The Vital Role of the CAMTA Gene Family in Phoebe bournei in Response to Drought, Heat, and Light Stress
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of PbCAMTA Genes
2.2. Display of the Motif, Domain, and Gene Structure of PbCAMTA Gene Family
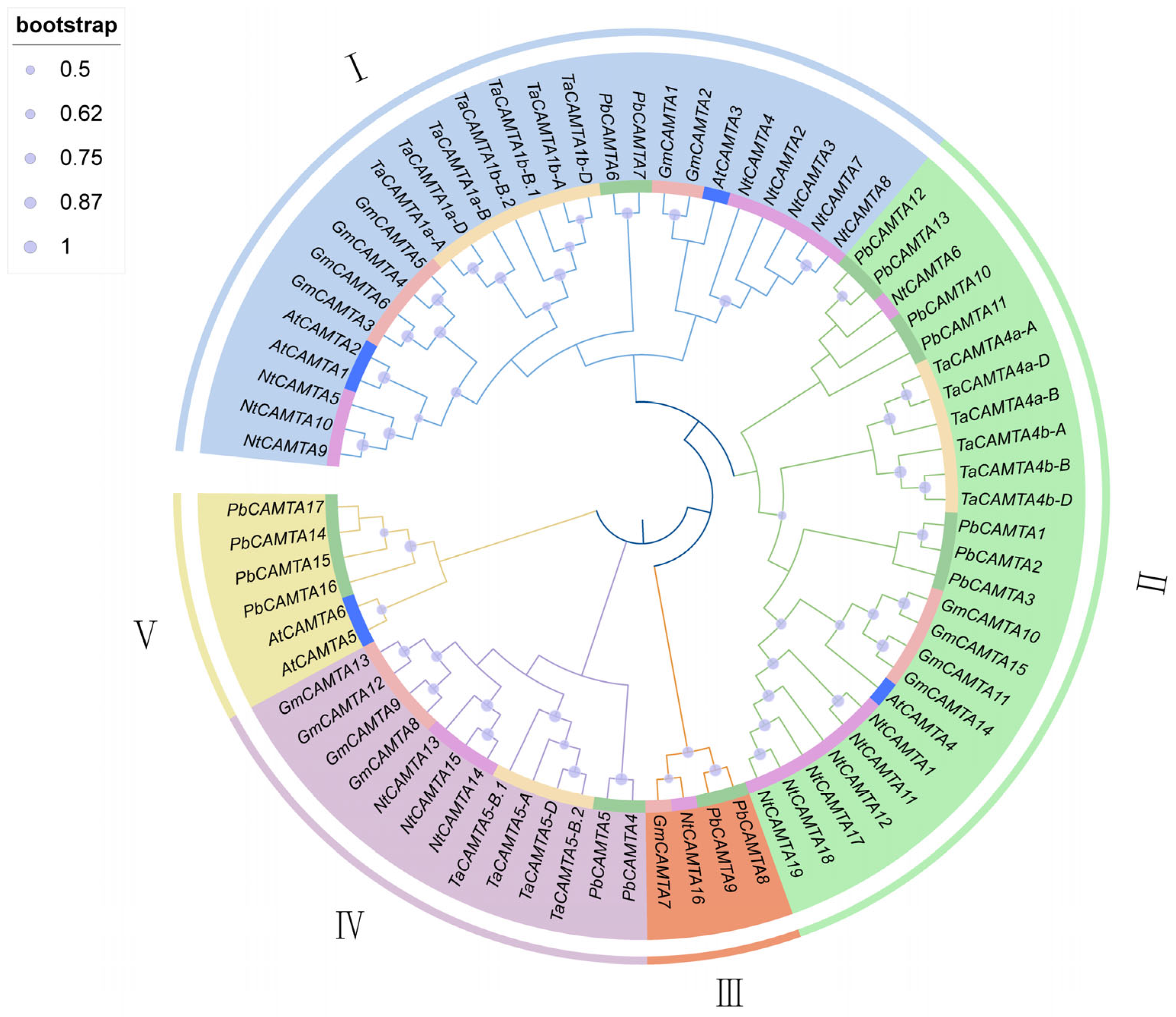
2.3. Phylogenetic Analysis of PbCAMTAs
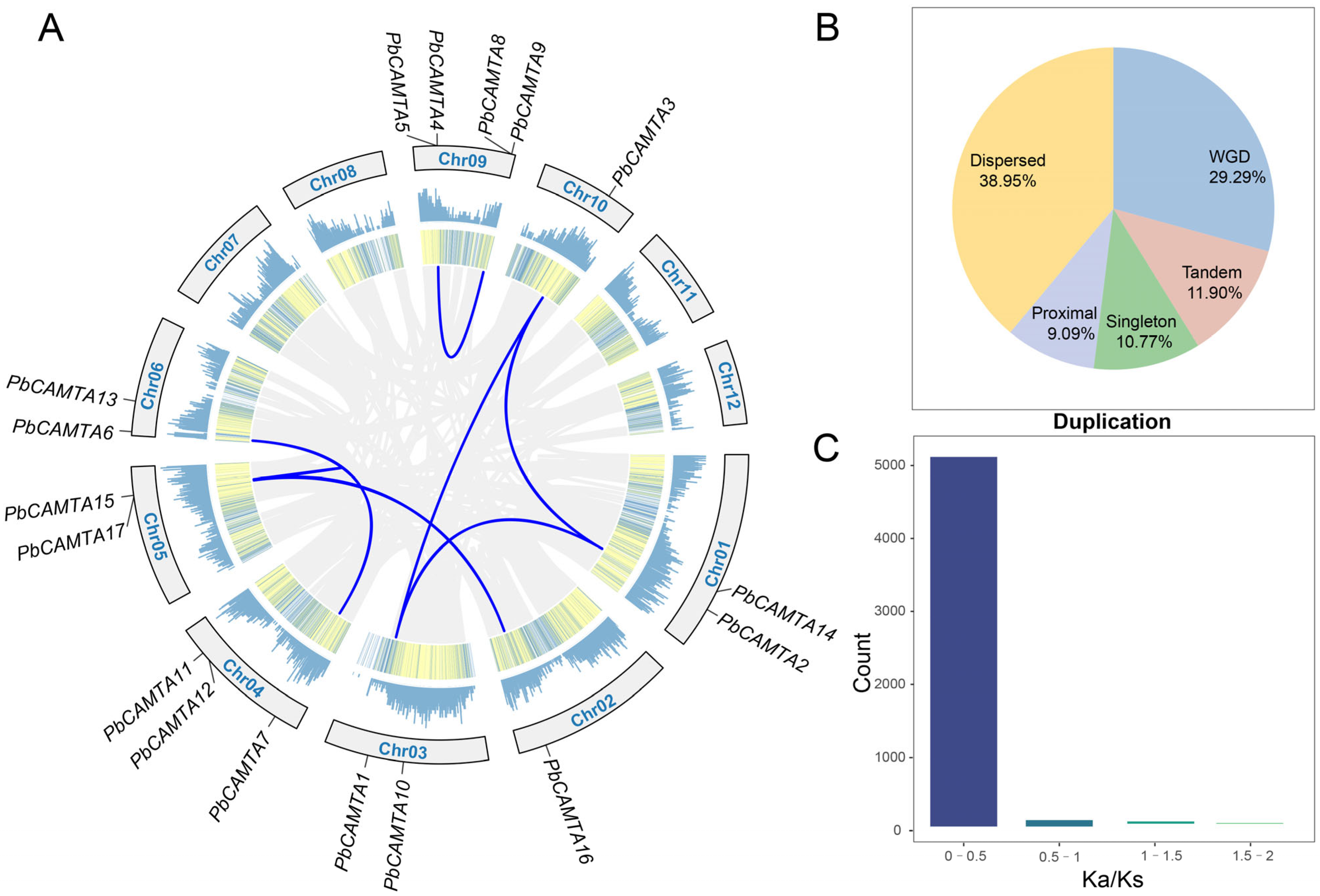
2.4. Chromosome Distribution of PbCAMTAs and Genomic Amplification in P. bournei
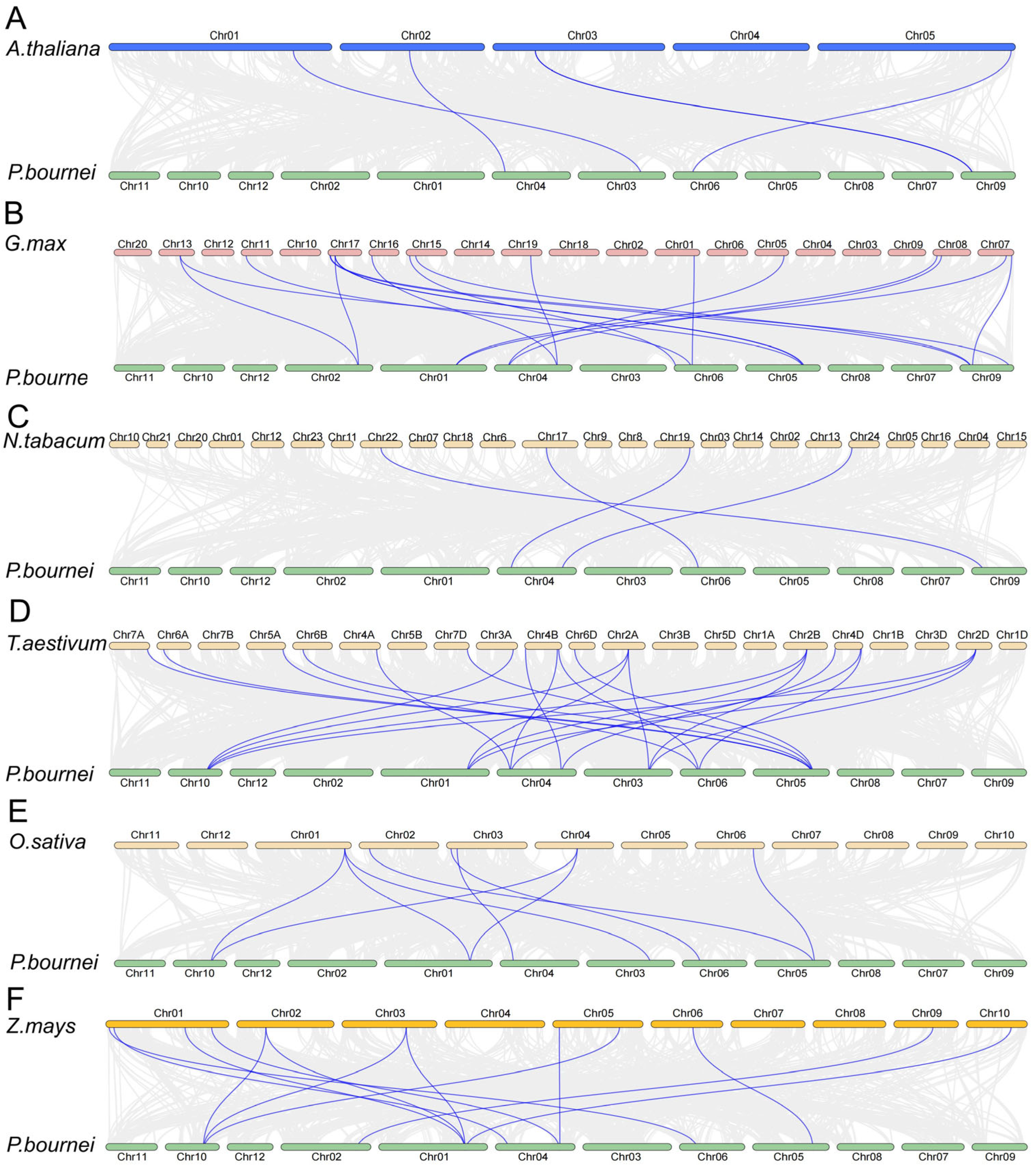
2.5. Syntenic Analysis of PbCAMTAs Genes
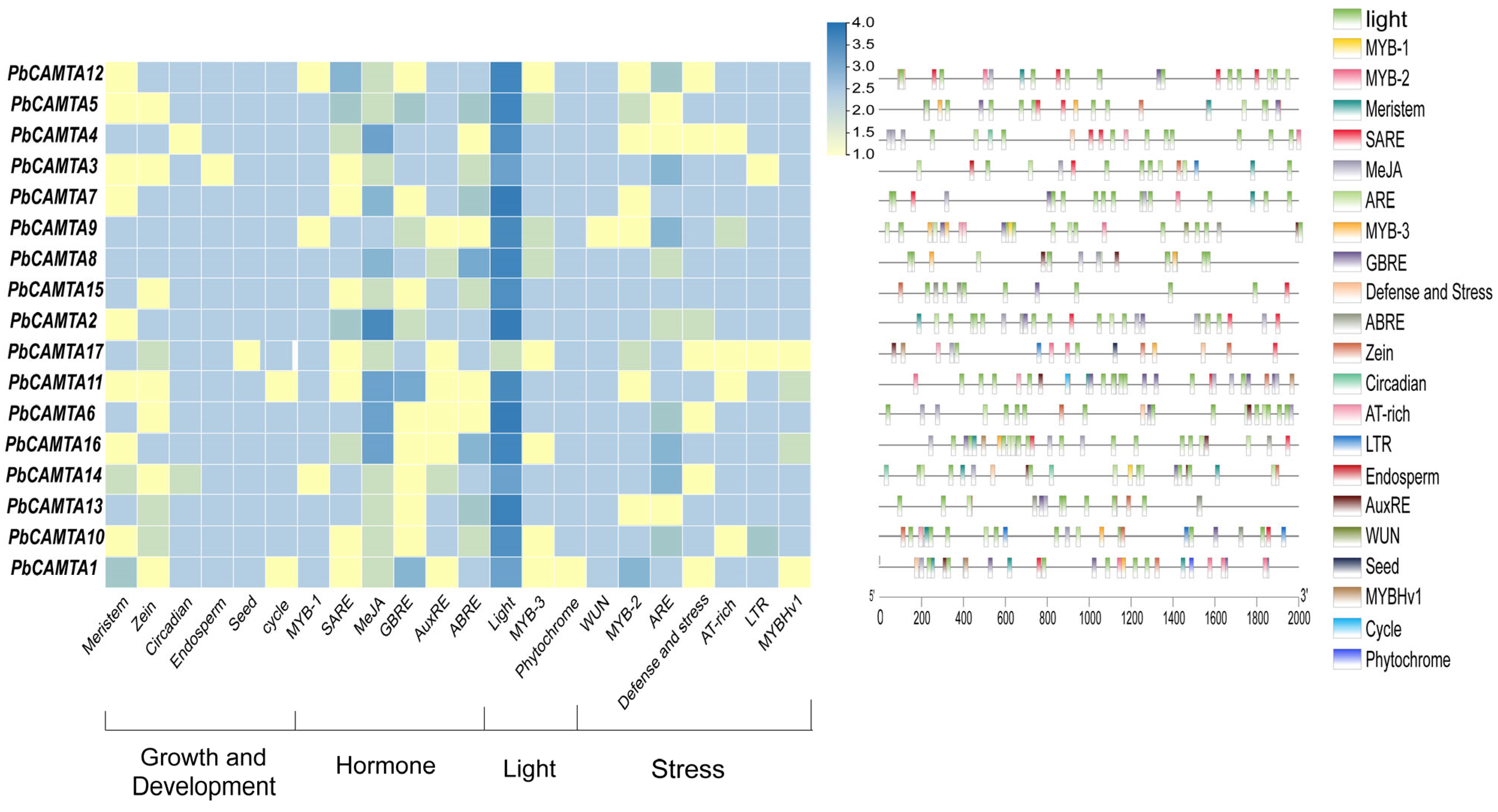
2.6. Cis-Regulatory Element Prediction of Promoters in 17 PbCAMTA Genes
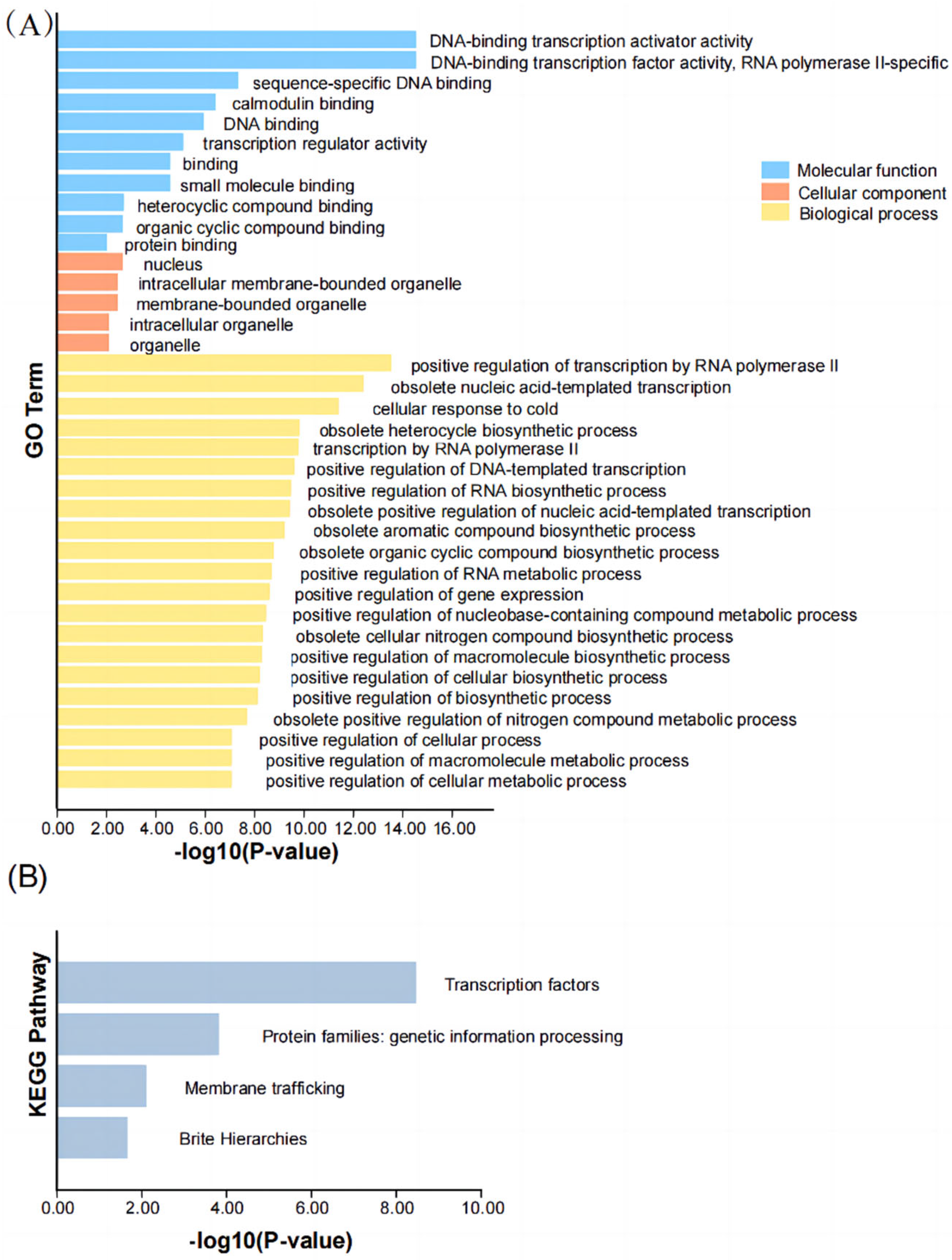
2.7. Gene Ontology (GO) and KEGG Analysis in PbCAMTA
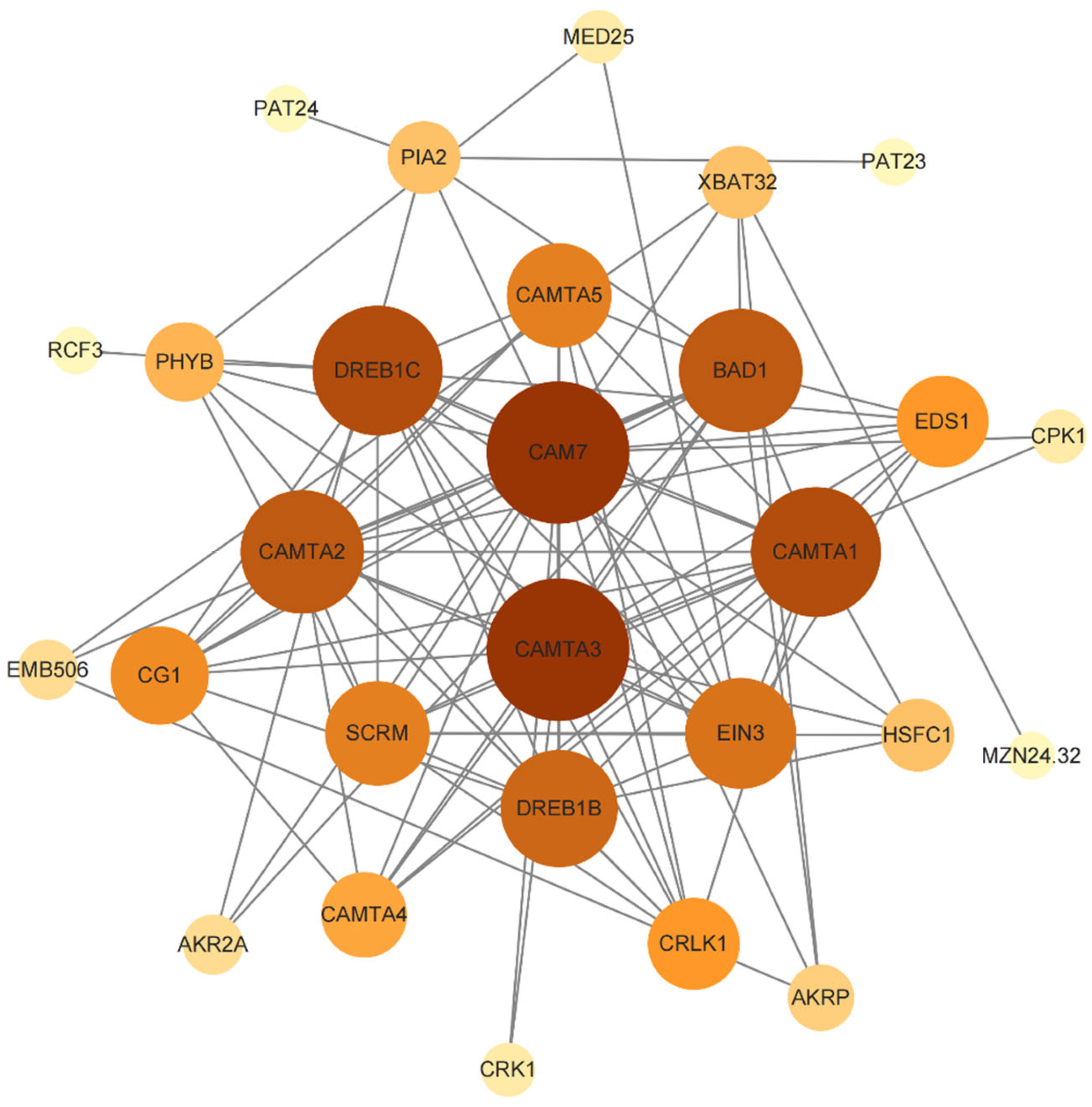
2.8. Protein–Protein Interaction Network of PbCAMTA Proteins
2.9. Expression Patterns of PbCAMTA Genes in Response to Heat, Drought, and Light Treatments
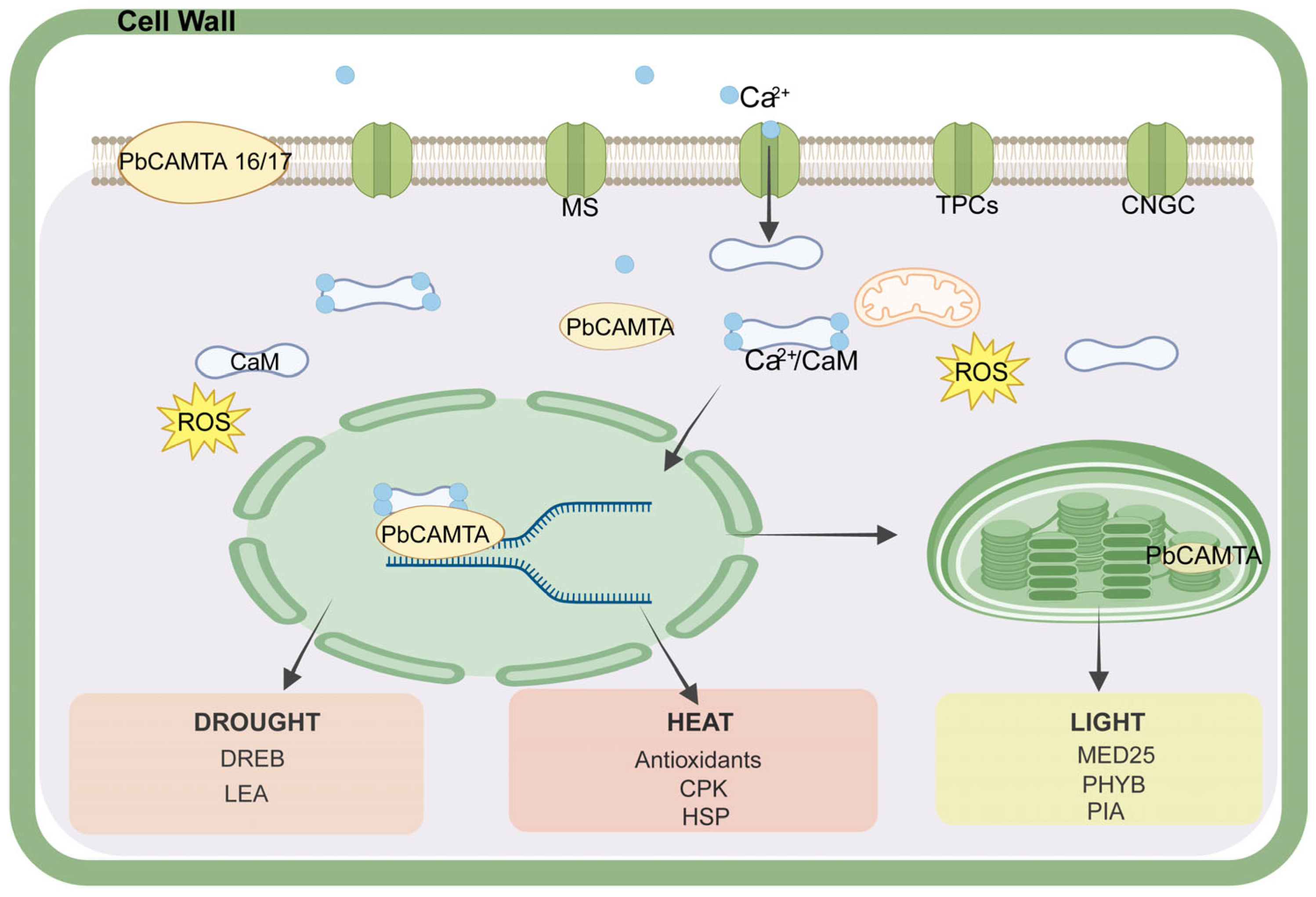
3. Discussion
4. Materials and Methods
4.1. Identification and Analysis of CAMTA Gene Families in P. bournei
4.2. Physical and Chemical Characteristics of P. bournei
4.3. Analysis of the Phylogenetic Tree and Motif, Domain, and Gene Structures of PbCAMTAs
4.4. Phylogenetic Analysis between PbCAMTAs and CAMTAs of Other Species
4.5. Collinearity Analysis and Duplication Events Analysis of CAMTA
4.6. GO and KEGG Analysis
4.7. Protein–Protein Interaction Network of PbCAMTAs
4.8. Abiotic Stress Treatment
4.9. RNA Extraction and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Liu, L.; Sun, S.; Li, Y.; Jia, L.; Ye, S.; Yu, Y.; Dossa, K.; Luan, Y. Leaf-transcriptome profiles of phoebe bournei provide insights into temporal drought stress responses. Front. Plant Sci. 2022, 13, 1010314. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, J.; Tang, T. Determining the optimal density of Phoebe bournei plantations based on dynamic programming under close-to-nature management measures. Sustainability 2022, 14, 847. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, J.; Zhong, P.; Liang, L.; Dang, H.; Wang, G. The impact of natural regeneration of Phoebe bournei in Anfu County, Jiangxi Province, on community diversity and soil nutrient characteristics. Forests 2023, 14, 1783. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Wu, X.-H.; Grossnickle, S.C.; Chen, L.