OsMBF1a Facilitates Seed Germination by Regulating Biosynthesis of Gibberellic Acid and Abscisic Acid in Rice
Abstract
:1. Introduction
2. Results
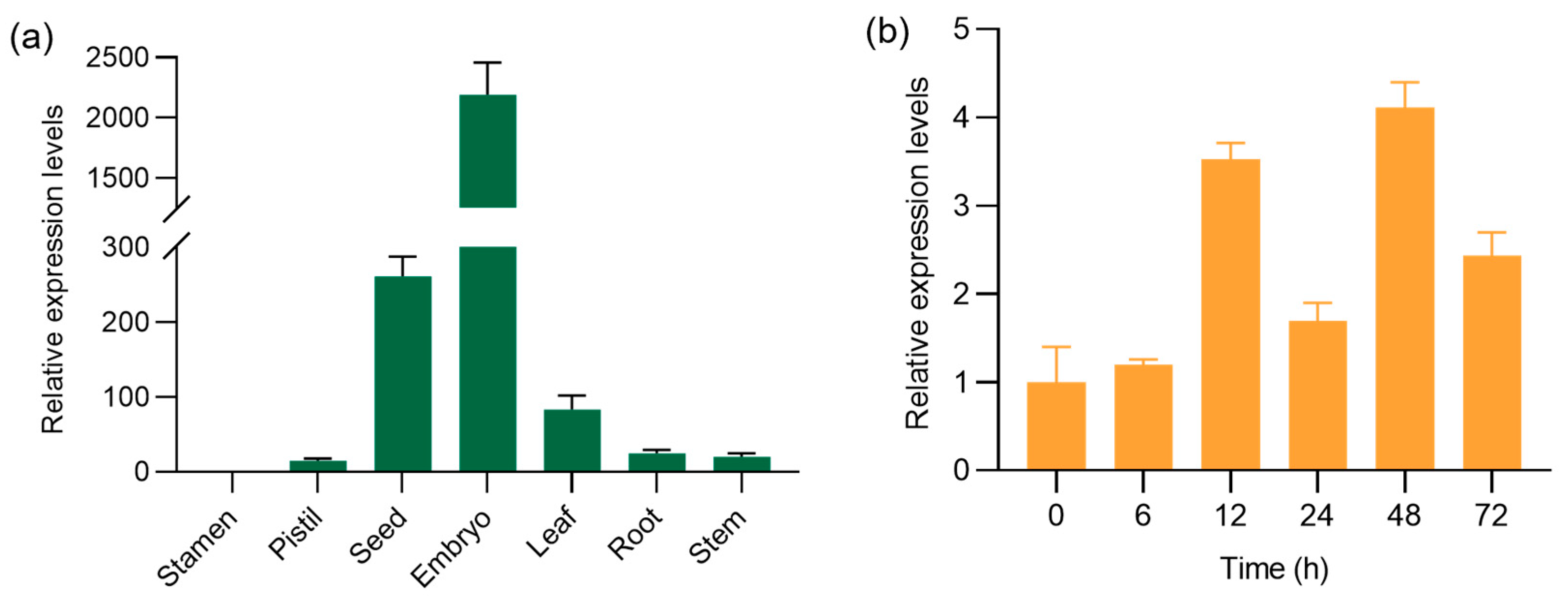
2.1. OsMBF1a Is Ubiquitously Expressed in Rice and Is Induced during Seed Germination
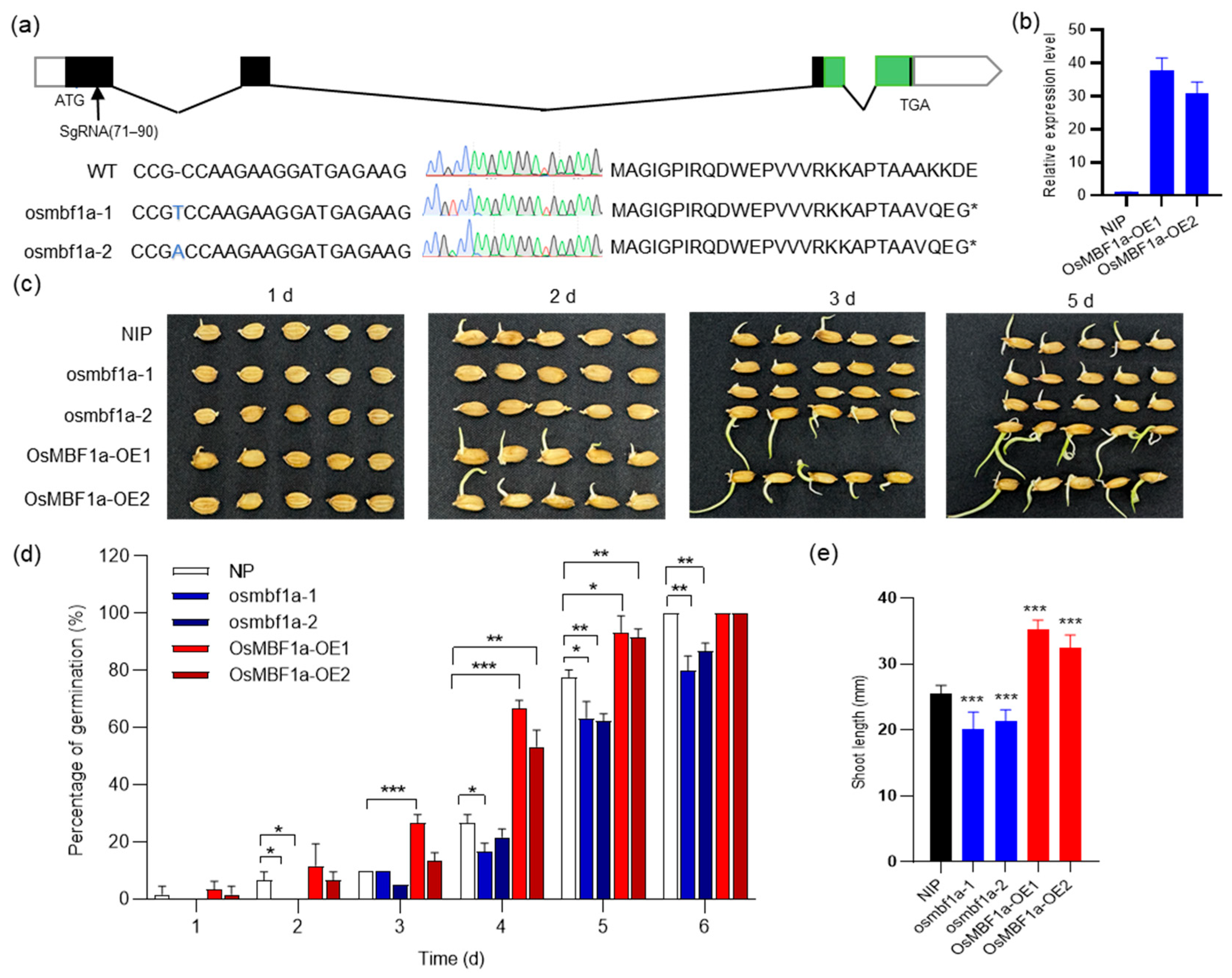
2.2. OsMBF1a Positively Regulates Seed Germination in Rice
2.3. OsMBF1a Modulates Terpenoid-Related Metabolic Pathways
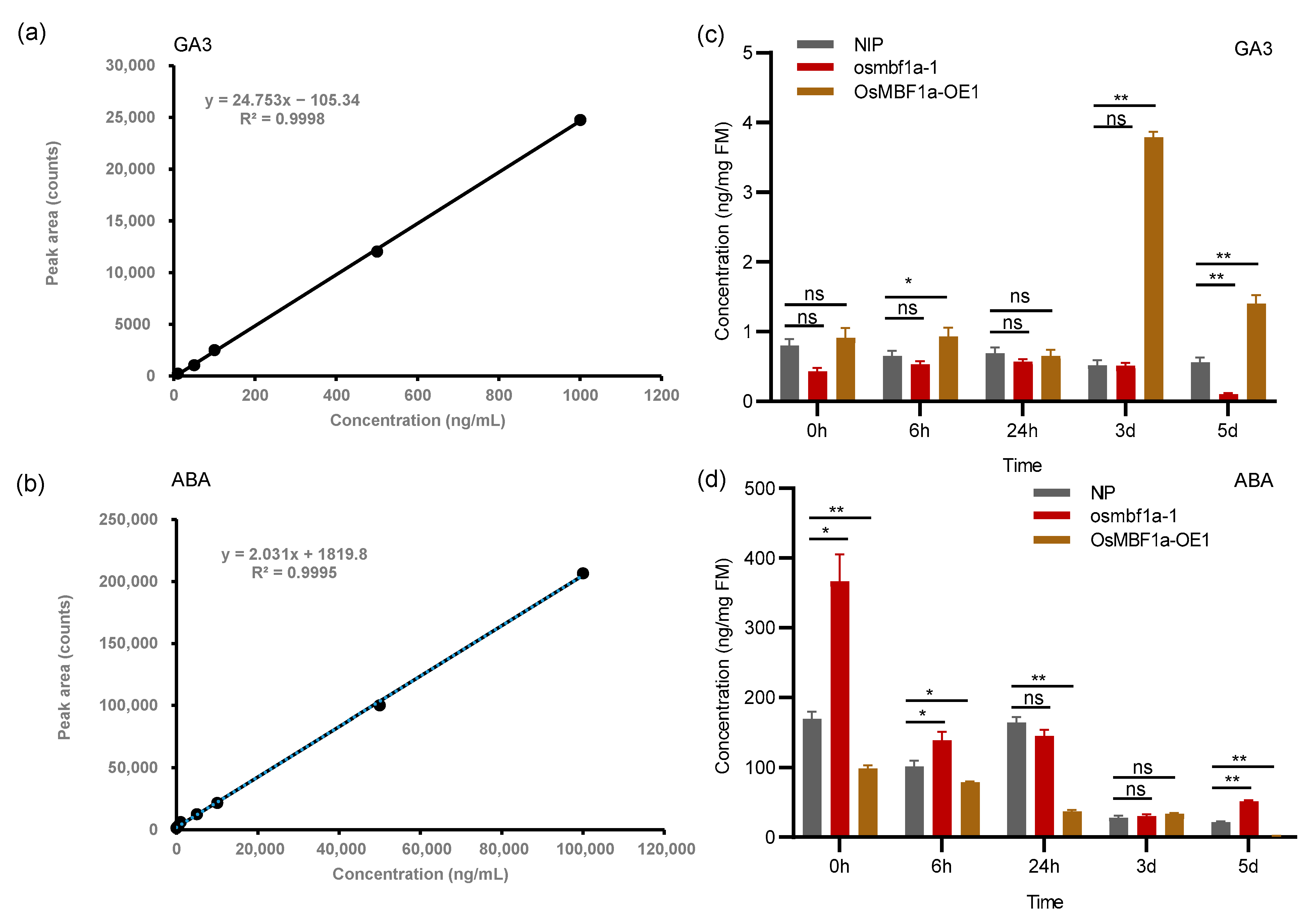
2.4. OsMBF1a Regulated Endogenous ABA and GA Levels in Rice Seed
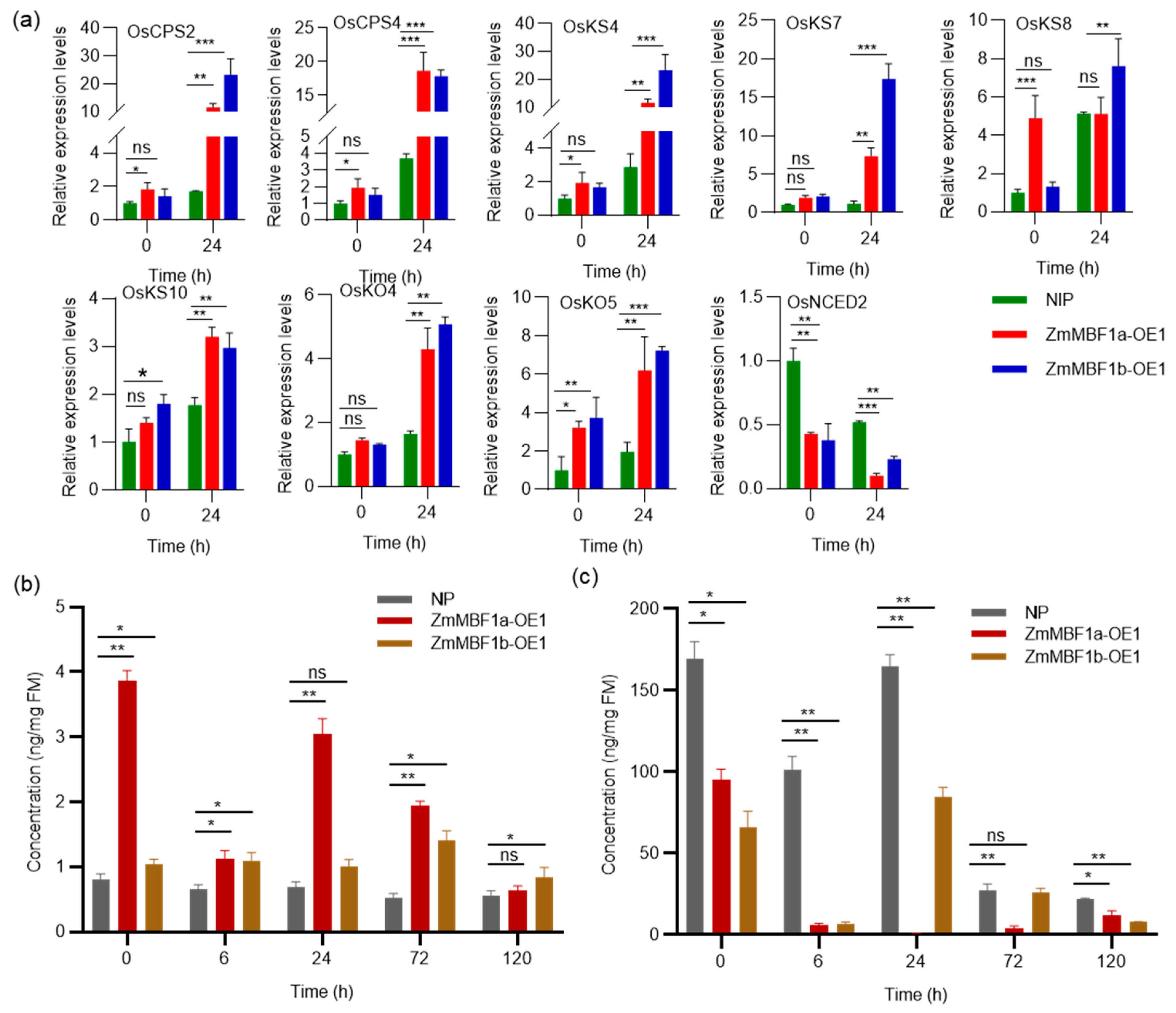
2.5. Overexpressing ZmMBF1a and ZmMBF1b in Rice Enhances Seed Germination
2.6. ZmMBF1a and ZmMBF1b Overexpression Modulates GA and ABA in Rice
3. Discussion
4. Materials and Methods
4.1. Construction and Planting Materials
4.2. Phenotypic Analysis of Seed Germination
4.3. RNA Extraction and RT-qPCR
4.4. RNA-Seq Analyses
4.5. DAP-qPCR Analyses
4.6. Detection of GA3 and ABA Content
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed Germination and Vigor: Ensuring Crop Sustainability in a Changing Climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Leubner-Metzger, G. The Biomechanics of Seed Germination. J. Exp. Bot. 2016, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.; Xie, Q.; He, Z. