, G.; Schönleber, A.; Sapozhnikov, A.N.; Kashaev, A.A. On the symmetry of optically isotropic modulated lazurites from the Baikal region. Ferroelectrics 2004, 305, 95–98. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Rastsvetaeva, R.K.; Sapozhnikov, A.N. Average structure of incommensurately modulated monoclinic lazurite. Crystallogr. Rep. 2006, 51, 589–595. [Google Scholar] [CrossRef]
- Bolotina, N.B. Isotropic lazurite: A cubic single crystal with an incommensurate three-dimensional modulation of the structure. Crystallogr. Rep. 2006, 51, 968–976. [Google Scholar] [CrossRef]
- Bolotina, N. Forms and origin of structure modulation in lazurites. Philos. Mag. 2007, 87, 2679–2685. [Google Scholar] [CrossRef]
- Tauson, V.L.; Sapozhnikov, A.N.; Kaneva, E.V.; Lipko, S.V. Reversion of incommensurate modulation in cubic lazurite: Example of reversible forced equilibrium? Nat. Resour. 2014, 5, 761–771. [Google Scholar] [CrossRef][Green Version]
- Demyanets, L.N. The growth of germanium bromide sodalite crystals under hydrothermal conditions. Crystallogr. Rep. 1997, 42, 1053–1055. [Google Scholar]
- Weippert, V.; Chau, T.; Witthaut, K.; Eisenburger, L.; Johrendt, D. BaGe8As14: A semiconducting sodalite-type compound. Chem. Commun. 2021, 57, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.T.; Haworth, K.E. Synthesis and thermal decomposition of sodalites Na8[SiAlO4]6·(XO4)2, X = CI, Mn. J. Chem. Soc. Chem. Commun. 1991, 10, 734–735. [Google Scholar] [CrossRef]
- Vidal, L.; Paillaud, J.; Gabelica, Z. A novel monoclinic AlPO4-sodalite formed in the presence of dimethylformamide as template and solvent. Microporous Mesoporous Mater. 1998, 24, 189–197. [Google Scholar] [CrossRef]
- Depmeier, W.; Yamamoto, A. Powder profile refinement of a commensurately modulated aluminate sodalite. Mater. Sci. Forum 1991, 79, 763–768. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Tauson, V.L.; Matveeva, L.N. Discrete change of the modulation wave in the structure of cubic lazurite from the Baikal Lake area during its annealing. Zap. Vseross. Mineral. Obsh. 2001, 130, 121–132. (In Russian) [Google Scholar]
- Petersen, H.; Robben, L.; Šehović, M.; Gesing, T.M. Synthesis, temperature-dependent X-ray diffraction and Raman spectroscopic characterization of the sodalite to nosean phase transformation of |Na7.7(1)(MnO4)1.7(2)(H2O)0.8(2)|[AlSiO4]6. Microporous Mesoporous Mater. 2017, 242, 144–151. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Chukanov, N.V.; Schäfer, C. Growth twins and deformation twins of sodalite-type microporous compounds. Microporous Mesoporous Mater. 2022, 344, 112193. [Google Scholar] [CrossRef]
- Borhade, A.V.; Dholi, A.G. Synthesis and crystal structure of chlorate-enclathrated in aluminogermanate sodalite Na8[AlGeO4]6(ClO3)2. Mater. Sci. Pol. 2013, 31, 246–252. [Google Scholar] [CrossRef]
- Yoshimori, T.; Asano, Y.; Toriumi, Y.; Shiota, T. Investigation on the drying and decomposition of sodium oxalate. Talanta 1978, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Robben, L. Cage reactions in sodalites—A phenomenological approach using cellular automata. Microporous Mesoporous Mater. 2020, 294, 109874. [Google Scholar] [CrossRef]
- Borhade, A.V.; Dholi, A.G.; Kshirsagar, T.A. Synthesis and characterization of a new aluminogermanate thiocyanate aluminogermanate sodalite Na8[AlGeO4]6(SCN)2. Russ. J. Phys. Chem. A 2020, 94, 370–375. [Google Scholar] [CrossRef]
- Borhade, A.V.; Wakchaure, S.G.; Dholi, A.G.; Kshirsagar, T.A. Hydrothermal synthesis, characterization, and thermal properties of alumino silicate azide sodalite, Na8[AlSiO4]6(N3)2. Russ. J. Phys. Chem. A 2017, 91, 1183–1189. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Ron, G.; Weissman, A. The thermal decomposition of potassium permanganate and related substances. Part I. Chemical aspects. J. Chem. Soc. A 1971, 1821–1826. [Google Scholar] [CrossRef]
- Šehović, M.; Robben, L.; Gesing, T.M. Carbon dioxide uptake in nitrite-sodalite: Reaction kinetics and template ordering of the carbonate-nosean formation. Z. Krist. Cryst. Mater. 2015, 230, 263–269. [Google Scholar] [CrossRef]
- Gobeltz-Hautecoeur, N.; Demortier, A.; Lede, B.; Lelieur, J.P.; Duhayon, C. Occupancy of the sodalite cages in the blue ultramarine pigments. Inorg. Chem. 2002, 41, 2848–2854. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubrovinsky, L.S. The S3− ion is stable in geological fluids at elevated temperatures and pressures. Science 2011, 331, 1052–1054. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubessy, J. Stability and abundance of the trisulfur radical ion S3− in hydrothermal fluids. Earth Planet. Sci. Lett. 2015, 411, 298–309. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Mangone, A.; Acquafredda, P. Blue coloured haüyne from Mt. Vulture (Italy) volcanic rocks: SEM-EDS and Raman investigation of natural and heated crystals. J. Raman Spectrosc. 2022, 53, 1–13. [Google Scholar] [CrossRef]
- Ballirano, O.; Maras, A. Crystal chemical and structural characterization of an unusual CO3-bearing sodalite-group mineral. Eur. J. Mineral. 2005, 17, 805–812. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Ivanova, A.G.; Chukanov, N.V.; Verin, I.A. Crystal structure of alloriite. Dokl. Earth Sci. 2007, 415, 815–819. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Pekov, I.V.; Giester, G.; Pushcharovsky, D.Y. Sulfite analogue of alloriite from Sacrofano, Latium, Italy: Crystal chemistry and specific features of genesis. Geol. Ore Depos. 2021, 63, 793–804. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Varlamov, D.A.; Pekov, I.V.; Belakovskiy, D.I.; Britvin, S.N.; Van, K.V.; Ermolaeva, V.N.; Vozchikova, S.A.; Pushcharovsky, D.Y. Steudelite, (Na3)[(K,Na)17Ca7]Ca4(Al24Si24O96)(SO3)6F6·4H2O, a new cancrinite-group mineral with afghanite-type framework topology. Phys. Chem. Miner. 2022, 49, 1. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Chukanov, N.V.; Varlamov, D.A.; Vigasina, M.F.; Pekov, I.V.; Ksenofontov, D.A.; Pushcharovsky, D.Y. Sulfite-bearing analogue of marinellite. Zap. Vseross. Mineral. Obsh. 2022, 151, 84–100. [Google Scholar] [CrossRef]
- Buhl, J.-C. Hydrothermal synthesis and characterization of nitrite sodalite single crystals. J. Cryst. Growth 1991, 108, 143–149. [Google Scholar] [CrossRef]
- Matussek, T.; Buhl, J.-C. Stability and thermal behaviour of BH4-anions inside pores of sodium-potassium-tetrahydroborate aluminosilicate sodalite (Na1–xKx)8[AlSiO4]6(BH4)2; x~0.5. React. Kinet. Catal. Lett. 2009, 97, 281–288. [Google Scholar] [CrossRef]
- Gilbert, M.R. Pressureless sintering of sodalite waste-forms for the immobilization of pyroprocessing wastes. Mater. Res. Soc. Symp. Proc. 2015, 1744, 61–66. [Google Scholar] [CrossRef]
- Richmann, M.K.; Reed, D.T.; Kropf, A.J.; Aase, S.B.; Lewis, M.A. EXAFS/XANES studies of plutonium-loaded sodalite/glass waste forms. J. Nucl. Mater. 2001, 297, 303–312. [Google Scholar] [CrossRef]
- Pierce, E.M.; Lilova, K.; Missimer, D.M.; Lukens, W.W.; Wu, L.; Fitts, J.; Rawn, C.; Huq, A.; Leonard, D.N.; Eskelsen, J.R.; et al. Structure and thermochemistry of perrhenate sodalite and mixed guest perrhenate/pertechnetate sodalite. Environ. Sci. Technol. 2017, 51, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Anenburg, M.; Le Losq, C. Perrhenate sodalite growth from alkali silicate melts by noble metal catalysis. SN Appl. Sci. 2019, 1, 372. [Google Scholar] [CrossRef]
- Lilova, K.; Pierce, E.M.; Wu, L.; Jubb, A.M.; Subramani, T.; Navrotsky, A. Energetics of salt-bearing sodalites, Na8Al6Si6O24X2 (X = SO4, ReO4, Cl, I): A treatment option for pertechnetate-enriched nuclear waste streams. ACS Earth Space Chem. 2020, 4, 2153–2161. [Google Scholar] [CrossRef]
- Dickson, J.O.; Harsh, J.B.; Flury, M.; Pierce, E.M. Immobilization and exchange of perrhenate in sodalite and cancrinite. Microporous Mesoporous Mater. 2015, 214, 115–120. [Google Scholar] [CrossRef][Green Version]
- Dickson, J.O.; Harsh, J.B.; Flury, M.; Lukens, W.W.; Pierce, E.M. Competitive incorporation of perrhenate and nitrate into sodalite. Environ. Sci. Technol. 2014, 48, 12851–12857. [Google Scholar] [CrossRef]
- Petersen, H.; Robben, L.; Gesing, T.M. On the nature of the phase transitions of aluminosilicate perrhenate sodalite. Z. Krist. Cryst. Mater. 2020, 235, 213–223. [Google Scholar] [CrossRef]
- Luksic, S.A.; Riley, B.J.; Parker, K.E.; Hrma, P. Sodalite as a vehicle to increase Re retention in waste glass stimulant during vitrification. J. Nucl. Mater. 2016, 479, 331–337. [Google Scholar] [CrossRef]
- Dickson, J.O.; Harsh, J.B.; Lukens, W.W.; Pierce, E.M. Perrhenate incorporation into binary mixed sodalites: The role of anion size and implications for technetium-99 sequestration. Chem. Geol. 2015, 395, 138–143. [Google Scholar] [CrossRef]
- Hirabayashi, D.; Tanada, Y.; Sugiyama, T.; Enokida, Y. Low-temperature conversion of spent adsorbent to iodine sodalite by a mechanochemical route. J. Am. Inst. Chem. Eng. 2012, 58, 2441–2447. [Google Scholar] [CrossRef]
- Sheppard, G.P.; Hriljac, J.A.; Maddrell, E.R.; Hyatt, N.C. Silver zeolites: Iodide occlusion and conversion to sodalite—A potential 129I waste form? Mat. Res. Soc. Symp. Proc. 2006, 932, 1–8. [Google Scholar] [CrossRef]
- Vance, E.R.; Gregg, D.J.; Grant, C.; Stopic, A.; Maddrell, E.R. Silver iodide sodalite for 129I immobilization. J. Nucl. Mater. 2016, 480, 177–181. [Google Scholar] [CrossRef]
- Maddrell, E.; Gandy, A.; Stennett, M. The durability of iodide sodalite. J. Nucl. Mater. 2014, 449, 168–172. [Google Scholar] [CrossRef]
- Maddrell, E.R.; Vance, E.R.; Grant, C.; Aly, Z.; Stopic, A.; Palmer, T.; Harrison, J.; Gregg, D.J. Silver iodide sodalite—Wasteform/Hip canister interactions and aqueous durability. J. Nucl. Mater. 2019, 517, 71–79. [Google Scholar] [CrossRef]
- Lepry, W.C.; Riley, B.J.; Crum, J.V.; Rodriguez, C.P.; Pierce, D.A. Solution-based approaches for making high-density sodalite waste forms to immobilize spent electrochemical salts. J. Nucl. Mater. 2013, 442, 350–359. [Google Scholar] [CrossRef]
- De Angelis, G.; Nannicini, R.; Martini, F.; Mazzocchia, C.; Modica, G. Different methods to synthesize sodalite, as a matrix for conditioning chloride spent salts from pyroprocesses. Radiochim. Acta 2008, 96, 303–310. [Google Scholar] [CrossRef]
