Exosomal miRNAs Differentiate Chronic Total Occlusion from Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Participants
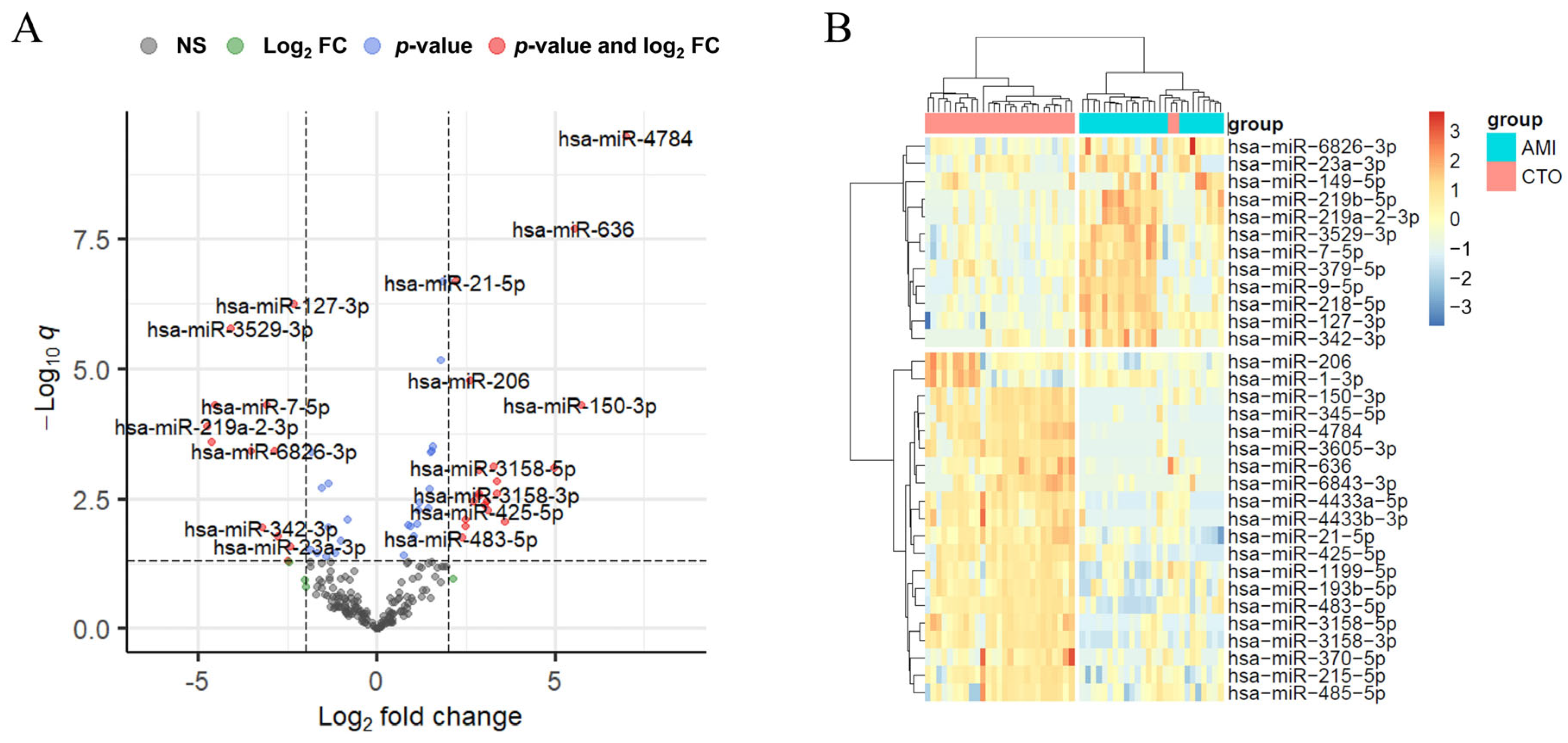
2.2. Differential Expression of miRNAs in Exosomes of CTO and AMI Patients
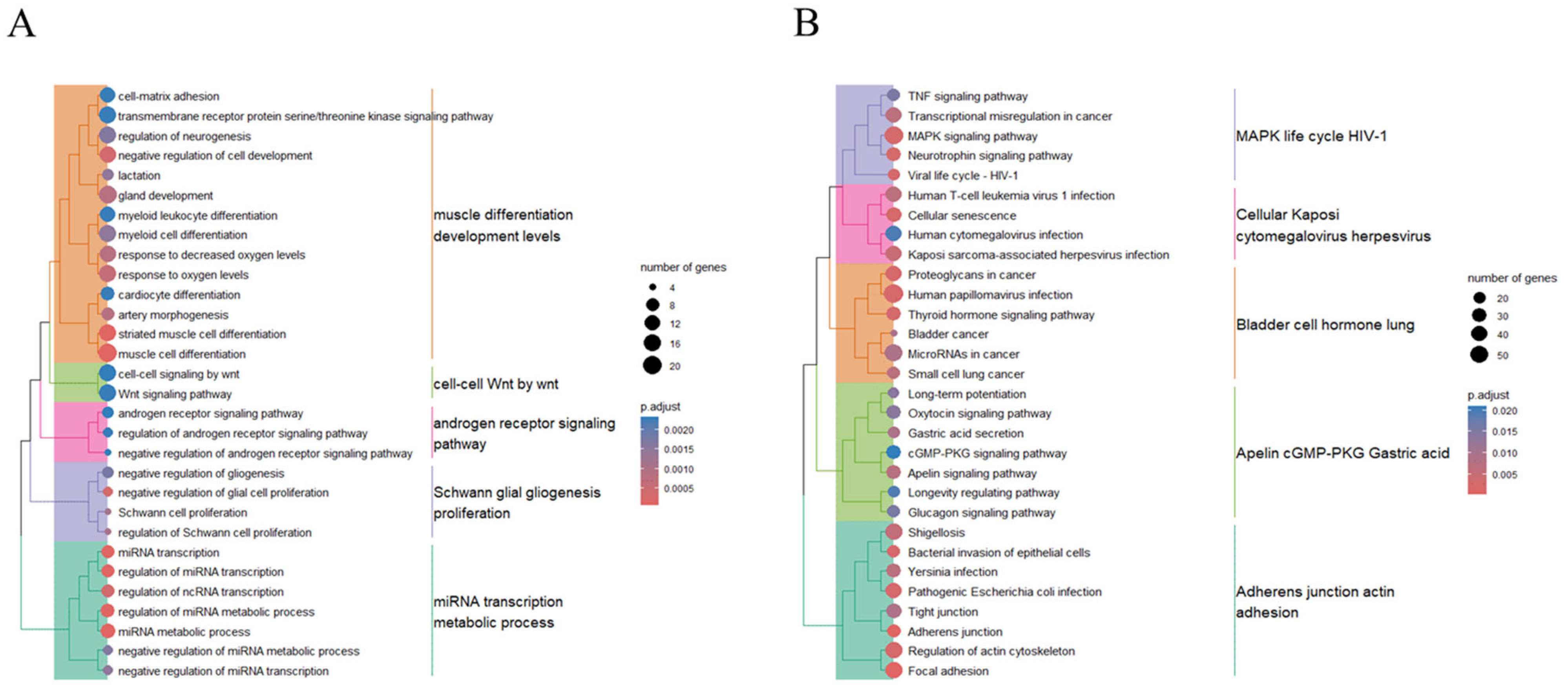
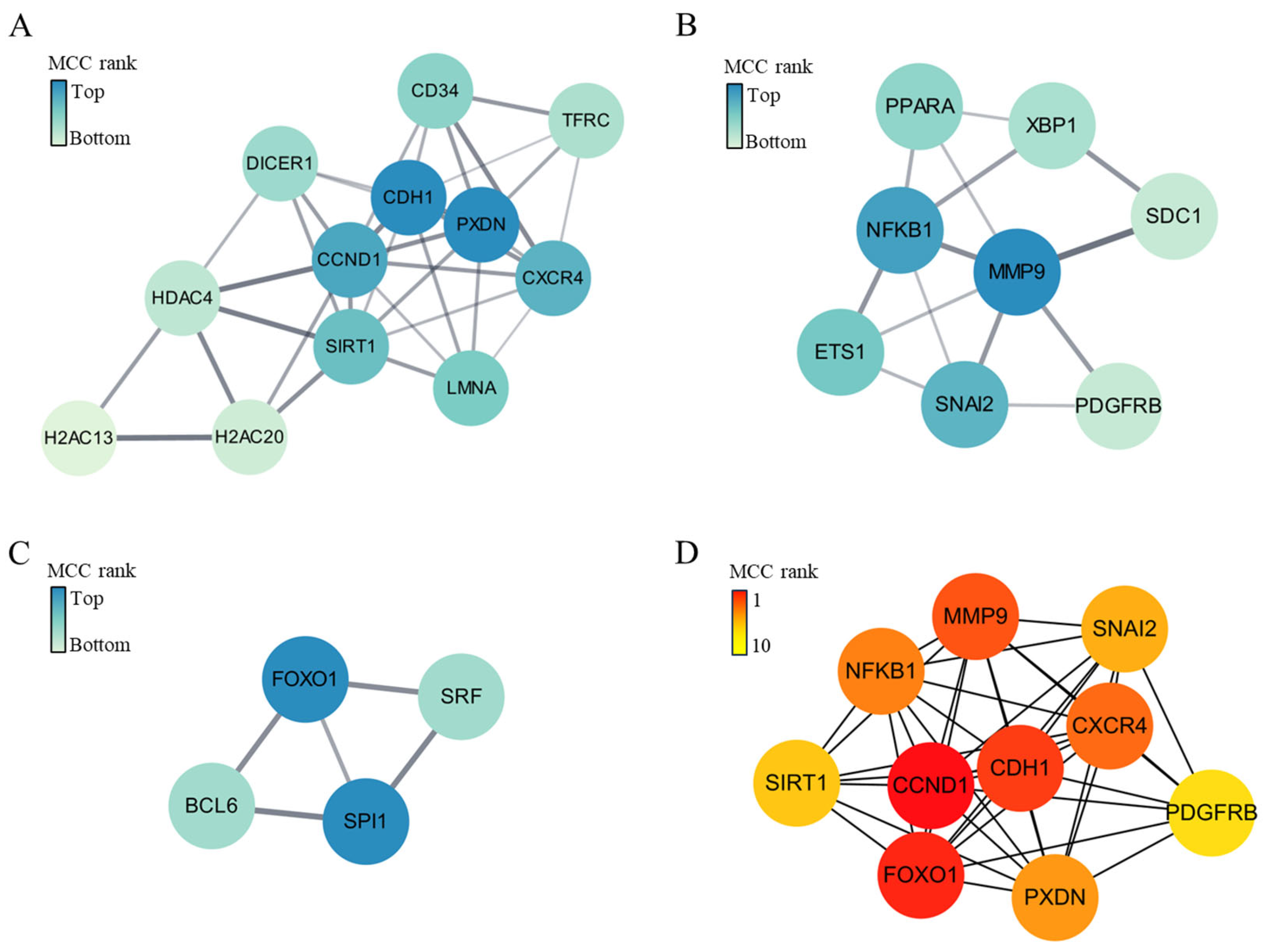
2.3. Enrichment Analyses of Biological Processes and Pathways, and the Interaction Networks of Genes Targeted by Upregulated Exosomal miRNAs in CTO Patients
2.4. Enrichments of Biological Processes and Pathways, and the Interaction Network of Genes Targeted by Downregulated Exosomal miRNAs in CTO
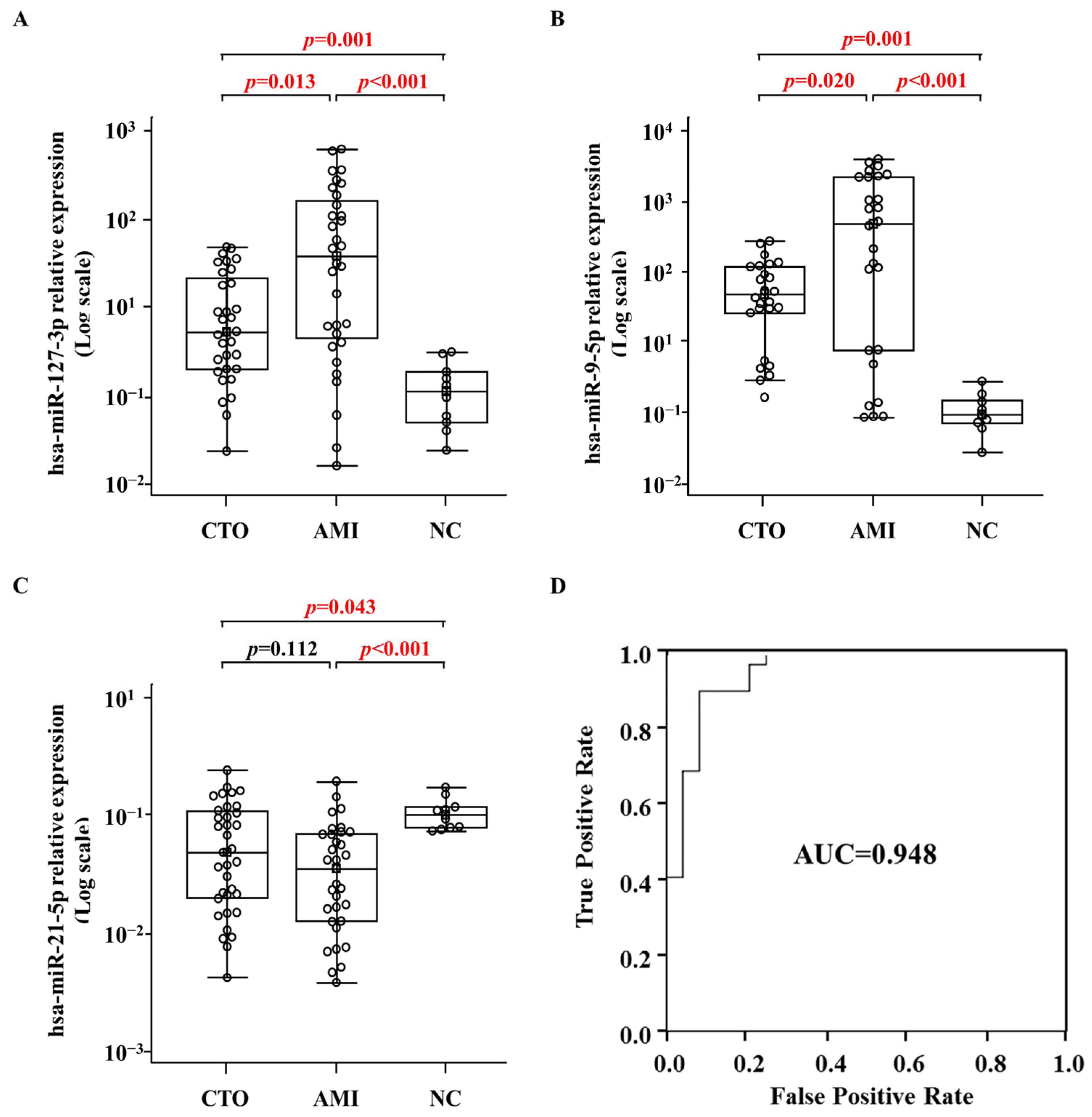
2.5. Validation of the miRNA Expression Levels in Normal Control, AMI, and CTO Samples via qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Study Subjects and Plasma Preparation
4.2. Isolation and Characterization of Exosomes
4.3. Procedures for Small RNA Sequencing
4.4. Differentially Expressed Gene Analysis and Target Gene Analysis
4.5. miRNA qRT-PCR Analysis
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Behnes, M.; Mashayekhi, K.; Bufe, A.; Meyer-Gessner, M.; El-Battrawy, I.; Akin, I. Prognostic Impact of Percutaneous Coronary Intervention of Chronic Total Occlusion in Acute and Periprocedural Myocardial Infarction. J. Clin. Med. 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Ramunddal, T.; Hoebers, L.P.; Henriques, J.P.; Dworeck, C.; Angeras, O.; Odenstedt, J.; Ioanes, D.; Olivecrona, G.; Harnek, J.; Jensen, U.; et al. Prognostic Impact of Chronic Total Occlusions: A Report From SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc. Interv. 2016, 9, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, I.; Vidal-Puig, A.J. Fed-EXosome: Extracellular vesicles and cell-cell communication in metabolic regulation. Essays Biochem. 2018, 62, 165–175. [Google Scholar] [CrossRef]
