Abstract
Dietary regulation has been recognized for its profound impact on human health. The convergence of cardiovascular, kidney, and metabolic disorders at the pathophysiological level has given rise to cardiovascular–kidney–metabolic (CKM) syndrome, which constitutes a significant global health burden. Maternal dietary nutrients play a crucial role in fetal development, influencing various programmed processes. This review emphasizes the effects of different types of dietary interventions on each component of CKM syndrome in both preclinical and clinical settings. We also provide an overview of potential maternal dietary strategies, including amino acid supplementation, lipid-associated diets, micronutrients, gut microbiota-targeted diets, and plant polyphenols, aimed at preventing CKM syndrome in offspring. Additionally, we discuss the mechanisms mediated by nutrient-sensing signals that contribute to CKM programming. Altogether, we underscore the interaction between maternal dietary interventions and the risk of CKM syndrome in offspring, emphasizing the need for continued research to facilitate their clinical translation.
1. Introduction
Metabolic syndrome is a cluster of risk factors that significantly increases the likelihood of developing cardiovascular disease (CVD). Traditionally, metabolic syndrome focuses on a specific set of metabolic disturbances, including insulin resistance, obesity, and dyslipidemia. However, the American Heart Association has proposed a more comprehensive framework that incorporates a holistic view of health by integrating cardiovascular and kidney issues along with metabolic factors. This integrated approach results in the concept of cardiovascular–kidney–metabolic (CKM) syndrome [1]. CKM syndrome represents a clinical intersection where the interconnected nature of cardiovascular, kidney, and metabolic health is recognized and addressed. The complex interplay among these systems underscores the need for tailored interventions that address their interconnected nature.
Modern dietary patterns are closely linked to the high prevalence of metabolic syndrome, cardiovascular disease, and kidney disease [2]. Contemporary diets often include excessive calories, heavily processed foods, and high amounts of salt, trans fats, and added sugars. Significant changes in the frequency, quantity, and quality of dietary intake contribute to maladaptation and the development of various chronic diseases. Conversely, precise dietary management is crucial for managing these health conditions and extending lifespans [3].
Maternal dietary nutrients profoundly shape fetal development [4,5]. Disparities in maternal diets are linked to the onset of various adult-onset diseases, including each component of the CKM syndrome [6,7,8]. This concept is widely recognized as the developmental origins of health and disease (DOHaD) [9,10]. A notable example is the Dutch famine study, in which it was found that maternal undernutrition during pregnancy is linked to an increased risk of adult offspring developing coronary heart disease, hyperlipidemia, obesity, kidney disease, and hypertension—all features of CKM syndrome [11]. Furthermore, emerging evidence suggests that interventions during critical developmental stages can mitigate or even reverse the adverse effects associated with developmental programming, a process known as reprogramming [12]. This highlights the potential for regulating maternal diet to function as a reprogramming strategy for preventing disorders associated with DOHaD, including the CKM syndrome.
Thus, the objective of this review is to evaluate the influence of maternal diet regulation on offspring outcomes by synthesizing existing human and animal data, with a specific focus on CKM syndrome.
2. Results and Discussion
2.1. Dietary Intervention and CKM Syndrome
In light of the omnipresence of dietary nutrient-mediated signaling throughout the human body [13], it is recognized that diet-derived impacts on health and disease extend across all organ systems [14]. To date, several dietary interventions have been shown to improve the different components of CKM syndrome [2].
2.1.1. Cardiovascular Disease
Diet is tightly connected to cardiovascular health [3,14]. For example, the Western diet is associated with an increased risk of CVD, while several other diets are linked to a reduced risk. These include the Mediterranean diet, the DASH diet (dietary approaches to stop hypertension), vegetarian diet, and plant-based diets.