- Giacobbo, F.; da Ros, M.; Macerata, E.; Mariani, M.; Giola, M.; de Angelis, G.; Capone, M.; Fedeli, C. An experimental study on Sodalite and SAP matrices for immobilization of spent chloride salt waste. J. Nucl. Mater. 2018, 499, 512–527. [Google Scholar] [CrossRef]
- Capone, M.; Fedeli, C.; de Angelis, G.; da Ros, M.; Giacobbo, F.; Giola, M.; Macerata, E.; Mariani, M. A study on sodalite pellets as matrix for spent chloride salts confinement. J. Mater. Res. Soc. 2017, 1, 4261–4267. [Google Scholar] [CrossRef]
- Riley, B.J.; Lepry, W.C.; Crum, J.V. Solution-Derived Sodalite Made with Si- and Ge-Ethoxide Precursors for Immobilizing Electrorefiner Salt. J. Nucl. Mater. 2016, 468, 140–146. [Google Scholar] [CrossRef]
- Riley, B.J.; Vienna, J.D.; Frank, S.M.; Kroll, J.O.; Peterson, J.A.; Canfield, N.L.; Zhu, Z.; Zhang, J.; Kruska, K.; Schreiber, D.K.; et al. Glass binder development for a glass-bonded sodalite ceramic waste form. J. Nucl. Mater. 2017, 489, 42–63. [Google Scholar] [CrossRef]
- Borhade, A.V.; Dholi, A.G.; Wakchaure, G.; Tope, D.R. Chemical modification of coal fly ash into iodate sodalite and its use for the removal of Cd2+, Pb2+, and Zn2+ from their aqueous solutions. Desalination Water Treat. 2012, 50, 157–169. [Google Scholar] [CrossRef]
- Esfandiana, H.; Parvinia, M.; Khoshandama, B.; Samadi-Maybodi, A. Removal of diazinon from aqueous solutions in batch systems using Cu-modified sodalite zeolite: An application of response surface methodology. Int. J. Eng. Trans. Appl. 2015, 28, 1552–1563. [Google Scholar] [CrossRef]
- Canfield, G.M.; Bizimis, M.; Latturner, S.E. Sodalite ion exchange in polyethylene oxide oligomer solvents. J. Mater. Chem. 2007, 17, 4530–4534. [Google Scholar] [CrossRef]
- van den Berg, A.W.C. Opportunities and Limitations of Hydrogen Storage in Zeolitic Clathrates. Ph.D. Thesis, Technische Universiteit Delft, Delft, The Netherlands, 2006; 245p. [Google Scholar]
- van den Berg, A.W.C.; Bromley, S.T.; Flikkema, E.; Wojdel, J.; Maschmeyer, T.; Jansen, J.C. Molecular-dynamics analysis of the diffusion of molecular hydrogen in all-silica sodalite. J. Chem. Phys. 2004, 120, 10285–10289. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, A.W.C.; Bromley, S.T.; Flikkema, E.; Jansen, J.C. Effect of cation distribution on self-diffusion of molecular hydrogen in Na3Al3Si3O12 sodalite: A molecular dynamics study. J. Chem. Phys. 2004, 121, 10209–10216. [Google Scholar] [CrossRef]
- van den Berg, A.W.C.; Bromley, S.T.; Jansen, J.C. Thermodynamic limits on hydrogen storage in sodalite framework materials: A molecular mechanics investigation. Microporous Mesoporous Mater. 2005, 78, 63–71. [Google Scholar] [CrossRef]
- Weitkamp, J.; Ernst, S.; Cubero, F.; Wester, F. Nitrido-sodalite Zn6[P12N24] as a material for reversible hydrogen encapsulation. Adv. Mater. 1997, 9, 247–248. [Google Scholar] [CrossRef]
- Dincă, M.; Dailly, A.; Tsay, C.; Long, J.R. Expanded sodalite-type metal–organic frameworks: Increased stability and H2 adsorption through ligand-directed catenation. Inorg. Chem. 2008, 47, 11–13. [Google Scholar] [CrossRef]
- Dincă, M.; Dailly, A.; Liu, Y.; Brown, C.M.; Neumann, D.A.; Long, J.R. Hydrogen storage in microporous metal-organic framework with exposed Mn2+ coordination sites. J. Am. Chem. Soc. 2006, 128, 16876–16883. [Google Scholar] [CrossRef]
- Luo, J.H.; Xu, H.W.; Liu, Y.; Zhao, Y.S.; Daemen, L.L.; Brown, C.; Timofeeva, T.V.; Ma, S.Q.; Zhou, H.C. Hydrogen adsorption in a highly stable porous rare-earth metal-organic framework: Sorption properties and neutron diffraction studies. J. Am. Chem. Soc. 2008, 130, 9626–9627. [Google Scholar] [CrossRef]
- Prasad, T.K.; Hong, D.H.; Suh, M.P. High gas sorption and metal-ion exchange of microporous metal-organic frameworks with incorporated imide groups. Chem. Eur. J. 2010, 16, 14043–14050. [Google Scholar] [CrossRef]
- Sumida, K. Design and Synthesis of Metal-Organic Frameworks for Hydrogen Storage and Carbon Dioxide Capture. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2012. [Google Scholar]
- Yang, S.J.; Im, J.H.; Nishihara, H.; Jung, H.; Lee, K.; Kyotani, T.; Park, C.R. General relationship between hydrogen adsorption capacities at 77 and 298 K and pore characteristics of the porous adsorbents. J. Phys. Chem. C 2012, 116, 10529–10540. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Prasad, P.P.M.H.; Latheef, A.S.A.; Pillai, A. Synthesis and fine tuning of MOF for hydrogen storage. In Recent Trends in the Application of Metal-Organic Frameworks; Chandrasekhar, A., Jacob, G., Eds.; IntechOpen Limited: London, UK, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Leonard, A.D.; Hudson, J.L.; Fan, H.; Booker, R.; Simpson, L.J.; O’Neill, K.J.; Parilla, P.A.; Heben, M.J.; Pasquali, M.; Kittrell, C.; et al. Nanoengineered carbon scaffolds for hydrogen storage. J. Am. Chem. Soc. 2009, 131, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.V.; Polyakov, M.S. Hybrid materials based on carbon nanotubes and polyaromatic molecules: Methods of functionalization and sensor properties. Macroheterocycles 2020, 13, 91–112. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Meng, M.; Zhong, D.-C.; Huang, Y.-L.; Jiang, L.; Lu, T.-B. Counter-cation modulation of hydrogen and methane storage in a sodalite-type porous metal–organic framework. Chem. Commun. 2012, 48, 12002–12004. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Horike, S.; Kaye, S.S.; Herm, Z.R.; Queen, W.L.; Brown, C.M.; Grandjean, F.; Long, G.J.; Dailly, A.; Long, J.R. Hydrogenstorage and carbon dioxide capture in an iron-based sodalite-type metal–organic framework (Fe-BTT) discovered via high-throughput methods. Chem. Sci. 2010, 1, 184–191. [Google Scholar] [CrossRef]
- Gygi, D.; Bloch, E.D.; Mason, J.A.; Hudson, M.R.; Gonzalez, M.I.; Siegelman, R.L.; Darwish, T.A.; Queen, W.L.; Brown, C.M.; Long, J.R. Hydrogen Storage in the Expanded Pore Metal–Organic Frameworks M2(dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Mater. 2016, 26, 1128–1138. [Google Scholar] [CrossRef]
- Buhl, J.-C.; Schomborg, L.; Rüscher, C.H. Tetrahydroborate sodalite nanocrystals: Low temperature synthesis and thermally controlled intra-cage reactions for hydrogen release of nano- and micro crystals. Microporous Mesoporous Mater. 2010, 132, 210–218. [Google Scholar] [CrossRef]
- Buhl, J.-C.; Rüscher, C.H.; Schomborg, L.; Stemme, F. Nanocrystalline NaBH4-enclathrated zeolite SOD: A model for the improvement of safeness and reactivity of boron hydride based hydrogen storage systems. Clean Technol. 2010, 236–239. [Google Scholar]
- Buhl, J.-C.; Gesing, T.M.; Rüscher, C.H. Synthesis, crystal structure and thermal stability of tetrahydroborate sodalite Na8[AlSiO4]6(BH4)2. Microporous Mesoporous Mater. 2005, 80, 57–63. [Google Scholar] [CrossRef]
- Rüscher, C.H.; Stemme, F.; Schomborg, L.; Buhl, J.-C. Low-temperature hydrogen release from borontetrahydride-sodalite and its reloading: Observations in in-situ and ex-situ TIR experiments. Ceram. Environ. Energy Appl. 2010, 217, 65–70. [Google Scholar]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Study on the thermal decomposition of NaBH4 catalyzed by ZrCl4. Int. J. Hydrogen Energy 2017, 42, 22432–22437. [Google Scholar] [CrossRef]
- Martelli, P.; Caputo, R.; Remhof, A.; Mauron, P.; Borgschulte, A.; Züttel, A. Stability and decomposition of NaBH4. J. Phys. Chem. 2010, 114, 7173–7177. [Google Scholar] [CrossRef]
- Buhl, J.-C.; Stemme, F.; Poltz, I. Hydrothermal stability of NaBH4 enclathrated sodalites with aluminosilicate and gallosilicate framework. Microporous Mesoporous Mater. 2009, 126, 276–282. [Google Scholar] [CrossRef]
- Buhl, J.-C. NaBH4 Sodalites, synthesized by modified methods: (1) autothermal synthesis and (2) crossover reaction from gel to melt flow. Adv. Chem. Eng. Sci. 2017, 7, 108–124. [Google Scholar] [CrossRef][Green Version]
- Zheng, Z.; Guliants, V.V.; Misture, S. Sodalites as ultramicroporous frameworks for hydrogen separation at elevated temperatures: Thermal stability, template removal, and hydrogen accessibility. J. Porous Mater. 2009, 16, 343–347. [Google Scholar] [CrossRef]
- De Luca, G.; Pullumbi, P.; Barbieri, G.; Famà, A.D.; Bernardo, P.; Drioli, E. Gusev and Suter calculation of the diffusion coefficients of light gases in silicalite-1 membrane and silica-sodalite zeolite. Sep. Purif. Technol. 2004, 36, 215–228. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.; Wang, H. Synthesis of nanocrystalline sodalite with organic additives. Mater. Lett. 2008, 62, 4028–4030. [Google Scholar] [CrossRef]
- Asgari, M.; Jawahery, S.; Bloch, E.D.; Hudson, M.R.; Flacau, R.; Vlaisavljevich, B.; Long, J.R.; Brown, C.M.; Queen, W.L. An experimental and computational study of CO2 adsorption in the sodalite-type M-BTT (M = Cr, Mn, Fe, Cu) metal–organic frameworks featuring open metal sites. Chem. Sci. 2018, 9, 4579–4588. [Google Scholar] [CrossRef]
- Eterigho-Ikelegbe, O.; Bada, S.; Daramola, M.O.; Falcon, R. Synthesis of high purity hydroxy sodalite nanoparticles via poreplugging hydrothermal method for inorganic membrane development: Effect of synthesis variables on crystallinity, crystal size and morphology. Mater. Today Proc. 2021, 38, 675–681. [Google Scholar] [CrossRef]
- Qin, J.-S.; Du, D.-Y.; Li, W.-L.; Zhang, J.-P.; Li, S.-L.; Su, Z.-M.; Wang, X.-L.; Xu, Q.; Shao, K.-Z.; Lan, Y.-Q. N-rich zeolite-like metal–organic framework with sodalite topology: High CO2 uptake, selective gas adsorption and efficient drug delivery. Chem. Sci. 2012, 3, 2114–2118. [Google Scholar] [CrossRef]
- Asgari, M.; Semino, R.; Schouwink, P.A.; Kochetygov, I. Understanding how ligand functionalization influences CO2 and N2 adsorption in a sodalite metaloorganic framework. Chem Mater. 2020, 32, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.; Hu, S.; Peng, S.; Xu, C.; Lu, A. Dehydrated Na6[AlSiO4]6 sodalite as a promising SO2 sorbent material: A first principles thermodynamics prediction. J. Am. Ceram. Soc. 2019, 103, 3663–3672. [Google Scholar] [CrossRef]
- Navarro, J.A.R.; Barea, E.; Salas, J.M.; Masciocchi, N.; Galli, S.; Sironi, A.; Ania, C.O.; Parra, J.B. H2, N2, CO, and CO2 sorption properties of a series of robust sodalite-type microporous coordination polymers. Inorg. Chem. 2006, 45, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
