Overexpression of PER2 Promotes De Novo Fatty Acid Synthesis, Fatty Acid Desaturation, and Triglyceride Accumulation in Bovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
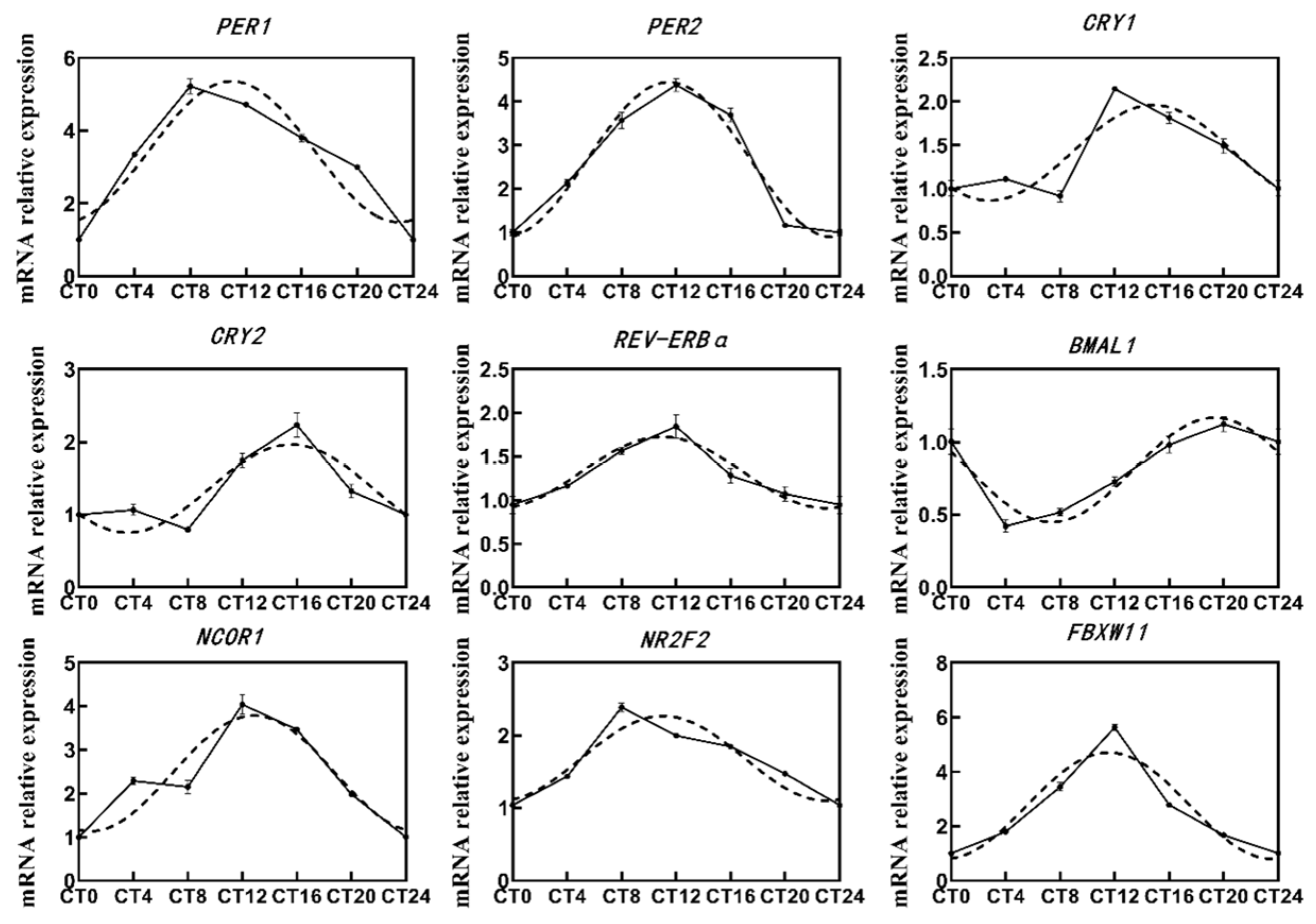
2.1. Gene Expression Pattern
2.2. Transfection Efficiency of PER2
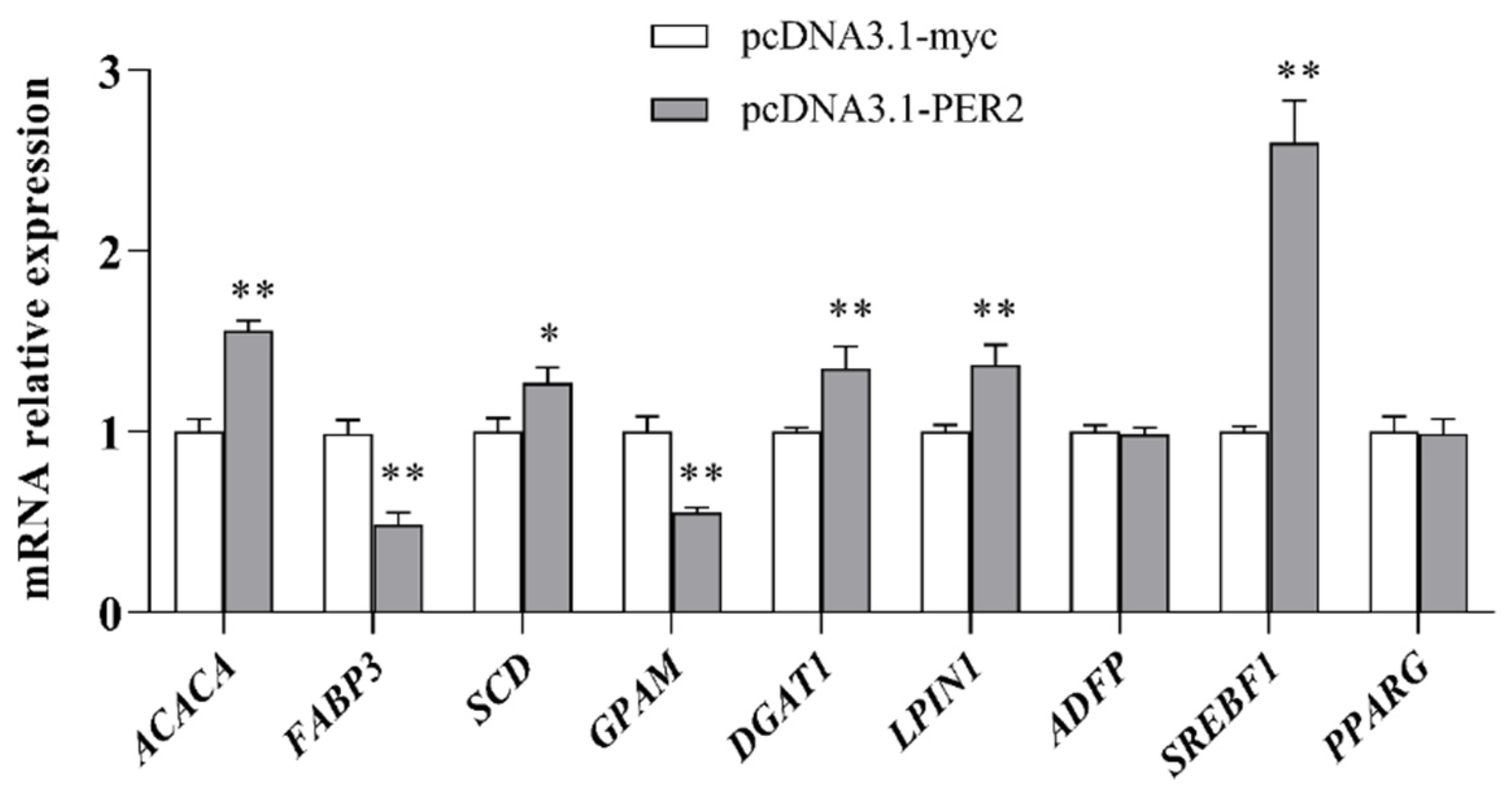
2.3. Lipogenic Genes mRNA Expression
2.4. Protein Abundance and Milk Fat Synthesis
2.5. Fatty Acid Content
3. Discussion
3.1. Circadian Oscillations of Genes
3.2. Fatty Acids Content
3.3. Milk Fat Metabolism
4. Materials and Methods
4.1. Cell Culture
4.2. Eukaryotic Expression Plasmid Construction
4.3. Cell Transfection
4.4. RNA Extraction and qRT-PCR
4.5. Protein Extraction and Western Blotting
4.6. Triacylglycerol Content Determination
4.7. Immunocytofluorescence
4.8. Fatty Acid Measurements
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowrey, P.L.; Takahashi, J.S. Genetics of circadian rhythms in Mammalian model organisms. Adv. Genet. 2011, 74, 175–230. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.M.; Plaut, K.; Kalyesubula, M.; Shamay, A.; Sabastian, C.; Wein, Y.; Bar-Shira, E.; Reicher, N.; Mabjeesh, S.J. Mammary core clock gene expression is impacted by photoperiod exposure during the dry period in goats. J. Appl. Anim. Res. 2018, 46, 1214–1219. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Khan, M.J.; Gao, J.; Loor, J.J. Clock circadian regulator (CLOCK) gene network expression patterns in bovine adipose, liver, and mammary gland at 3 time points during the transition from pregnancy into lactation. J. Dairy Sci. 2015, 98, 4601–4612. [Google Scholar] [CrossRef] [PubMed]
- Milk Components, Nutrition, and Circadian Rhythms of Cows. Available online: https://api.semanticscholar.org/CorpusID:246824962 (accessed on 24 June 2024).