- Bakhshian Nik, A.; Hutcheson, J.D.; Aikawa, E. Extracellular Vesicles As Mediators of Cardiovascular Calcification. Front. Cardiovasc. Med. 2017, 4, 78. [Google Scholar] [CrossRef]
- Blaser, M.C.; Aikawa, E. Roles and Regulation of Extracellular Vesicles in Cardiovascular Mineral Metabolism. Front. Cardiovasc. Med. 2018, 5, 187. [Google Scholar] [CrossRef]
- Krohn, J.B.; Aikawa, E.; Aikawa, M.; Hutcheson, J.D.; Sahoo, S.; Fish, J.E. Editorial: Extracellular vesicles in cardiovascular inflammation and calcification. Front. Cardiovasc. Med. 2022, 9, 1077124. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Ai, X.; Kilic, E.; Hermann, D.M.; Venkataramani, V.; Bahr, M.; Doeppner, T.R. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-beta/Smad2/3 pathway. Cell Death Dis. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Seara, F.A.C.; Maciel, L.; Kasai-Brunswick, T.H.; Nascimento, J.H.M.; Campos-de-Carvalho, A.C. Extracellular Vesicles and Cardiac Aging. Adv. Exp. Med. Biol. 2023, 1418, 33–56. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Laffont, B.; Rayner, K.J. MicroRNAs in the Pathobiology and Therapy of Atherosclerosis. Can. J. Cardiol. 2017, 33, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Marzano, F.; Salvatore, M.; Basile, C.; Buonocore, D.; Parlati, A.L.M.; Nardi, E.; Asile, G.; Abbate, V.; Colella, A.; et al. MicroRNAs: Diagnostic, prognostic and therapeutic role in heart failure-a review. ESC Heart Fail. 2023, 10, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef]