- Fasolin, S.; Romano, M.; Boldrini, S.; Ferrario, A.; Fabrizio, M.; Armelao, L.; Barison, S. Single-step process to produce alumina supported hydroxy-sodalite zeolite membranes. J. Mater Sci. 2019, 54, 2049–2058. [Google Scholar] [CrossRef]
- Yang, G.; Guo, H.; Kang, Z.; Feng, S.; Zhao, L.; Mintova, S. Sandwich-type H2/CO2 membranes comprising of graphene oxide and sodalite crystals with adjustable morphology and size. Microporous Mesoporous Mater. 2020, 300, 110120. [Google Scholar] [CrossRef]
- Eterigho-Ikelegbe, O.; Bada, S.O.; Daramola, M.O. Preparation and evaluation of nanocomposite sodalite/α-Al2O3 tubular membranes for H2/CO2 separation. Membranes 2020, 10, 312. [Google Scholar] [CrossRef]
- Eden, C.L.; Daramola, M.O. Evaluation of silica sodalite infused polysulfone mixed matrix membranes during H2/CO2 separation. Mater. Today Proc. 2021, 38, 522–527. [Google Scholar] [CrossRef]
- Lee, S.-R.; Son, Y.-H.; Julbe, A.; Choy, J.-H. Vacuum seeding and secondary growth route to sodalite membrane. Thin Solid Films 2006, 495, 92–96. [Google Scholar] [CrossRef]
- Julbe, A.; Motuzas, J.; Cazevielle, F.; Volle, G.; Guizard, C. Synthesis of sodalite/αAl2O3 composite membranes by microwave heating. Sep. Purif. Technol. 2003, 32, 139–149. [Google Scholar] [CrossRef]
- Guo, H.; Kong, G.; Yang, G.; Pang, J.; Kang, Z.; Feng, S.; Zhao, L.; Fan, L.; Zhu, L.; Vicente, A.; et al. Cross-linking between sodalite nanoparticles and graphene oxide in composite membranes to trigger high gas permeance, selectivity, and stability in hydrogen separation. Angew. Chem. Int. Ed. 2020, 59, 6284–6288. [Google Scholar] [CrossRef]
- Yang, G.; Guo, H.; Kang, Z.; Zhao, L.; Feng, S.; Jiao, F.; Mintova, S. Green hydrogen separation from nitrogen by mixed-matrix membranes consisting of nanosized sodalite crystals. ChemSusChem 2019, 12, 4529–4537. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bao, Y.; Song, C.; Yang, W.; Liu, J.; Lin, L. Microwave-assisted hydrothermal synthesis of hydroxy-sodalite zeolite membrane. Microporous Mesoporous Mater. 2004, 75, 173–181. [Google Scholar] [CrossRef]
- van Niekerk, A.; Zaha, J.; Breytenbach, J.C.; Krieg, H.M. Direct crystallisation of a hydroxy sodalite membrane without seeding using a conventional oven. J. Membr. Sci. 2007, 300, 156–164. [Google Scholar] [CrossRef]
- Nabavi, M.S.; Mohammadi, T.; Kazemimoghadam, M. Hydrothermal synthesis of hydroxysodalite zeolite membrane: Separation of H2/CH4. Ceram. Int. 2014, 40, 5889–5896. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of hydroxy sodalite films as novel water selective membranes. J. Membr. Sci. 2009, 326, 153–160. [Google Scholar] [CrossRef]
- Khajavi, S.; Kapteijn, F.; Jansen, J.C. Synthesis of thin defect-free hydroxy sodalite membranes: New candidate for activated water permeation. J. Membr. Sci. 2007, 299, 63–72. [Google Scholar] [CrossRef]
- Bayati, B.; Babaluo, A.A.; Namini, P.A. Synthesis and seeding time effect on the inter-crystalline structure of hydroxy-sodalite zeolite membranes by single gas (H2 and N2) permeation. Iran. J. Chem. Chem. Eng. 2009, 28, 1–12. [Google Scholar] [CrossRef]
- Kalantari, N.; Vaezi, M.J.; Yadollahi, M.; Babaluo, A.A.; Bayati, B.; Kazemzadeh, A. Synthesis of nanostructure hydroxy sodalite composite membranes via hydrothermal method: Support surface modification and synthesis method effects. Asia-Pac. J. Chem. Eng. 2015, 10, 45–55. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Production of ultra pure water by desalination of seawater using a hydroxyl sodalite membrane. J. Membr. Sci. 2010, 356, 52–57. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Performance of hydroxy sodalite membranes as absolute water selective materials under acidic and basic conditions. J. Membr. Sci. 2010, 356, 1–6. [Google Scholar] [CrossRef]
- Wei, X.-L.; Pan, W.-Y.; Li, X.; Pan, M.; Huo, C.-F.; Yang, R.; Chao, Z.-S. MCM-22 zeolite-induced synthesis of thin sodalite zeolite membranes. Chem. Mater. 2020, 32, 333–340. [Google Scholar] [CrossRef]
- Workneh, S.; Shukla, A. Synthesis of sodalite octahydrate zeolite-clay composite membrane and its use in separation of SDS. J. Membr. Sci. 2008, 309, 189–195. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Zhang, Y. Desilication mechanism and kinetics of synthesized hydroxy-sodalite in high-alkali sodium aluminate solutions. React. Kinet. Mech. Catal. 2019, 127, 489–504. [Google Scholar] [CrossRef]
- Ntshangase, N.C.; Sadare, O.O.; Daramola, M.O. Effect of silica sodalite functionalization and PVA coating on performance of sodalite infused PSF membrane during treatment of acid mine drainage. Membranes 2021, 11, 315. [Google Scholar] [CrossRef]
- Li, D.; Zhu, H.Y.; Ratinac, K.R.; Ringer, S.P.; Wang, H. Synthesis and characterization of sodalite–polyimide nanocomposite membranes. Microporous Mesoporous Mater. 2009, 126, 14–19. [Google Scholar] [CrossRef]
- Yu, H.; Shen, J.; Li, J.; Sun, X.; Han, W.; Liu, X.; Wang, L. Preparation, characterization and adsorption properties of sodalite pellets. Mater. Lett. 2014, 32, 259–262. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fijałkowska, G.; Nosal, A.; Franus, M.; Panek, R. Adsorption mechanism of poly(vinyl alcohol) on the surfaces of synthetic zeolites: Sodalite, Na-P1 and Na-A. Adsorption 2019, 25, 567–574. [Google Scholar] [CrossRef]
- Daramola, M.O.; Silinda, B.; Masondo, S.; Oluwasina, O.O. Polyethersulphone-sodalite (PES-SOD) mixed-matrix membranes: Prospects for acid mine drainage (AMD) treatment. J. South. Afr. Inst. Min. Metall. 2015, 115, 1221–1228. [Google Scholar] [CrossRef]
- Cano, N.F.; Ayta, W.E.F.; Watanabe, S. The electronic and optical properties of sodalite (Na8Al6Si6O24Cl2) from first principles. Solid State Commun. 2010, 150, 195–197. [Google Scholar] [CrossRef]
- Pan, L.; Liu, W.; Chen, W.; Yan, K.; Yang, H.; Yu, J. Crystal structure and band gap studies of sodalite: Experimental and calculated results. J. Mol. Struct. 2016, 1106, 59–63. [Google Scholar] [CrossRef]
- Grajciar, L. PbS clusters embedded in sodalite zeolite cavities of different compositions: Unraveling the structural evolution and optical properties using ab initio calculations. J. Phys. Chem. C 2016, 120, 27050–27065. [Google Scholar] [CrossRef]
- Gmelin, C.G. Üeber die künstliche Darstellung einer dem Ultramarin ähnlichen Farbe. Naturwiss. Abhandl. 1828, 2, 191–224. (In German) [Google Scholar]
- Deer, W.A.; Howie, R.A.; Wise, W.S.; Zussman, J. Rock-forming minerals. Volume 4B. Framework silicates: Silica minerals. In Feldspathoids and the Zeolites; The Geological Society: London, UK, 2004. [Google Scholar]
- Tauson, V.L.; Goettlicher, J.; Sapozhnikov, A.N.; Mangold, S.; Lustenberg, R.E. Sulfur speciation in lazurite-type minerals (Na,Ca)8[Al6Si6O24](SO4,S)2 and their annealing products: A comparative XPS and XAS study. Eur. J. Mineral. 2012, 24, 133–152. [Google Scholar] [CrossRef]
- Hettmann, K.; Wenzel, T.; Marks, M.; Markl, G. The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals. Am. Mineral. 2012, 97, 1653–1661. [Google Scholar] [CrossRef]
- Paterson, I. A Dictionary of Colour: A Lexicon of the Language of Colour; Thorogood: London, UK, 2004; 520p. [Google Scholar]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium—A Dictionary and Optical Microscopy of Historical Pigments; Routledge: London, UK, 2004; 512p. [Google Scholar] [CrossRef]
- Eckert, B.; Steudel, R. Molecular spectra of sulfur molecules and solid sulfur allotropes. Top. Curr. Chem. 2003, 231, 31–97. [Google Scholar] [CrossRef]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef]
- Platonov, A.N.; Tarashchan, A.N.; Belichenko, V.P.; Povarennikh, A.S. Spectroscopic study of sulfide sulfur in some framework aluminosilicates. Const. Prop. Miner. 1971, 5, 61–72. (In Russian) [Google Scholar]
- Samoilovich, M.I. An ESR study of sulfur-bearing radical ions in minerals. Geokhimiya 1971, 4, 477–483. (In Russian) [Google Scholar]
- Evsyunin, V.G.; Sapozhnikov, A.N.; Kashaev, A.A.; Rastsvetaeva, R.R. Crystal structure of triclinic lazurite. Crystallogr. Rep. 1997, 42, 938–945. [Google Scholar]
- Steudel, R. Inorganic polysulfides Sn2− and radical anions Sn∙−. In Elemental Sulfur und Sulfur-Rich Compounds II. Topics in Current Chemistry; Steudel, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 231. [Google Scholar]
- Climent-Pascual, E.; de Paz, J.R.; Rodri, E.; Suard, E.; Sa, R. Synthesis and characterization of the ultramarine-type analog Na8-x[Si6Al6O24]·(S–2,S–3,CO3)(1–2). Inorg. Chem. 2009, 48, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Fleet, M.E.; Liu, X.; Harmer, S.L.; Nesbitt, H.W. Chemical state of sulfur in natural and synthetic lazurite by S K-edge XANES and X-ray photoelectron spectroscopy. Can. Mineral. 2005, 43, 1589–1603. [Google Scholar] [CrossRef]
- Chivers, T.; Elder, P.J.W. Ubiquitous trisulfur radical anion: Fundamentals and applications in materials science, electrochemistry, analytical chemistry and geochemistry. Chem. Soc. Rev. 2013, 42, 5996–6005. [Google Scholar] [CrossRef] [PubMed]
- Rejmak, P. Computational refinement of the puzzling red tetrasulfur chromophore in ultramarine pigments. Phys. Chem. Chem. Phys. 2020, 22, 22684–22698. [Google Scholar] [CrossRef]
- Wong, M.W.; Steudel, R. Structure and spectra of tetrasulfur S4—an ab initio MO study. Chem. Phys. Lett. 2003, 379, 162–169. [Google Scholar] [CrossRef]
- Rolfe, J. Emission spectra of S2−, Se2−, and SeS− ions in KI Crystals. J. Chem. Phys. 1968, 49, 4193–4197. [Google Scholar] [CrossRef]
- Hålenius, U. Absorption of light by exchange coupled pairs of tetrahedrally coordinated divalent manganese in the helvite-genthelvite solid solution. Period. Mineral. 2011, 80, 105–111. [Google Scholar] [CrossRef]
- Blumentritt, F.; Fritsch, E. Photochromism and photochromic gems: A review and some new data (Part 1). J. Gemmol. 2021, 37, 780. [Google Scholar] [CrossRef]
- Agamah, C.; Vuori, S.; Colinet, P.; Norrbo, I.; de Carvalho, J.M.; Key, L.; Nakamura, O.; Lindblom, J.; van Goethem, L.; Emmermann, A. Hackmanite—The natural glow-in-the-dark material. Chem. Mater. 2020, 32, 8895–8905. [Google Scholar] [CrossRef]
