Synthesis, the Reversible Isostructural Phase Transition, and the Dielectric Properties of a Functional Material Based on an Aminobenzimidazole–Iron Thiocyanate Complex
Abstract
:1. Introduction
2. Results and Discussion
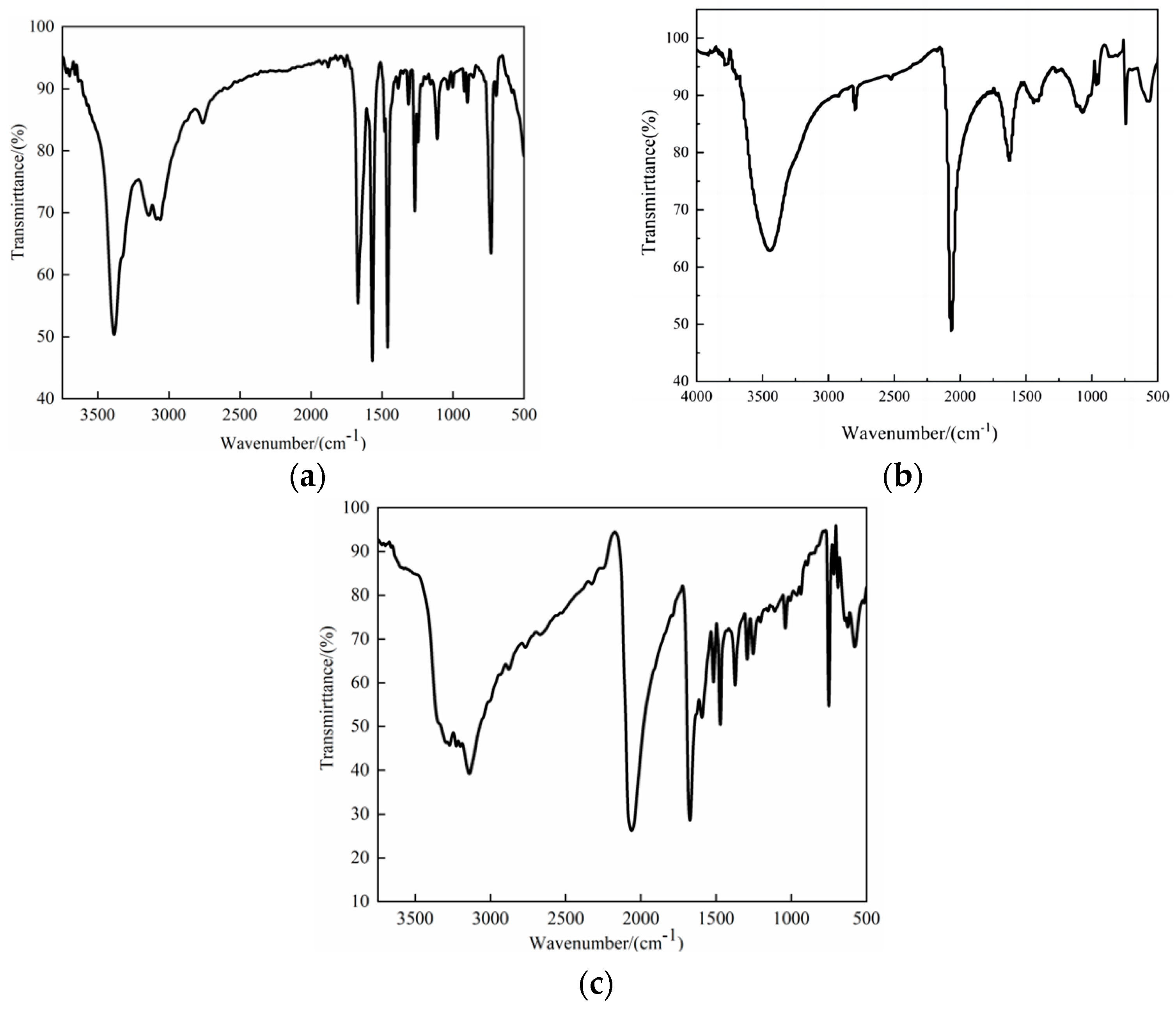
2.1. IR Spectroscopy
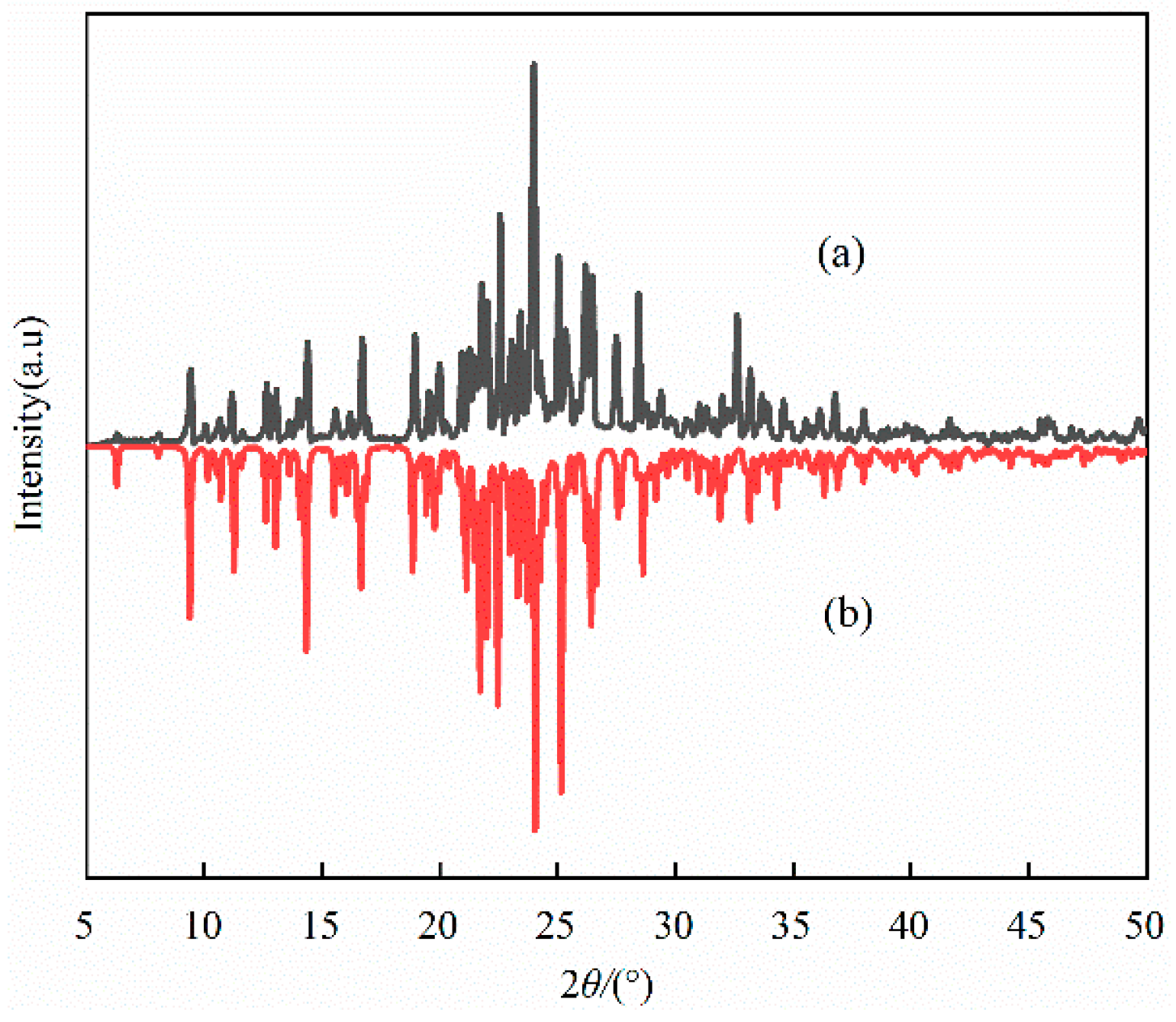
2.2. Powder XRD Analysis
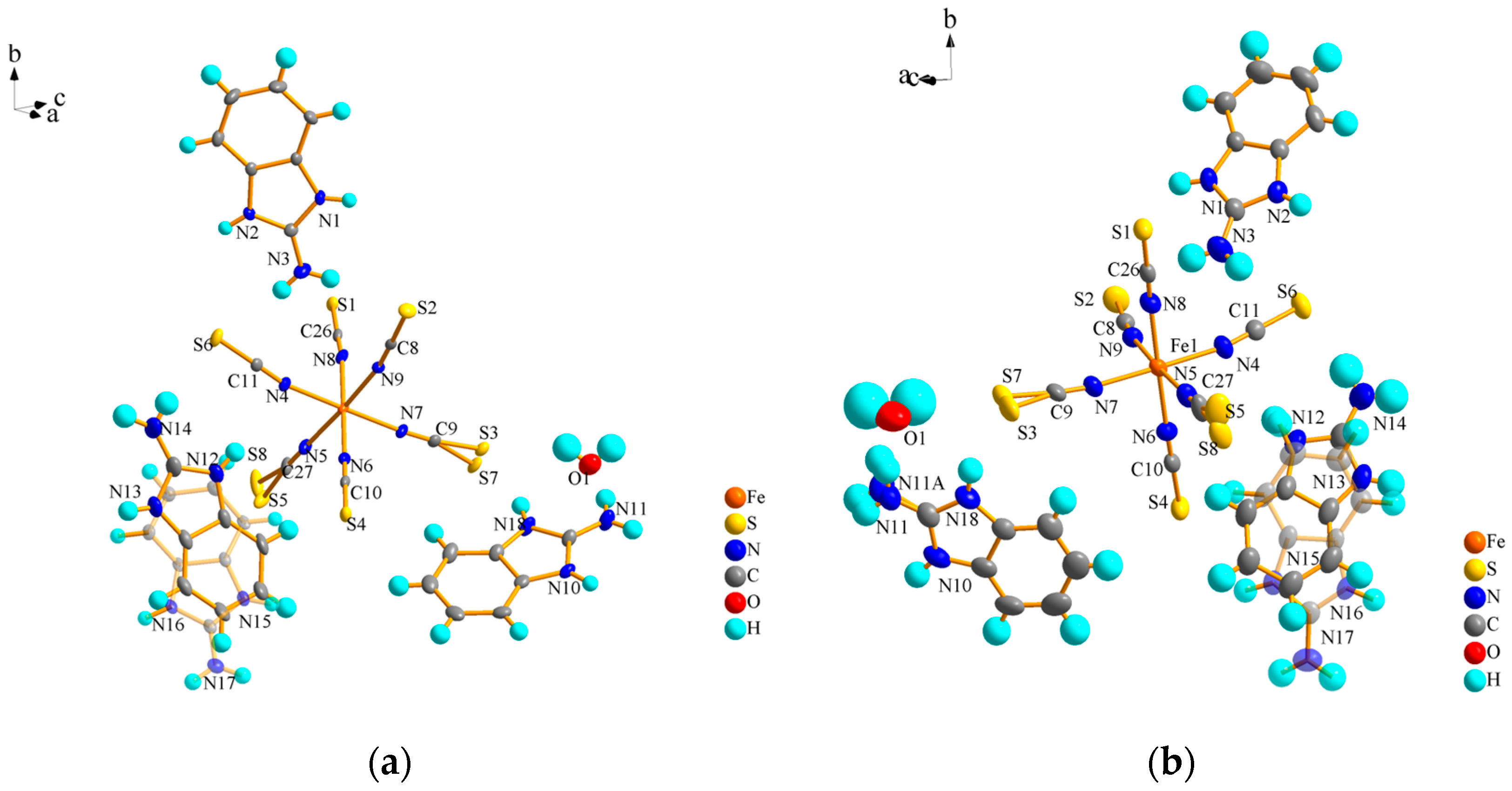
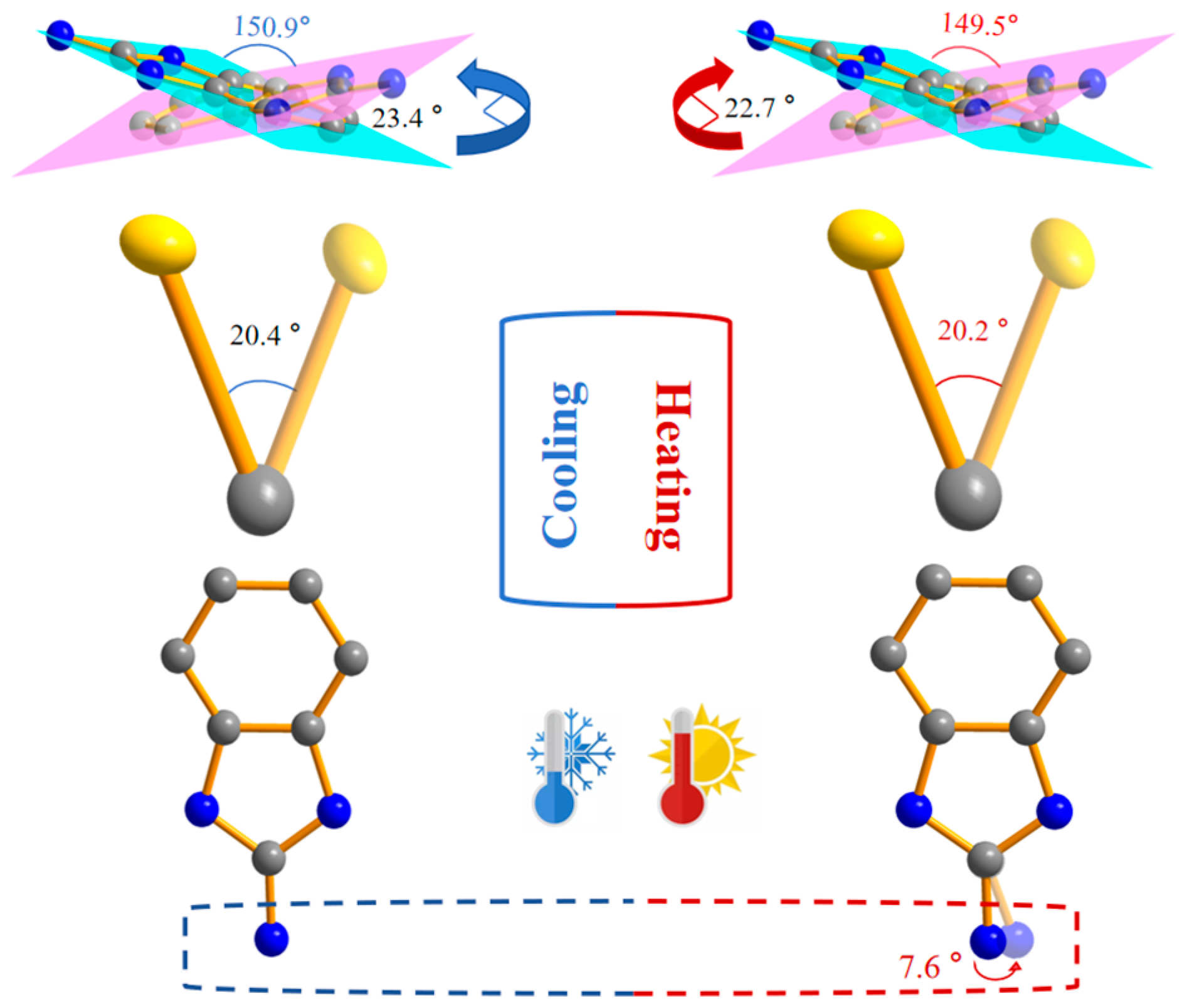
2.3. Crystal Structure Analysis
2.4. Hirshfeld Surface Analysis
2.5. UV-Vis Spectral Analysis
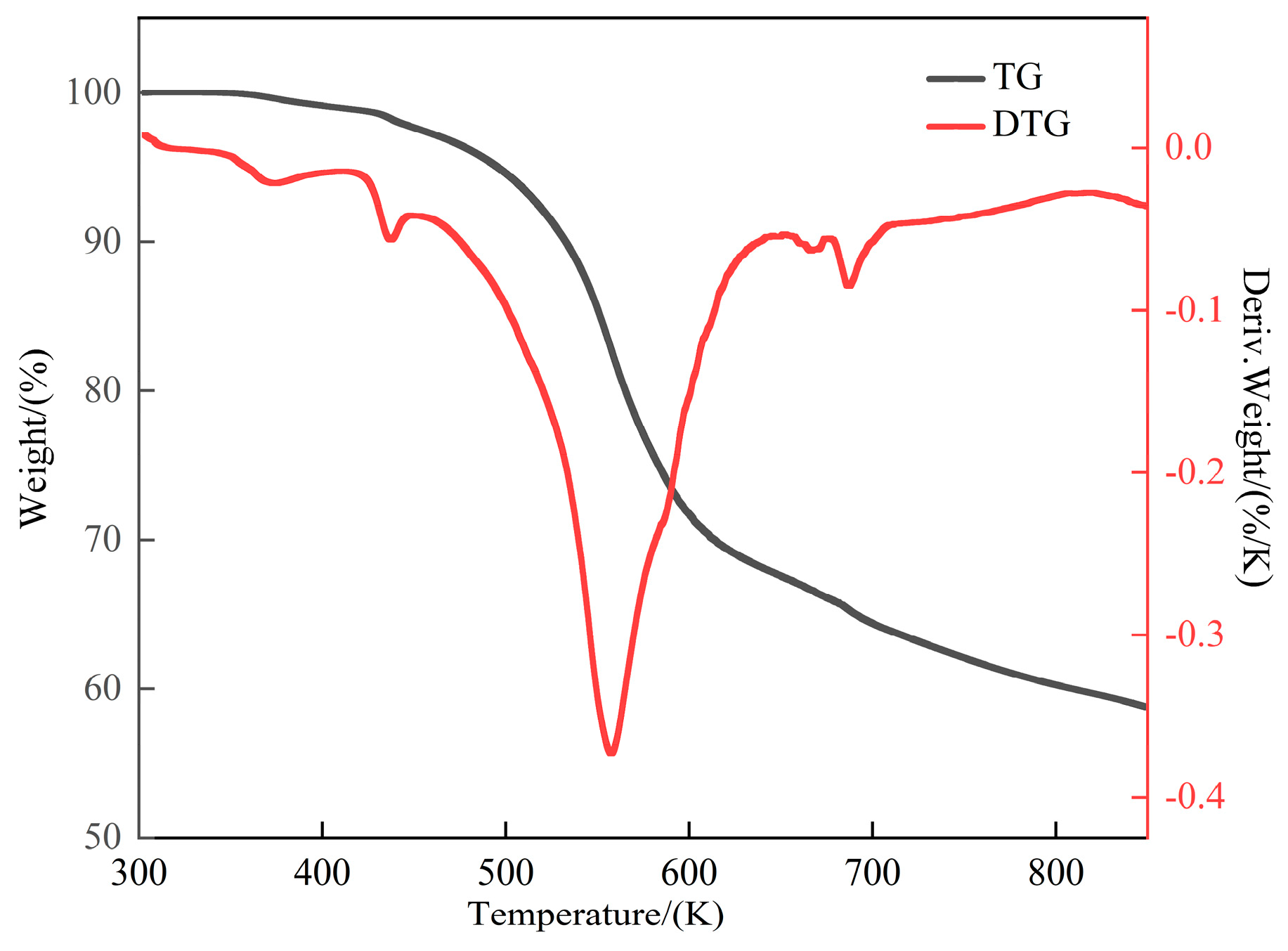
2.6. TGA
2.7. DSC Analysis
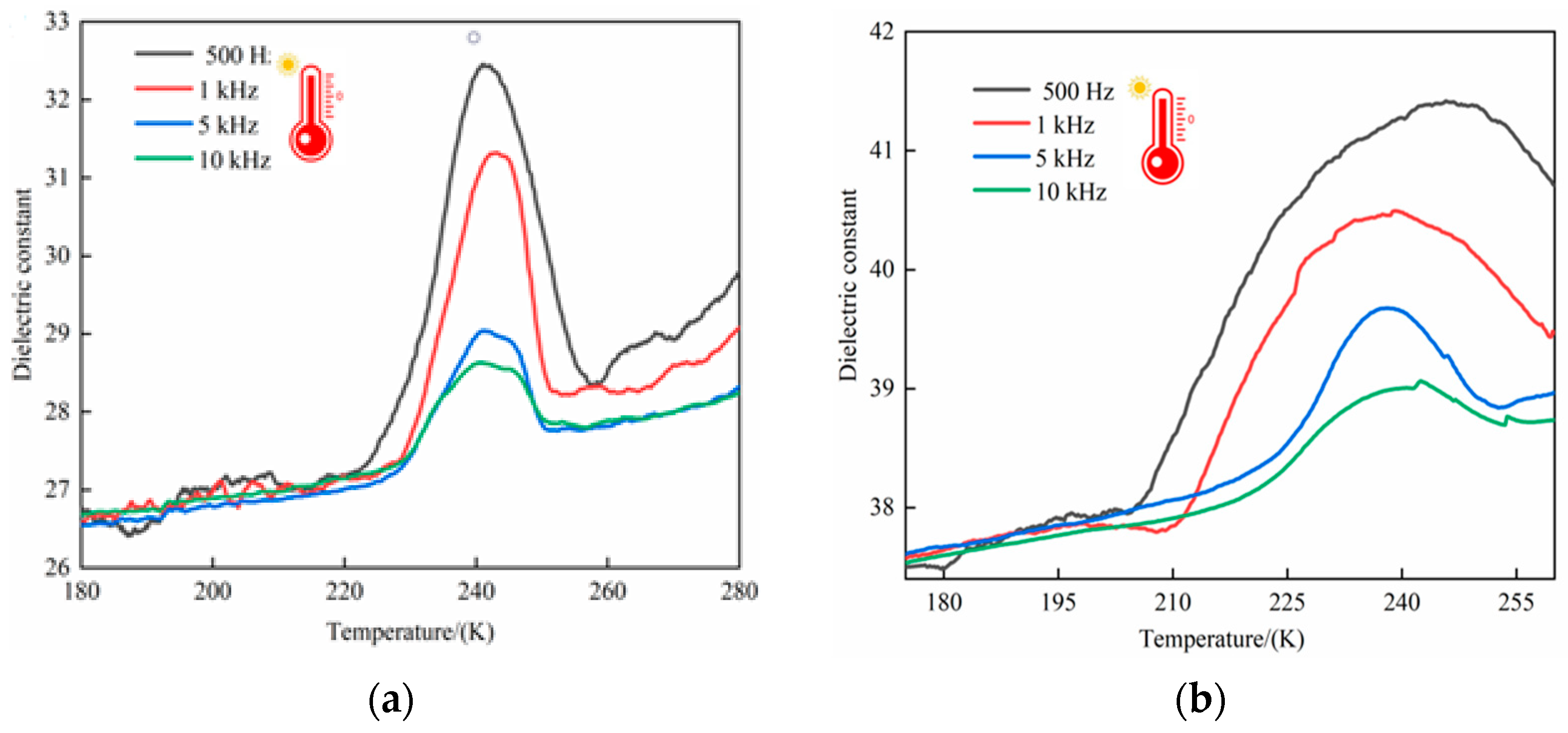
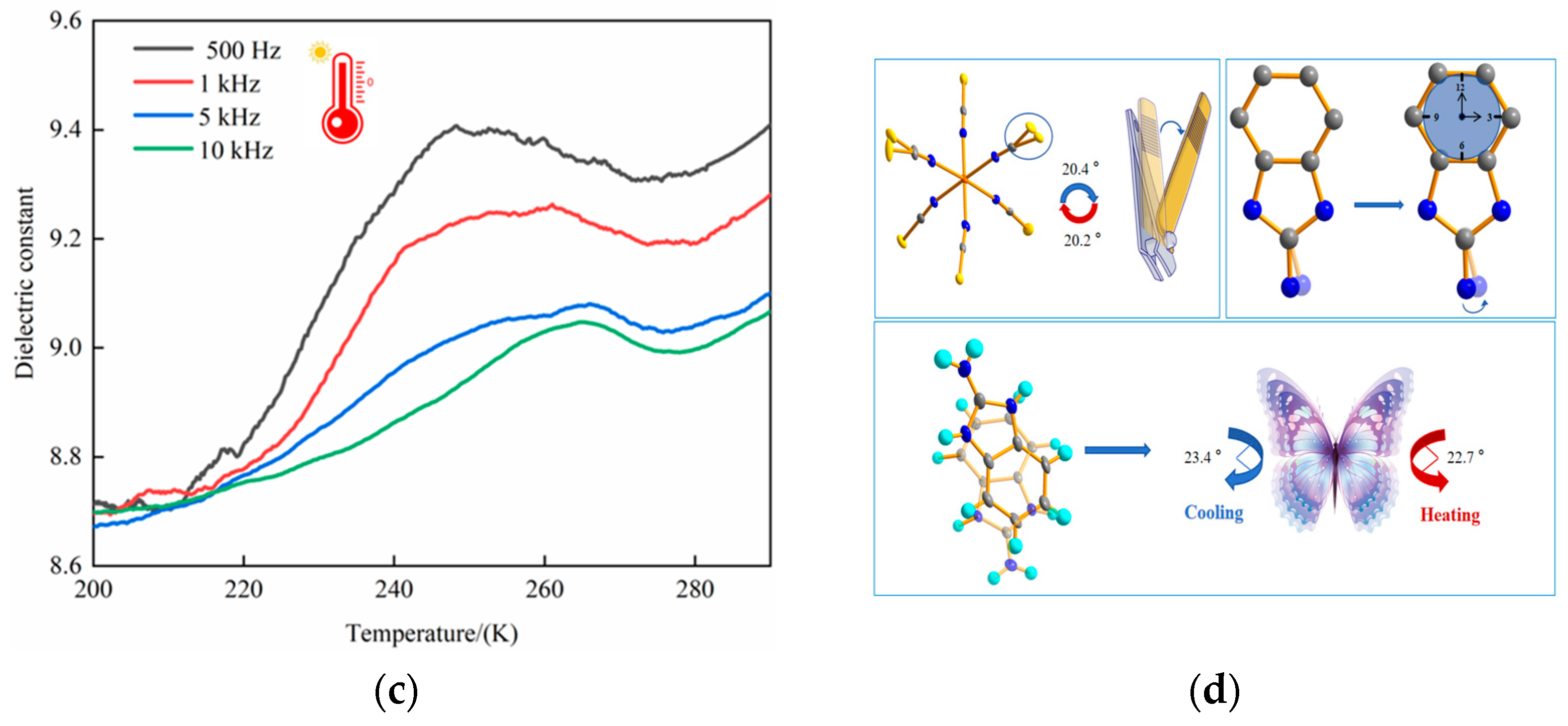
2.8. Dielectric Property Tests
3. Materials and Methods
3.1. Reagents and Instruments
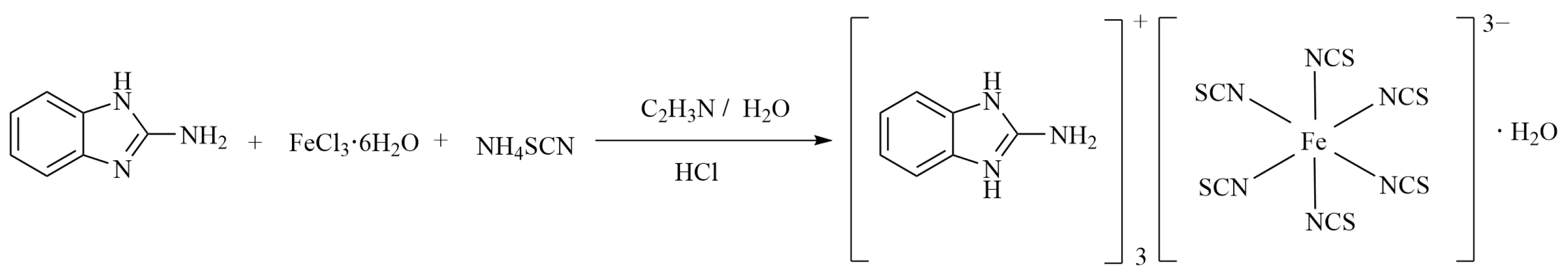
3.2. Synthesis of Compound 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.; Cheng, C.; Zhang, W.-Y.; Ye, Q.; Fu, D.-W. Semiconducting Organic—Inorganic Hybrid Material with Distinct Switchable Dielectric Phase Transition. J. Phys. Chem. C 2018, 122, 20989–20995. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Tang, Y.-Y.; Gu, Z.-X.; Wang, P.; Chen, X.-G.; Lv, H.-P.; Li, P.-F.; Jiang, Q.; Gu, N.; Ren, S.; et al. Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science 2024, 383, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.P.; Ding, N.; Wang, N.; Ye, H.-Y.; Shi, C.; Dong, S. Fast switching of spontaneous polarization in a microporous molecular rotor ferroelectric. Inorg. Chem. Front. 2023, 10, 61–66. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Li, T. Crystal structure, thin film preparation, theoretical and experimental study the nonlinear optical properties of a novel copper complex. Inorg. Chem. Commun. 2021, 12, 108–115. [Google Scholar] [CrossRef]
- Ravisankar, V.; Ramesh, V.; Girisun, T.C.S.; Sridevi, D.V.; Gunasekaran, B. Synthesis, growth, structural, physicochemical, linear and nonlinear optical properties of new hybrid [(Ba(C10H20O5)2)(Mn(SCN)4)] single crystal. Appl. Phys. A 2021, 127, 885. [Google Scholar] [CrossRef]
