Neuroinflammation and Brain Health Risks in Veterans Exposed to Burn Pit Toxins
Abstract
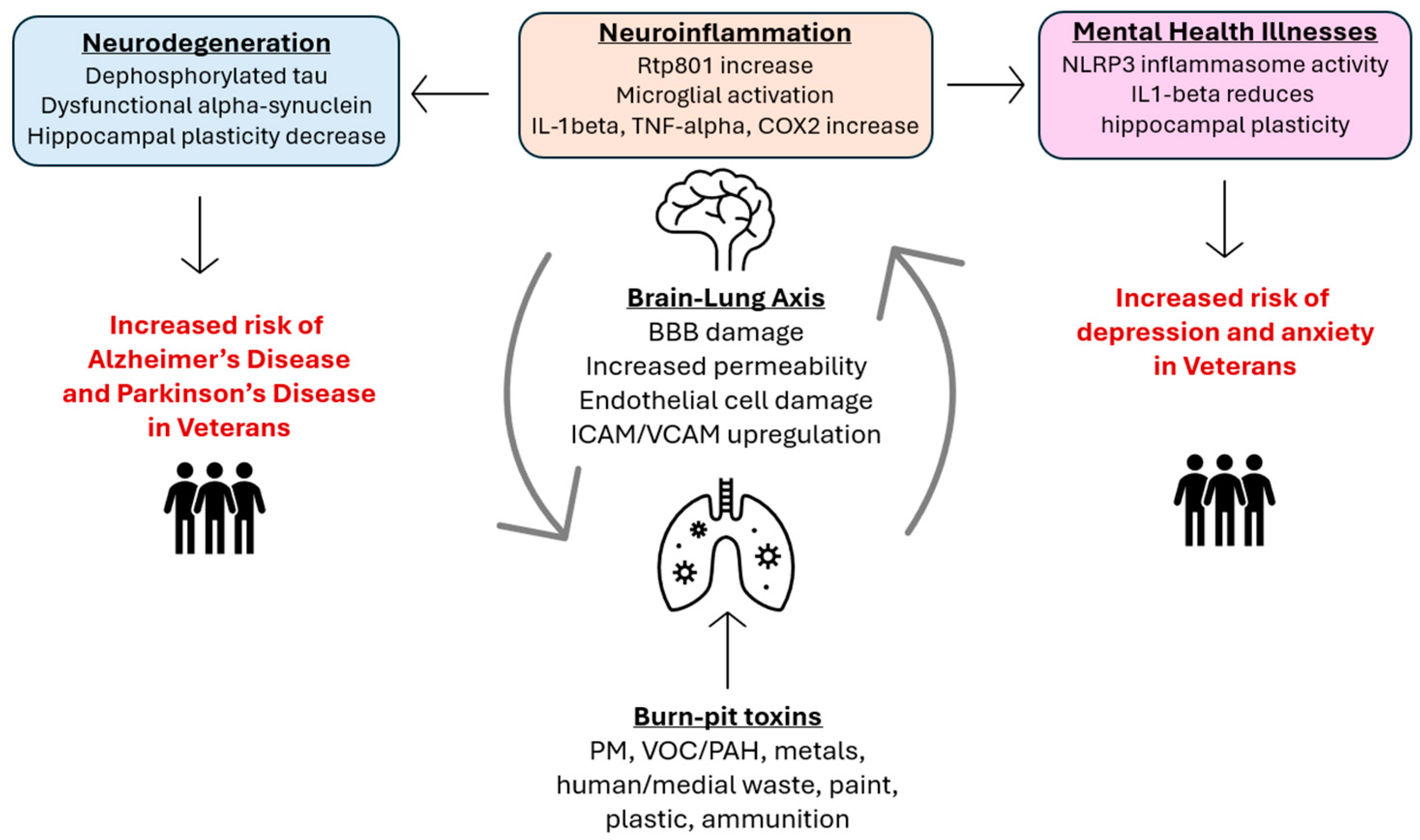
:1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Inconsistencies and Biases in the Literature
3. The Lung–Brain Axis
4. Toxin-Induced Neuroinflammation
5. Toxin-Induced Neurodegeneration/Cognitive Impairment
6. The Impacts of Air Pollution on Veteran’s Mental Wellbeing
7. Risks for Veterans and Other Communities
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riedl, M.A. The effect of air pollution on asthma and allergy. Curr. Allergy Asthma Rep. 2008, 8, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandström, T.; Blomberg, A.; Newby, D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Azzarelli, B.; Acuna, H.; Garcia, R.; Gambling, T.M.; Osnaya, N.; Monroy, S.; Tizapantzi, M.D.R.; Carson, J.L.; Villarreal-Calderon, A.; et al. Air Pollution and Brain Damage. Toxicol. Pathol. 2002, 30, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Tomei, F.; Rosati, M.V.; Ciarrocca, M.; Baccolo, T.P.; Gaballo, M.; Caciari, T.; Tomao, E. Plasma Cortisol Levels and Workers Exposed to Urban Pollutants. Ind. Health 2003, 41, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, H.; Cui, S.; Han, B.; Zhou, L.; Zhang, N.; Su, X.; Niu, Y.; Chen, W.; Chen, R.; et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard. Mater. 2019, 369, 180–190. [Google Scholar] [CrossRef]
- Woodall, B.D.; Yamamoto, D.P.; Gullett, B.K.; Touati, A. Emissions from Small-Scale Burns of Simulated Deployed U.S. Military Waste. Environ. Sci. Technol. 2012, 46, 10997–11003. [Google Scholar] [CrossRef]
- Hoisington, A.J.; Stearns-Yoder, K.A.; Kovacs, E.J.; Postolache, T.T.; Brenner, L.A. Airborne Exposure to Pollutants and Mental Health: A Review with Implications for United States Veterans. Curr. Environ. Health Rep. 2024, 11, 168–183. [Google Scholar] [CrossRef]
- Penuelas, V.L.; Lo, D.D. Burn pit exposure in military personnel and the potential resulting lung and neurological pathologies. Front. Environ. Health 2024, 3, 1364812. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering: EASE ’14, London, UK, 13–14 May 2014; Association for Computing Machinery: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Yoshida, T.; Mett, I.; Bhunia, A.K.; Bowman, J.; Perez, M.; Zhang, L.; Gandjeva, A.; Zhen, L.; Chukwueke, U.; Mao, T.; et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat. Med. 2010, 16, 767–773. [Google Scholar] [CrossRef]
- Romaní-Aumedes, J.; Canal, M.; Martín-Flores, N.; Sun, X.; Pérez-Fernández, V.; Wewering, S.; Fernández-Santiago, R.; Ezquerra, M.; Pont-Sunyer, C.; Lafuente, A.A.; et al. Parkin loss of function contributes to RTP801 elevation and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2014, 5, e1364. [Google Scholar] [CrossRef] [PubMed]