- Jensen, A.; Petersen, O.V. Tugtupite: A gemstone from Greenland. Gems Gemmol. 1982, 19, 90–94. [Google Scholar] [CrossRef]
- Warner, T.E.; Andersen, J.H. The effects of sulfur intercalation on the optical properties of artificial ‘hackmanite’, Na8[Al6Si6O24]Cl1.8S0.1; ‘sulfosodalite’, Na8[Al6Si6O24]S; and natural tugtupite, Na8[Be2Al2Si8O24](Cl,S)2–δ. Phys. Chem. Miner. 2012, 39, 163–168. [Google Scholar] [CrossRef]
- Curutchet, A.; le Bahers, T. Modeling the photochromism of S-doped sodalites using DFT, TDDFT, and SAC-CI methods. Inorg. Chem. 2017, 56, 414–423. [Google Scholar] [CrossRef]
- Colinet, P.; Gheeraert, A.; Curutchet, A.; le Bahers, T. On the spectroscopic modeling of localized defects in sodalites by TD-DFT. J. Phys. Chem. C 2020, 124, 8949–8957. [Google Scholar] [CrossRef]
- Hassib, A.; Beckman, O.; Annersten, H. Photochromic properties of natural sodalite. J. Phys. D Appl. Phys. 1977, 334, 771–777. [Google Scholar] [CrossRef]
- Pizani, P.S.; Terrile, M.C.; Farach, H.A.; Poole, C.P. Color centers in sodalite. Am. Mineral. 1985, 70, 1186–1192. [Google Scholar]
- Norrbo, I.; Gluchowski, P.; Hyppänen, I.; Laihinen, T.; Laukkanen, P.; Mäkelä, J.; Mamedov, F.; Santos, H.S.; Sinkkonen, J.; Tuomisto, M.; et al. Mechanisms of tenebrescence and persistent luminescence in synthetic hackmanite Na8Al6Si6O24(Cl,S)2. ACS Appl. Mater. Interfaces 2016, 8, 11592–11602. [Google Scholar] [CrossRef]
- Goettlicher, J.; Kotelnikov, A.; Suk, N.; Kovalski, A.; Vitova, T.; Steininger, R. Sulfur K X-ray absorption near edge structure spectroscopy on the photochrome sodalite variety hackmanite. Z. Kristallogr. 2013, 228, 157–171. [Google Scholar] [CrossRef]
- Zahoransky, T.; Friis, H.; Marks, M.A.W. Luminescence and tenebrescence of natural sodalites: A chemical and structural study. Phys. Chem. Miner. 2016, 43, 459–480. [Google Scholar] [CrossRef]
- Sidike, A.; Sawuti, A.; Wang, X.-M.; Zhu, H.-J.; Kobayashi, S.; Kusachi, I.; Yamashita, N. Fine structure in photoluminescence spectrum of S2− center in sodalite. Phys. Chem. Miner. 2007, 34, 477–484. [Google Scholar] [CrossRef]
- Gaft, M.; Panczer, G.; Nagli, L.; Yeates, H. Laser-induced timeresolved luminescence of tugtupite, sodalite and hackmanite. Phys. Chem. Miner. 2009, 36, 127–141. [Google Scholar] [CrossRef]
- Kaiheriman, M.; Maimaitinaisier, A.; Rehiman, A.; Sidike, A. Photoluminescence properties of green and red luminescence from natural and heat-treated sodalite. Phys. Chem. Miner. 2014, 41, 227–235. [Google Scholar] [CrossRef]
- Kirk, R.J. The luminescence and tenebrescence of natural and synthetic sodalite. Am. Miner. 1955, 40, 22–31. [Google Scholar]
- Ballentyne, D.W.G.; Bye, K.L. The nature of photochromism in chlorosodalites from optical data. J. Phys. D Appl. Phys. 1970, 3, 1438–1443. [Google Scholar] [CrossRef]
- Warner, T.E. Artificial Hackmanite Na8[Al6Si6O24]Cl1.8S0.1 by a Structure-Conversion Method with Annealing under a Reducing Atmosphere, Synthesis, Properties and Mineralogy of Important Inorganic Materials; Wiley: Hoboken, NJ, USA, 2011; pp. 240–253. [Google Scholar]
- Taylor, M.J.; Marshall, D.J.; Forrester, P.A.; McLaughlan, S.D. Colour centres in sodalites and their use in storage displays. Radio Electro Eng. 1970, 40, 17–25. [Google Scholar] [CrossRef]
- Finch, A.A.; Friis, H.; Maghrabi, M. Defects in sodalite-group minerals determined from X-ray-induced luminescence. Phys. Chem. Miner. 2016, 43, 481–491. [Google Scholar] [CrossRef]
- Lezhnina, M.M.; Kynast, U.H. NIR- and upconverted luminescence from rare-earth sodalites. Phys. Solid State 2005, 47, 1485–1488. [Google Scholar] [CrossRef]
- Lezhnina, M.; Laeri, F.; Benmouhadi, L.; Kynast, U. Efficient near-infrared emission from sodalite derivatives. Adv. Mater. 2006, 18, 280–283. [Google Scholar] [CrossRef]
- Kaiheriman, M.; Sidike, A.; Maimaitinasier, A.; Reheman, A.; Rouzi, B. Photoluminescence properties of Tb3+-doped sodalite under VUV–UV light excitation. J. Lumin. 2015, 157, 411–415. [Google Scholar] [CrossRef]
- Cano, N.F.; Blak, A.R.; Watanabe, S. Correlation between electron paramagnetic resonance and thermoluminescence in natural sodalite. Phys. Chem. Miner. 2010, 37, 57–64. [Google Scholar] [CrossRef]
- Chen, C.; Cai, P.; Qin, L.; Wang, J.; Bi, S.; Huang, Y.; Seo, H.J. Luminescence properties of sodalite-type Zn4B6O13:Mn2+. J. Lumin. 2018, 199, 154–159. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of a sodalite membrane reactor in esterification—Coupling reaction and separation. Catal. Today 2010, 156, 132–139. [Google Scholar] [CrossRef]
- Aghaeinejad-Meybodi, A.; Mousavi, S.M.; Shahabi, A.A.; Kakroudi, M.R. CFD modeling of methanol to light olefins in a sodalite membrane reactor using SAPO-34 catalyst with in situ steam removal. Comb. Chem. High Throughput Screen. 2021, 24, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, F.; Khezri, S.H.; Azizian, J. Hydroxy sodalite zeolite as a recyclable catalyst for the green synthesis of tetrahydrobenzo[b]pyrans via one-pot three-component condensation reaction. Iran. J. Catal. 2015, 5, 253–259. [Google Scholar]
- Bijsterbosch, J.W.; Das, A.; Kerkhof, F. Clean technology in the production of epichlorohydrin. J. Clean. Prod. 1994, 2, 181–184. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Zhang, J.; Jin, Q.; Luo, G. Evaluation of an improved epichlorohydrin synthesis from dichloropropanol using a microchemical system, Chinese. J. Chem. Eng. 2015, 23, 1123–1130. [Google Scholar] [CrossRef]
- Shanbhag, G.V.; Choi, M.; Kim, J.; Ryoo, R. Mesoporous sodalite: A novel, stable solid catalyst for base-catalyzed organic transformations. J. Catal. 2009, 264, 88–92. [Google Scholar] [CrossRef]
- Hiyoshi, N. Nanocrystalline sodalite: Preparation and application to epoxidation of.2-cyclohexen-1-one with hydrogen peroxide. Appl. Catal. A Gen. 2012, 419, 164–169. [Google Scholar] [CrossRef]
- Sachse, A.; Galarneau, A.; di Renzo Francois Fajula, F.; Coq, B. Synthesis of zeolite monoliths for flow continuous processes. The case of sodalite as a basic catalyst. Chem. Mater. 2010, 22, 4123–4125. [Google Scholar] [CrossRef]
- Makgaba, C.P.; Daramola, M.O. Transesterification of waste cooking oil to biodiesel over calcined hydroxy sodalite (HS) catalyst: A preliminary investigation. In Proceedings of the 2015 International Conference on Sustainable Energy and Environmental Engineering, Bangkok, Thailand, 25–26 October 2015; Atlantic Press: Amsterdam, The Netherlands, 2015; pp. 52–56. [Google Scholar]
- Rahimnejad, M.; Hassaninejad, S.K.; Pourali, S.M. Preparation of template-free sodalite nanozeolite–chitosan-modified carbon paste electrode for electrocatalytic oxidation of ethanol. J. Iran. Chem. Soc. 2015, 12, 413–425. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Adlii, A.; Jumah, M.N.B.; Othman, S.I.; Alruhaimi, R.S.; Salama, Y.F.; Allam, A.A. Sustainable conversion of waste corn oil into biofuel over different forms of synthetic muscovite based K+/Na+ sodalite as basic catalysts; characterization and mechanism. Mater. Res. Express 2021, 8, 065502. [Google Scholar] [CrossRef]
- Mani, P.; Devadas, S.; Gurusamy, T.; Karthik, P.E.; Ratheesh, B.P.; Ramanujam, K.; Mandal, S. Sodalite-type Cu-based three-dimensional metal–organic framework for efficient oxygen reduction reaction. Chem. Asian J. 2019, 14, 4814–4818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Han, C.-Y.; Dang, Q.-Q.; Wang, Y.-H.; Zhang, X.-M. Solvent-free heterogeneous catalysis for cyanosilylation in a modified sodalite-type Cu(II)-MOF. Adv. R. Soc. Chem. 2015, 5, 24293–24298. [Google Scholar] [CrossRef]
- Chen, W.; Maugé, F.; Gestel, J.V.; Nie, H.; Li, D.; Long, X. Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sulfide catalysts. J. Catal. 2013, 304, 47–62. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Prins, R. Hydrodesulfurization with classic Co–MoS2 and Ni–MoS2/c-Al2O3 and new Pt–Pd on mesoporous zeolite catalysts. Catal. Today 2010, 150, 213–217. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.Y.; Luo, M.F.; Lu, J.Q. Remarkable enhancement of dichloromethane oxidation over potassium-promoted Pt/Al2O3 catalysts. J. Catal. 2014, 311, 314–324. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, W.; Ke, Q.; Zhang, S.; Jin, H.; Hu, J.; Wang, S.; Tang, T. Study of hydrodesulfurization of 4,6-DM-DBT over Pd supported on mesoporous USY zeolite. Appl. Catal. A 2012, 433, 251–257. [Google Scholar] [CrossRef]
- Xue, D.; Liu, F.; Gao, H.; Yang, L.; Wu, Y.; Xue, W.; Li, Z.L.F. The synthesis, characterization and catalytic performance of platinum encapsulated mesopotous sodalite material. Rev. Roum. Chim. 2018, 63, 337–346. [Google Scholar]
- Gao, H.; Liu, F.; Xue, D.; Han, R.; Li, F. Study on sulfur-tolerant benzene hydrogenation catalyst based on Pt-encapsulated sodalite zeolite. React. Kinet. Mech. Catal. 2018, 124, 891–903. [Google Scholar] [CrossRef]
- Jiao, F.; Guo, H.; Chai, Y.; Awala, H.; Mintova, S.; Liu, C. Synergy between a sulfur-tolerant Pt/Al2O3 sodalite core–shell catalyst and a CoMo/Al2O3 catalyst. J. Catal. 2018, 368, 89–97. [Google Scholar] [CrossRef]
- Choi, M.; Lee, D.-H.; Na, K.; Yu, B.-W.; Ryoo, R. High catalytic activity of palladium(II)-exchanged mesoporous sodalite and NaA zeolite for bulky aryl coupling reactions: Reusability under aerobic conditions. Angew. Chem. Int. Ed. 2009, 48, 3673–3676. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.-J.; Chang, X.; Zhao, J.; Tian, H.; Yang, C.; Li, M.; Fu, Q.; Mu, R.; Gong, J. Activation and spillover of hydrogen on sub-1 nm palladium nanoclusters confined within sodalite zeolite for the semi-hydrogenation of alkynes. Angew. Chem. Int. Ed. 2019, 58, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Morozumi, K.; Elangovan, S.P.; Tanada, H.; Ando, H.; Okubo, T. Potassium-doped sodalite: A tectoaluminosilicate for the catalytic material towards continuous combustion of carbonaceous matters. App. Catal. B Environ. 2008, 77, 294–299. [Google Scholar] [CrossRef]