- Zheng, H.; Meng, Y.S.; Zhou, G.L.; Duan, C.; Sato, O.; Hayami, S.; Luo, Y.; Liu, T. Simultaneous Modulation of Magnetic and Dielectric Transition via Spin-Crossover-Tuned Spin Arrangement and Charge Distribution. Angew. Chem. Intl. Ed. 2018, 57, 8468–8472. [Google Scholar] [CrossRef]
- Zheng, X.D.; Huang, Y.L.; Tong, Y.P. A pair of homochiral complexes generated via spontaneous resolution: Synthesis, structures and dielectric properties. Inorg. Chem. Acta 2018, 48, 454–459. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.L.; Tan, Y.H.; Tang, Y.-Z.; Fan, X.-W.; Luo, J.-L.; Wang, F.-X.; Wan, M.-Y. High-Temperature Ferroelasticity and Photoluminescence in a 2D Monolayer Perovskite Compound: (C5NH8Br)2PbBr4. Inorg. Chem. 2023, 62, 10847–10853. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Ying, T.T.; Tan, Y.H.; Tang, Y.-Z.; Liao, J.; Wang, L.-J.; Wang, F.-X.; Wan, M.-Y. 2-Chloroethylamine·trifluoromethanesulfonate combined with 18-crown-6: A ferroelectric with excellent dielectric switching properties. Dalton Trans. 2023, 52, 11196–11202. [Google Scholar] [CrossRef] [PubMed]
- Trzebiatowska, M.; Kowalska, D.A.; Gusowski, M.A.; Jach, E.; Ciżman, A. Dielectric switching in correlation with the structural phase transitions in tetrapropylammonium perchlorate. Phys. Chem. Chem. Phys. 2023, 25, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Chen, X.X.; Ye, Z.M.; Zhang, W.X.; Chen, X.M. High- and low-temperature dual ferroelasticity in a new hybrid crystal:(Me3NCH2CH2OH)4[Ni(NCS)6]. Sci. China Mater. 2022, 65, 263–267. [Google Scholar] [CrossRef]