- Ellisen, L.W. Smoking and emphysema: The stress connection. Nat. Med. 2010, 16, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sisqués, L.; Sancho-Balsells, A.; Solana-Balaguer, J.; Campoy-Campos, G.; Vives-Isern, M.; Soler-Palazón, F.; Anglada-Huguet, M.; López-Toledano, M.; Mandelkow, E.-M.; Alberch, J.; et al. RTP801/REDD1 contributes to neuroinflammation severity and memory impairments in Alzheimer’s disease. Cell Death Dis. 2021, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Adivi, A.; Lucero, J.; Simpson, N.; McDonald, J.D.; Lund, A.K. Exposure to traffic-generated air pollution promotes alterations in the integrity of the brain microvasculature and inflammation in female ApoE-/- mice. Toxicol. Lett. 2021, 339, 39–50. [Google Scholar] [CrossRef]
- Gárate-Vélez, L.; Escudero-Lourdes, C.; Salado-Leza, D.; González-Sánchez, A.; Alvarado-Morales, I.; Bahena, D.; Labrada-Delgado, G.J.; Rodríguez-López, J.L. Anthropogenic Iron Oxide Nanoparticles Induce Damage to Brain Microvascular Endothelial Cells Forming the Blood-Brain Barrier. J. Alzheimers Dis. 2020, 76, 1527–1539. [Google Scholar] [CrossRef]
- Kang, Y.J.; Tan, H.Y.; Lee, C.Y.; Cho, H. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci. 2021, 8, 2101251. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.; Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008, 68, 117–127. [Google Scholar] [CrossRef]
- Sama, P.; Long, T.C.; Hester, S.; Tajuba, J.; Parker, J.; Chen, L.-C.; Veronesi, B. The Cellular and Genomic Response of an Immortalized Microglia Cell Line (BV2) to Concentrated Ambient Particulate Matter. Inhal. Toxicol. 2007, 19, 1079–1087. [Google Scholar] [CrossRef]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004, 18, 1618–1620. [Google Scholar] [CrossRef]
- Zhang, P.; Hatter, A.; Liu, B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol. Lett. 2007, 173, 88–100. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood–brain Barrier: Structural Components and Function Under Physiologic and Pathologic Conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Chen, L.; Yokel, R.A.; Hennig, B.; Toborek, M. Manufactured Aluminum Oxide Nanoparticles Decrease Expression of Tight Junction Proteins in Brain Vasculature. J. Neuroimmune Pharmacol. 2008, 3, 286–295. [Google Scholar] [CrossRef]
- Witkowska, A.M. Soluble ICAM-1: A marker of vascular inflammation and lifestyle. Cytokine 2005, 31, 127–134. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Xicota, L.; Lagarde, J.; Eysert, F.; Grenier-Boley, B.; Rivals, I.; Botté, A.; Forlani, S.; Landron, S.; Gautier, C.; Gabriel, C.; et al. Modifications of the endosomal compartment in fibroblasts from sporadic Alzheimer’s disease patients are associated with cognitive impairment. Transl. Psychiatry 2023, 13, 54. [Google