- Manique, M.C.; Lacerda, L.V.; Alves, A.K.; Bergmann, C.P. Biodiesel production using coal fly ash-derived sodalite as a heterogeneous catalyst. Fuel 2017, 190, 268–273. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.-S.; Yang, H.; Tan, Y.-X.; Zhang, J. Hybrid zeolitic imidazolate frameworks with catalytic active TO4 (T = Mo6+ or W6+) building blocks. Angew. Chem. Int. Ed. 2011, 50, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fu, H.-R.; Kang, Y.; Zhang, J. New approach towards zeolitic tetrazolate-imidazolate frameworks (ZTIFs) with uncoordinated N-heteroatom sites for high CO2 uptake. Chem. Commun. 2014, 50, 12065–12068. [Google Scholar] [CrossRef]
- Chen, J.-B.; Cui, T.-J.; Lin, G.; Wang, F.; Zhang, J. Sodalite-type metal-organic zeolite with uncoordinated N-sites as potential anticancer drug 5-fluorouracil (5-FU) delivery platform. Inorg. Chem. Commun. 2019, 109, 107560. [Google Scholar] [CrossRef]
- Peng, F.; Sun, Y.; Pickard, C.J.; Needs, R.J.; Wu, Q.; Ma, Y. Hydrogen clathrate structures in rare earth hydrides at high pressures: Possible route to room-temperature superconductivity. Phys. Rev. Lett. 2017, 119, 107001. [Google Scholar] [CrossRef]
- Somayazulu, M.; Ahart, M.; Mishra, A.K.; Geballe, Z.M.; Baldini, M.; Meng, Y.; Struzhkin, V.V.; Hemley, R.J. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 2019, 122, 027001. [Google Scholar] [CrossRef]
- Wang, H.; Tse, J.S.; Tanaka, K.; Iitaka, T.; Ma, Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Nat. Acad. Sci. USA 2012, 109, 6463–6466. [Google Scholar] [CrossRef]
- Fu, J.; Song, T.; Liang, X.; Zhao, G.; Liu, Z. Room temperature ferromagnetic half metal in Mn doped cluster-assembled sodalite phase of III-N compounds. J. Magn. Magn. Mater. 2020, 499, 166295. [Google Scholar] [CrossRef]
- Nakano, T.; Ishida, Y.; Hanazawa, A.; Nozue, Y. Antiferromagnetic phase transition of K-Rb alloy nanoclusters incorporated in sodalite. J. Korean Phys. Soc. 2013, 62, 2197–2201. [Google Scholar] [CrossRef]
- Nakano, T.; Suehiro, R.; Hanazawa, A.; Watanabe, K.; Watanabe, I.; Amato, A.; Pratt, F.L.; Nozue, Y. μSR Study on antiferromagnetism of alkali-metal clusters incorporated in zeolite sodalite. J. Phys. Soc. Jpn. 2010, 79, 073707-1–073707-4. [Google Scholar] [CrossRef]
- Nakano, T. Antiferromagnetic orderings of alkali-metal nanoclusters arrayed in sodalite crystal studied by μSR and other microscopic probes. J. Comput. Chem. Jpn. 2020, 19, 57–63. [Google Scholar] [CrossRef]
- Igarashi, M.; Nakano, T.; Goto, A.; Hashi, K.; Shimizu, T.; Hanazawa, A.; Nozue, Y. NMR property of rubidium loaded sodalite. J. Phys. Chem. Solids 2012, 73, 1534–1537. [Google Scholar] [CrossRef]
- Nakamura, K.; Koretsune, T.; Arita, R. Ab initio derivation of the low-energy model for alkali-cluster-loaded sodalites. Phys. Rev. B 2009, 80, 174420. [Google Scholar] [CrossRef]
- Srdanov, V.I.; Stucky, G.D.; Lippmaa, E.; Engelhardt, G. Evidence for an antiferromagnetic transition in a zeolite-supported cubic lattice of F centers. Phys. Rev. Lett. 1998, 80, 2449–2454. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Iversen, B.B.; Blaha, P.; Schwarz, K. Electronic structure of the sodium and potassium electrosodalites (Na/K)8(AlSiO4)6. Phys. Rev. B 2001, 64, 195102. [Google Scholar] [CrossRef]
- Tou, H.; Maniwa, Y.; Mizoguchi, K.; Damjanovic, L.; Srdanov, V.I. NMR studies on antiferromagnetism in alkali-electro-sodalite. J. Magn. Magn. Mater. 2001, 230, 1098–1100. [Google Scholar] [CrossRef]
- Komada, N.; Westrum, E.F., Jr.; Hemingway, B.S.; Zolotov, M.Y.; Semenov, Y.V.; Khodakovsky, I.L.; Anovitz, L.M. Thermodynamic properties of sodalite at temperatures from 15 K to 1000 K. J. Chem. Thermodyn. 1995, 27, 1119–1132. [Google Scholar] [CrossRef]
- Schliesser, J.; Lilova, K.; Pierce, E.M.; Wub, L.; Missimer, D.M.; Woodfield, B.F.; Navrotsky, A. Low temperature heat capacity and thermodynamic functions of anion bearing sodalites Na8Al6Si6O24X2 (X = SO4, ReO4, Cl, I). J. Chem. Thermodyn. 2017, 114, 14–24. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Helffrich, G.R.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaAlSiO4–NaCl. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef]
- Kimura, R.; Nghia, D.T.; Wakabayashi, J.; Elangovan, S.P.; Ogura, M.; Okubo, T. Nepheline synthesized from sodalite as diesel-soot combustion catalyst: Structure-property relationship study for an enhanced water tolerance. Bull. Chem. Soc. Jpn. 2012, 85, 527532. [Google Scholar] [CrossRef]
- Pekov, I.V.; Turchkova, A.G.; Lovskaya, E.V.; Chukanov, N.V. Zeolites of Alkaline Massifs; Ekost: Moscow, Russia, 2004. (In Russian) [Google Scholar]
- Hassan, I.; Antao, S.M.; Parise, J.B. Sodalite: High-temperature structures obtained from synchrotron radiation and Rietveld refinements. Am. Mineral. 2004, 89, 359–364. [Google Scholar] [CrossRef]
- Khajavi, S.; Sartipi, S.; Gascon, J.; Jansen, J.C.; Kapteijn, F. Thermostability of hydroxy sodalite in view of membrane applications. Microporous Mesoporous Mater. 2010, 132, 510–517. [Google Scholar] [CrossRef]
- Henderson, C.M.B.; Taylor, D. The thermal expansion of aluminate- and .aluminogermanate-sodalites. Mineral. Mag. 1978, 43, 429–431. [Google Scholar] [CrossRef][Green Version]
- Gesing, T.M. Structure and properties of tecto-gallosilicates II. Sodium chloride, bromide and iodide sodalites. Z. Kristallogr. 2007, 222, 289–296. [Google Scholar] [CrossRef]
- Gesing, T.M.; Schmidt, B.C.; Murshed, M.M. Temperature dependent structural and spectroscopic studies of sodium gallosilicate nitrite sodalite. Mater. Res. Bull. 2010, 45, 1618–1624. [Google Scholar] [CrossRef]
- Leardini, L.; Martucci, A.; Cruciani, G. The unusual thermal expansion of pure silica sodalite probed by in situ time-resolved synchrotron powder diffraction. Microporous Mesoporous Mater. 2012, 151, 163–171. [Google Scholar] [CrossRef]
- Martucci, A.; Leardini, L.; Alberti, A. Negative thermal expansion in trioxane silica sodalite (TRSS). Acta Cryst. 2011, A67, C248–C249. [Google Scholar] [CrossRef]
- Antao, S.; Hassan, I. Thermal analyses of sodalite, tugtupite, danalite and helvite. Can. Mineral. 2002, 40, 163–172. [Google Scholar] [CrossRef]
- Golovina, N.I.; Chukanov, N.V.; Raevskii, A.V.; Atovmyan, L.O. Prephase state in crystals of Prephase state in 2-bromo-2-nitropropane-1,3-diol: Structural aspects and IR spectra. J. Struct. Chem. 2000, 41, 238–242. [Google Scholar] [CrossRef]
- Golovina, N.I.; Raevskii, A.V.; Fedorov, B.S.; Gusakovskaya, I.G.; Trofimova, R.F.; Chukanov, N.V.; Atovmyan, L.O. Experimental study of structure-energy changes in molecules and crystals of 2,2-dinitropropane-1,3-diol caused by temperature variations. J. Solid State Chem. 2001, 151, 296–308. [Google Scholar] [CrossRef]
- Golovina, N.I.; Raevskii, A.V.; Fedorov, B.S.; Chukanov, N.V.; Shilov, G.V.; Leonova, L.S.; Tarasov, V.P.; Erofeev, L.N. Temperature-dependet structure-energetic changes in crystals of compounds with pyly(hydroxymethyl) grouping. J. Solid State Chem. 2002, 164, 301–312. [Google Scholar] [CrossRef]
- Zakharov, V.V.; Chukanov, N.V.; Larikova, T.S.; Shilov, G.V.; Korepin, A.G.; Pivkina, A.N.; Monogarov, K.A.; Korsunskiy, B.L.; Korchagin, D.V.; Aldoshin, S.M. Effect of polymorphic phase transitions on stability of energetic compounds. Thermal transformations of 2,4,6-tris(2,2,2-trinitroethylnitramino)-1,3,5-triazine. Russ. Chem. Bull. Int. Ed. 2020, 69, 118–124. [Google Scholar] [CrossRef]
- Zakharov, V.V.; Chukanov, N.V.; Shilov, G.V.; Malkov, G.V.; Larikova, T.S.; Nedel’ko, V.V.; Korepin, A.G.; Korsunskiy, B.L. Phase transformations of 2,4,6-tris(2,2,2-trinitroethylamino)-1,3,5-triazine. Russ. J. Phys. Chem. B 2021, 15, 622–629. [Google Scholar] [CrossRef]
- Robben, L.; Abrahams, I.; Fischer, M.; Hull, S.; Dove, M.T.; Gesing, T.M. Low-temperature anharmonicity and symmetry breaking in the sodalite |Na8I2 |[AlSiO4]6. Z. Kristallogr. 2018, 233, 1–11. [Google Scholar] [CrossRef]
- Depmeier, W. Aluminate sodalite Ca8[Al12O24](WO4)2 at room temperature. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, 40, 226–231. [Google Scholar] [CrossRef]
- Robben, L.; Wolpmann, M.; Bottke, P.; Petersen, H.; Šehović, M.; Gesing, T.M. Disordered but primitive gallosilicate hydro-sodalite: Structure and thermal behaviour of a framework with novel cation distribution. Microporous Mesoporous Mater. 2018, 256, 206–213. [Google Scholar] [CrossRef]
- Hermeler, G.; Buhl, J.-C.; Hoffmann, W. The influence of carbonate on the synthesis of an intermediate phase between sodalite and cancrinite. Catal. Today 1991, 8, 415–426. [Google Scholar] [CrossRef]
- Grader, C.; Buhl, J.-C. The intermediate phase between sodalite and cancrinite: Synthesis of nano-crystals in the presence of Na2CO3/TEA and its thermal- and hydrothermal stability. Microporous Mesoporous Mater. 2013, 171, 110–117. [Google Scholar] [CrossRef]
- Buhl, J.-C. Synthesis of a sulfate enclathrated zeolite with intermediate framework structure between sodalite and cancrinite. Z. Anorg. Allg. Chem. 2017, 643, 1030–1036. [Google Scholar] [CrossRef]
- Petersen, H.; Zhao, H.; Robben, L.; Kolb, U.; Gesing, T.M. An average structure model of the intermediate phase between sodalite and cancrinite. Z. Krist. Cryst. Mater. 2019, 234, 351–361. [Google Scholar] [CrossRef]
- Heiden, F.; Nielsen, U.G.; Warner, T.E. Synthesis and thermal stability of the sodalite Na6Zn2[Al6Si6O24](SO4)2 and its reaction with hydrogen. Microporous Mesoporous Mater. 2012, 161, 91–97. [Google Scholar] [CrossRef]
- Poltz, I.; Robben, L.; Buhl, J.-C.; Gesing, T.M. Synthesis, crystal structure and temperature-dependent behavior of gallogermanate tetrahydroborate sodalite |Na8(BH4)2|[GaGeO4]6. Microporous Mesoporous Mater. 2013, 174, 54–61. [Google Scholar] [CrossRef]
- Buhl, J.-C.; Luger, S. The properties of salt-filled sidalites. I. Thermal decomposition reactions of hydroxoborate sodalite. Thermochim. Acta 1990, 168, 253–259. [Google Scholar] [CrossRef]
- Buhl, J.-C. The properties of salt-filled sodalites. Part 3. Synthesis and thermal behaviour of basic and non-basic carbonate enclathrated sodalites. Thermochim. Acta 1993, 219, 205–214. [Google Scholar] [CrossRef]
- Braunbarth, C.M.; Behrens, P.; Felsche, J.; van de Goor, G. Phase transitions and thermal behaviour of silica sodalites. Solid State Ion. 1997, 101–103, 1273–1277. [Google Scholar] [CrossRef]