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular Mechanisms Underlying Abscisic Acid/Gibberellin Balance in the Control of Seed Dormancy and Germination in Cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, J.; Xu, F.; Chu, J.; Yan, C.; Schläppi, M.R.; Wang, Y.; Chu, C. Expression Patterns of ABA and GA Metabolism Genes and Hormone Levels during Rice Seed Development and Imbibition: A Comparison of Dormant and Non-Dormant Rice Cultivars. J. Genet. Genom. 2014, 41, 327–338. [Google Scholar] [CrossRef]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid Cleavage by NCED5 Fine-tunes ABA Accumulation and Affects Seed Dormancy and Drought Tolerance with Other NCED Family Members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef]
- Tan, B.-C.; Joseph, L.M.; Deng, W.-T.; Liu, L.; Li, Q.-B.; Cline, K.; McCarty, D.R. Molecular Characterization of the Arabidopsis 9-Cis Epoxycarotenoid Dioxygenase Gene Family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Lee, H.G.; Lee, K.; Seo, P.J. The Arabidopsis MYB96 Transcription Factor Plays a Role in Seed Dormancy. Plant Mol. Biol. 2015, 87, 371–381. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.G.; Yoon, S.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 Transcription Factor Is a Positive Regulator of ABSCISIC ACID-INSENSITIVE4 in the Control of Seed Germination. Plant Physiol. 2015, 168, 677–689. [Google Scholar] [CrossRef]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early Abscisic Acid Signal Transduction Mechanisms: Newly Discovered Components and Newly Emerging Questions. Genes Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, S.C. Arabidopsis SnRK2.3/SRK2I Plays a Positive Role in Seed Germination under Cold Stress Conditions. Environ. Exp. Bot. 2023, 212, 105399. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Min, M.-K.; Choi, E.-H.; Kim, N.; Moon, S.-J.; Yoon, I.; Kwon, T.; Jung, K.-H.; Kim, B.-G. The Protein Phosphatase 2C Clade A Protein OsPP2C51 Positively Regulates Seed Germination by Directly Inactivating OsbZIP10. Plant Mol. Biol. 2017, 93, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, C.; Pang, J.; Zhang, X.; Yang, C.; Xia, G.; Tian, Y.; He, C. OsLOL1, a C2C2-Type Zinc Finger Protein, Interacts with OsbZIP58 to Promote Seed Germination through the Modulation of Gibberellin Biosynthesis in Oryza Sativa. Plant J. 2014, 80, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Feng, J.; Zhang, L.; Zhang, J.; Mispan, M.S.; Cao, Z.; Beighley, D.H.; Yang, J.; Gu, X.-Y. Map-Based Cloning of Seed Dormancy1-2 Identified a Gibberellin Synthesis Gene Regulating the Development of Endosperm-Imposed Dormancy in Rice. Plant Physiol. 2015, 169, 2152–2165. [Google Scholar] [CrossRef]
- Jaimes-Miranda, F.; Chávez Montes, R.A. The Plant MBF1 Protein Family: A Bridge between Stress and Transcription. J. Exp. Bot. 2020, 71, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Yamazaki, K. Structure and Expression Analysis of Three Subtypes of Arabidopsis MBF1 Genes. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2004, 1680, 1–10. [Google Scholar] [CrossRef]