- Hakimzadeh, N.; Nossent, A.Y.; van der Laan, A.M.; Schirmer, S.H.; de Ronde, M.W.; Pinto-Sietsma, S.J.; van Royen, N.; Quax, P.H.; Hoefer, I.E.; Piek, J.J. Circulating MicroRNAs Characterizing Patients with Insufficient Coronary Collateral Artery Function. PLoS ONE 2015, 10, e0137035. [Google Scholar] [CrossRef][Green Version]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc. Res. 2006, 70, 240–253. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Segers, V.F.; Davis, M.E.; MacGillivray, C.; Gannon, J.; Molkentin, J.D.; Robbins, J.; Lee, R.T. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat. Med. 2007, 13, 970–974. [Google Scholar] [CrossRef]
- Vilahur, G.; Juan-Babot, O.; Pena, E.; Onate, B.; Casani, L.; Badimon, L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell Cardiol. 2011, 50, 522–533. [Google Scholar] [CrossRef]
- Yao, H.; Han, X.; Han, X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am. J. Cardiovasc. Drugs 2014, 14, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Giri, H.; Li, J.; Rezaie, A.R. The Cardioprotective Signaling Activity of Activated Protein C in Heart Failure and Ischemic Heart Diseases. Int. J. Mol. Sci. 2019, 20, 1762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shang, Z.; Liu, A. Angiotensin-(3-7) alleviates isoprenaline-induced cardiac remodeling via attenuating cAMP-PKA and PI3K/Akt signaling pathways. Amino Acids 2021, 53, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Kong, W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell Signal 2020, 66, 109485. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liang, D.; Liu, D.K.; Lin, L.; Zhang, L.; Yang, W.Q. The role of the ERK signaling pathway in promoting angiogenesis for treating ischemic diseases. Front. Cell Dev. Biol. 2023, 11, 1164166. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, J.; Wu, R.; Yuan, J.; Ge, J. Integrated Analysis of Angiogenesis Related lncRNA-miRNA-mRNA in Patients With Coronary Chronic Total Occlusion Disease. Front. Genet. 2022, 13, 855549. [Google Scholar] [CrossRef]
- Du, S.Y.; Huang, X.X.; Li, N.M.; Lv, C.Y.; Lv, C.H.; Wei, M.L.; Gao, Z.; Zhang, Y.P. MiR-127-3p inhibits proliferation of ovarian cancer in rats through down-regulating MAPK4. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10383–10390. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, X.; Fang, Y.; Nie, Y.; Cai, S.; Chen, Y.; Mo, D. mir-127-3p inhibits the proliferation of myocytes by targeting KMT5a. Biochem. Biophys. Res. Commun. 2018, 503, 970–976. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Xiong, L.L.; Xue, L.L.; Deng, Y.P.; Du, R.L.; Hu, Q.; Xu, Y.; Yang, S.J.; Wang, T.H. MiR-127-3p targeting CISD1 regulates autophagy in hypoxic-ischemic cortex. Cell Death Dis. 2021, 12, 279. [Google Scholar] [CrossRef]
- Koens, L.; Qin, Y.; Leung, W.Y.; Corver, W.E.; Jansen, P.M.; Willemze, R.; Vermeer, M.H.; Tensen, C.P. MicroRNA profiling of primary cutaneous large B-cell lymphomas. PLoS ONE 2013, 8, e82471. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, R.; Li, X.; Zhang, W.; Zhan, Y.; Lang, Z.; Tao, Q.; Yu, J.; Yu, S.; Yu, Z.; et al. Circular RNA cVIM promotes hepatic stellate cell activation in liver fibrosis via miR-122-5p/miR-9-5p-mediated TGF-beta signaling cascade. Commun. Biol. 2024, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, T.; Jiang, Z.; Gai, C.; Yu, S.; Xin, D.; Li, T.; Liu, D.; Wang, Z. The miR-9-5p/CXCL11 pathway is a key target of hydrogen