- He, L.; Zhou, L.; Shi, P.-P.; Ye, Q.; Fu, D.-W. One-dimensional cadmium thiocyanate perovskite ferroelastics tuned by halogen substitution. Chem. Mater. 2019, 31, 10236–10242. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.R.; Brandão, P.; Jana, A.D. Role of imidazole edge to edge supramolecular interaction in the crystal packing of Cu(II)(SCN−)2(imidazole)2 complex: A novel variety of supramolecular interaction revealed by CCDC database analysis and explored through DFT computational studies. J. Mol. Struct. 2021, 12, 278–291. [Google Scholar] [CrossRef]
- Sadhu, M.H.; Kumar, S.B. Synthesis, characterization and structures of copper(II), nickel(II), cobalt(II) and cadmium(II) complexes involving N4–donor ligand and cyanate as coligand: Formation of 1D and 2D crystallographic networks by short intermolecular interactions. Polyhedron 2017, 12, 419–429. [Google Scholar] [CrossRef]
- Karoui, S.; Chouaib, H.; Kamoun, S. Synthesis, X-ray powder diffraction study, thermal analysis, Hirshfeld surface analysis and optical properties of new crystalline polymer: {(C2H10N2)(MnCl(NCS)2)2}n. J. Mol. Struct. 2021, 1223, 128933. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, T.; Lun, M.M.; Zhang, Y.; Chen, L.; Fu, D. Facile Control of Ferroelectricity Driven by Ingenious Interaction Engineering. Small 2023, 19, e2301364. [Google Scholar] [CrossRef]
- Wan, M.Y.; Tang, Y.Z.; Tan, Y.H.; Wang, F.-X.; Li, Y.-N.; Wang, L.-J.; Liao, J.; Wang, M.-N. Excellent Switchable Properties, Broad-Band Emission, Ferroelectricity, and High T c in a Two-Dimensional Hybrid Perovskite: (4,4-DCA)2PbBr4 Exploited by H/F Substitution. Inorg. Chem. 2023, 62, 12525–12533. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Zhang, Z.-X.; Su, C.-Y.; Zhang, Y.; Fu, D.-W. Methylation Design Strategy to Trigger a Dual Dielectric Switch and Improve the Phase Transition Temperature. Inorg. Chem. 2020, 59, 16635–16643. [Google Scholar] [CrossRef]