Western diets and diets high in branched chain amino acids (BCAAs) are well-known risk factors for CVD [15,16]. Conversely, certain diet interventions are able to diminish adverse factors for CVD and improve cardiovascular outcomes [2]. Calorie restriction can enhance healthy aging. A 3-month fasting-mimicking diet (low in sugars, calories, and protein but high in unsaturated fats) has been reported to reduce bodyweight and total body fat, lower blood pressure (BP), and lessen dyslipidemia, all of which are risk factors for CVD [17]. Another study revealed that carbohydrate restriction in cases with type 2 diabetes (T2DM) improved most biomarkers of CVD risk after 1 year [18]. Studies have further demonstrated that several types of diets have a positive effect on reducing BP, including a vegetarian diet [19], the DASH diet [20], Mediterranean diet [21], and fermented/probiotic diet [22]. The DASH diet emphasizes the consumption of vegetables, fruits, legumes, nuts, whole grains, lean protein, and low-fat dairy products [20]. Similarly, the Mediterranean diet is distinguished by high intakes of grains, vegetables, fruits, fish, legumes, extra virgin olive oil, nuts, and the moderate consumption of red wine [21]. In particular, the Mediterranean diet can alter microbial composition and function to benefit cardiovascular health and prevent CVD [23,24].
2.1.2. Chronic Kidney Disease
In advanced chronic kidney disease (CKD), patients frequently face protein–energy wasting due to multiple interrelated factors. Decreased appetite, often caused by metabolic alterations and systemic inflammation, impairs food intake. Additionally, gastrointestinal nutrient absorption is compromised, hindering the efficient processing and utilization of nutrients [25,26,27]. The accumulation of nitrogen-containing waste products, such as urea and creatinine, further aggravates this condition by inducing metabolic imbalances and reducing appetite. Coupled with increased muscle and fat wasting due to impaired protein synthesis and energy balance, these issues necessitate targeted dietary adjustments to support nutritional needs and overall health in this population.
Protein restriction is recommended in patients with CKD, as it retards the rate of renal function decline [28]. Likewise, the restriction of dietary sodium is recommended for the management of CKD and its associated risks [29]. As hyperkalemia and hyperphosphatemia are common complications, restricting dietary potassium and phosphorus intake is often recommended for patients with CKD [26]. Additional calcium and vitamin D supplementation may be offered to patients with advanced CKD. Furthermore, the DASH diet, plant-based diet, and the Mediterranean diet are currently being shown to play a potential role in delaying CKD progression. All together, these studies confirm that optimized diets play a pivotal role in alleviating CKD [30].
2.1.3. Obesity and Diabetes
Overnutrition contributes to obesity and diabetes. Consuming a diet high in fats and excessive amounts of BCAAs, methionine, and tryptophan contribute to weight gain and obesity in rodent models, but human studies are limited [31]. Conversely, dietary interventions can be used to combat obesity-associated disorders. Calorie restriction, with or without time-restricted eating, showed similar bodyweight-lowering effects in patients with obesity [32]. The ketogenic diet is defined by its low-carbohydrate, high-fat, and normal protein composition, which triggers the production of ketone bodies by mimicking the breakdown of a fasting state. A meta-analysis revealed that the ketogenic diet had a positive effect on decreases in blood glucose, lipid control, and weight loss among patients with T2DM [33]. Additionally, plant-rich diets and the Mediterranean diet are associated with a lower risk of T2DM and obesity [34,35]. Moreover, daily high-fiber supplementation combined with fecal microbiota transplantation (FMT) alleviates insulin resistance in patients with obesity or metabolic syndrome [36].
2.1.4. Dyslipidemia and Fatty Liver
Dyslipidemia has a decisive role in the development of non-alcoholic fatty liver disease (NAFLD), which is the result of metabolic disorders such as obesity, insulin resistance, and metabolic syndrome. The accumulation of free fatty acids and lipid metabolites within liver cells disturbs insulin signaling, resulting in the development of NAFLD [37]. Dietary cholesterol is a key factor in activating the inflammatory pathways underlying NAFLD [38], while regulating dietary composition may benefit both NAFLD and dyslipidemia. A dose–response relationship between the degree of calorie restriction and beneficial effects on liver function and weight loss was demonstrated [39]. A meta-analysis suggests that the Mediterranean diet may be an effective diet therapy for NAFLD [39]. Other plant-based diets, such as the DASH and vegetarian diets, are also beneficial, although more data are needed to establish the roles of the ketogenic diet and intermittent fasting in NAFLD [40].