-H.; Yu, X.-X.; El-Kassaby, Y.A.; Feng, J.-L. Formula fertilization promotes Phoebe bournei robust seedling cultivation. Forests 2020, 11, 781. [Google Scholar] [CrossRef]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14, S401–S417. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. Signal transduction. The calcium entry pas de deux. Science 2000, 287, 1604–1605. [Google Scholar] [CrossRef]
- Zielinski, R.E. Calmodulin and calmodulin-binding proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 697–725. [Google Scholar] [CrossRef]
- Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 37–42. [Google Scholar] [CrossRef]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef]
- Snedden, W.A.; Fromm, H. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2001, 151, 35–66. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Shariq Iqbal, M.; Singh, S.P.; Buaboocha, T. Ca2+/Calmodulin Complex Triggers CAMTA Transcriptional Machinery under Stress in Plants: Signaling Cascade and Molecular Regulation. Front. Plant Sci. 2020, 11, 598327. [Google Scholar] [CrossRef]
- Xiao, P.; Feng, J.W.; Zhu, X.T.; Gao, J. Evolution Analyses of CAMTA Transcription Factor in Plants and Its Enhancing Effect on Cold-tolerance. Front. Plant Sci. 2021, 12, 758187. [Google Scholar] [CrossRef]
- Liu, C.; Tang, D. Comprehensive identification and expression analysis of CAMTA gene family in Phyllostachys edulis under abiotic stress. PeerJ 2023, 11, e15358. [Google Scholar] [CrossRef]
- Rahman, H.; Yang, J.; Xu, Y.P.; Munyampundu, J.P.; Cai, X.Z. Phylogeny of Plant CAMTAs and Role of AtCAMTAs in Nonhost Resistance to Xanthomonas oryzae pv. oryzae. Front. Plant Sci. 2016, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Costa e Silva, O.D. CG-1, a parsley light-induced DNA-binding protein. Plant Mol. Biol. 1994, 25, 921–924. [Google Scholar] [CrossRef]
- Breeden, L.; Nasmyth, K. Cell cycle control of the yeast HO gene: Cis- and Trans-acting regulators. Cell 1987, 48, 389–397. [Google Scholar] [CrossRef]
- Bouché, N.; Scharlat, A.; Snedden, W.; Bouchez, D.; Fromm, H. A Novel Family of Calmodulin-binding Transcription Activators in Multicellular Organisms. J. Biol. Chem. 2002, 277, 21851–21861. [Google Scholar] [CrossRef]