sulfide-mediated inhibition of neuroinflammation in hypoxic ischemic brain injury. Neural Regen. Res. 2024, 19, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Iordache, F.; Petcu, A.I.; Pisoschi, A.M.; Stanca, L.; Geicu, O.I.; Bilteanu, L.; Curutiu, C.; Amuzescu, B.; Serban, A.I. PCR Array Profiling of miRNA Expression Involved in the Differentiation of Amniotic Fluid Stem Cells toward Endothelial and Smooth Muscle Progenitor Cells. Int. J. Mol. Sci. 2023, 25, 302. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, F.; Yang, Q.; Zhang, Y.; Cai, K.; Zhang, J.; Xia, M.; Wang, Y.; Wang, X.; Gui, Y.; et al. Upregulation of miR-21-5p rescues the inhibition of cardiomyocyte proliferation induced by high glucose through negative regulation of Rhob. J. Dev. Orig. Health Dis. 2023, 14, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Pravalika, J.; Manjula, P.; Farooq, U. Gender and CVD- Does It Really Matters? Curr. Probl. Cardiol. 2023, 48, 101604. [Google Scholar] [CrossRef]
- Di Mario, C.; Werner, G.S.; Sianos, G.; Galassi, A.R.; Buttner, J.; Dudek, D.; Chevalier, B.; Lefevre, T.; Schofer, J.; Koolen, J.; et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): Consensus document from the EuroCTO Club. EuroIntervention 2007, 3, 30–43. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Vestad, B.; Llorente, A.; Neurauter, A.; Phuyal, S.; Kierulf, B.; Kierulf, P.; Skotland, T.; Sandvig, K.; Haug, K.B.F.; Ovstebo, R. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: A variation study. J. Extracell. Vesicles 2017, 6, 1344087. [Google Scholar] [CrossRef]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]



| Variable | NGS | Validation (qPCR) | |||||
|---|---|---|---|---|---|---|---|