- Su, C.-Y.; Zhang, Z.-X.; Zhang, W.-Y.; Shi, P.-P.; Fu, D.-W.; Ye, Q. Unique Design Strategy for Dual Phase Transition That Successfully Validates Dual Switch Implementation in the Dielectric Material. Inorg. Chem. 2020, 59, 4720–4728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shi, P.-P.; Zheng, X.; Geng, F.-J.; Ye, Q.; Fu, D.-W. Molecular design of high-temperature organic dielectric switches. Chem. Commun. 2018, 54, 13111–13114. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J.; Bratcher, P.E.; Chandler, J.D.; Kilgore, M.B.; Min, E.; LiPuma, J.J.; Hondal, R.J.; Nichols, D.P. The thiocyanate analog selenocyanate is a more potent antimicrobial pro-drug that also is selectively detoxified by the host. Free Radic. Biol. Med. 2020, 146, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Numata, Y.; Sanehira, Y.; Ishikawa, R.A.; Shirai, H.; Miyasaka, T. Thiocyanate Containing Two-Dimensional Cesium Lead Iodide Perovskite, Cs2PbI2(NCS)2: Characterization, Photovoltaic Application, and Degradation Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 42363–42371. [Google Scholar] [CrossRef]
- Echeverría, J. Intermolecular Interactions between Thiocyanato Ligands in Metal Complexes. Cryst. Growth Des. 2021, 21, 1636–1644. [Google Scholar] [CrossRef]
- Peng, H.; Xu, Z.K.; Du, Y.; Li, P.; Wang, Z.; Xiong, R.; Liao, W. The First Enantiomeric Stereogenic Sulfur-Chiral Organic Ferroelectric Crystals. Angew. Chem. Intl. Ed. 2023, 62, e202306732. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Lun, M.M.; Teri, G.; Xie, L.-Y.; Fu, D.-W.; Guo, Q. Fluorescence Emission Is Highly Structure-Dependent in Hybrid Lead Halides. Inorg. Chem. 2023, 62, 7186–7194. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.; Choi, J.H. Synthesis, Crystal Structure, Infrared Spectroscopy and Hirshfeld Surface Analysis of Cis-(Thiocyanato-κN)(1,4,8,11-Tetraazacyclotetradecane-κN4)Chromium(III)(µ-1,3-Thiocyanato-κN,S)Trichloridozincate. J. Coord. Chem. 2021, 74, 969–982. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, Z.X.; Su, C.Y.; Zhang, T.; Fu, D.-W.; Zhang, Y. A-site cation with high vibrational motion in ABX3 perovskite effectively induces dielectric phase transition. Dalton Trans. 