Scholar] [CrossRef]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Dorans, K.S.; Gold, D.R.; Schwartz, J.; Koutrakis, P.; Washko, G.R.; O’connor, G.T.; Mittleman, M.A. Long-Term Exposure to Traffic Emissions and Fine Particulate Matter and Lung Function Decline in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2015, 191, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Cacciottolo, M.; Wang, X.; Driscoll, I.; Woodward, N.; Saffari, A.; Reyes, J.; Serre, M.L.; Vizuete, W.; Sioutas, C.; Morgan, T.E.; et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl. Psychiatry 2017, 7, e1022. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Xu, X.; Weil, Z.M.; Chen, G.; Sun, Q.; Rajagopalan, S.; Nelson, R.J. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry 2011, 16, 987–995. [Google Scholar] [CrossRef]
- Nabi, M.; Tabassum, N. Role of Environmental Toxicants on Neurodegenerative Disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Finnegan, P.; Chen, W. Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Niño, S.A.; Morales-Martínez, A.; Chi-Ahumada, E.; Carrizales, L.; Salgado-Delgado, R.; Pérez-Severiano, F.; Díaz-Cintra, S.; Jiménez-Capdeville, M.E.; Zarazua, S. Arsenic Exposure Contributes to the Bioenergetic Damage in an Alzheimer’s Disease Model. ACS Chem. Neurosci. 2019, 10, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, B.; Ke, T.; Li, S.; Yang, X.; Aschner, M.; Chen, P. Mechanisms of Metal-Induced Mitochondrial Dysfunction in Neurological Disorders. Toxics 2021, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Monnet-Tschudi, F.; Zurich, M.G.; Boschat, C.; Corbaz, A.; Honegger, P. Involvement of Environmental Mercury and Lead in the Etiology of Neurodegenerative Diseases. Rev. Environ. Health 2006, 21, 105–118. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Heng, Y.; Yuan, Y.H.; Chen, N.H. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2017, 265, 30–37. [Google Scholar] [CrossRef]
- Abraham, J.H.; Eick-Cost, A.; Clark, L.L.; Hu, Z.; Baird, C.P.; DeFraites, R.; Tobler, S.K.; Richards, E.E.; Sharkey, J.M.; Lipnick, R.J.; et al. A Retrospective Cohort Study of Military Deployment and Postdeployment Medical Encounters for Respiratory Conditions. Mil. Med. 2014, 179, 540–546. [Google Scholar] [CrossRef]
- Hill, W.; Lim, E.L.; Weeden, C.E.; Lee, C.; Augustine, M.; Chen, K.; Kuan, F.-C.; Marongiu, F.; Evans, E.J.; Moore, D.A.; et al. Lung adenocarcinoma promotion by air pollutants. Nature 2023, 616, 159–167. [Google Scholar] [CrossRef]
- MacMahon, J.; Bruun, D.; Lein, P. Military Burn Pits: A Toxic Legacy of War; UC Davis School of Veterinary Medicine: Davis, CA, USA, 2023. [Google Scholar]
- Poulsen, A.H.; Hvidtfeldt, U.A.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Christensen, J.H.; Geels, C.; Raaschou-Nielsen, O. Components of particulate matter air-pollution and brain tumors. Environ. Int. 2020, 144, 106046. [Google Scholar] [CrossRef] [PubMed]