| AMI | CTO | p-Value | CTO | AMI | NC | p-Value | |
| No. | 24 | 29 | - | 35 | 35 | 10 | - |
| Sex (Male/Female) | 20/4 | 22/7 | 0.504 | 30/5 | 30/5 | 8/2 | 0.894 |
| Age (years) | 63.5 (52.5–72) | 64.0 (61.0–69.5) | 0.714 | 64 (59–69) | 63 (51–67) | 65 (63–68) | 0.692 |
| Smoking (%) | 12 (50.0%) | 11 (37.9%) | 0.378 | 15 (42.9%) | 19 (54.3%) | 2 (20%) | 0.149 |
| Body mass index (kg/m2) | 24.25 ± 0.38 | 25.70 ± 0.55 | 0.054 | 25.72 ± 0.61 | 24.26 ± 0.36 | 26.92 ± 0.73 | 0.009 |
| Hypertension (%) | 15 (62.5%) | 22 (75.9%) | 0.292 | 27 (77.1%) | 20 (57.1%) | 9 (90%) | 0.064 |
| Diabetes (%) | 11 (45.8%) | 22 (75.9%) | 0.025 | 23 (65.7%) | 15 (42.9%) | 3 (30%) | 0.057 |
| Dyslipidemia (%) | 9 (37.5%) | 20 (69.0%) | 0.022 | 23 (65.7%) | 17 (48.6%) | 9 (90%) | 0.046 |
| Chronic kidney disease (%) | 4 (16.7%) | 6 (20.7%) | 0.709 | 7 (20%) | 4 (11.4%) | 0 (0%) | 0.234 |
| Previous PCI (%) | 3 (12.5%) | 11 (37.9%) | 0.037 | 13 (37.1%) | 4 (11.4%) | 1 (10%) | 0.022 |
| Previous MI (%) | 2 (8.3%) | 6 (20.7%) | 0.211 | 8 (22.9%) | 3 (8.6%) | 0 (0%) | 0.089 |
| Previous CVA (%) | 5 (20.8%) | 7 (24.1%) | 0.775 | 8 (22.9%) | 1 (2.9%) | 1 (10%) | 0.039 |
| Previous PAD (%) | 1 (4.2%) | 5 (17.2%) | 0.135 | 4 (11.4%) | 1 (2.9%) | 0 (0%) | 0.228 |
| hs-CRP (mg/dL) | 0.33 (0.09–1.15) | 0.17 (0.07–0.60) | 0.537 | 0.16 (0.07–0.48) | 0.19 (0.07–0.77) | 0.09 (0.06–0.1) | 0.099 |
| LDL-cholesterol (mg/dL) | 108.5 (77.3–128.0) | 110.0 (79.5–132.5) | 0.782 | 94 (61–116) | 114 (94–130) | 103.5 (62.8–120.8) | 0.070 |
| Creatinine (mg/dL) | 1.00 (0.93–1.28) | 0.90 (0.85–1.10) | 0.070 | 1(0.9–1.1) | 1.1 (0.95–1.2) | - | 0.482 |
| Pre LVEF (%) | 52.0 (43.3–61.5) | 56.0 (43.5–60.5) | 0.761 | 53 (46–59) | 47 (44–52) | - | 0.081 |