2.2. Maternal Dietary Intervention for Offspring CKM Syndrome: Human Evidence
As elucidated in this review, there are clear correlations between diet regulation and the prevention of CKM syndrome. Maternal diets have a decisive role in fetal development, impacting the health and disease outcomes of offspring [41]. Dietary interventions started during pregnancy are essential to prevent downstream complications for both mothers and their children; however, evidence with respect to these interventions in humans, particularly concerning CKM syndrome, remains limited.
A healthy diet for a pregnant woman should consist of a diverse range of nutrient-rich whole foods, including vegetables, fruits, whole grains, and healthy fats with omega-3 fatty acids sourced from nuts, fish, and seeds [42]. It is advisable to prioritize these foods over less nutritious, heavily processed options. However, maternal nutritional requirements vary based on individual characteristics. In addition to assessing pre-pregnancy dietary quality, it is important to consider factors such as maternal body size, age, gestational age, activity level, multiple pregnancies, and medical conditions. Pregnant women should avoid diets that excessively restrict any macronutrient category. The Mediterranean diet, DASH diet, and the Nordic diet are considered optimal during pregnancy, while high-protein, low-carbohydrate diets, such as ketogenic and modified Atkins diets, should be avoided [42].
Special populations of pregnant women with distinct nutritional requirements include adolescent girls, and those with gestational diabetes mellitus (GDM), overweight/obesity, preeclampsia, and underweight conditions. Developing tailored strategies for these groups is highly recommended to meet the specific nutritional needs of each condition effectively. For example, nutrition therapy, based on carbohydrate restriction, is the foundation for the treatment of gestational diabetes mellitus (GDM). However, restricting dietary carbohydrates can lead to an increase in dietary fat intake. Accordingly, various dietary strategies for GDM have been reported, including low glycemic index (GI) diets, energy restriction, adjustments in carbohydrate levels, and modifications in the quality or quantity of fats or proteins. A meta-analysis of 18 randomized controlled trials (RCTs) and 8 dietary patterns for GDM nutrition revealed that improving nutritional quality and intake positively impacted outcomes related to maternal glycemia and birth weight [43].
Prior work indicates that fetal and newborn anthropometry was the most observed outcome related to maternal diets and child health [44]. Although some reports suggested that a mother’s adherence to Mediterranean dietary patterns was associated with a decreased risk of offspring obesity [45,46,47], there were inconsistent findings observed by others [48,49]. However, no evidence was reported regarding how maternal diet regulation protects offspring against other phenotypes of CKM syndrome.
2.3. Maternal Dietary Interventions as Reprogramming Strategies
In reprogramming strategies, the goal is to reverse or delay adverse programmed processes and foster normal development. Included in these strategies are nutritional interventions, lifestyle modifications, pharmacological therapy, and exercise. Given the limited information available from human studies, animal models with precise control over dietary regulation are crucial for discovering suitable maternal dietary interventions to prevent offspring CKM syndrome before implementing them in humans.
In this review, we were restricted to dietary interventions during gestation and breastfeeding periods as reprogramming strategies aiming at averting offspring CKM syndrome in all sorts of animal models [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105], as detailed in Table 1, Table 2, Table 3, Table 4 and Table 5. Rats are the most commonly used species, followed by mice and rabbits. It should be noted that the developmental window is not uniform across different species and organ systems. In contrast to humans, kidney development in rodents progresses until approximately postnatal weeks 1–2 [106]. As a result, nutritional interventions during gestation and breastfeeding can help to preserve nephrogenesis and enhance nephron numbers, thereby mitigating kidney diseases originating from developmental factors [106].