- Ripley, S.; Maher, B.A.; Hatzopoulou, M.; Weichenthal, S. Within-city spatial variations in PM2.5 magnetite nanoparticles and brain cancer incidence in Toronto and Montreal, Canada. Sci. Rep. 2024, 14, 12136. [Google Scholar] [CrossRef]
- Zundel, C.G.; Ryan, P.; Brokamp, C.; Heeter, A.; Huang, Y.; Strawn, J.R.; Marusak, H.A. Air pollution, depressive and anxiety disorders, and brain effects: A systematic review. NeuroToxicology 2022, 93, 272–300. [Google Scholar] [CrossRef]
- Xia, C.-Y.; Guo, Y.-X.; Lian, W.-W.; Yan, Y.; Ma, B.-Z.; Cheng, Y.-C.; Xu, J.-K.; He, J.; Zhang, W.-K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Morimoto, S.S. The inflammation hypothesis in geriatric depression. Int. J. Geriatr. Psychiatry 2011, 26, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Duman, R.S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, I.; Zhang, S.; Kirkbride, J.B.; Osborn, D.P.J.; Hayes, J.F. Air Pollution (Particulate Matter) Exposure and Associations with Depression, Anxiety, Bipolar, Psychosis and Suicide Risk: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2019, 127, 126002. [Google Scholar] [CrossRef]
- Newbury, J.B.; Stewart, R.; Fisher, H.L.; Beevers, S.; Dajnak, D.; Broadbent, M.; Pritchard, M.; Shiode, N.; Heslin, M.; Hammoud, R.; et al. Association between air pollution exposure and mental health service use among individuals with first presentations of psychotic and mood disorders: Retrospective cohort study. Br. J. Psychiatry 2021, 219, 678–685. [Google Scholar] [CrossRef]
- Rentschler, J.; Leonova, N. Global air pollution exposure and poverty. Nat. Commun. 2023, 14, 4432. [Google Scholar] [CrossRef] [PubMed]
- Jbaily, A.; Zhou, X.; Liu, J.; Lee, T.-H.; Kamareddine, L.; Verguet, S.; Dominici, F. Air pollution exposure disparities across US population and income groups. Nature 2022, 601, 228–233. [Google Scholar] [CrossRef]
- Chen, L.C.; Lippmann, M. Effects of Metals within Ambient Air Particulate Matter (PM) on Human Health. Inhal. Toxicol. 2009, 21, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef]
- Malkov, A.; Lvanov, A.I.; Popova, I.; Mukhtarov, M.; Gubkina, O.; Waseem, T.; Bregestovski, P.; Zilberter, Y. Reactive Oxygen Species Initiate a Metabolic Collapse in Hippocampal Slices: Potential Trigger of Cortical Spreading Depression. J. Cereb. Blood Flow Metab. 2014, 34, 1540–1549. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia. Brain Sci. 2020, 10, 742. [Google Scholar] [CrossRef]
- Wisnivesky, J.P.; Teitelbaum, S.L.; Todd, A.C.; Boffetta, P.; Crane, M.; Crowley, L.; de la Hoz, R.E.; Dellenbaugh, C.; Harrison, D.; Herbert, R.; et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: A cohort study. Lancet 2011, 378, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Bith-Melander, P.; Ratliff, J.; Poisson, C.; Jindal, C.; Choi, Y.M.; Efird, J.T. Slow burns: A qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann. Psychiatry Clin. Neurosci. 2021, 4, 1042. [Google Scholar] [PubMed]