2021, 50, 3841–3847. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, W.; Gao, L.; Gan, X.; Sun, X.; Cui, Z.; Cai, H.-L.; Wu, X.S. A high-temperature organic-inorganic ferroelectric with outstanding switchable dielectric characteristics. RSC Adv. 2017, 7, 47933–47937. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, G.; Wang, W.; Hu, F.; Liu, C.L.; Luo, D.; Liu, D.S. A semiconducting organic-inorganic hybrid metal halide [(C6H15ClNO)2CdBr4] with switchable dielectric and large phase transition thermal hysteresis. ChemistrySelect 2019, 4, 3921–3925. [Google Scholar] [CrossRef]
- Portela, S.; Fernández, I. Nature of the hydrogen bond enhanced halogen bond. Molecules 2021, 26, 1885. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dong, X.X.; Xu, Q.; Tan, Y.H.; Tang, Y.Z. Nopinic acid is an unprecedented homochiral single-component organic ferroelectric. Appl. Mater. Today 2020, 20, 10–27. [Google Scholar] [CrossRef]
- Deng, B.-W.; Yang, Z.; Ding, K.; Li, J.; Lun, M.-M.; Fu, D.-W.; Zhang, Z.-X. Homochiral Chemistry Strategy to Trigger Second-Harmonic Generation and Dual Dielectric Switches. Inorg. Chem. 2023, 62, 11701–11707. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Ying, T.T.; Zhao, Y.R.; Tang, Y.Z.; Tan, Y.-H.; Li, Q.-L.; Liu, W.-F.; Wan, M.-Y.; Wang, F.-X. Zero-Dimensional Sn-Based Enantiomeric Phase-Transition Materials with High-Tc and Dielectric Switching. Chem. A Eur. J. 2023, 29, e202301499. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Huang, C.R.; Xu, Z.K.; Hu, W.; Li, P.-F.; Xiong, R.-G.; Wang, Z.-X. Photochromic Single-Component Organic Fulgide Ferroelectric with Photo-Triggered Polarization Response. J. Am. Chem. Soc. Au 2023, 3, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Zhang, H.; Huang, X.Q.; Liu, Y.-L. A high-temperature halide perovskite molecular ferroelastic with evident dielectric switching. Inorg. Chem. Front. 2021, 8, 1197–1204. [Google Scholar] [CrossRef]