- Quist, M.A.; LeBlanc, S.J.; Hand, K.J.; Lazenby, D.; Miglior, F.; Kelton, D.F. Milking-to-Milking Variability for Milk Yield, Fat and Protein Percentage, and Somatic Cell Count. J. Dairy Sci. 2008, 91, 3412–3423. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Zhang, T.; He, Q.; Shi, H.; Luo, J.; Loor, J.J. Insulin-induced gene 1 and 2 isoforms synergistically regulate triacylglycerol accumulation, lipid droplet formation, and lipogenic gene expression in goat mammary epithelial cells. J. Dairy Sci. 2019, 102, 1736–1746. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Y.; Ge, Y.; Li, W.; Cao, Y.; Qu, Y.; Liu, S.; Guo, Y.; Fu, S.; Liu, J. Sodium butyrate promotes milk fat synthesis in bovine mammary epithelial cells via GPR41 and its downstream signalling pathways. Life Sci. 2020, 259, 118375. [Google Scholar] [CrossRef]
- Alameen, A.O.; Abdelatif, A.M.; Elnageeb, M.E. Circadian variations of thermoregulation, blood constituents and hormones in crossbred dairy cows in relation to level of milk production. Eur. J. Heart Fail. 2014, 4, 466–480. [Google Scholar] [CrossRef]
- Casey, T.M.; Plaut, K. Lactation biology symposium: Circadian clocks as mediators of the homeorhetic response to lactation. J. Anim. Sci. 2012, 90, 744–754. [Google Scholar] [CrossRef]
- Cagampang, F.R.; Bruce, K.D. The role of the circadian clock system in nutrition and metabolism. Br. J. Nutr. 2012, 108, 381–392. [Google Scholar] [CrossRef]
- Wang, M.; Jing, Y.; Hu, L.; Gao, J.; Ding, L.; Zhang, J. Recent advances on the circadian gene PER2 and metabolic rhythm of lactation of mammary gland. Anim. Nutr. 2015, 1, 257–261. [Google Scholar] [CrossRef]
- McQueen, C.M.; Schmitt, E.E.; Sarkar, T.R.; Elswood, J.; Metz, R.P.; Earnest, D.; Rijnkels, M.; Porter, W.W. PER2 regulation of mammary gland development. Development 2018, 145, dev157966. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, M.; Bu, D.; Ma, L.; Liu, F.; Xue, C.; Du, C.; Aboragah, A.; Loor, J.J. Ruminal Microbes Exhibit a Robust Circadian Rhythm and Are Sensitive to Melatonin. Front. Nutr. 2021, 8, 760578. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, Y.; Cortes, I.M.; Coleman, D.N.; Dai, H.; Liang, Y.; Parys, C.; Fernandez, C.; Wang, M.; Loor, J.J. Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells. Food Funct. 2020, 11, 883–894. [Google Scholar] [CrossRef]
- Casey, T.M.; Crodian, J.; Erickson, E.; Kuropatwinski, K.K.; Gleiberman, A.S.; Antoch, M.P. Tissue-specific changes in molecular clocks during the transition from pregnancy to lactation in mice. Biol. Reprod. 2014, 90, 127. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Sun, Y.; He, W.; Luo, M.; Zhou, Y.; Chang, G.; Ren, W.; Wu, K.; Li, X.; Shen, J.; Zhao, X.; et al. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumor Biol. 2015, 36, 4133–4141. [Google Scholar] [CrossRef]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 12071–12076. [Google Scholar] [CrossRef]
- Le Martelot, G.; Claudel, T.; Gatfield, D.; Schaad, O.; Kornmann, B.; Lo, S.G.; Moschetta, A.; Schibler, U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS. Biol. 2009, 7, e1000181. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Tian, H.; Li, J.; Zhang, X.; Chen, Z.; Li, M.; Loor, J.J. Rapid communication: Lipid metabolic gene expression and triacylglycerol accumulation in goat mammary epithelial cells are decreased by inhibition of SREBP-1. J. Anim. Sci. 2018, 96, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Delezie, J.; Challet, E. Interactions between metabolism and circadian clocks: Reciprocal disturbances. Ann. N. Y. Acad. Sci. 2011, 1243, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef]