- Teresinski, H.J.; Hau, B.; Symonds, K.; Kilburn, R.; Munro, K.A.; Doner, N.M.; Mullen, R.; Li, V.H.; Snedden, W.A. Arabidopsis calmodulin-like proteins CML13 and CML14 interact with proteins that have IQ domains. Plant Cell Environ. 2023, 46, 2470–2491. [Google Scholar] [CrossRef]
- Finkler, A.; Ashery-Padan, R.; Fromm, H. CAMTAs: Calmodulin-binding transcription activators from plants to human. FEBS Lett. 2007, 581, 3893–3898. [Google Scholar] [CrossRef] [PubMed]
- Kakar, K.U.; Nawaz, Z.; Cui, Z.; Cao, P.; Jin, J.; Shu, Q.; Ren, X. Evolutionary and expression analysis of CAMTA gene family in Nicotiana tabacum yielded insights into their origin, expansion and stress responses. Sci. Rep. 2018, 8, 10322. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gilmour, S.J.; Chao, L.; Park, S.; Thomashow, M.F. Arabidopsis CAMTA Transcription Factors Regulate Pipecolic Acid Biosynthesis and Priming of Immunity Genes. Mol. Plant 2020, 13, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Gain, H.; Nandi, D.; Kumari, D.; Das, A.; Dasgupta, S.B.; Banerjee, J. Genome-wide identification of CAMTA gene family members in rice (Oryza sativa L.) and in silico study on their versatility in respect to gene expression and promoter structure. Funct. Integr. Genom. 2022, 22, 193–214. [Google Scholar] [CrossRef]
- Yue, R.; Lu, C.; Sun, T.; Peng, T.; Han, X.; Qi, J.; Yan, S.; Tie, S. Identification and expression profiling analysis of calmodulin-binding transcription activator genes in maize (Zea mays L.) under abiotic and biotic stresses. Front. Plant Sci. 2015, 6, 576. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, H.; Hu, X.; Zhu, Y.; Chen, Y.; Shen, C.; Wang, H.; Poovaiah, B.W.; Du, L. Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant Soil 2014, 386, 205–221. [Google Scholar] [CrossRef]
- Pant, P.; Iqbal, Z.; Pandey, B.K.; Sawant, S.V. Genome-wide comparative and evolutionary analysis of Calmodulin-binding Transcription Activator (CAMTA) family in Gossypium species. Sci. Rep. 2018, 8, 5573. [Google Scholar] [CrossRef]
- Cai, P.; Lan, Y.; Gong, F.; Li, C.; Xia, F.; Li, Y.; Fang, C. Identification and Molecular Characterization of the CAMTA Gene Family in Solanaceae with a Focus on the Expression Analysis of Eggplant Genes under Cold Stress. Int. J. Mol. Sci. 2024, 25, 2064. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Zhang, G.; Shang, L.; Wang, Q.; Hong, S.; Ma, Q.; Gu, C. Identification of CAMTA Gene Family in Heimia myrtifolia and Expression Analysis under Drought Stress. Plants 2022, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Z.; Cao, X.; Duan, W.; Wei, C.; Zhang, C.; Jiang, D.; Li, M.; Chen, K.; Qiao, Y.; et al. Genome-Wide Analysis of Calmodulin Binding Transcription Activator (CAMTA) Gene Family in Peach (Prunus persica L. Batsch) and Ectopic Expression of PpCAMTA1 in Arabidopsis camta2,3 Mutant Restore Plant Development. Int. J. Mol. Sci. 2022, 23, 10500. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Gao, S.; Zhang, S.; Wang, W.; Fang, Z.; Liu, S.; Wang, X.; Zhao, C.; Tang, Y. Systematic Analysis and Identification of Drought-Responsive Genes of the CAMTA Gene Family in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 4542. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; An, C.; Park, S.; Gilmour, S.J.; Wang, L.; Renna, L.; Brandizzi, F.; Grumet, R.; Thomashow, M.F. CAMTA-Mediated Regulation of Salicylic Acid Immunity Pathway Genes in Arabidopsis Exposed to Low Temperature and Pathogen Infection. Plant Cell 2017, 29, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, M.A.; Çetin, D.; Filiz, E.; Srivastava, V. Genome-wide exploration and analysis of plant stress-responsive CAMTA transcription factor genes in Brachypodium distachyon and their expression patterns under environmental challenges. S. Afr. J. Bot. 2024, 166, 208–217. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. An early ethylene up-regulated gene encoding a calmodulin-binding protein involved in plant senescence and death. J. Biol. Chem. 2000, 275, 38467–38473. [Google Scholar] [CrossRef]
- Pandey, N.; Ranjan, A.; Pant, P.; Tripathi, R.K.; Ateek, F.; Pandey, H.P.; Patre, U.V.; Sawant, S.V. CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genom. 2013, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Jameel, A.; Qiang, W.D.; Ahmad, N.; Liu, W.C.; Wang, F.W.; Li, H.Y. Overexpression of GmCAMTA12 Enhanced Drought Tolerance in Arabidopsis and Soybean. Int. J. Mol. Sci. 2019, 20, 4849. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, M.; Xing, F.; Mao, G.; Wang, Y.; Dai, Y.; Niu, M.; Yuan, H. Identification and Expression Analysis of CAMTA Genes in Tea Plant Reveal Their Complex Regulatory Role in Stress Responses. Front. Plant Sci. 2022, 13, 910768. [Google Scholar] [CrossRef]