- Suzuki, N.; Bajad, S.; Shuman, J.; Shulaev, V.; Mittler, R. The Transcriptional Co-Activator MBF1c Is a Key Regulator of Thermotolerance in Arabidopsis Thaliana. J. Biol. Chem. 2008, 283, 9269–9275. [Google Scholar] [CrossRef]
- Suzuki, N.; Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Mittler, R. Enhanced Tolerance to Environmental Stress in Transgenic Plants Expressing the Transcriptional Coactivator Multiprotein Bridging Factor 1c. Plant Physiol. 2005, 139, 1313–1322. [Google Scholar] [CrossRef]
- Qin, D.; Wang, F.; Geng, X.; Zhang, L.; Yao, Y.; Ni, Z.; Peng, H.; Sun, Q. Overexpression of Heat Stress-Responsive TaMBF1c, a Wheat (Triticum aestivum L.) Multiprotein Bridging Factor, Confers Heat Tolerance in Both Yeast and Rice. Plant Mol. Biol. 2015, 87, 31–45. [Google Scholar] [CrossRef]
- Arce, D.P.; Tonón, C.; Zanetti, M.E.; Godoy, A.V.; Hirose, S.; Casalongué, C.A. The Potato Transcriptional Co-Activator StMBF1 Is up-Regulated in Response to Oxidative Stress and Interacts with the TATA-Box Binding Protein. J. Biochem. Mol. Biol. 2006, 39, 355–360. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, H.; Singer, S.D.; Yan, X.; Guo, R.; Wang, X. The Grape VvMBF1 Gene Improves Drought Stress Tolerance in Transgenic Arabidopsis Thaliana. Plant Cell Tissue Organ Cult. 2014, 118, 571–582. [Google Scholar] [CrossRef]