Notably, dietary interventions employed for CKM syndrome may yield contrasting or potentially adverse outcomes on the developmental origins of CKM syndrome. For instance, dietary intervention with caloric restriction improves metabolic syndrome [107], kidney disease [108], and cardiovascular disease [109]. However, maternal caloric restriction has resulted in various components of CKM syndrome in adult rat offspring, including obesity, hypertension, kidney disease, hyperleptinemia, and hyperinsulinism [52,67,74,110]. Comparable adverse outcomes in offspring have been noted in both cows and ewes [111,112].
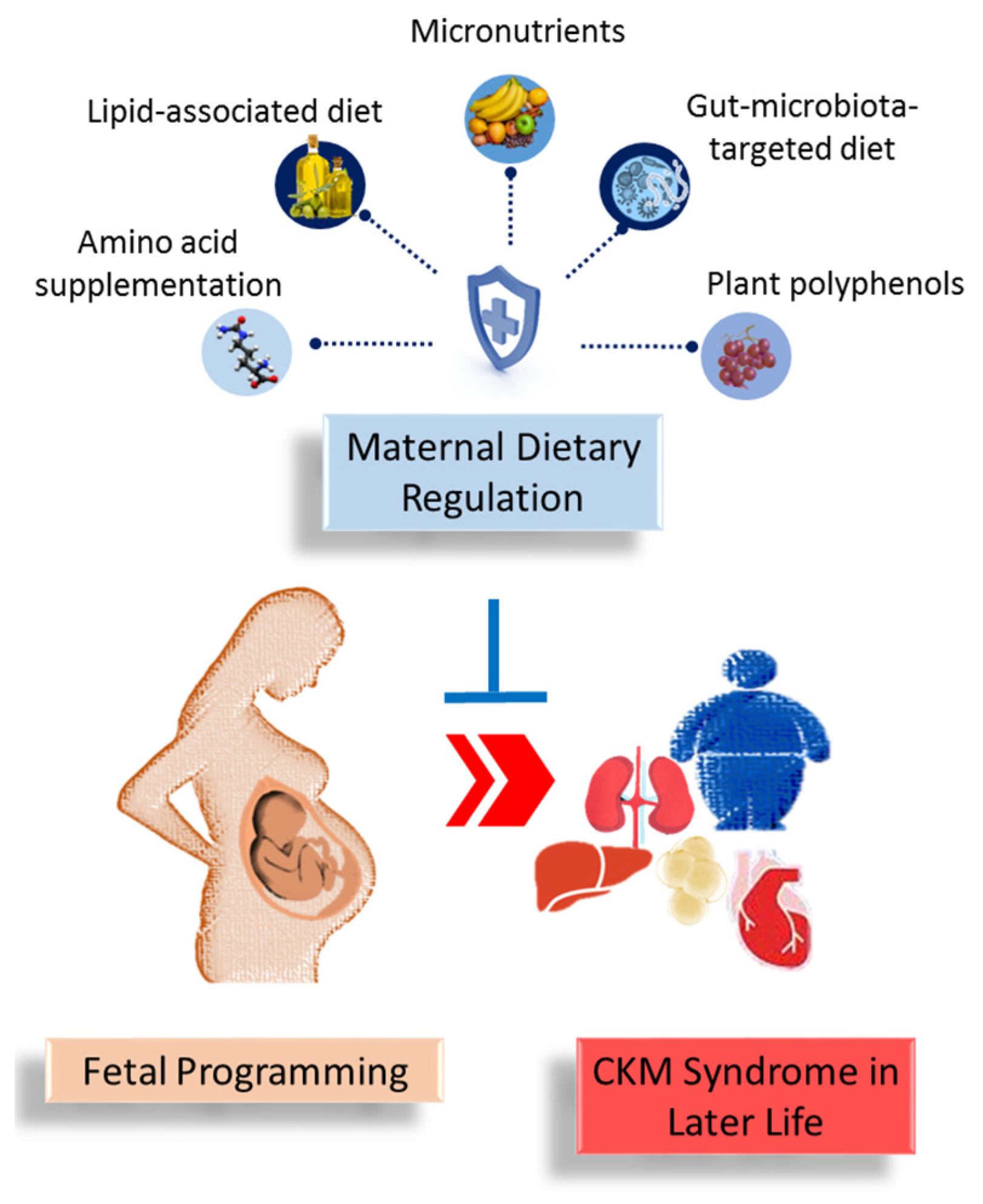
Dietary interventions during pregnancy and lactation are grouped into amino acid supplementation, lipid-associated diet, micronutrients, gut-microbiota-targeted diet, and plant polyphenols. Each will be discussed in detail in turn.