- Yang, F.; Dong, F.S.; Hu, F.H.; Liu, Y.W.; Chai, J.F.; Zhao, H.; Lv, M.Y.; Zhou, S. Genome-wide identification and expression analysis of the calmodulin-binding transcription activator (CAMTA) gene family in wheat (Triticum aestivum L.). BMC Genet. 2020, 21, 105. [Google Scholar] [CrossRef]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef]
- Rahman, H.; Xu, Y.-P.; Zhang, X.-R.; Cai, X.-Z. Brassica napus Genome Possesses Extraordinary High Number of CAMTA Genes and CAMTA3 Contributes to PAMP Triggered Immunity and Resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2016, 7, 581. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, H.; Chen, D.; Zhang, H.; Sun, M.; Chen, S.; Qin, Z.; Ding, Z.; Dai, S. Cysteine-rich receptor-like protein kinases: Emerging regulators of plant stress responses. Trends Plant Sci. 2023, 28, 776–794. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, R.; Singh, A.; Chattopadhyay, S. Calmodulin7 plays an important role as transcriptional regulator in Arabidopsis seedling development. Plant Cell 2008, 20, 1747–1759. [Google Scholar] [CrossRef]
- Abbas, N.; Maurya, J.P.; Senapati, D.; Gangappa, S.N.; Chattopadhyay, S. Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell 2014, 26, 1036–1052. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, H.; Pan, Y.; Lyu, M.; Yang, Z.; Kou, X.; Deng, X.W.; Zhong, S. Sensory circuitry controls cytosolic calcium-mediated phytochrome B phototransduction. Cell 2023, 186, 1230–1243.e14. [Google Scholar] [CrossRef]
- Kim, T.; Samraj, S.; Jiménez, J.; Gómez, C.; Liu, T.; Begcy, K. Genome-wide identification of heat shock factors and heat shock proteins in response to UV and high intensity light stress in lettuce. BMC Plant Biol. 2021, 21, 185. [Google Scholar] [CrossRef]
- Yoo, J.; Cho, M.H.; Lee, S.W.; Bhoo, S.H. Phytochrome-interacting ankyrin repeat protein 2 modulates phytochrome A-mediated PIF3 phosphorylation in light signal transduction. J. Biochem. 2016, 160, 243–249. [Google Scholar] [CrossRef]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to arabidopsis stomatal differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Nodzon, L.A.; Xu, W.H.; Wang, Y.; Pi, L.Y.; Chakrabarty, P.K.; Song, W.Y. The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant J. 2004, 40, 996–1006. [Google Scholar] [CrossRef]