- Suzuki, N.; Sejima, H.; Tam, R.; Schlauch, K.; Mittler, R. Identification of the MBF1 Heat-response Regulon of Arabidopsis thaliana. Plant J. 2011, 66, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Hommel, M.; Khalil-Ahmad, Q.; Jaimes-Miranda, F.; Mila, I.; Pouzet, C.; Latché, A.; Pech, J.C.; Bouzayen, M.; Regad, F. Over-Expression of a Chimeric Gene of the Transcriptional Co-Activator MBF1 Fused to the EAR Repressor Motif Causes Developmental Alteration in Arabidopsis and Tomato. Plant Sci. 2008, 175, 168–177. [Google Scholar] [CrossRef]
- Di Mauro, M.F.; Iglesias, M.J.; Arce, D.P.; Valle, E.M.; Arnold, R.B.; Tsuda, K.; Yamazaki, K.; Casalongué, C.A.; Godoy, A.V. MBF1s Regulate ABA-Dependent Germination of Arabidopsis Seeds. Plant Signal. Behav. 2012, 7, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Zheng, H.; Fan, J.; Wang, J.; Saba, T.; Wang, K.; Wu, J.; Wu, H.; Zhong, Y.; Chen, G.; et al. Genome-Wide Characterization of the MBF1 Gene Family and Its Expression Pattern in Different Tissues and Stresses in Zanthoxylum Armatum. BMC Genom. 2022, 23, 652. [Google Scholar] [CrossRef]
- Song, S.; Wang, G.; Wu, H.; Fan, X.; Liang, L.; Zhao, H.; Li, S.; Hu, Y.; Liu, H.; Ayaad, M.; et al. OsMFT2 Is Involved in the Regulation of ABA Signaling-mediated Seed Germination through Interacting with OsbZIP23/66/72 in Rice. Plant J. 2020, 103, 532–546. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Li, Z.; Qiao, J.; Quan, R.; Wang, J.; Huang, R.; Qin, H. SALT AND ABA RESPONSE ERF1 Improves Seed Germination and Salt Tolerance by Repressing ABA Signaling in Rice. Plant Physiol. 2022, 189, 1110–1127. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; He, D.; Wang, K.; Yang, P. Mutations on Ent-Kaurene Oxidase 1 Encoding Gene Attenuate Its Enzyme Activity of Catalyzing the Reaction from Ent-Kaurene to Ent-Kaurenoic Acid and Lead to Delayed Germination in Rice. PLoS Genet. 2020, 16, e1008562. [Google Scholar] [CrossRef]
- Yaish, M.W.; El-kereamy, A.; Zhu, T.; Beatty, P.H.; Good, A.G.; Bi, Y.-M.; Rothstein, S.J. The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef]
- Yu, R.-M.; Suo, Y.-Y.; Yang, R.; Chang, Y.-N.; Tian, T.; Song, Y.-J.; Wang, H.-J.; Wang, C.; Yang, R.-J.; Liu, H.-L.; et al. StMBF1c Positively Regulates Disease Resistance to Ralstonia Solanacearum via It’s Primary and Secondary Upregulation Combining Expression of StTPS5 and Resistance Marker Genes in Potato. Plant Sci. 2021, 307, 110877. [Google Scholar] [CrossRef]
- Yu, B.; Liang, Y.; Qin, Q.; Zhao, Y.; Yang, C.; Liu, R.; Gan, Y.; Zhou, H.; Qiu, Z.; Chen, L.; et al. Transcription Cofactor CsMBF1c Enhances Heat Tolerance of Cucumber and Interacts with Heat-Related Proteins CsNFYA1 and CsDREB2. J. Agric. Food Chem. 2024, 72, 15586–15600. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Guan, L.; Yin, Y.; Wang, Y.; Shi, H.; Li, W.; Zhang, D.; Song, R.; Hu, T.; Zhan, X. Genome-Wide Analysis of MBF1 Family Genes in Five Solanaceous Plants and Functional Analysis of SlER24 in Salt Stress. Int. J. Mol. Sci. 2023, 24, 13965. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qi, H.; Gao, L.; Luo, S.; Damaris, R.N.; Ke, Y.; Wu, W.; Yang, P. Identifying RNA Modifications by Direct RNA Sequencing Reveals Complexity of Epitranscriptomic Dynamics in Rice. Genom. Proteom. Bioinform. 2023, 21, 788–804. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, R.C.; Huang, S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell 2016, 165, 1280–1292, Erratum in Cell 2016, 166, 1598. [Google Scholar] [CrossRef]
- Xin, P.; Guo, Q.; Li, B.; Cheng, S.; Yan, J.; Chu, J. A Tailored High-Efficiency Sample Pretreatment Method for Simultaneous Quantification of 10 Classes of Known Endogenous Phytohormones. Plant Commun. 2020, 1, 100047. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, Z.; Guo, J.; Han, X.; Ji, X.; Ke, M.; Yu, F.; Yang, P. OsMBF1a Facilitates Seed Germination by Regulating Biosynthesis of Gibberellic Acid and Abscisic Acid in Rice. Int. J. Mol. Sci. 2024, 25, 9762. https://doi.org/10.3390/ijms25189762
Wang X, Chen Z, Guo J, Han X, Ji X, Ke M, Yu F, Yang P. OsMBF1a Facilitates Seed Germination by Regulating Biosynthesis of Gibberellic Acid and Abscisic Acid in Rice. International Journal of Molecular Sciences. 2024; 25(18):9762. https://doi.org/10.3390/ijms25189762
Chicago/Turabian StyleWang, Xin, Ziyun Chen, Jinghua Guo, Xiao Han, Xujian Ji, Meicheng Ke, Feng Yu, and Pingfang Yang. 2024. "OsMBF1a Facilitates Seed Germination by Regulating Biosynthesis of Gibberellic Acid and Abscisic Acid in Rice" International Journal of Molecular Sciences 25, no. 18: 9762. https://doi.org/10.3390/ijms25189762