- Xiong, Y. Solubility constants of hydroxyl sodalite at elevated temperatures evaluated from hydrothermal experiments: Applications to nuclear waste isolation. Appl. Geochem. 2016, 74, 138–143. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Aoki, S.; Nagase, T. Thermal behavior of modulated haüyne from Eifel, Germany: In situ high–temperature single–crystal X–ray diffraction study. J. Mineral. Pet. Sci. 2018, 113, 51–55. [Google Scholar] [CrossRef]
- Bokiy, G.B.; Borutskiy, B.E. (Eds.) Minerals V(2): Feldspathoids; Nauka: Moscow, Russa, 2003. (In Russian) [Google Scholar]
- Johnson, G.M.; Mead, P.J.; Weller, M.T. Synthesis of a range of anion-containing gallium and germanium sodalites. Microporous Mesoporous Mater. 2000, 38, 445–460. [Google Scholar] [CrossRef]
- Nenoff, T.M.; Harrison, W.T.A.; Gier, T.E.; Keder, N.L.; Zaremba, C.M.; Srdanov, V.I.; Nicol, J.M.; Stucky, G.D. Structural and chemical investigations of Na3(ABO4)3·4H2O-Type sodalite Phases. Inorg. Chem. 1994, 33, 2472–2480. [Google Scholar] [CrossRef]
- Newsam, J.M.; Jorgensen, J.D. Gallosilicate sodalite—further syntheses and structural details. Zeolites 1987, 7, 569–573. [Google Scholar] [CrossRef]
- McCusker, L.B.; Meier, W.M.; Suzuki, K.; Shin, S. The crystal structure of a sodium gallosilicate sodalite. Zeolites 1986, 6, 388–391. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, S.M.A.I.V. Infrared spectroscopy as a tool for the analysis of framework topology and extraframework components in microporous cancrinite- and sodalite-related aluminosilicates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287, 121993. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2014; 1716p. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; New York, NY, USA; London, UK, 2016; 1109p. [Google Scholar] [CrossRef]
- Ling, Z.C.; Wang, A.; Jolliff, B.L. Mineralogy and geochemistry of four lunar soils by laser-Raman study. Icarus 2011, 211, 101–113. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Rastsvetaeva, R.K.; Zubkova, N.V.; Vigasina, M.F.; Pekov, I.V.; Zolotarev, A.A.; Mikhailova, J.A.; Aksenov, S.M. Spectroscopic characterization of extra-framework hydrated proton complexes with the extremely strong hydrogen bonds in microporous silicate minerals. J. Raman Spectrosc. 2024, 55, 581–597. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O–H stretching frequencies and O–H···O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, X.; Liu, L.-P.; Zhang, W.-P. A novel chemosynthetic method for the production of nitrate sodalite. Mater. Lett. 2010, 64, 2667–2669. [Google Scholar] [CrossRef]
- Borhade, V.; Wakchaure, S.G.; Dholi, A.G. One pot synthesis and crystal structure of aluminosilicate mixed chloro-iodo sodalite. Indian J. Phys. 2010, 84, 133–141. [Google Scholar] [CrossRef]
- Lau, C.; Brück, S.; Mai, H.-J.; Kynast, U. Incorporation of tungsten trioxide into faujasites and sodalites by solid-state reactions. Microporous Mesoporous Mater. 2001, 47, 339–344. [Google Scholar] [CrossRef]
- Peng, H.; Ding, M.; Vaughan, J. The anion effect on zeolite LTA to sodalite phase transformation. Ind. Eng. Chem. Res. 2018, 57, 10292–10303. [Google Scholar] [CrossRef]
- Warner, T.E.; Bancells, M.M.; Lund, P.B.; Lund, F.W.; Ravnsbæk, D.B. On the thermal stability of manganese(II) sulfate and its reaction with zeolite A to form the sodalite Na6Mn2[Al6Si6O24](SO4)2. J. Solid State Chem. 2019, 277, 434–440. [Google Scholar] [CrossRef]
- Beagley, B.; Henderson, C.M.B.; Taylor, D. The crystal structures of aluminosilicate-sodalites: X-ray diffraction studies and computer modelling. Mineral. Mag. 1982, 46, 459–464. [Google Scholar] [CrossRef]
- Brenchley, M.E.; Weller, M.T. Synthesis and structures of M8[ALSiO4]6·(XO4)2, M = Na, Li, K.; X = Cl, Mn sodalites. Zeolites 1994, 14, 682–686. [Google Scholar] [CrossRef]
- Borhade, A.V.; Kshirsagar, T.A.; Dholi, A.G. Eco-friendly synthesis of aluminosilicate bromo sodalite from waste coal fly ash for the removal of copper and methylene blue dye. Arab. J. Sci. Eng. 2017, 42, 4479–4491. [Google Scholar] [CrossRef]
- Nielsen, N.C.; Bildsøe, H.; Jakobsen, H.J.; Norby, P. 7Li, 23Na, and 27Al quadrupolar interactions in some aluminosilicate sodalites from MAS n.m.r. spectra of satellite transitions. Zeolites 1991, 11, 622–632. [Google Scholar] [CrossRef]
- Stein, A.; Ozin, G.A.; Macdonald, P.M.; Stucky, G.D.; Jelinek, R. Silver, sodium halosodalites: Class A sodalites. J. Am. Chem. Soc. 1992, 114, 5171–5186. [Google Scholar] [CrossRef]
- Pierce, E.M.; Lukens, W.W.; Fitts, J.P.; Jantzen, C.M.; Tang, G. Experimental determination of the speciation, partitioning, and release of perrhenate as a chemical surrogate for pertechnetate from a sodalite-bearing multiphase ceramic waste form. Appl. Geochem. 2014, 42, 47–59. [Google Scholar] [CrossRef]
- Mead, P.J.; Weller, M.T. Synthesis, structure, and characterization of halate sodalites: M8[AlSiO4]6(XO3)x(OH)2−x; M = Na, Li, or K.; X =Cl, Br, or I. Zeolites 1995, 15, 561–568. [Google Scholar] [CrossRef]
- Murshed, M.M.; Baer, A.J.; Gesing, T.M. Isomorphous framework cation substitution in the alumosilicatesodalites: Synthesis, structural and spectroscopic studies of nitrite containing phases. Z. Krist. 2008, 223, 575–583. [Google Scholar] [CrossRef]
- Latturner, S.E.; Sachleben, J.; Iversen, B.B.; Hanson, J.; Stucky, G.D. Covalent guest−framework interactions in heavy metal sodalites: Structure and properties of thallium and silver sodalite. J. Phys. Chem. B 1999, 103, 7135–7144. [Google Scholar] [CrossRef]
- Baerlocher, C.; Meier, W.M. Synthese und Kristallstruktur von Tetramethylammonium-Sodalith. Helv. Chim. Acta 1969, 52, 1853–1860. [Google Scholar] [CrossRef]
- Chong, S.; Peterson, J.; Nam, J.; Riley, B.; McCloy, J. Synthesis and characterization of iodosodalite. J. Am. Ceram. Soc. 2017, 100, 2273–2284. [Google Scholar] [CrossRef]
- Fejes, P.; Kiricsi, I.; Kovács, K.; Lázár, K.; Marsi, I.; Oszkó, A.; Rockenbauer, A.; Schay, Z. Incorporation of iron in sodalite structures and their transformation into other iron containing zeolites. Synthesis of Fe-NaA (LTA). Appl. Catal. A Gen. 2002, 223, 147–160. [Google Scholar] [CrossRef]
- Paillaud, J.-L.; Marichal, C.; Roux, M.; Baerlocher, C.; Chézeau, J.M. Tripling of the unit cell volume of the non-centrosymmetric AlPO4-SOD after Dehydration: A structural study of a reversible process. J. Phys. Chem. B 2005, 109, 11893–11899. [Google Scholar] [CrossRef]
- Roux, M.; Marichal, C.; Paillaud, J.-L.; Fernandez, C.; Baerlocher, C.; Chézeau, J.-M. Structural investigation by multinuclear solid state NMR and X-ray diffraction of as-synthesized, dehydrated, and calcined AlPO4-SOD. J. Phys. Chem. B 2001, 105, 9083–9092. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Lok, B.M.; Patton, R.L.; Wilson, S.T. Aluminophosphate Molecular Sieves and the Periodic Table; Elsevier: Amsterdam, The Netherlands, 1986; pp. 103–112. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Han, S.; Smith, J.V.; Pluth, J.J.; Richardson, J.W. Crystal structure of MAPO-20 sodalite; theoretical analysis of three-color ordering of Mg, Al and P in a sodalite unit. Eur. J. Mineral. 1990, 2, 787–798. [Google Scholar] [CrossRef]
- Brenchley, M.E.; Weller, M.T. Synthesis and structure of sulfide aluminate sodalites. J. Mater. Chem. 1992, 2, 1003. [Google Scholar] [CrossRef]
- Ponomarev, V.I.; Kheiker, D.M.; Belov, N.V. Crystal structure of tetracalcium trialuminate—The aluminate analog of sodalite. Sov. Phys. Crystallogr. 1971, 15, 799–801. [Google Scholar]
- Dann, S.E.; Weller, M.T. The preparation, characterisation and structure of Ca8[AlO2]12Te2 and Cd8[AlO2]12Te2; two new members of the sodalite family. J. Mater. Chem. 1998, 8, 1029–1031. [Google Scholar] [CrossRef]
- Depmeier, W. Structure of cubic aluminate sodalite Ca8[Al12O24](WO4)2 in comparison with its orthorhombic phase and with cubic Sr8[Al12O24](CrO4)2. Acta Crystallogr. Sect. B Struct. Sci. 1988, 44, 201–207. [Google Scholar] [CrossRef]
- van Smaalen, S.; Dinnebier, R.; Katzke, H.; Depmeier, W. Structural characterization of the high-temperature phase transitions in Ca8[Al12O24](MoO4)2 aluminate sodalite using X-ray powder diffraction. J. Solid State Chem. 1997, 129, 130–143. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Parise, J.B. Chromate aluminate sodalite, Ca8[Al12O24](CrO4)2: Phase transitions and high-temperature structural evolution of the cubic phase. Can. Mineral. 2004, 42, 1047–1056. [Google Scholar] [CrossRef]
- Saalfeld, H.; Depmeier, W. Silicon-Free Compounds with Sodalite Structure. Krist. Tech. 1972, 7, 229–233. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T. The structures of strontium tellurite and strontium telluride aluminate sodalites studied by powder neutron diffraction, EXAFS, IR and MAS NMR spectroscopies. J. Mater. Chem. 1996, 6, 1717. [Google Scholar] [CrossRef]
- Depmeier, W.; Bührer, W. Aluminate sodalites: Sr8[Al12O24](MoO4)2 (SAM) at 293, 423, 523, 623 and 723 K and Sr8[Al12O24](WO4)2 (SAW) at 293 K. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 197–206. [Google Scholar] [CrossRef]
- Depmeier, W.; Schmid, H.; Setter, N.; Werk, M.L. Structure of cubic aluminate sodalite, Sr8[Al12O24](CrO4)2. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 2251–2255. [Google Scholar] [CrossRef]
- Többens, D.M.; Depmeier, W. Superstructure of strontium chromate aluminate sodalite at low temperatures. Z. Krist. Cryst. Mater. 2001, 216, 586–590. [Google Scholar] [CrossRef]
- Többens, D.M.; Depmeier, W. The intermediate phase of strontium chromate aluminate sodalite. Z. Krist. Cryst. Mater. 2001, 216, 611–615. [Google Scholar] [CrossRef]
- Scheikowski, M.; Müller-Buschbaum, H. Zur Kristallchemie der Blei-Lanthanoid-Oxoaluminate. Zur Kenntnis von Pb2HoAl3O8 und Pb2LuAl3O8. Z. Anorg. Allg. Chem. 1993, 619, 1755–1758. [Google Scholar] [CrossRef]
- King, R.S.P.; Dann, S.E.; Elsegood, M.R.J.; Kelly, P.F.; Mortimer, R.J. The synthesis, full characterisation and utilisation of template-free silica sodalite, a novel polymorph of silica. Chem. Eur. J. 2009, 15, 5441–5443. [Google Scholar] [CrossRef] [PubMed]
- Moteki, T.; Chaikittisilp, W.; Shimojima, A.; Okubo, T. Silica sodalite without occluded organic matters by topotactic conversion of lamellar precursor. J. Am. Chem. Soc. 2008, 130, 15780–15781. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Sakai, R.; Enomoto, S.; Mino, T.; Sugimura, N.; Gotoh, T.; Wada, H.; Shimojima, A.; Kuroda, K. Encapsulation of Cu nanoparticles in nanovoids of plate-like silica sodalite through interlayer condensation of Cu2+ ion-exchanged layered silicate RUB-15. Dalton Trans. 2020, 49, 8067–8074. [Google Scholar] [CrossRef]