- Yoo, J.; Shin, D.H.; Cho, M.H.; Kim, T.L.; Bhoo, S.H.; Hahn, T.R. An ankyrin repeat protein is involved in anthocyanin biosynthesis in Arabidopsis. Physiol. Plant 2011, 142, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Fontes, C.G.; Dawson, T.E.; Jardine, K.; McDowell, N.; Gimenez, B.O.; Anderegg, L.; Negrón-Juárez, R.; Higuchi, N.; Fine, P.V.A.; Araújo, A.C.; et al. Dry and hot: The hydraulic consequences of a climate change-type drought for Amazonian trees. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20180209. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Lafourcade, B.; Araujo, M.B. Consequences of climate change on the tree of life in Europe. Nature 2011, 470, 531–534. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Sharma, S.; Prasad, S.M.; Dubey, N.K.; Ramawat, N. Plant Life under Changing Environment: Responses and Management; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Yang, T.; Poovaiah, B.W. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J. Biol. Chem. 2002, 277, 45049–45058. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, T.; Xu, L.; Pi, E.; Wang, S.; Wang, H.; Shen, C. Genome-wide identification of CAMTA gene family members in Medicago truncatula and their expression during root nodule symbiosis and hormone treatments. Front. Plant Sci. 2015, 6, 459. [Google Scholar] [CrossRef]
- Noman, M.; Aysha, J.; Ketehouli, T.; Yang, J.; Du, L.; Wang, F.; Li, H. Calmodulin binding transcription activators: An interplay between calcium signalling and plant stress tolerance. J. Plant Physiol. 2021, 256, 153327. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, X.; Zhong, G.; Wang, W.; Hake, K.; Matschi, S.; Lederer, S.; Hoehenwarter, W.; Sun, Q.; Lee, J.; et al. CAMTA3 repressor destabilization triggers TIR domain protein TN2-mediated autoimmunity in the Arabidopsis exo70B1 mutant. Plant Cell 2024, 36, 2021–2040. [Google Scholar] [CrossRef]
- Chinpongpanich, A.; Phean-O-Pas, S.; Thongchuang, M.; Qu, L.-J.; Buaboocha, T. C-terminal extension of calmodulin-like 3 protein from Oryza sativa L.: Interaction with a high mobility group target protein. Acta Biochim. Biophys. Sin. 2015, 47, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Niu, Y.; Zhang, J.; Zhou, Y.; Ma, Z.; Huang, X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 413–424. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.