| FU LVEF (%) | 58.5 (46.0–66.8) | 50.0 (46.0–56.0) | 0.262 | 50 (46.5–56.25) | 53 (47–62) | - | 0.579 |
| Target vessel (culprit lesion) | |||||||
| LAD | 12 (50.0%) | 10 (34.5%) | 0.220 | 12 (34.3%) | 14 (40%) | - | 0.446 |
| RCA | 6 (25.0%) | 5 (17.2%) | 6 (17.1%) | 9 (25.7%) | - | ||
| LCX | 6 (25.0%) | 14 (48.3%) | 17 (48.6%) | 12 (34.3%) | - | ||
| Coronary artery disease (LM excepted) | |||||||
| 1VD | 9 (37.5%) | 2 (6.9%) | <0.001 | 6 (17.1%) | 17 (48.6%) | - | 0.001 |
| 2VD | 12 (50%) | 10 (34.5%) | 12 (34.3%) | 14 (40%) | - | ||
| 3VD | 3 (12.5%) | 17 (58.6%) | 17 (48.6%) | 4 (11.4%) | - | ||
| Calcification (%) | 4 (16.7%) | 19 (65.5%) | <0.001 | ||||
| MACE | 1 (4.2%) | 6 (20.7%) | 0.077 | ||||
| TVR (%) | 0 (0%) | 3 (10.3%) | 0.105 | ||||
| Death (%) | 1 (4.2%) | 1 (3.4%) | 0.891 | ||||
| miRNA | AMI | CTO | glmQLFTest | ||||
|---|---|---|---|---|---|---|---|
| logFC | logCPM | F | p-Value | FDR | |||
| hsa-miR-3605-3p | 6.72 ± 8.63 | 11.67 ± 12.11 | 4.97 | 10.84 | 18.01 | <0.001 | 0.001 |
| hsa-miR-345-5p | 7.23 ± 9.42 | 10.8 ± 10.65 | 3.58 | 10.02 | 10.87 | 0.002 | 0.009 |
| hsa-miR-3158-3p | 8.14 ± 8.85 | 11.5 ± 11.17 | 3.36 | 10.74 | 14.48 | <0.001 | 0.003 |
| hsa-miR-1-3p | 12.48 ± 12.74 | 15.58 ± 16.5 | 3.09 | 14.84 | 13.42 | 0.001 | 0.004 |
| hsa-miR-485-5p | 8.85 ± 9.35 | 11.7 ± 13.29 | 2.86 | 10.98 | 14.47 | <0.001 | 0.003 |
| hsa-miR-215-5p | 9.83 ± 10.85 | 12.68 ± 13.33 | 2.85 | 11.97 | 17.59 | <0.001 | 0.001 |
| hsa-miR-206 | 10.63 ± 10.17 | 13.22 ± 13.67 | 2.59 | 12.54 | 31.28 | <0.001 | <0.001 |
| hsa-miR-1199-5p | 8.26 ± 8.93 | 10.74 ± 10.88 | 2.49 | 10.07 | 11.17 | 0.001 | 0.008 |
| hsa-miR-483-5p | 9.37 ± 10.47 | 11.74 ± 11.36 | 2.38 | 11.08 | 8.92 | 0.004 | 0.018 |