2.3.1. Amino Acid Supplementation
Macronutrients utilized as dietary interventions primarily focus on amino acid supplementation. Amino acids serve as building blocks for a diverse array of structural proteins within the body, thereby playing crucial roles in organogenesis and fetal development. Among them, the most frequently utilized as reprogramming interventions are citrulline and taurine (Table 1).
Citrulline is a non-essential amino acid that can be metabolized into arginine. Both amino acids are purported to increase nitric oxide (NO) production and provide benefits for cardiovascular disease [113]. Supplementation with maternal citrulline has been shown to safeguard adult offspring from hypertension and kidney disease across various models of developmental programming, including, as follows: streptozotocin (STZ)-induced diabetes [51]; maternal caloric restriction [52]; co-administration of NG-nitro-L-arginine-methyl ester (L-NAME, NO synthase inhibitor) and dexamethasone [53]; maternal CKD [54]; antenatal dexamethasone exposure [55]; and spontaneously hypertensive rat (SHR, a genetic hypertensive rat model) [56]. While post-weaning arginine supplementation has been shown to enhance hypertension, insulin sensitivity, and beta cell function in adult rat offspring [114,115], the reprogramming effects of maternal arginine supplementation remain unexplored. Citrulline supplementation is more effective than arginine in generating NO because citrulline can bypass hepatic metabolism and undergo renal conversion to arginine [116]. Hence, before its implementation in humans, further exploration is warranted to enhance our understanding of how maternal citrulline supplementation can prevent developmental programming in various animal models.
Taurine is another amino acid commonly supplemented during gestation. As indicated in Table 1, perinatal use of taurine has been extensively studied in various components of CKM syndrome, including obesity, dyslipidemia, diabetes, hypertension, and kidney disease [57,58,59,60,61,62,63,64]. Taurine, the most plentiful sulfur-containing amino acid [117], is primarily obtained through dietary sources, although it can also be synthesized from cysteine. Maternal taurine supplementation can improve offspring obesity primed by maternal dyslipidemia [61] and a maternal high-fructose/high-fat diet [62]. Additionally, taurine supplementation perinatally protected offspring against hypertension in stroke-prone spontaneously hypertensive rats (SHRSP) and SHRs [59,63]. Using a non-obese diabetic (NOD) mice model, the use of taurine during pregnancy and lactation delayed the onset time of diabetes from 30 to 38 weeks in male offspring and from 18 to 30 weeks in female offspring [64]. In maternal CKD, the protective actions of perinatal taurine supplementation on offspring hypertension and kidney disease are closely associated with the modulation of gut microbiota [58]. Taurine supplementation led to an increased presence of Dehalobacterium, Bifidobacterium, and Asteroleplasma genera, alongside a decrease in Erisipelactoclostridium [58]. The replenishment of Bifidobacterium levels, which had declined due to maternal CKD, was linked to taurine’s probiotic properties, which contribute towards preventing hypertension [58]. Using the same model, gestational supplements with cysteine or tryptophan also showed beneficial effects on offspring hypertension complicated by maternal CKD [64,65]. Glycine is a simple amino acid that is not essential in the human diet. In a rat model, glycine supplementation during gestation averted hypertension in progeny born to dams that experienced protein restriction [50]. Moreover, maternal BCAA supplementation has been shown to protect adult rat offspring against hypertension, obesity, and diabetes in maternal caloric restriction and high-fat diet models, respectively [67,68].