-K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mizoi, J.; Ohori, T.; Moriwaki, T.; Kidokoro, S.; Todaka, D.; Maruyama, K.; Kusakabe, K.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. GmDREB2A;2, a Canonical DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN2-Type Transcription Factor in Soybean, Is Posttranslationally Regulated and Mediates Dehydration-Responsive Element-Dependent Gene Expression. Plant Physiol. 2012, 161, 346–361. [Google Scholar] [CrossRef]
- Yu, L.-H.; Wu, S.-J.; Peng, Y.-S.; Liu, R.-N.; Chen, X.; Zhao, P.; Xu, P.; Zhu, J.-B.; Jiao, G.-L.; Pei, Y.; et al. Arabidopsis 1/11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnol. J. 2016, 14, 72–84. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef]
- Kim, C.; Kwon, Y.; Jeong, J.; Kang, M.; Lee, G.S.; Moon, J.H.; Lee, H.-J.; Park, Y.-I.; Choi, G. Phytochrome B photobodies are comprised of phytochrome B and its primary and secondary interacting proteins. Nat. Commun. 2023, 14, 1708. [Google Scholar] [CrossRef]
- Kim, J.; Bordiya, Y.; Kathare, P.K.; Zhao, B.; Zong, W.; Huq, E.; Sung, S. Phytochrome B triggers light-dependent chromatin remodelling through the PRC2-associated PHD finger protein VIL1. Nat. Plants 2021, 7, 1213–1219. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, J.; Han, S.; Chong, S.L.; Meng, G.; Song, M.; Wang, Y.; Zhou, S.; Liu, C.; Lou, L.; et al. The chromosome-scale genome of Phoebe bournei reveals contrasting fates of terpene synthase (TPS)-a and TPS-b subfamilies. Plant Commun. 2022, 3, 100410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Day, R.W.; Quinn, G.P. Comparisons of Treatments after an Analysis of Variance in Ecology. Ecol. Monogr. 1989, 59, 433–463. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Size/aa | Molecular Weight/kDa | Theoretical PI | Grand Average of Hydropathicity | Instability Index | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| PbCAMTA1 | OF24973 | 1050 | 117.00 | 5.95 | −0.538 | 40.02 | cytoplasm |
| PbCAMTA2 | OF11451 | 1059 | 117.88 | 5.39 | −0.464 | 42.68 | nucleus |
| PbCAMTA3 | OF06278 | 1157 | 129.66 | 5.83 | −0.553 | 44.52 | nucleus |
| PbCAMTA4 | OF03012 | 964 | 108.86 | 6.59 | −0.377 | 82.89 | cytoplasm |
| PbCAMTA5 | OF03004 | 720 | 79.77 | 5.27 | −0.255 | 34.69 | nucleus |
| PbCAMTA6 | OF19359 | 1088 | 121.79 | 5.79 | −0.525 | 44.20 | nucleus |