- Rok, M.; Ciżman, A.; Zarychta, B.; Zaręba, J.K.; Trzebiatowska, M.; Mączka, M.; Stroppa, A.; Yuan, S.; Phillips, A.E.; Bator, G. Cyano-bridged perovskite[(CH3)3NOH]2[KM(CN)6], [M: Fe(iii), and Co(iii)] for high-temperature multi-axial ferroelectric applications with enhanced thermal and nonlinear optical performance. J. Mater. Chem. C 2020, 8, 17491–17501. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Zhou, L.; Shi, P.-P.; Ye, Q.; Fu, D.-W. H/F substituted perovskite compounds with above-room-temperature ferroelasticity: [(CH3)4P][Cd(SCN)3] and [(CH3)3PCH2F][Cd(SCN)3. Chem. Commun. 2019, 55, 8418–8421. [Google Scholar] [CrossRef]
- Abdelkader, M.M.; Abdelmohsen, M. Hydrogen-bonded and supramolecular ferroelectricity in a new hybrid (C12H25NH3)2CoCl4. Mater. Res. Express 2018, 6, 025608. [Google Scholar] [CrossRef]
- Zhou, L.; Li, R.X.; Shi, P.-P.; Ye, Q.; Fu, D.-W. Successive Phase Transitions and Dual Dielectric Switching in an Organic-Inorganic Hybrid Perovskite. Inorg. Chem. 2020, 59, 18174–18180. [Google Scholar] [CrossRef]
- Xu, Y.L.; Zhang, J.; Chen, L.S.; Zeng, Y.-Y.; Zhou, J.-R.; Ni, C.-L.; Zheng, W.-X. Crystal structure, spectroscopic, non-linear optical, magnetic properties and DFT studies of bis(2-aminopyridinium) tetrachlorocobaltate(II). J. Mol. Struct. 2020, 12, 128902. [Google Scholar] [CrossRef]
- Gong, J.M.; Shao, T.; Huang, P.Z.; Su, C.-Y.; Chen, M.; Fu, D.-W.; Lu, H.-F. Reversible Phase Transition and Second-Harmonic Response Based on a Zero-Dimensional Organic-Inorganic Hybrid Compound. J. Phys. Chem. C 2022, 126, 15274–15279. [Google Scholar] [CrossRef]
- You, X.; Yao, J.; Wei, Z. Tin-based organic-inorganic hybrid semiconductors with reversible phase transition and dielectric anomaly. Dalton Trans. 2020, 49, 7252–7257. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Qattan, I.A.; Ahmad, A.A.; Al Bataineh, Q.M.; Al Abed, H.I.; Albataineh, Z.; Telfah, A.; Sabirianov, R.F. Theoretical and experimental overview of structural, dielectric, crystallographic, electronic, optical, and physical tensors of α-DIPAB and iodine-doped α-DIPAB molecular ferroelectric crystals. J. Electron. Mater. 2020, 49, 7112–7132. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Li, Z.; Qian, K.; Sao, F. High-temperature ferroelastic phase transition in a perovskite-like complex: [Et4N]2[PbBr3]2. RSC Adv. 2019, 9, 10364–10370. [Google Scholar] [CrossRef] [PubMed]
- An, L.-C.; Zhao, C.; Zhao, Y.; Zhang, Y.; Li, K.; Stroppa, A.; Li, W.; Bu, X.-H. Chiral 1D Hybrid Metal Halides with Piezoelectric Energy Harvesting and Sensing Properties. Small Struct. 2023, 4, 2300135. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Zhang, W.Y.; Shi, P.-P.; Ye, Q.; Fu, D.-W. Smart and efficient opto-electronic dual response material based on two-dimensional perovskite crystal/thin film. J. Mater. Chem. C 2020, 8, 1953–1961. [Google Scholar] [CrossRef]
- Gao, H.; Chen, Y.-D.; Zhang, T.; Ge, J.-Z.; Fu, D.-W.; Zhang, Y. Homochiral Chemistry Strategy To Trigger Dielectric Switching and Second-Harmonic Generation Response on Spirocyclic Derivatives. Inorg. Chem. 2022, 61, 10872–10879. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Yoo, J.C. Loop-mediated isothermal amplification using a lab-on-a-disc device with thin-film phase change material. Appl. Biochem. Biotechnol. 2018, 186, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, Z.; Shi, P.; Zhang, W.; Ye, Q.; Fu, D. High-temperature dielectric switch and second harmonic generation integrated in a stimulus responsive material. Chin. Chem. Lett. 2021, 32, 539–542. [Google Scholar] [CrossRef]
- Wang, P.; Chen, M.-K.; Tong, Y.-Q.; Yin, S.Q.; Huang, B. Structural phase transition and dielectric relaxation in an organic-inorganic hybrid compound: [(CH3)3NH]4[Fe(SCN)6]Cl. CrystEngComm 2022, 24, 7083–7088. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.Y.; Shi, P.P.; Ye, Q.; Fu, D.W. Switchable Dielectric Phase Transition Triggered by Pendulum-Like Motion in an Ionic Co-crystal. Chem. Asian J. 2018, 13, 2916–2922. [Google Scholar] [CrossRef] [PubMed]