- Hatanaka, F.; Matsubara, C.; Myung, J.; Yoritaka, T.; Kamimura, N.; Tsutsumi, S.; Kanai, A.; Suzuki, Y.; Sassone-Corsi, P.; Aburatani, H.; et al. Genome-wide profiling of the core clock protein BMAL1 targets reveals a strict relationship with metabolism. Mol. Cell. Biol. 2010, 30, 5636–5648. [Google Scholar] [CrossRef]
- Rey, G.; Cesbron, F.; Rougemont, J.; Reinke, H.; Brunner, M.; Naef, F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011, 9, e1000595. [Google Scholar] [CrossRef]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef]
- Xu, W.; van Knegsel, A.; Saccenti, E.; van Hoeij, R.; Kemp, B.; Vervoort, J. Metabolomics of Milk Reflects a Negative Energy Balance in Cows. J. Proteome Res. 2020, 19, 2942–2949. [Google Scholar] [CrossRef]
- Wenk, M.R. Lipidomics: New tools and applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; Hrabé, D.A.M.; Kronenberg, F.; Meitinger, T.; Mewes, H.W.; Wichmann, H.E.; Weinberger, K.M.; Adamski, J.; et al. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef]
- Gooley, J.J. Applications of Circadian Metabolomics. Curr. Metabolomics 2014, 2, 2–14. [Google Scholar] [CrossRef]
- Milk Components, Nutrition, and Circadian Rhythms of Cows. Available online: https://mnnutritionconf.umn.edu/sites/mnnutritionconf.umn.edu/files/2021-09/RN%209%20MAN%20Salfer%20Final_Hubbard.pdf (accessed on 24 June 2024).
- Langmesser, S.; Tallone, T.; Bordon, A.; Rusconi, S.; Albrecht, U. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol. Biol. 2008, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A. Collective timekeeping among cells of the master circadian clock. J. Endocrinol. 2016, 230, R27–R49. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Circadian rhythms and memory formation: Regulation by chromatin remodeling. Front. Molec. Neurosci. 2012, 5, 37. [Google Scholar] [CrossRef]
- Akashi, M.; Takumi, T. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat. Struct. Mol. Biol. 2005, 12, 441–448. [Google Scholar] [CrossRef]
- Liu, A.C.; Tran, H.G.; Zhang, E.E.; Priest, A.A.; Welsh, D.K.; Kay, S.A. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008, 4, e1000023. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, X.; Yang, J.; Huang, L.; Li, R.; Wu, Q.; Jiang, X. Proteomics analysis of G protein-coupled receptor kinase 4-inhibited cellular growth of HEK293 cells. J. Proteom. 2019, 207, 103445. [Google Scholar] [CrossRef]
- Sissaoui, S.; Yu, J.; Yan, A.; Li, R.; Yukselen, O.; Kucukural, A.; Zhu, L.J.; Lawson, N.D. Genomic Characterization of Endothelial Enhancers Reveals a Multifunctional Role for NR2F2 in Regulation of Arteriovenous Gene Expression. Circ. Res. 2020, 126, 875–888. [Google Scholar] [CrossRef]
- Yang, X.; Downes, M.; Yu, R.T.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006, 126, 801–810. [Google Scholar] [CrossRef]
- Adamovich, Y.; Aviram, R.; Asher, G. The emerging roles of lipids in circadian control. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, E.; Ishihara, A.; Tamai, S.; Nemoto, A.; Iwase, K.; Hiwasa, T.; Shibata, S.; Takiguchi, M. Time of day and nutrients in feeding govern daily expression rhythms of the gene for sterol regulatory element-binding protein (SREBP)-1 in the mouse liver. J. Biol. Chem. 2010, 285, 33028–33036. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.; Lange, D.; Baler, R.; Anzulovich, A. SREBP-1 as a transcriptional integrator of circadian and nutritional cues in the liver. J. Biol. Rhythms 2005, 20, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Mabjeesh, S.J.; Gal-Garber, O.; Shamay, A. Effect of Photoperiod in the Third Trimester of Gestation on Milk Production and Circulating Hormones in Dairy Goats. J. Dairy Sci. 2007, 90, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, F.; Migliavacca, E.; Naldi, A.; Baruchet, M.; Canella, D.; Le Martelot, G.; Guex, N.; Desvergne, B. Genome-wide analysis of SREBP1 activity around the clock reveals its combined dependency on nutrient and circadian signals. PLoS Genet. 2014, 10, e1004155. [Google Scholar] [CrossRef]
- Lee, J.N.; Wang, Y.; Xu, Y.O.; Li, Y.C.; Tian, F.; Jiang, M.F. Characterisation of gene expression related to milk fat synthesis in the mammary tissue of lactating yaks. J. Dairy Res. 2017, 84, 283–288. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Jacobs, A.A.A.; Dijkstra, J.; Liesman, J.S.; VandeHaar, M.J.; Lock, A.L.; van Vuuren, A.M.; Hendriks, W.H.; van Baal, J. Effects of short- and long-chain fatty acids on the expression of stearoyl-CoA desaturase and other lipogenic genes in bovine mammary epithelial cells. Animal 2013, 7, 1508–1516. [Google Scholar] [CrossRef]
- Ma, L.; Corl, B.A. Transcriptional regulation of lipid synthesis in bovine mammary epithelial cells by sterol regulatory element binding protein-1. J. Dairy Sci. 2012, 95, 3743–3755. [Google Scholar] [CrossRef]
- McFadden, J.W.; Corl, B.A. Activation of AMP-activated protein kinase (AMPK) inhibits fatty acid synthesis in bovine mammary epithelial cells. Biochem. Biophys. Res. Commun. 2009, 390, 388–393. [Google Scholar] [CrossRef]
- Frolov, A.; Cho, T.H.; Murphy, E.J.; Schroeder, F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry 1997, 36, 6545–6555. [Google Scholar] [CrossRef] [PubMed]
- Vogel Hertzel, A.; Bernlohr, D.A. The Mammalian Fatty Acid-binding Protein Multigene Family: Molecular and Genetic Insights into Function. Trends Endocrinol. Metab. 2000, 11, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, H.D.; Hurley, W.L.; Davis, C.L. Identification and characterization of a fatty acid binding protein in bovine mammary gland. Comp. Biochem. Phyciology B-Biochem. Mol. Biol. 1986, 85, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hussain, M.M. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J. Biol. Chem. 2007, 282, 24707–24719. [Google Scholar] [CrossRef]
- Chua, E.C.; Shui, G.; Lee, I.T.; Lau, P.; Tan, L.C.; Yeo, S.C.; Lam, B.D.; Bulchand, S.; Summers, S.A.; Puvanendran, K.; et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 14468–14473. [Google Scholar] [CrossRef]
- Mu, T.; Hu, H.; Ma, Y.; Feng, X.; Zhang, J.; Gu, Y. Regulation of Key Genes for Milk Fat Synthesis in Ruminants. Front. Nutr. 2021, 8, 765147. [Google Scholar] [CrossRef]
- Shi, H.B.; Yu, K.; Luo, J.; Li, J.; Tian, H.B.; Zhu, J.J.; Sun, Y.T.; Yao, D.W.; Xu, H.F.; Shi, H.P.; et al. Adipocyte differentiation-related protein promotes lipid accumulation in goat mammary epithelial cells. J. Dairy Sci. 2015, 98, 6954–6964. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; Wang, M.; Liang, Y.; Sun, Y.; Chen, Z.; Zhang, H.; Karrow, N.A.; Yang, Z.; Mao, Y. Polymorphisms in Fatty Acid Desaturase 2 Gene Are Associated with Milk Production Traits in Chinese Holstein Cows. Animals 2020, 10, 671. [Google Scholar] [CrossRef]
- Brenna, J.T.; Kothapalli, K. New understandings of the pathway of long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care. 2022, 25, 60–66. [Google Scholar] [CrossRef]