| hsa-miR-21-5p | 9.61 ± 9.13 | 11.82 ± 11.15 | 2.21 | 11.19 | 47.81 | <0.001 | <0.001 |
| hsa-miR-127-3p | 10.87 ± 10.42 | 8.53 ± 7.87 | −2.35 | 10.04 | 43.57 | <0.001 | <0.001 |
| hsa-miR-3529-3p | 11.11 ± 11.78 | 7.03 ± 7.34 | −4.11 | 10.07 | 39.34 | <0.001 | <0.001 |
| hsa-miR-9-5p | 12.19 ± 12.26 | 7.59 ± 8.24 | −4.62 | 11.11 | 22.1 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.-H.; Park, J.K.; Bang, J.-H.; Kim, D.; Moon, I.; Kong, M.G.; Park, H.-W.; Choi, H.-O.; Seo, H.-S.; Cho, Y.H.; et al. Exosomal miRNAs Differentiate Chronic Total Occlusion from Acute Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 10223. https://doi.org/10.3390/ijms251810223
Son J-H, Park JK, Bang J-H, Kim D, Moon I, Kong MG, Park H-W, Choi H-O, Seo H-S, Cho YH, et al. Exosomal miRNAs Differentiate Chronic Total Occlusion from Acute Myocardial Infarction. International Journal of Molecular Sciences. 2024; 25(18):10223. https://doi.org/10.3390/ijms251810223
Chicago/Turabian StyleSon, Ji-Hye, Jeong Kyu Park, Ji-Hong Bang, Dongeon Kim, Inki Moon, Min Gyu Kong, Hyun-Woo Park, Hyung-Oh Choi, Hye-Sun Seo, Yoon Haeng Cho, and et al. 2024. "Exosomal miRNAs Differentiate Chronic Total Occlusion from Acute Myocardial Infarction" International Journal of Molecular Sciences 25, no. 18: 10223. https://doi.org/10.3390/ijms251810223
APA StyleSon, J.-H., Park, J. K., Bang, J.-H., Kim, D., Moon, I., Kong, M. G., Park, H.-W., Choi, H.-O., Seo, H.-S., Cho, Y. H., Chang, H. S., & Suh, J. (2024). Exosomal miRNAs Differentiate Chronic Total Occlusion from Acute Myocardial Infarction. International Journal of Molecular Sciences, 25(18), 10223. https://doi.org/10.3390/ijms251810223