Table 1.
Effects of maternal amino acid supplementation on offspring CKM phenotypes in animal models.
Table 1.
Effects of maternal amino acid supplementation on offspring CKM phenotypes in animal models.
| Dietary Intervention | Dose | Periods Pregnancy/Lactation | Model | Species | Prevented CKM Phenotypes | Age at Measure (Weeks) | Ref. |
|---|---|---|---|---|---|---|---|
| Glycine | 3% | Yes/No | Protein restriction | Rat | Hypertension | 4 | [50] |
| Citrulline | 0.25% | Yes/Yes | STZ-induced diabetes | Rat | Hypertension and kidney disease | 12 | [51] |
| Citrulline | 0.25% | Yes/Yes | Caloric restriction | Rat | Kidney disease | 12 | [52] |
| Citrulline | 0.25% | Yes/Yes | Maternal L-NAME exposure | Rat | Hypertension | 12 | [53] |
| Citrulline | 0.25% | Yes/Yes | Maternal CKD | Rat | Hypertension | 12 | [54] |
| Citrulline | 0.25% | Yes/Yes | Prenatal dexamethasone exposure | Rat | Hypertension and kidney disease | 16 | [55] |
| Citrulline | 0.25% | Yes/Yes | SHR | Rat | Hypertension | 50 | [56] |
| Taurine | 3% | Yes/Yes | Maternal high sugar diet | Rat | Hypertension | 8 | [57] |
| Taurine | 3% | Yes/Yes | Maternal CKD | Rat | Hypertension and kidney disease | 12 | [58] |
| Taurine | 3% | Yes/Yes | SHRSP | Rat | Hypertension | 12 | [59] |
| Taurine | 3% | Yes/Yes | STZ-induced diabetes | Rat | Hypertension | 16 | [60] |
| Taurine | 3% | Yes/Yes | Maternal dyslipidemia | Rat | Obesity, dyslipidemia, and hypertension | 16 | [61] |
| Taurine | 1.5% | Yes/Yes | Maternal high-fructose/high-fat diet | Rat | Obesity | 21 | [62] |
| Taurine | 3% | Yes/Yes | SHR | Rat | Hypertension | 22 | [63] |
| Taurine | 2.5% | Yes/Yes | NOD | Mouse | Diabetes | 50 | [64] |
| Cysteine | 8 mmol/kg/day | Yes/No | Maternal CKD | Rat | Hypertension | 12 | [65] |
| Tryptophan | 200 mg/kg BW/day | Yes/No | Maternal CKD | Rat | Hypertension | 12 | [66] |
| BCAAs | NA | Yes/No | Caloric restriction | Rat | Hypertension | 16 | [67] |
| Leucine | 1.5% chow | Yes/Yes | High-fat diet | Mouse | Obesity and diabetes | 16 | [68] |
BCAA, branched chain amino acid; STZ, streptozotocin; L-NAME, NG-nitro–L-arginine methyl ester; CKD, chronic kidney disease; SHR, spontaneously hypertensive rat; SHRSP, stroke-prone spontaneously hypertensive rat; NOD, non-obese diabetic; NA, not available.
2.3.2. Lipid-Associated Diet
Dietary fat, the most caloric-rich macronutrient, breaks down into fatty acids that perform essential physiological functions. Generally, the consumption of saturated fatty acids and trans fats is associated with a heightened risk of CVD. In contrast, monounsaturated and polyunsaturated fatty acids (PUFAs) are associated with a decreased risk of CVD [118]. As shown in Table 2, maternal consumption diets high in saturated fats resulted in obesity, diabetes, hypertension, dyslipidemia, and fatty liver in adult offspring [68,69,77,78,81]. Conversely, PUFA supplementation has been used in reprogramming interventions against offspring CKM syndrome. To date, three reports have demonstrated that PUFA supplementation during gestation and lactation has beneficial effects against hypertension [69,70], CVD [70], and fatty liver [71] in adult rat offspring. Conjugated linoleic acid (CLA) primarily originates from dietary PUFAs, especially linoleic acid, which is present in various foods. Supplementation with CLA during gestation and breastfeeding has been shown to protect adult rat offspring from hypertension induced by a high-fat diet [69]. Despite recommendations for pregnant and breastfeeding women to consume PUFAs [119], a meta-analysis involving 3644 children revealed that maternal supplementation with omega-3 PUFAs during pregnancy does not significantly impact obesity risk [120]. Consequently, the potential beneficial or detrimental effects of individual PUFAs used as dietary supplements during gestation on the offspring’s risk of CKM syndrome remain undecided.