- He, Z.; Zhang, R.; Jiang, F.; Zhang, H.; Zhao, A.; Xu, B.; Jin, L.; Wang, T.; Jia, W.; Jia, W.; et al. FADS1-FADS2 genetic polymorphisms are associated with fatty acid metabolism through changes in DNA methylation and gene expression. Clin. Epigenetics 2018, 10, 113. [Google Scholar] [CrossRef]
- Bionaz, M.; Thering, B.J.; Loor, J.J. Fine metabolic regulation in ruminants via nutrient-gene interactions: Saturated long-chain fatty acids increase expression of genes involved in lipid metabolism and immune response partly through PPAR-alpha activation. Br. J. Nutr. 2012, 107, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Wang, M.Z.; Ouyang, J.L.; Li, P.F.; Loor, J.J. Rapid Communication: Period2 gene silencing increases the synthesis of alphas-casein protein in bovine mammary epithelial cells. J. Anim. Sci. 2017, 95, 4510–4513. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Zhao, L.J.; Li, W.D.; Wang, X.Y.; Ma, T.T.; Yang, L.D.; Gao, L.; Li, C.M.; Zhang, M.H.; Yang, D.; et al. Circadian clock gene BMAL1 controls testosterone production by regulating steroidogenesis-related gene transcription in goat Leydig cells. J. Cell. Physiol. 2021, 236, 6706–6725. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome proliferator-activated receptor-gamma activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef]
- Xu, H.F.; Luo, J.; Zhao, W.S.; Yang, Y.C.; Tian, H.B.; Shi, H.B.; Bionaz, M. Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J. Dairy Sci. 2016, 99, 783–795. [Google Scholar] [CrossRef]




| Fatty Acid | pcDNA3.1-myc | pcDNA3.1-PER2 | SEM | p-Value |
|---|---|---|---|---|
| C14:0 | 0.004 | 0.003 | 0.000 | 0.407 |
| C16:0 | 0.022 a | 0.035 b | 0.003 | 0.014 |
| C18:0 | 0.026 b | 0.017 a | 0.002 | 0.007 |
| C18:1n9t | 0.038 a | 0.052 b | 0.002 | 0.002 |
| C18:1n9c | 0.036 a | 0.045 b | 0.002 | 0.015 |
| C18:2n6t | 0.001 | 0.002 | 0.000 | 0.715 |
| C22:1n9 | 0.009 b | 0.003 a | 0.001 | 0.000 |
| C24:0 | 0.004 | 0.003 | 0.000 | 0.174 |
| Acetic acid | 17.703 b | 11.635 a | 1.279 | 0.009 |
| Propionic acid | 0.748 | 0.641 | 0.085 | 0.276 |
| Isobutyric acid | 0.664 | 0.714 | 0.093 | 0.613 |
| Butyric acid | 0.822 | 0.654 | 0.106 | 0.189 |
| Isovaleric acid | 0.250 | 0.263 | 0.081 | 0.881 |
| Valeric acid | 0.149 | 0.139 | 0.031 | 0.769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Jing, Y.; Hu, L.; Xi, Z.; Lu, Z.; Loor, J.J.; Wang, M. Overexpression of PER2 Promotes De Novo Fatty Acid Synthesis, Fatty Acid Desaturation, and Triglyceride Accumulation in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 9785. https://doi.org/10.3390/ijms25189785
Chen Y, Jing Y, Hu L, Xi Z, Lu Z, Loor JJ, Wang M. Overexpression of PER2 Promotes De Novo Fatty Acid Synthesis, Fatty Acid Desaturation, and Triglyceride Accumulation in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences. 2024; 25(18):9785. https://doi.org/10.3390/ijms25189785
Chicago/Turabian StyleChen, Yifei, Yujia Jing, Liangyu Hu, Zanna Xi, Zhiqi Lu, Juan J. Loor, and Mengzhi Wang. 2024. "Overexpression of PER2 Promotes De Novo Fatty Acid Synthesis, Fatty Acid Desaturation, and Triglyceride Accumulation in Bovine Mammary Epithelial Cells" International Journal of Molecular Sciences 25, no. 18: 9785. https://doi.org/10.3390/ijms25189785
APA StyleChen, Y., Jing, Y., Hu, L., Xi, Z., Lu, Z., Loor, J. J., & Wang, M. (2024). Overexpression of PER2 Promotes De Novo Fatty Acid Synthesis, Fatty Acid Desaturation, and Triglyceride Accumulation in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences, 25(18), 9785. https://doi.org/10.3390/ijms25189785