- Sato, M.; Kojima, E.; Uehara, H.; Miyake, M. Si,Al solid solution in sodalite: Synthesis, 29Si NMR and X-ray structure. Stud. Surf. Sci. Catal. 1997, 105, 509–516. [Google Scholar] [CrossRef]
- Richardson, J.W.; Pluth, J.J.; Smith, J.V.; Dytrych, W.J.; Bibby, D.M. Conformation of ethylene glycol and phase change in silica sodalite. J. Phys. Chem. 1988, 92, 243–247. [Google Scholar] [CrossRef]
- Hong, S.B.; Camblor, M.A.; Davis, M.E. Host–guest Interactions in pure-silica and aluminosilicate sodalites containing ethylene glycol as a guest molecule. J. Am. Chem. Soc. 1997, 119, 761–770. [Google Scholar] [CrossRef]
- Bibby, D.M.; Dale, M.P. Synthesis of silica-sodalite from non-aqueous systems. Nature 1985, 317, 157–158. [Google Scholar] [CrossRef]
- Murshed, M.M.; Gesing, T.M. Gallium substitution in the alumosilicate framework: Synthesis and structural studies of hydro sodalites. Z. Kristallogr. 2008, 223, 178–185. [Google Scholar] [CrossRef]
- Johnson, G.M.; Weller, M.T. A powder neutron diffraction study of lithium-substituted gallosilicate and aluminogermanate halide sodalites. Inorg. Chem. 1999, 38, 2442–2450. [Google Scholar] [CrossRef]
- Murshed, M.M.; Gesing, T.M. Isomorphous gallium substitution in the alumosilicate sodalite framework: Synthesis and structural studies of chloride and bromide containing phases. Z. Krist. 2007, 222, 341–349. [Google Scholar] [CrossRef]
- Borhade, A.V.; Dholi, A.G.; Wakchaure, S.G. Synthesis, characterization and crystal structure of gallosilicate perchlorate sodalite. Int. J. Chem. 2010, 2, 2–15. [Google Scholar] [CrossRef][Green Version]
- Borhade, A.V.; Wakchaure, S.G. Synthesis and characterization of gallosilicate sodalite containing NO2– ions. Int. J. Chem. Biol. Eng. 2010, 3, 51–56. [Google Scholar]
- Buhl, J.-C.; Gesing, T.M.; Höfs, T.; Rüscher, C.H. Synthesis and crystal structure of gallosilicate- and aluminogermanate tetrahydroborate sodalites Na8[GaSiO4]6(BH4)2 and Na8[AlGeO4]6(BH4)2. J. Solid State Chem. 2006, 179, 3877–3882. [Google Scholar] [CrossRef]
- Borhade, A.V.; Wakchaure, S.G. Synthesis, crystal structure and characterization of Na2.02Ag5.98[GaSiO4]6(NO2)2 and Na3.12K4.88[GaSiO4]6(NO2)2 sodalites. Indian J. Pure Appl. Phys. 2011, 49, 563–567. [Google Scholar]
- Armstrong, J.A.; Weller, M.T. New sodalite frameworks; synthetic tugtupite and a beryllosilicate framework with a 3:1 Si:Be ratio. Dalton Trans. 2006, 24, 2998–3005. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T. Synthesis and structure of cadmium chalcogenide beryllosilicate sodalites. Inorg. Chem. 1996, 35, 555–558. [Google Scholar] [CrossRef]
- Nenoff, T.M.; Harrison, W.T.A.; Gier, T.E.; Stucky, G.D. Room-temperature synthesis and characterization of new ZnPO and ZnAsO sodalite open frameworks. J. Am. Chem. Soc. 1991, 113, 378–379. [Google Scholar] [CrossRef]
- Holden, M.A.; Cubillas, P.; Attfield, M.P.; Gebbie, J.T.; Anderson, M.W. Growth mechanism of microporous zincophosphate sodalite revealed by in situ atomic force microscopy. J. Am. Chem. Soc. 2012, 134, 13066–13073. [Google Scholar] [CrossRef]
- Gier, T.E.; Harrison, W.T.A.; Stucky, G.D. The synthesis and structure of some new sodalites: The lithium haloberyllophosphates and -arsenates. Angew. Chem. Int. Ed. 1991, 30, 1169–1171. [Google Scholar] [CrossRef]
- Wiebcke, M.; Sieger, P.; Felsche, J.; Engelhardt, G.; Behrens, P.; Schefer, J. Sodium aluminogermanate hydroxosodalite hydrate Na6+x[Al6Ge6O24](OH)x·nH2O (x = 1.6, n = 3.0): Synthesis, phase transitions and dynamical disorder of the hydrogen dihydroxide anion, H3O2?, in the cubic high-temperature form. Z. Anorg. Allg. Chem. 1993, 619, 1321–1329. [Google Scholar] [CrossRef]
- Fleet, M.E. Structures of sodium alumino-germanate sodalites [Na8(Al6Ge6O24)A2, A = Cl, Br, I]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 843–847. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Demyanets, L.N.; Uvarova, T.G.; Belov, N.V. Crystal structure of Ge Sodalite Na8Al6Ge6O24(OH)2. Kristallografiya 1982, 27, 995–996. (In Russian) [Google Scholar]
- Borhade, A.V.; Dholi, A.G.; Tope, D.R.; Wakchaure, S.G. Synthesis and characterization of perchlorate enclathrated aluminogermanate sodalite and its potassium and silver derivatives. J. Anal. Sci. Technol. 2012, 3, 95. [Google Scholar] [CrossRef]
- Bachmann, S.; Buhl, J.-C. Crystallization, characterization and structure of nitrite aluminogermanate sodalite Na8[AlGeO4]6(NO2)2. Microporous Mesoporous Mater. 1999, 28, 35–47. [Google Scholar] [CrossRef]
- Camblor, M.A.; Lobo, R.F.; Koller, H.; Davis, M.E. Synthesis and characterization of zincosilicates with the SOD topology. Chem. Mater. 1994, 6, 2193–2199. [Google Scholar] [CrossRef]
- Feng, P.; Bu, X.; Stucky, G.D. Hydrothermal syntheses and structural characterization of zeolite analogue compounds based on cobalt phosphate. Nature 1997, 388, 735–741. [Google Scholar] [CrossRef]
- Bu, X.; Gier, T.E.; Feng, P.; Stucky, G.D. Template control of framework topology and charge in new phosphate- and arsenate-based sodalite analogs. Microporous Mesoporous Mater. 1998, 20, 371–379. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. The ionothermal synthesis of cobalt aluminophosphate zeolite frameworks. J. Am. Chem. Soc. 2006, 128, 2204–2205. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, T.; Bu, X. Arsenate Zeolite analogues with 11 topological types. J. Am. Chem. Soc. 2001, 123, 8608–8609. [Google Scholar] [CrossRef]
- Poltz, I.; Robben, L.; Buhl, J.-C. Synthesis and characterization of B(OH)4·H2O enclathered gallogermanate sodalite. Acta Crystallogr. Sect. A 2010, 66, s237. [Google Scholar] [CrossRef]
- Bu, X.; Feng, P.; Gier, T.E.; Zhao, D.; Stucky, G.D. Hydrothermal synthesis and structural characterization of zeolite-like structures based on gallium and aluminum germanates. J. Am. Chem. Soc. 1998, 120, 13389–13397. [Google Scholar] [CrossRef]
- Vaughan, D.E.W.; Yennawar, H.P.; Perrotta, A.J. Synthesis and structure of large optically clear crystals of gallogermanate sodalite. Cryst. Growth Des. 2006, 6, 2072–2075. [Google Scholar] [CrossRef]
- Poltz, I.; Robben, L.; Buhl, J.-C.; Gesing, T.M. Synthesis, crystal structure and temperature-dependent properties of gallogermanate nitrite sodalite |Na8(NO2)2|[GaGeO4]6. Microporous Mesoporous Mater. 2015, 203, 100–105. [Google Scholar] [CrossRef]
- Fouassier, C.; Levasseur, A.; Joubert, J.C.; Muller, J.; Hagenmuller, P. Les Systémes B2O3·MO·MS boracites (M = Mg, Mn, Fe, Cd) et Sodalites (M = Co, Zn). Z. Anorg. Allg. Chem. 1970, 375, 202–208. [Google Scholar] [CrossRef]
- Pan, R.; Cheng, J.-W.; Yang, B.-F.; Yang, G.-Y. CsBxGe6–xO12 (x = 1): A zeolite sodalite-type borogermanate with a high Ge/B ratio by partial boron substitution. Inorg. Chem. 2017, 56, 2371–2374. [Google Scholar] [CrossRef]
- Zheng, S.-T.; Wu, T.; Zuo, F.; Chou, C.; Feng, P.; Bu, X. Mimicking zeolite to its core: Porous sodalite cages as hangers for pendant trimeric M3(OH) Clusters (M = Mg, Mn, Co, Ni, Cd). J. Am. Chem. Soc. 2012, 134, 1934–1937. [Google Scholar] [CrossRef]
- Zhang, T.-Z.; Zhang, Z.-M.; Lu, Y.; Fu, H.; Wang, E.-B. Expansion of sodalite-type metal–organic frameworks with heterometallic metal–oxo cluster and its cation exchange property. CrystEngComm 2013, 15, 459–462. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Fris, T. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. Int. Ed. 2010, 49, 9640–9643. [Google Scholar] [CrossRef]
- Martinez, V.; Karadeniz, B.; Biliškov, N.; Lončarić, I.; Muratović, S.; Žilić, D.; Avdoshenko, S.M.; Roslova, M.; Popov, A.A.; Užarević, K. Tunable fulleretic sodalite MOFs: Highly efficient and controllable entrapment of C60 fullerene via mechanochemistry. Chem. Mater. 2020, 32, 10626–10640. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A catalytically active zeolitic imidazolate framework of sodalite topology with unsubstituted linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef] [PubMed]
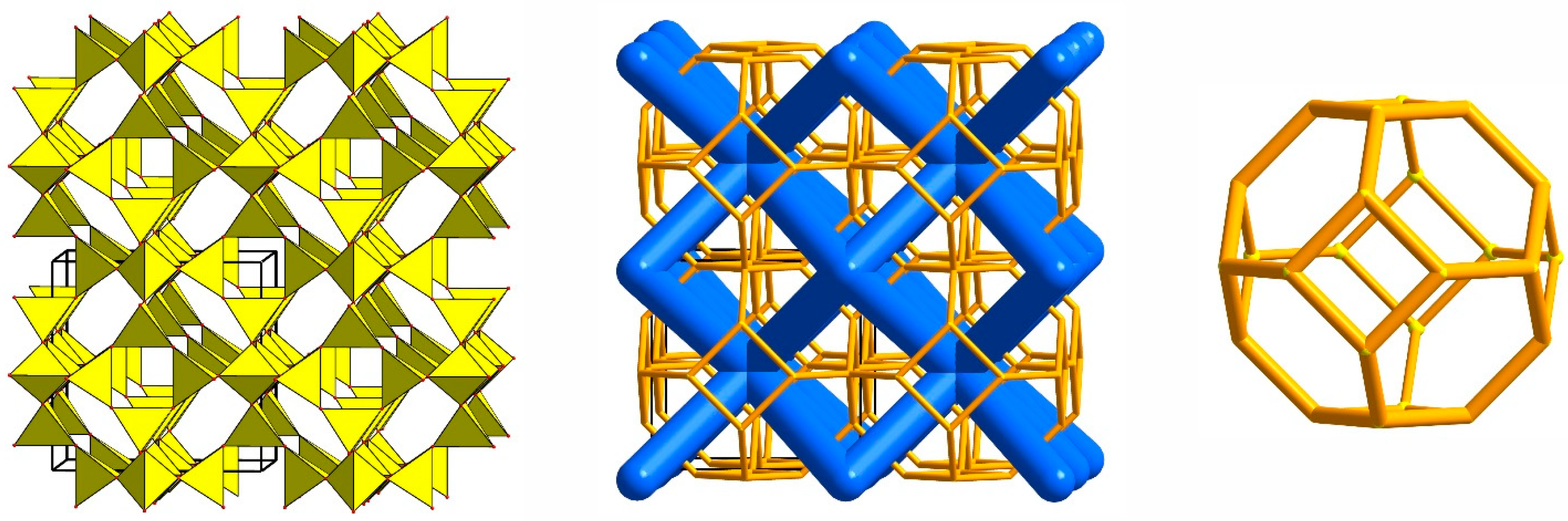
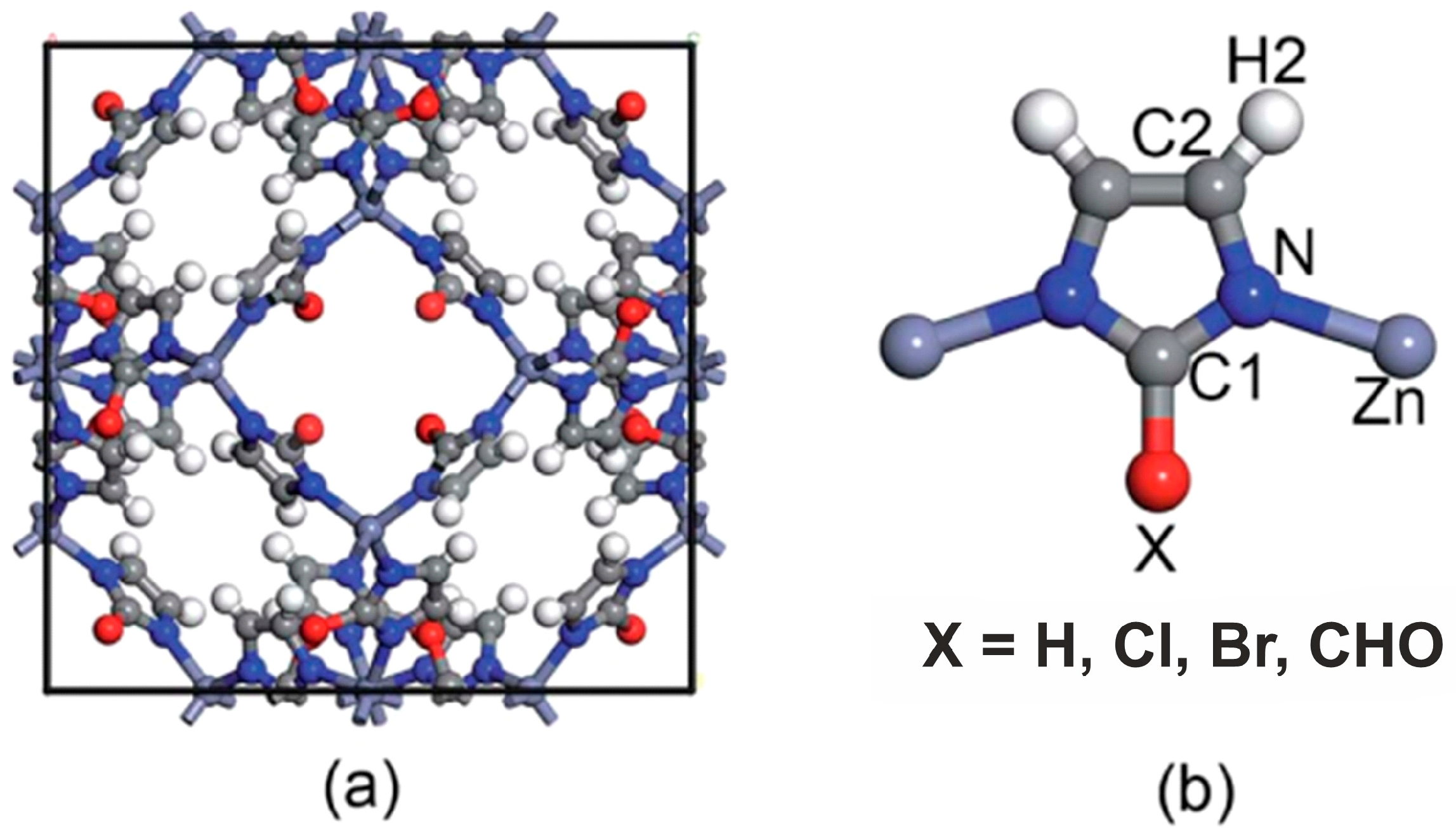
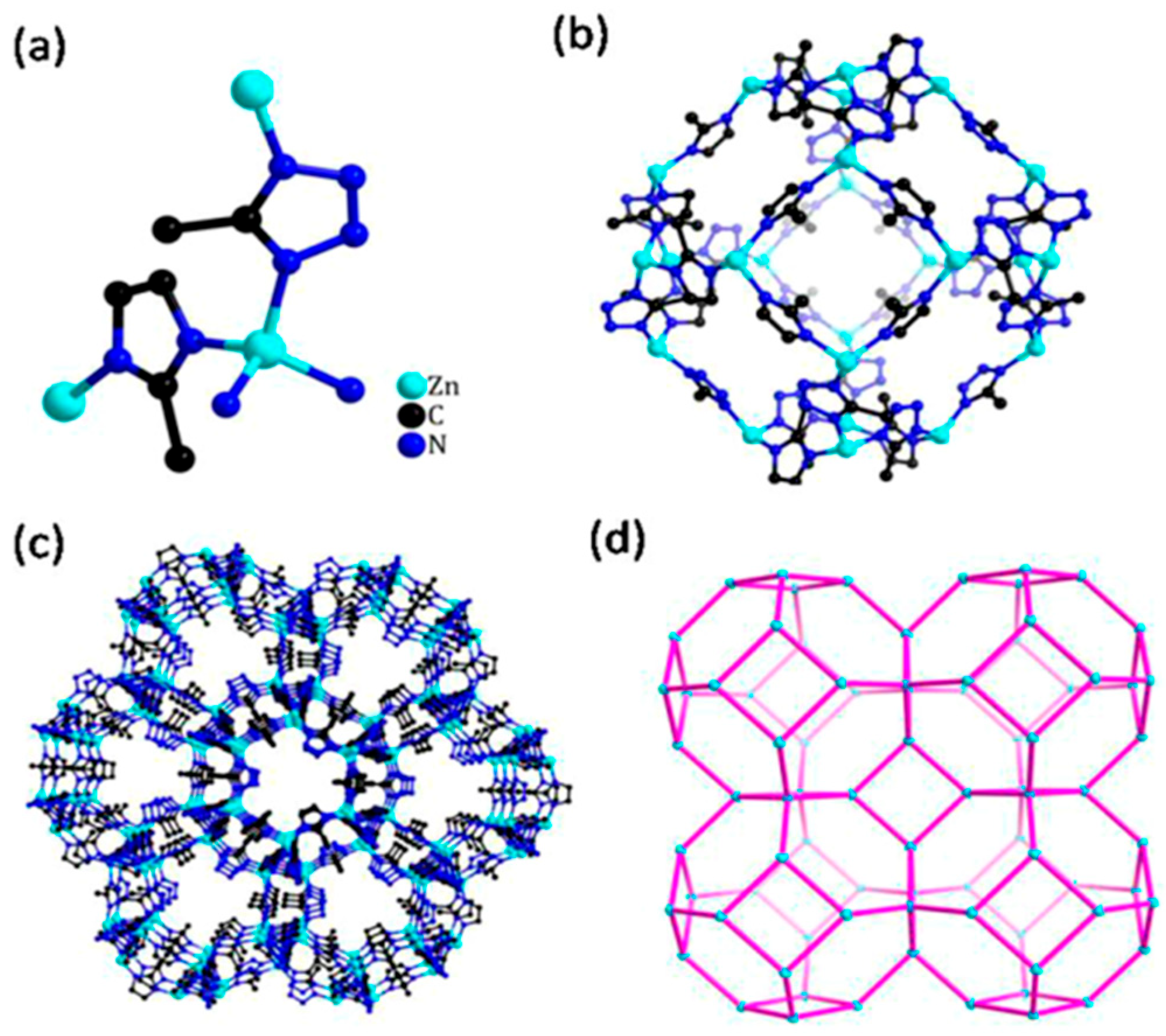
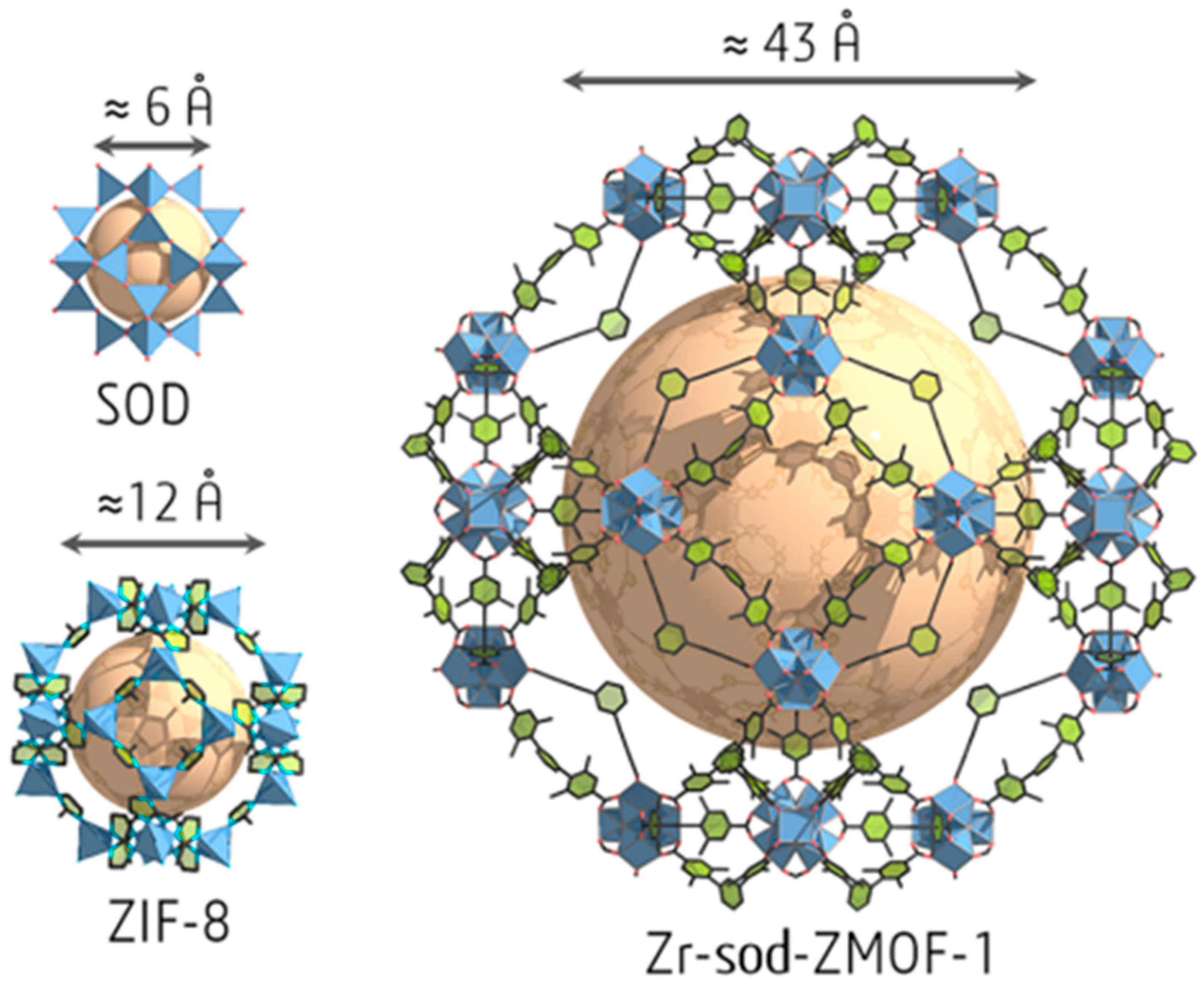
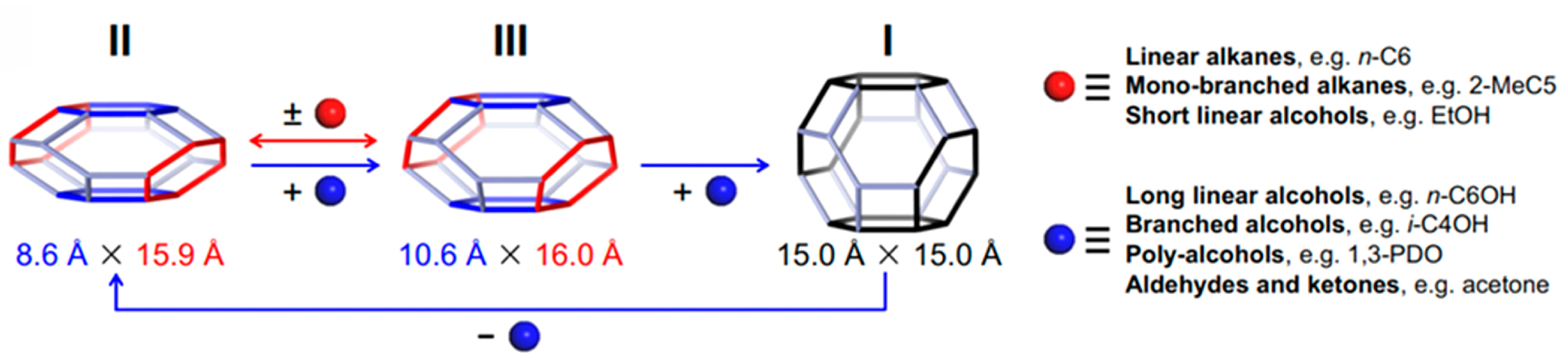





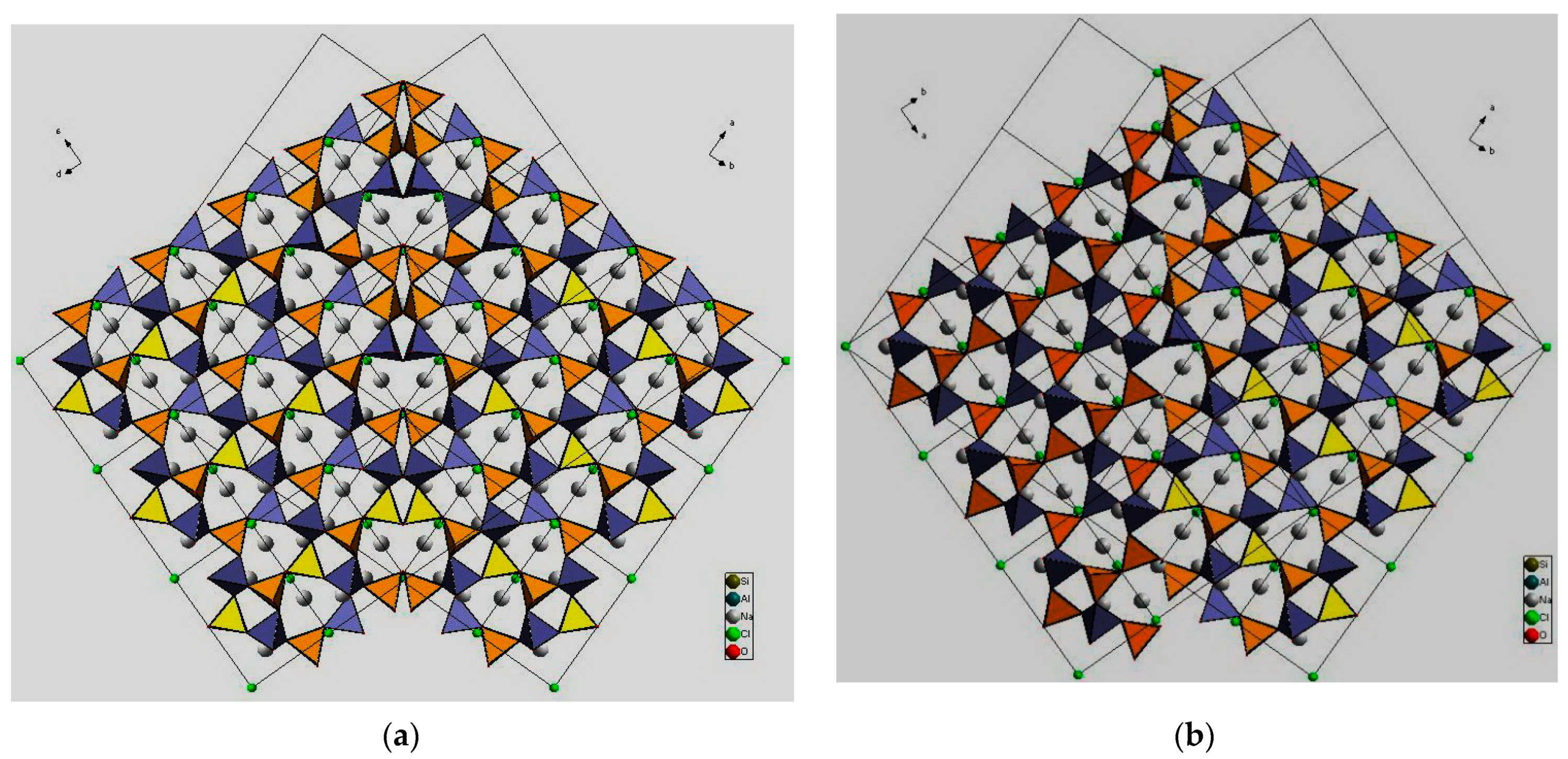

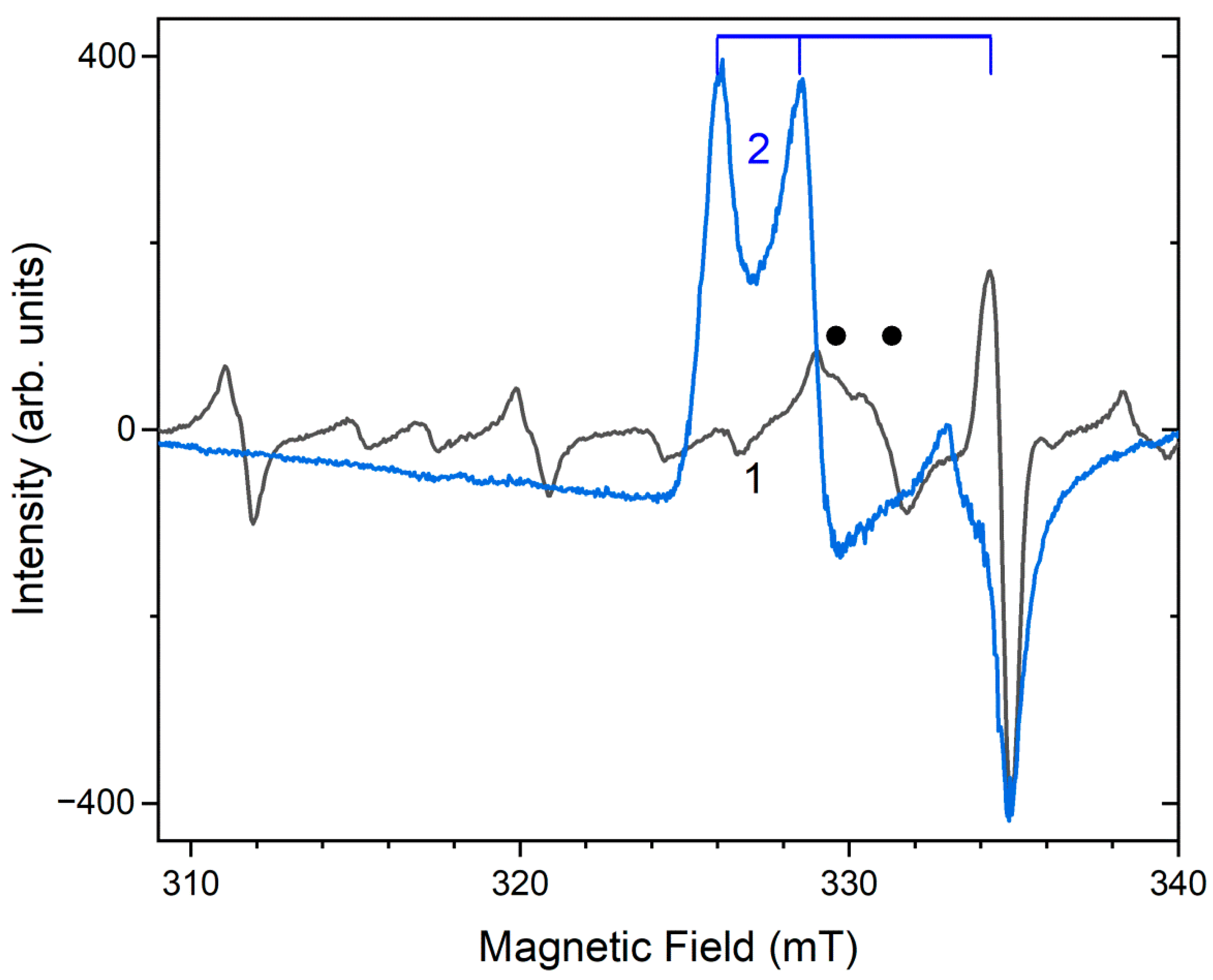
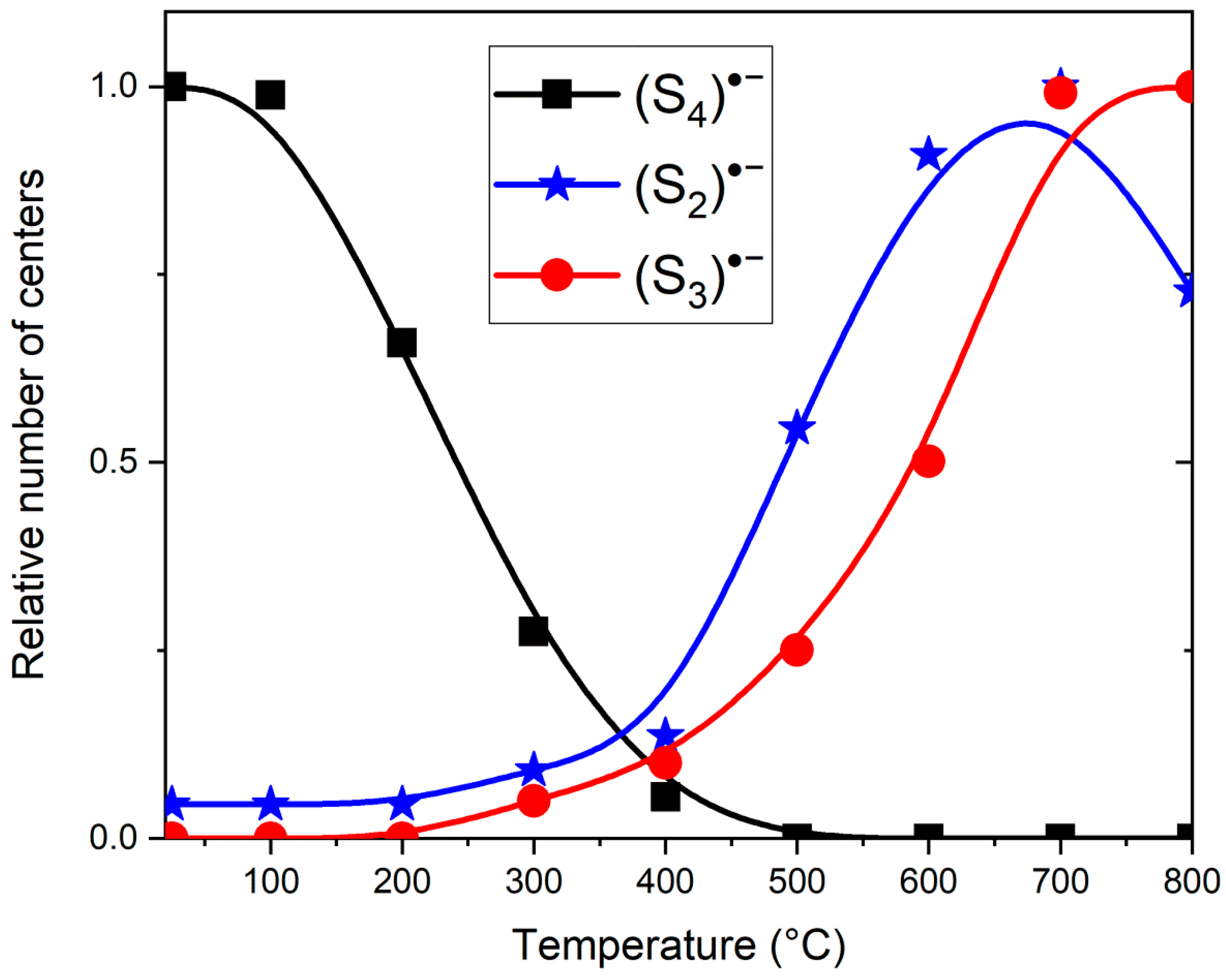


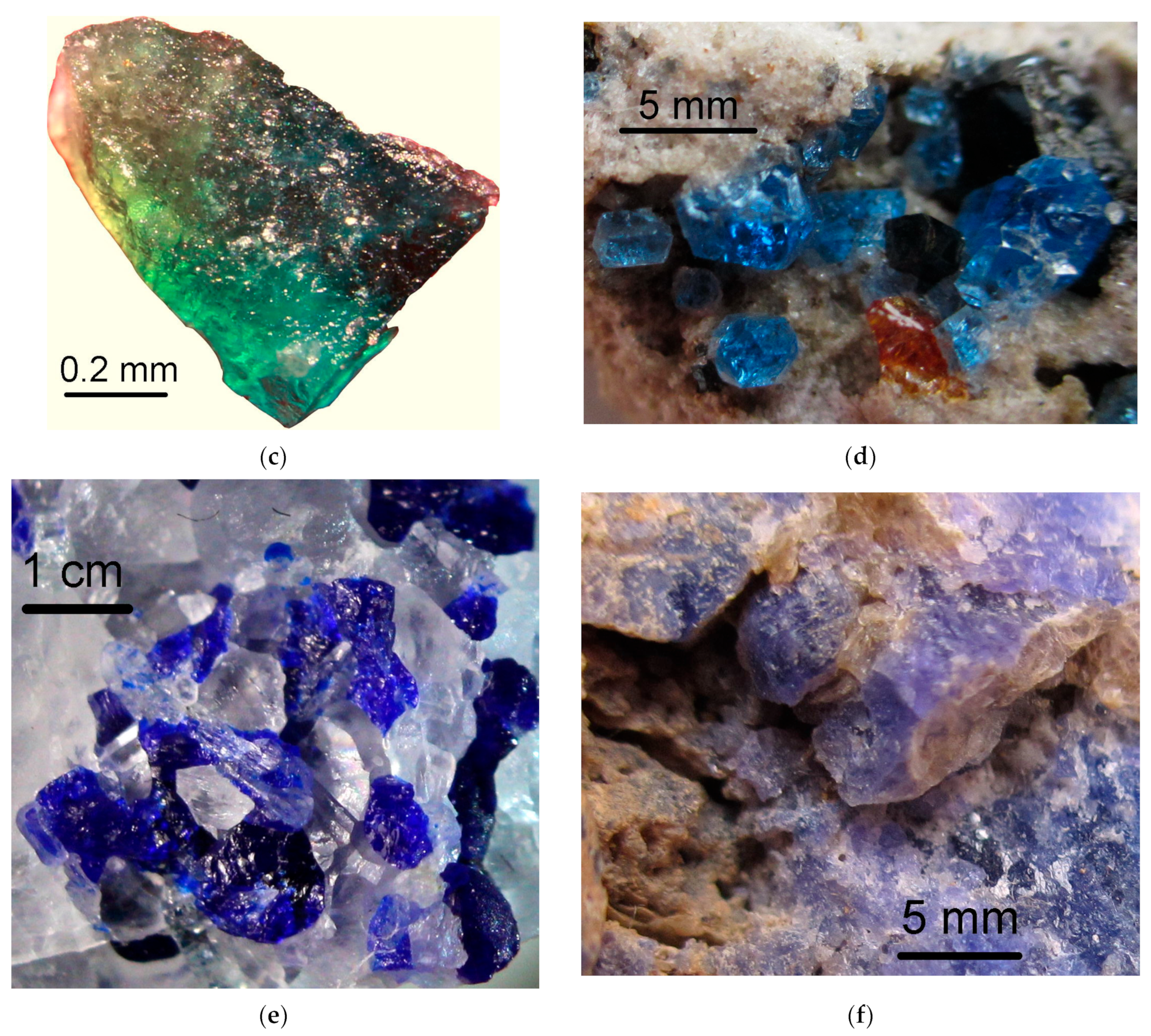
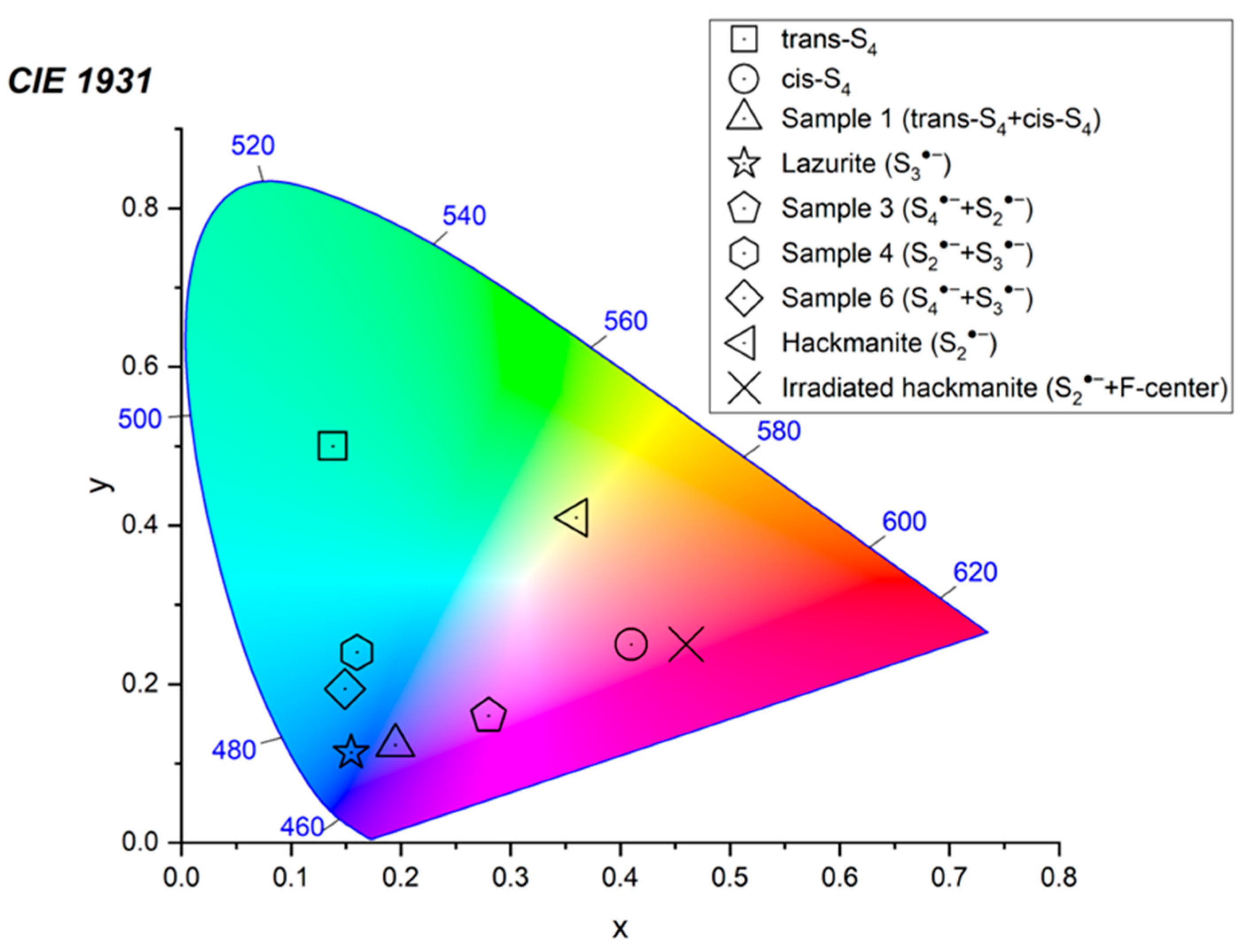


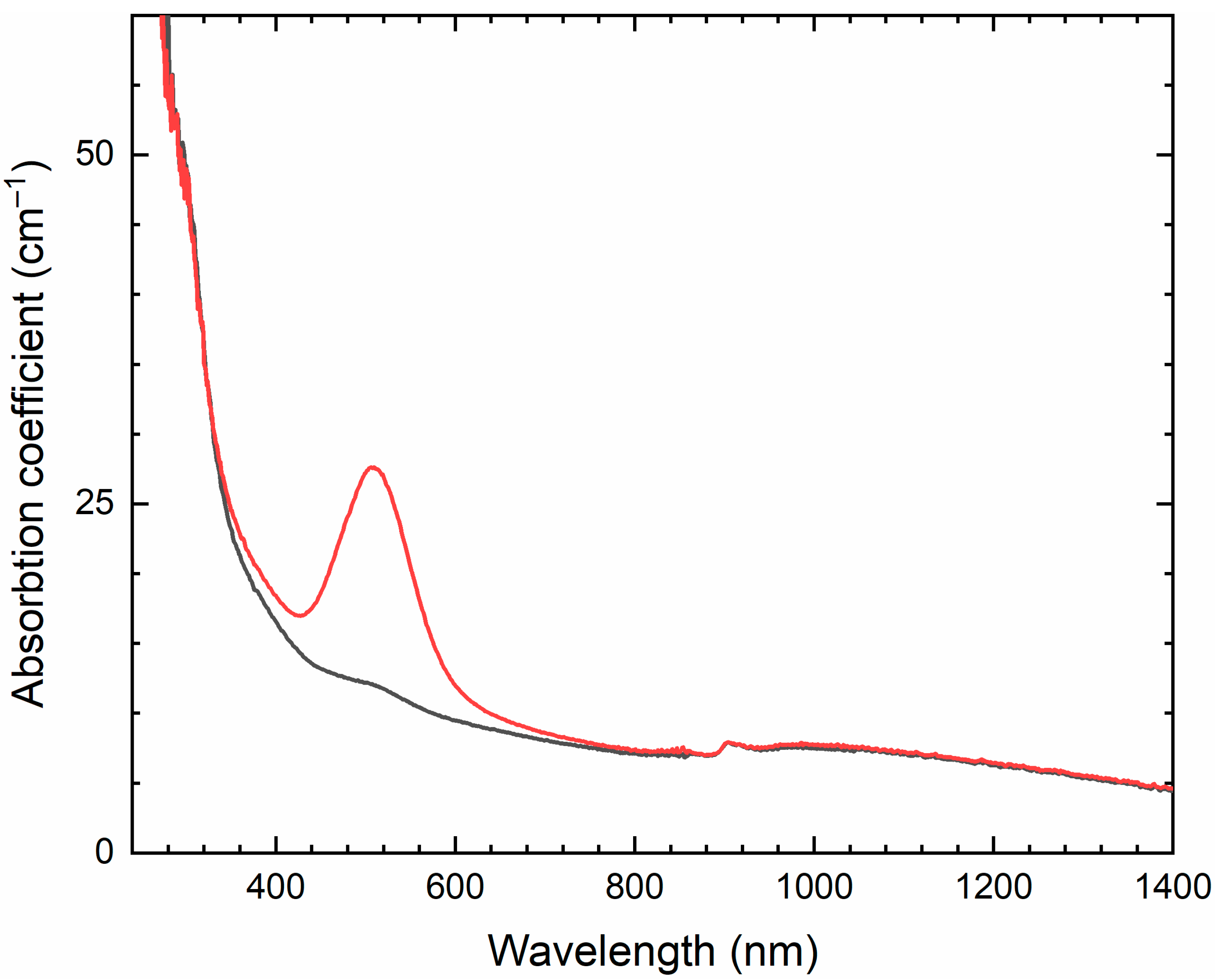



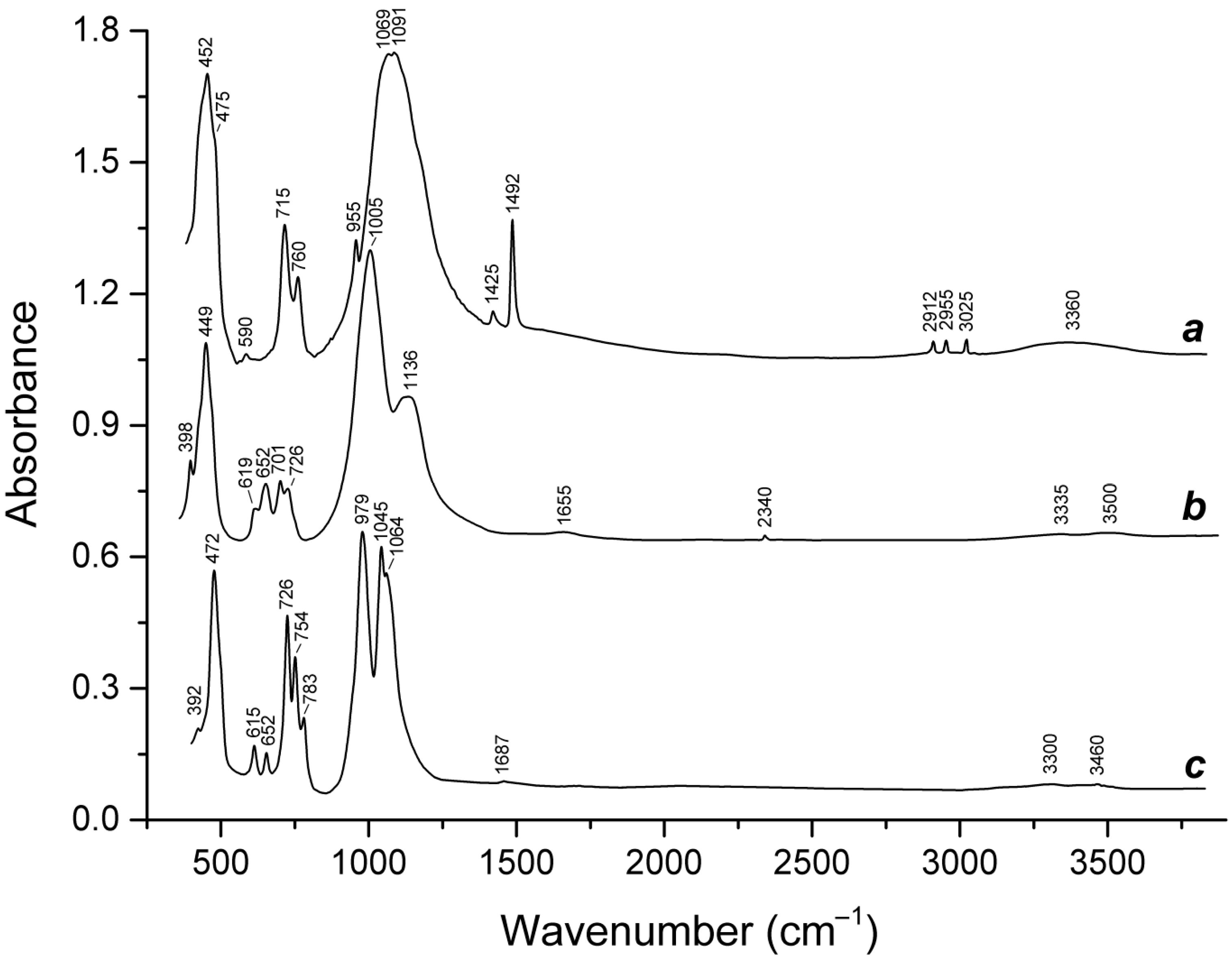

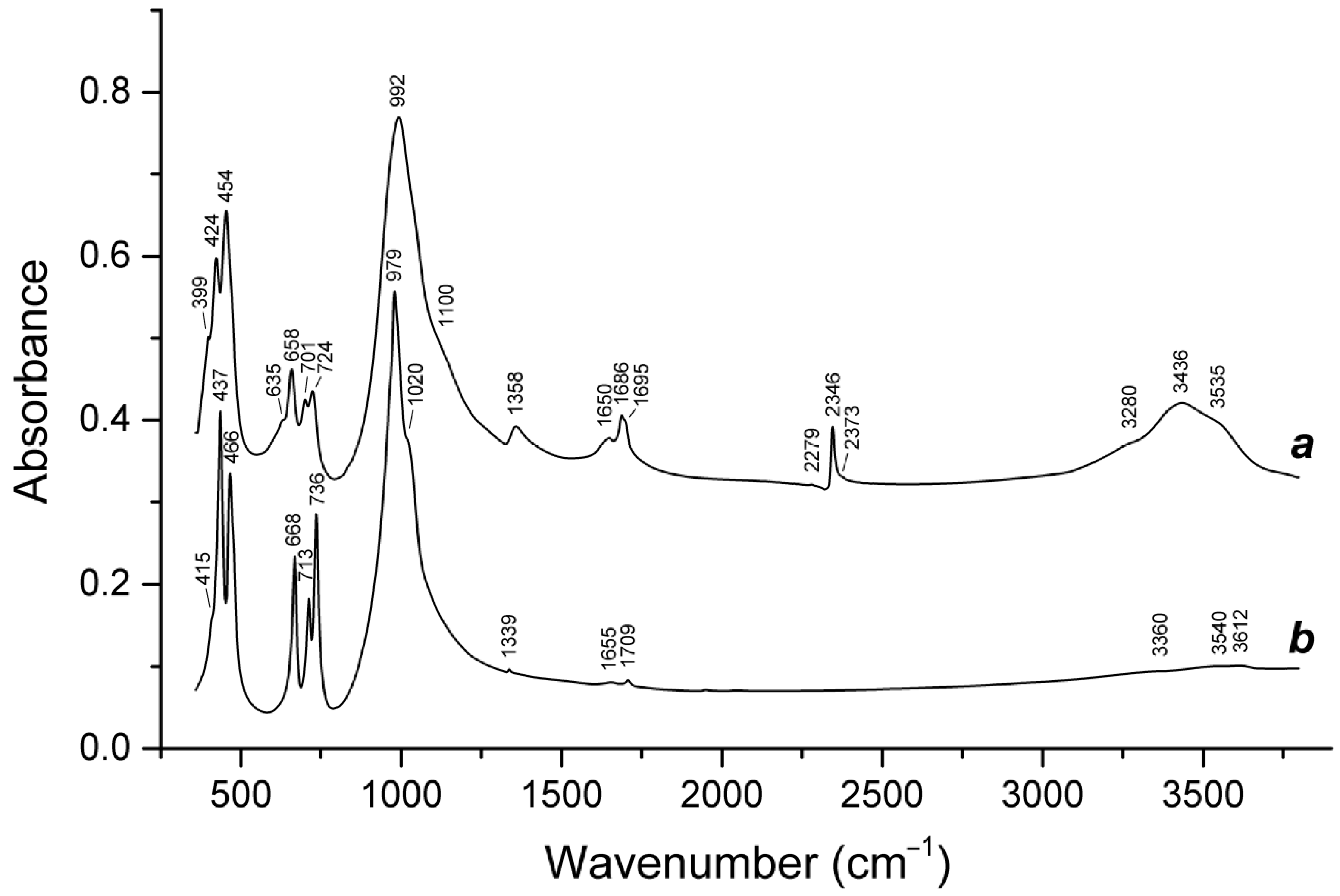
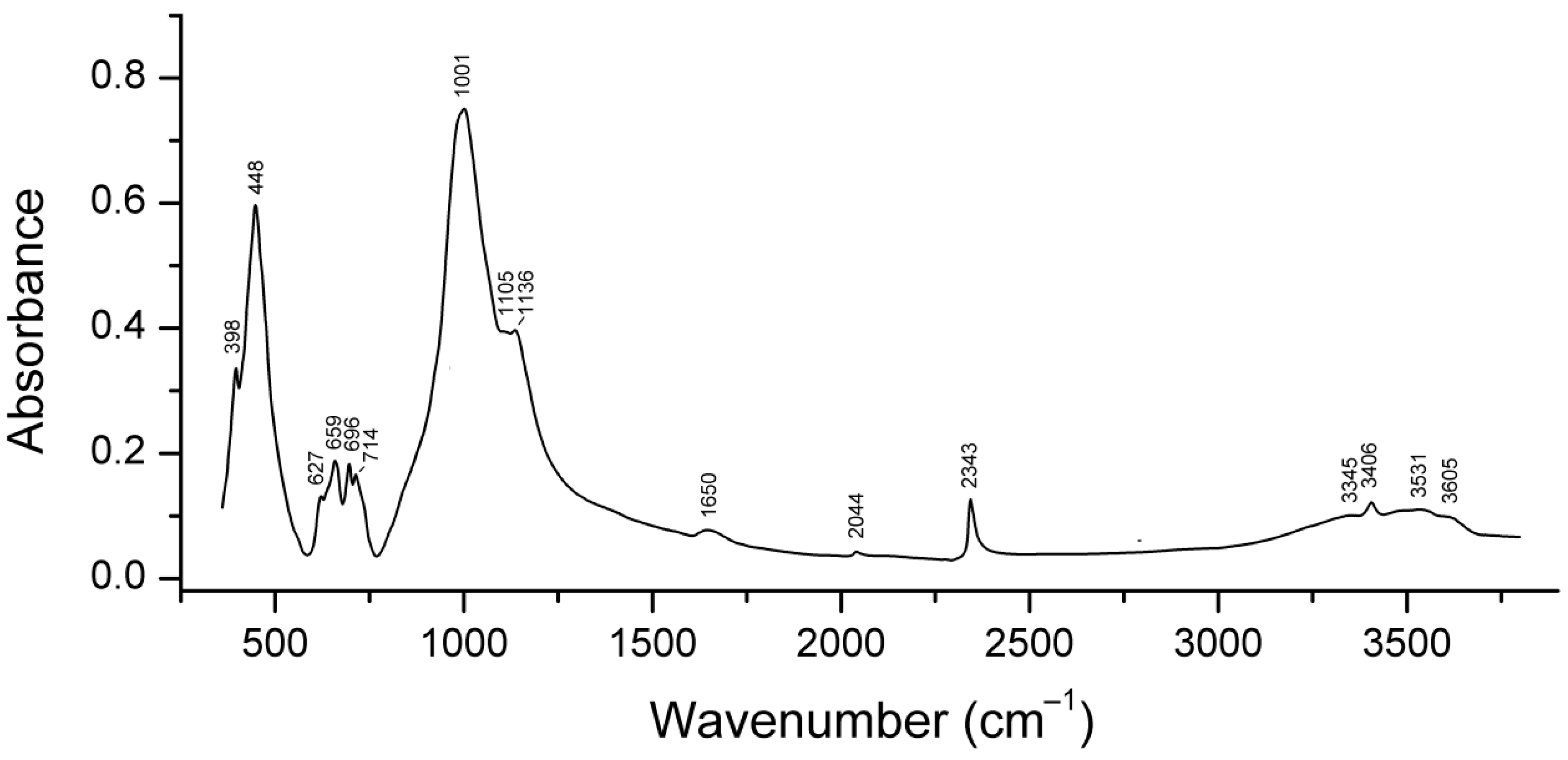

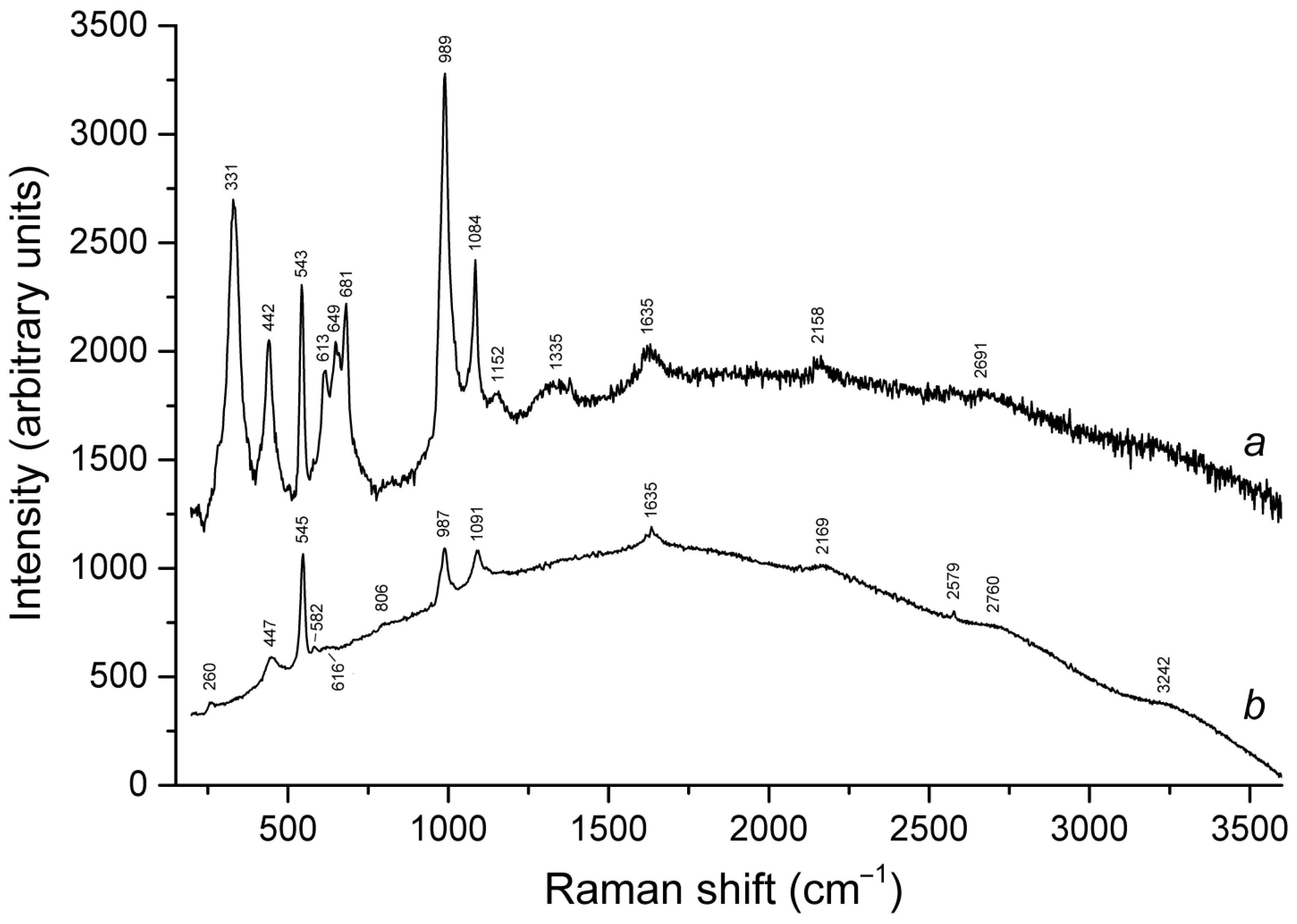

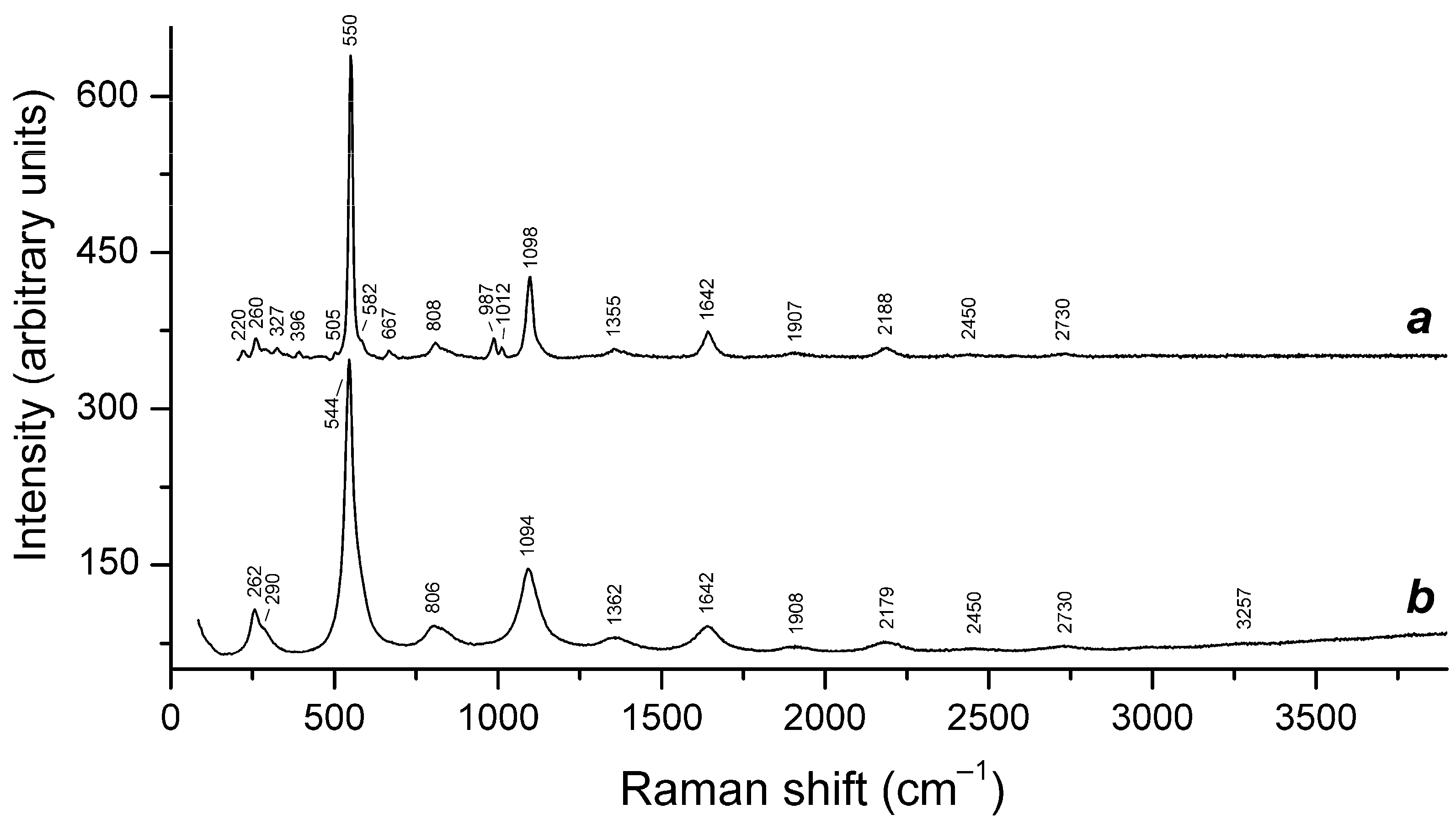
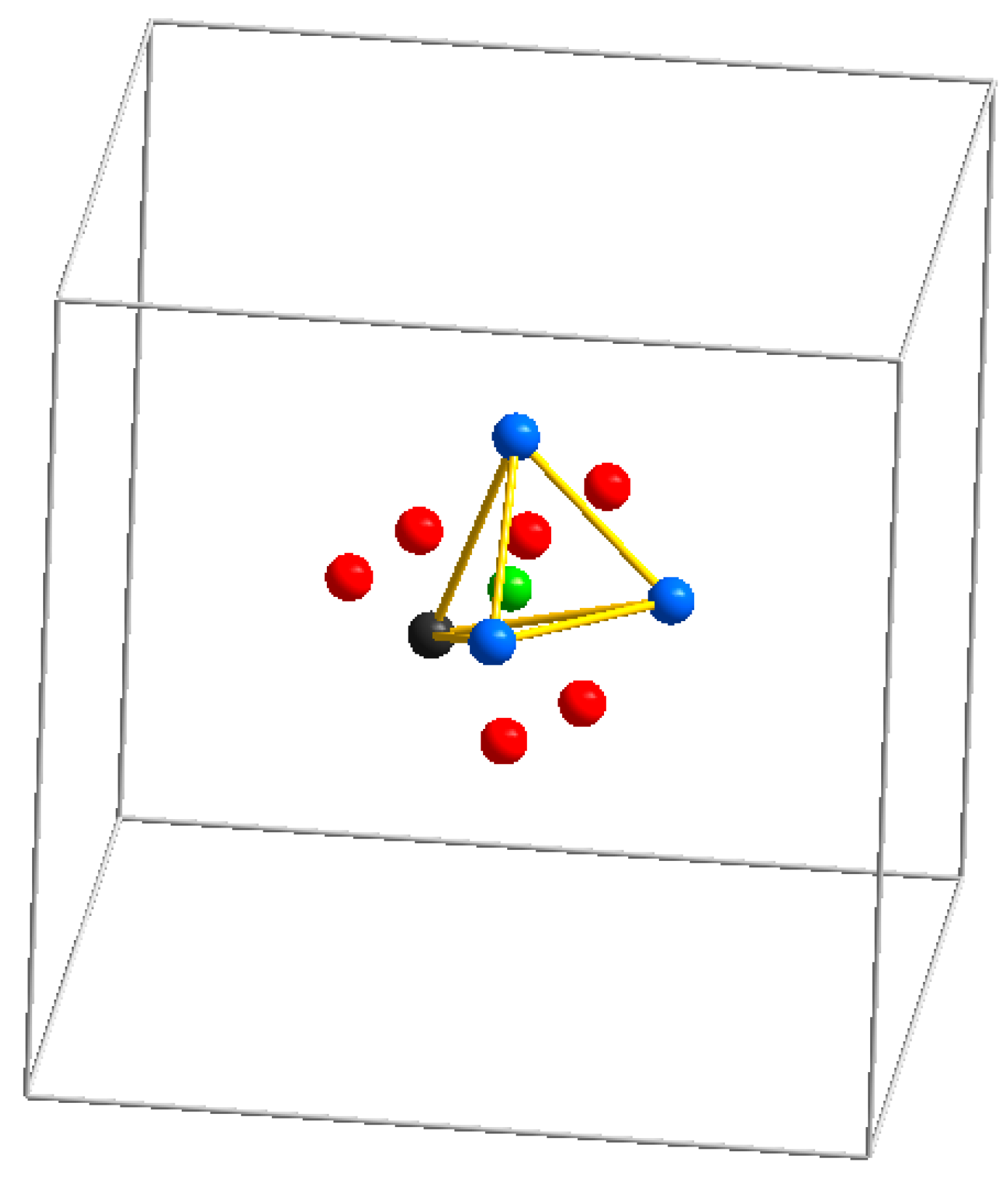
| Angle | T–O–T, Mean [°] | Range of T–X–T, Ind [°] | N | T–O, Mean [Å], All Samples |
|---|---|---|---|---|
| Al–O–P | no data | no data | no data | 1.63 |
| Si–O–Si | 150.6 | 141–160 | 4 | 1.60 |
| Al–O–Al | 144.3 | 128–166 | 16 | 1.74 |
| Al–O–Si | 142.0 | 135–156 | 32 | 1.67 |
| Al–O-Ge | 133.9 | 130–138 | 7 | 1.74 |
| Ge–O–Ge | 129.3 | 125–134 | 2 | 1.74 |
| Ga–O–Si | 130.2 | 130 | 2 | 1.72 |
| Be–O–Si | 129.9 | 123–144 | 9 | 1.61 |
| Ga–O–Ge | 129.7 | 125–137 | 5 | 1.79 |
| B–O–B | 124.7 | 120–128 | 5 | 1.47 |
| Extra-Framework Anions and Groups | r, Å | a, Å | V, Å3 |
|---|---|---|---|
| (OH)− | 1.32 | 8.885 | 701.4 |
| Cl– | 1.81 | 8.873 | 698.6 |
| Br– | 1.96 | 8.932 | 712.6 |
| (CO3)2− | 2.57 | 9.005 | 730.2 |
| (NO3)− | 2.60 | 8.997 | 728.3 |
| (SO4)2− | 2.98 | 9.072 | 746.6 |
| (WO4)2− | 3.40 | 9.148 | 765.5 |
| (MoO4)2− | 2.00 | 9.152 | 766.5 |
| (ReO4)2− | 2.60 | 9.153 | 766.8 |
| (AlF6)3−+H2O | 2.56 | 9.046 | 749.3 |
| N3− | 1.95 | 8.982 | 724.5 |
| (H2O)4 | 2.90 | 9.034 | 737.2 |
| h, k, l * | dmeas (Å) | dcalc (Å) | I (%) | h, k, l * | dmeas (Å) | dcalc (Å) | I (%) |
|---|---|---|---|---|---|---|---|
| 1 1 0 | 6.42 | 6.41 | 13.5 | 3 + n, 3, n | 2.060 | 2.060 | 3.8 |
| 1 + n, 1, n | 5.72 | 5.71 | 4.4 | 4 + n, 1 + n, 1 | 2.015 | 2.016 | 2.5 |
| 2 − 0.5, 1 − 0.5, 1 | 4.859 | 4.847 | 3.4 | 3 3 2 | 1.9328 | 1.9331 | 3.3 |