- Trembley, J.H.; So, S.W.; Nixon, J.P.; Bowdridge, E.C.; Garner, K.L.; Griffith, J.; Engles, K.J.; Batchelor, T.P.; Goldsmith, W.T.; Tomáška, J.M.; et al. Whole-body inhalation of nano-sized carbon black: A surrogate model of military burn pit exposure. BMC Res. Notes 2022, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Hendryx, M.; Fedorko, E.; Halverson, J. Pollution Sources and Mortality Rates Across Rural-Urban Areas in the United States. J. Rural Health 2010, 26, 383–391. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Rumrill, P.D., Jr.; Fitzgerald, S.M. Using narrative literature reviews to build a scientific knowledge base. Work 2001, 16, 165–170. [Google Scholar]
- Greve, H.J.; Mumaw, C.L.; Messenger, E.J.; Kodavanti, P.R.S.; Royland, J.L.; Kodavanti, U.P.; Block, M.L. Diesel exhaust impairs TREM2 to dysregulate neuroinflammation. J. Neuroinflammation 2020, 17, 351. [Google Scholar] [CrossRef]
- Mshigeni, S.K.; Moore, C.; Arkadie, N.L. The prevalence rate of smoking among Veterans: A forgotten epidemic. J. Mil. Veteran Fam. Health 2021, 7, 16–25. [Google Scholar] [CrossRef]
- National Academies of Sciences, Medicine Division, Board on Population Health, Public Health Practice; Committee on the Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]

| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study types |
|
|
| Year published | Literature published between 2008–July 2024 | Literature published before 2008 and after July 2024 |
| Language | Literature published in English | Literature published in non-English languages |
| Search results | Top 10 literature results from PubMed and Google Scholar databases using search query terms | Literature that were not in the top 10 search results |
| Content |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooks, A.W.; Sandri, B.J.; Nixon, J.P.; Nurkiewicz, T.R.; Barach, P.; Trembley, J.H.; Butterick, T.A. Neuroinflammation and Brain Health Risks in Veterans Exposed to Burn Pit Toxins. Int. J. Mol. Sci. 2024, 25, 9759. https://doi.org/10.3390/ijms25189759
Brooks AW, Sandri BJ, Nixon JP, Nurkiewicz TR, Barach P, Trembley JH, Butterick TA. Neuroinflammation and Brain Health Risks in Veterans Exposed to Burn Pit Toxins. International Journal of Molecular Sciences. 2024; 25(18):9759. https://doi.org/10.3390/ijms25189759
Chicago/Turabian StyleBrooks, Athena W., Brian J. Sandri, Joshua P. Nixon, Timothy R. Nurkiewicz, Paul Barach, Janeen H. Trembley, and Tammy A. Butterick. 2024. "Neuroinflammation and Brain Health Risks in Veterans Exposed to Burn Pit Toxins" International Journal of Molecular Sciences 25, no. 18: 9759. https://doi.org/10.3390/ijms25189759