| PbCAMTA7 | OF07073 | 1112 | 124.82 | 5.82 | −0.561 | 45.34 | nucleus |
| PbCAMTA8 | OF07605 | 982 | 110.74 | 8.42 | −0.518 | 36.32 | nucleus |
| PbCAMTA9 | OF07600 | 532 | 36.37 | 5.68 | −0.520 | 47.73 | nucleus |
| PbCAMTA10 | OF24143 | 340 | 55.07 | 4.46 | −0.564 | 39.56 | nucleus |
| PbCAMTA11 | OF17261 | 350 | 38.75 | 6.04 | −0.498 | 60.49 | chloroplast |
| PbCAMTA12 | OF00973 | 317 | 35.63 | 9.21 | −0.5115 | 40.97 | nucleus |
| PbCAMTA13 | OF21192 | 514 | 55.07 | 7.02 | −0.058 | 39.45 | chloroplast |
| PbCAMTA14 | OF20820 | 317 | 59.31 | 6.33 | −0.066 | 33.92 | nucleus |
| PbCAMTA15 | OF09478 | 539 | 69.12 | 6.37 | −0.207 | 32.58 | Plasma memberane |
| PbCAMTA16 | OF20135 | 635 | 70.07 | 6.59 | −0.219 | 31.69 | Plasma membrane |
| PbCAMTA17 | OF14546 | 600 | 66.96 | 6.32 | −0.002 | 28.67 | nucleus |
| Gene Name | Forward | Reverse |
|---|---|---|
| PbCAMTA1 | GAACCATGCCCATCGATCA | GATATTGCTGCCGTGCCTTG |
| PbCAMTA2 | GGTAGAACTGTTGGGGAGGC | TCCAATAGCAGCGCCTTTGA |
| PbCAMTA4 | AGGTACTTCGCTGGTTGGTG | GCCTCCAAAAGATCTGCCCA |
| PbCAMTA6 | TGCTGGTTACCTTGCGGAAT | CGCTCTGCAACTGTTTGGAC |
| PbCAMTA8 | CCGCCATCGAATGCATCAAG | CTGTAGCATCCGTCAGTCCC |
| PbCAMTA12 | ACGCATACCTAATCTGGCCG | ACATTGTGTTTGCGCAGCTT |
| PbCAMTA16 | AACGGCCATGTCTGTGTTGA | GGAACCCATCAGACTTGCCA |
| PbCAMTA17 | CTGCCCTATTGGCTGGGAAT | GCTCAACACAACGATCGCAA |
| EF1a | GCTCCTGGTCACCGTGAC | TCACGAGTTTGCCCGTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Li, M.; Yang, Z.; He, C.; Wu, Z.; Tong, Z.; Zhang, J.; Zhang, Y.; Cao, S. The Vital Role of the CAMTA Gene Family in Phoebe bournei in Response to Drought, Heat, and Light Stress. Int. J. Mol. Sci. 2024, 25, 9767. https://doi.org/10.3390/ijms25189767
Zheng K, Li M, Yang Z, He C, Wu Z, Tong Z, Zhang J, Zhang Y, Cao S. The Vital Role of the CAMTA Gene Family in Phoebe bournei in Response to Drought, Heat, and Light Stress. International Journal of Molecular Sciences. 2024; 25(18):9767. https://doi.org/10.3390/ijms25189767
Chicago/Turabian StyleZheng, Kehui, Min Li, Zhicheng Yang, Chenyue He, Zekai Wu, Zaikang Tong, Junhong Zhang, Yanzi Zhang, and Shijiang Cao. 2024. "The Vital Role of the CAMTA Gene Family in Phoebe bournei in Response to Drought, Heat, and Light Stress" International Journal of Molecular Sciences 25, no. 18: 9767. https://doi.org/10.3390/ijms25189767