| 2 0 0 | 4.538 | 4.534 | 5.8 | 4 2 2 | 1.8487 | 1.8508 | 2.2 |
| 2 − n, 1 − n, 1 | 4.139 | 4.134 | 4.5 | 4 + n, 2 − n, 2 | 1.8144 | 1.8154 | 1.9 |
| 2 1 0 | 4.058 | 4.055 | 3.6 | 5 1 0, 4, 3, 1 | 1.7781 | 1.7782 | 7.6 |
| 2, 1 − n, 1 − n | 3.961 | 3.962 | 4.2 | 4, 3 + n, 1 − n | 1.7482 | 1.7466 | 1.8 |
| 2 − 0.5, 1 + 0.5, 1 | 3.862 | 3.866 | 2.9 | 5 + n, 1 − n, 0 | 1.7188 | 1.7197 | 1.6 |
| 2 1 1 | 3.703 | 3.702 | 100 | 5 2 1 | 1.6553 | 1.6554 | 2.8 |
| 2 + n, 1 − n, 1 | 3.551 | 3.551 | 3 | 4 − n, 4, n | 1.6446 | 1.6449 | 2 |
| 2, 1 + n, 1 + n | 3.440 | 3.441 | 4.5 | 4 − n, 3 − n, 3 | 1.6253 | 1.6258 | 1.6 |
| 2+ n, 1 + n,1 | 3.339 | 3.339 | 4.3 | 4 4 0 | 1.6028 | 1.6029 | 7.2 |
| 2 2 0 | 3.203 | 3.206 | 3.2 | 4 − n, 3 + n, 3 | 1.5551 | 1.5596 | 3.8 |
| 2 − 0.5, 2 + 0.5, 0 | 3.111 | 3.110 | 2.9 | 4 4 2 | 1.5109 | 1.5112 | 3.5 |
| 2 + 0.5, 1 + 0.5, 1 | 2.943 | 2.942 | 2.9 | 6 1 1 | 1.4710 | 1.4709 | 4.3 |
| 3 1 0 | 2.868 | 2.867 | 13.9 | 4 + n, 4, 2 + n | 1.4586 | 1.4584 | 1.7 |
| 2, 2 − n, 2 − n | 2.816 | 2.814 | 4.2 | 5, 4 − n, 1 + n | 1.4199 | 1.4193 | 1.7 |
| 3 + n, 1 − n, 0 | 2.732 | 2.740 | 2.7 | 5 4 1 | 1.3988 | 1.3991 | 1.6 |
| 2 2 2 | 2.618 | 2.617 | 25.9 | 6 − n, 2, 2 + n | 1.3926 | 1.3927 | 1.6 |
| 2 + n, 2 + n, 2 | 2.441 | 2.441 | 3.8 | 6 2 2 | 1.3669 | 1.3669 | 5.4 |
| 3 2 1 | 2.423 | 2.423 | 7.9 | 6 3 1 | 1.3371 | 1.3369 | 2.2 |
| 4 0 0 | 2.267 | 2.267 | 6.9 | 4 4 4 | 1.3085 | 1.3087 | 3.5 |
| 4 − n, 1 + n, 1 | 2.211 | 2.211 | 3.3 | 7 1 0 | 1.2825 | 1.2823 | 2.2 |
| 4, 1 − n, 1 − n | 2.183 | 2.184 | 2.6 | 7, 2 − n, 1 − n | 1.2479 | 1.2477 | 1.4 |
| 4 1 1 | 2.137 | 2.137 | 16.1 | 7 2 1 | 1.2339 | 1.2339 | 3.7 |
| 3 + 2n, 2 + 2n, 1 | 2.092 | 2.100 | 2.7 | 7, 2 + n, 1 + n | 1.2184 | 1.2185 | 1.4 |
| Mineral | Parameters of the Basic Unit Cells a. b c (Å) α, β, γ (°) | Symmetry Group | Wave Vector |
|---|---|---|---|
| Cubic LRM-1 | acub = 9.087(3) | P23 | q ~ 0.30c* (in orthorhombic setting) |
| Cubic LRM-2 | acub = 9.077(1) | P23 | q ~ 0.43c* (in orthorhombic setting) |
| Orthorhombic LRM a~acub, b~acub√2 c~3acub√2 | a = 9.057 b = 12.843 c = 38.513 | Pnaa | q = 0.33c* |
| Monoclinic LRM a~acub, b~c~acub√2 | a = 9.069(1) b = 12.868(1) c = 12.872(1) γ = 90.19(1) | P11a(00δ)0 | q ~ 0.43c* |
| Triclinic LRM a~acub, b~acub√2 c~2acub√2 | a = 9.0523(4) b = 12.8806(6) c = 25.681(1) α = 89.988(2) β = 90.052(1) γ = 90.221(1) (T = 170 K) | P1 | q = 0.5c* |
| Raman Shift (cm−1) | Assignment | ||
|---|---|---|---|
| Initial Sample | Preheated Sample | Sample Heated at 800 °C in Air | |
| 219 | trans-S4 bending | ||
| 260 | 262 | S3•− bending A2 (ν2) and S6 (with D3d symmetry) bending | |
| 283 | Framework bending vibrations (resonance with a S6 bending mode?) | ||
| 298 | 302s | S4•− bending vibrations | |
| 330 | cis-S4 mixed ν4 mode (symmetric bending + stretching) | ||
| 380 w | cis-S4 mixed ν3 mode | ||
| 437 | 440w | SO4 [bending E (ν2) mode] and/or S6 (mixed mode) | |
| 461s | [(HS)Na4]3+ stretching vibrations | ||
| 477 | 474 | S6 stretching mode and/or mixed ν4 mode of trans-S4 | |
| 503 | Bending vibrations of the framework | ||
| 545 s | 548s | S3•− symmetric stretching (ν1) (possibly, overlapping with the stretching band of gauche-S4) | |
| 580 | 586 | S3•− antisymmetric stretching mode (ν3) | |
| 603 | S2•− stretching mode | ||
| 614 | SO42− [bending F2 (ν4) mode] | ||
| 645 | cis-S4 stretching | ||
| 682 | trans-S4 symmetric stretching ν3 mode | ||
| 724, 756w | 722w | O–C–O bending vibrations of oxalate anions | |
| 807 | 810 | S3•− combination mode (ν1 + ν2) | |
| 985 s | 983 | SO42− [symmetric stretching A1 (ν1) mode] (possibly, overlapping with the weak band of framework stretching vibrations) | |
| 1077w | CO32− symmetric stretching mode | ||
| 1088 s | 1097s | S3•− overtone (2 × ν1) [possibly, overlapping with the SO4•− stretching band (ν3−F2)] | |
| 1163 | S2•− overtone (2 × ν1) | ||
| 1279, 1381 | Symmetric stretching vibrations of CO2 molecules (Fermi doublet, resonance with the overtone of bending vibrations). | ||
| 1340 | 1342 | 1360 | Symmetric C–O stretching vibrations of CO2 molecules—involved in strong dipole–dipole interactions and/or symmetric C–O stretching vibrations of acid oxalate anions |
| 1480w | CO32− asymmetric stretching mode | ||
| 1609s | Antisymmetric C–O stretching vibrations of acid oxalate anions | ||
| 1631 | 1651 | S3•− overtone (3 × ν1) | |
| 1768, 1832 | C = O stretching vibrations of acid oxalate groups | ||
| 1891 | 1915 | S3•− combination mode (3 × ν1 + ν2) | |
| 2172 | 2191 | S3•− overtone (4 × ν1) | |
| 2428 w | S3•− combination mode (4 × ν2 + ν1) | ||
| 2553s | HS− stretching mode | ||
| 2575 w | H2S symmetric stretching mode | ||
| 2710 | 2737 | S3•− overtone (5 × ν1) | |
| 2964 w | S3•− combination mode (5 × ν1 + ν2) | ||
| 3025 | O–H stretching vibrations | ||
| 3247 w | S3•− overtone (6 × ν1) | ||
| Name | Simplified Formula | Sp. Gr. | Unit-Cell Parameters, Å | Ref. |
|---|---|---|---|---|
| Sodalite | Na8(Si6Al6O24)Cl2 | P-43n | a = 8.87–8.88 | [2] |
| Sapozhnikovite | Na8(Si6Al6O24)(HS)2 | P-43n | a = 8.9146 | [17] |
| Bolotinaite | (Na6K□)(Al6Si6O24)F·4H2O | I-43m | a = 9.027 | [21] |
| Haüyne | (Na,K)6Ca2(Al6Si6O24)(SO4)2 | P-43n | a = 9.09–9.13 | [4] |
| Lazurite | Na7Ca(Al6Si6O24)(SO4)S3•−·H2O | P-43n (for the sub-cell) | a = 9.08 (for the sub-cell) The structure is modulated | [7,20] |
| Lazurite-related mineral | Na8(Al6Si6O24)(S2−,SO42−,S3•−)1+x (H2O,CO2) | No data | No data | [23] |
| Nosean | Na8(Al6Si6O24)(SO4)·H2O | P-43n | a = 9.05–9.08 | [8] |
| Bicchulite | Ca8(Al8Si4O24)(OH)8 | I-43m | a = 8.82–8.83 | [9] |
| Kamaishilite | Ca8(Al8Si4O24)(OH)8 | No data (tetragonal) | a = 8.850, c = 8.770 | [10] |
| Valleyite | Ca8(Fe12O24)O2 | I-43m | a = 8.8852 | [11] |
| Vladimirivanovite | Na6+xCa2–x(Al6Si6O24) (SO4,S3•−)2·H2O | Pnaa | a = 9.053, b = 12.837, c = 38.445 | [12] |
| Wavenumber (cm−1) | Assignment |
|---|---|
| 448–454 | S52− stretching mode 2 |
| 530 | Stretching vibrations of trans-S4 |
| 580–585 | S3•− antisymmetric stretching mode (ν3) |
| 614–622 | SO42− bending vibrations [F2(ν4) mode] |
| 955 | C–N stretching mode of N(CH3)4+ |
| 1130–1160 | SO42− asymmetric stretching vibrations [F2(ν3) mode] |
| 1339–1358 | H···O stretching vibrations of hydrated proton complexes |
| 1400–1500 | CH3 bending modes of N(CH3)4+ |
| 1610–1660 | H2O bending vibrations |
| 1685–1720 | H3O+ bending vibrations |
| 2037–2040 | C=O stretching vibrations of COS |
| 2340–2343 | Antisymmetric stretching mode of CO2 |
| 2554 | Stretching vibrations of HS− |
| 2900–3030 | CH3 stretching modes of N(CH3)4+ |
| 3300–3600 | H2O stretching vibrations |
| 3600–3670 | Stretching vibrations of OH− |
| Raman Shift (cm−1) | Assignment |
|---|---|
| 219–223 | trans-S4 or S42− bending mode |
| 254–25 | S3•− bending mode (ν2) |
| 254–260 | Bending vibrations of the [ClNa4] and [(HS)Na4] clusters |
| 262 | S52− stretching mode? |
| 283–294w | Combination of low–frequency lattice modes involving Na+ cations and S6 bending mode |
| 298 | S4•− bending vibrations |
| 327–332w | cis-S4 mixed ν4 mode (combined symmetric bending + stretching vibrations) |
| 380 | cis-S4 mixed ν3 mode |
| 413–422 | S52− stretching mode 1 |
| 435 | S52− stretching mode 1 (a different conformation) |
| 436–447 | SO42− [the E(ν2) mode] |
| 454–466 | S52− stretching mode 2 |
| 459–464 | Stretching vibrations of the [ClNa4] and [(HS)Na4] clusters |
| 477–481 | S6 stretching mode and/or mixed ν4 mode of trans–S4 or S42− |
| 543–550s | S3•− symmetric stretching (ν1) and/or AlF6 stretching mode |
| 578–585sh | S3•− antisymmetric stretching (ν3) |
| 602–612 | S2•− stretching mode |
| 594–605 | Stretching vibrations of the [(S2−)Na4] cluster |
| 615–673 | HF translational modes ? |
| 613–625 | SO42− bending vibrations [F2(ν4) mode] |
| 645 | cis-S4 symmetric stretching mode |
| 649–652 | gauche-S4 symmetric stretching vibrations [A1(ν1) mode] |
| 667–684w | trans-S4 symmetric stretching ν3 mode |
| 802–814 | S3•− combination mode (ν1 + ν2) |
| 975–990 | SO42− symmetric stretching vibrations [A1(ν1) mode] |
| 1058 | CO32− symmetric stretching vibrations |
| 1074 | HF libration? |
| 1084–1098 | S3•− overtone (2’ν1) |
| 1135–1152w | SO42− asymmetric stretching vibrations [F2(ν3) mode], possibly, overlapping with S2•− overtone (2 × ν1) |
| 1160–1166w | S2•− overtone (2 × ν1) ? |
| 1271–1279w | CO2 Fermi resonance |
| 1335 | Overtone of the cis-S4 antisymmetric stretching mode (2 × ν3) |
| 1340 | Symmetric C–O stretching vibrations of CO2 molecules involved in strong dipole–dipole interactions with H2O molecules |
| 1349–1350 | H···O stretching vibrations of hydrated proton complexes |
| 1351–1363 | S3•− combination mode (2ν1 + ν2) |
| 1381 | CO2 Fermi resonance |
| 1442w | CO3 asymmetric stretching mode |
| 1632–1642 | S3•− overtone (3 × ν1) |
| 1894–1908w | S3•− combination mode (3 × ν2 + ν1) |
| 2168–2188 | S3•− overtone (4 × ν1) |
| 2420–2450w | S3•− combination mode (4 × ν2 + ν1) |
| 2553–2581 | HS− stretching mode |
| 2691 | cis-S4 antisymmetric stretching (4 × ν3) |
| 2712–2730w | S3•− overtone (5 × ν1) |
| 2904 | CH4 stretching vibrations |
| 2975w | S3•− combination mode (5 × ν1 + ν2) |
| 3242–3257w | S3•− overtone (6 × ν1) |
| 3243 | H3O+ stretching mode |
| 3495–3670 | H2O stretching vibrations |
| 3796 | S3•− overtone (7 × ν1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukanov, N.V.; Aksenov, S.M. Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review. Int. J. Mol. Sci. 2024, 25, 10218. https://doi.org/10.3390/ijms251810218
Chukanov NV, Aksenov SM. Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review. International Journal of Molecular Sciences. 2024; 25(18):10218. https://doi.org/10.3390/ijms251810218
Chicago/Turabian StyleChukanov, Nikita V., and Sergey M. Aksenov. 2024. "Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review" International Journal of Molecular Sciences 25, no. 18: 10218. https://doi.org/10.3390/ijms251810218
APA StyleChukanov, N. V., & Aksenov, S. M. (2024). Structural Features, Chemical Diversity, and Physical Properties of Microporous Sodalite-Type Materials: A Review. International Journal of Molecular Sciences, 25(18), 10218. https://doi.org/10.3390/ijms251810218