| D-H···A | d(D-A)/Å | % | ∠D-H···A/(°) | % |
|---|---|---|---|---|
| 100 K | ||||
| N14-H14···S6 | 3.3 | 7.1% | 164.6 | 4.7% |
| N3-H3A···S6 | 3.3 | 7.1% | 173.0 | 13.3% |
| C18-H18···S3 | 3.4 | 2.9% | 167.4 | 7.7% |
| O1-H1B···S3 | 3.2 | 17.1% | 143.2 | 16.5% |
| O1-H1C···S5 | 3.6 | 22.9% | 145.7 | 14.0% |
| N3-H3B···S2 | 3.4 | 2.9% | 166.2 | 6.5% |
| N2-H2···S1 | 3.4 | 2.9% | 157.8 | 1.9% |
| 293 K | ||||
| N14-H14···S6 | 3.3 | 8.6% | 163.2 | 11.3% |
| N3-H3A···S6 | 3.3 | 8.6% | 171.2 | 19.3% |
| C18-H18···S3 | 3.4 | 1.4% | 163.7 | 11.8% |
| O1-H1B···S3 | 3.2 | 18.6% | 119.8 | 32.0% |
| O1-H1C···S5 | 3.6 | 21.4% | 121.6 | 30.2% |
| N3-H3B···S2 | 3.4 | 1.4% | 166.9 | 15.1% |
| N2-H2···S1 | 3.5 | 11.4% | 156.4 | 4.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Abuduheni, A.; Yang, F.; Hu, H.; Liu, Z. Synthesis, the Reversible Isostructural Phase Transition, and the Dielectric Properties of a Functional Material Based on an Aminobenzimidazole–Iron Thiocyanate Complex. Int. J. Mol. Sci. 2024, 25, 9064. https://doi.org/10.3390/ijms25169064
Liu Y, Abuduheni A, Yang F, Hu H, Liu Z. Synthesis, the Reversible Isostructural Phase Transition, and the Dielectric Properties of a Functional Material Based on an Aminobenzimidazole–Iron Thiocyanate Complex. International Journal of Molecular Sciences. 2024; 25(16):9064. https://doi.org/10.3390/ijms25169064
Chicago/Turabian StyleLiu, Yang, Adila Abuduheni, Fang Yang, Hongzhi Hu, and Zunqi Liu. 2024. "Synthesis, the Reversible Isostructural Phase Transition, and the Dielectric Properties of a Functional Material Based on an Aminobenzimidazole–Iron Thiocyanate Complex" International Journal of Molecular Sciences 25, no. 16: 9064. https://doi.org/10.3390/ijms25169064