Table 2.
Effects of maternal lipid-associated diet on offspring CKM phenotypes in animal models.
Table 2.
Effects of maternal lipid-associated diet on offspring CKM phenotypes in animal models.
| Dietary Intervention | Dose | Periods Pregnancy/Lactation | Model | Species | Prevented CKM Phenotypes | Age at Measure (Weeks) | Ref. |
|---|---|---|---|---|---|---|---|
| Lipid-associated diet | |||||||
| Conjugated linoleic acid | 1% chow | Yes/Yes | Maternal high-fat diet | Rat | Hypertension | 18 | [69] |
| PUFA | 1.5 g/kg/day | Yes/Yes | Protein restriction | Rat | Hypertension and cardiovascular disease | 24 | [70] |
| PUFA | 8.78% chow | Yes/Yes | Maternal cafeteria diet | Rat | Fatty liver | 56 | [71] |
PUFA, polyunsaturated fatty acids.
2.3.3. Micronutrients
Micronutrients consist of vitamins and minerals. Vitamins C and E, as well as selenium, etc., have antioxidant properties and exhibit advantageous effects on human health [121]. Among the antioxidant supplements, vitamins C and E are the most commonly utilized. As a water-soluble antioxidant, vitamin C acts as a scavenger of free radicals and serves as a reducing agent [122]. On the other hand, vitamin E, being lipid-soluble, works by inhibiting various oxidative enzymes, thereby decreasing ROS production [123]. As shown in Table 3, supplementation with either vitamin C or E alone during pregnancy protected against offspring hypertension induced by maternal lipopolysaccharide (LPS) exposure [72,73]. Additionally, vitamin C and E, in combination with selenium and folic acid, protected against hypertension and CVD in adult rat offspring born to dams experiencing caloric restriction [74]. A causal link between maternal hypercholesterolemia and the development of atherosclerosis later in life has been established in rabbits [124]. Using this rabbit model, treatment with vitamin E demonstrated protective effects against the progression of atherosclerosis in adult rabbit offspring [75].
An essential water-soluble B vitamin, folic acid is widely present in various fruits and vegetables. It, along with choline and betaine, serves as a source of the coenzymes involved in one-carbon metabolism [125]. One-carbon metabolites work as methyl donors that are required for DNA methylation. One study showed that gestational supplementation with folic acid protected against hypertension and CVD in adult rat progeny born to dams experiencing protein restriction [76]. Feeding mice a high-fat diet and supplementing with choline before mating and during gestation had a protective effect on the development of obesity and fatty liver in offspring maintained on a high-fat diet [77,78]. Another report showed that maternal betaine supplementation attenuated dyslipidemia and fatty liver in adult rat offspring exposed to dexamethasone [79]. Although supplementation with folic acid and other methyl donors during gestation has been recommended to improve certain offspring outcomes [126], a diet high in folic acid or methyl donors may increase the offspring’s susceptibility to negative health outcomes later in life, including hypertension, hyperlipidemia, and insulin resistance [127,128]. It is important to note that vitamin supplements should be given only when there is a documented deficiency and not as a routine practice during pregnancy.

Table 3.
Effects of maternal micronutrient supplementation on offspring CKM phenotypes in animal models.
Table 3.
Effects of maternal micronutrient supplementation on offspring CKM phenotypes in animal models.
| Dietary Intervention | Dose | Periods Pregnany/Lactation | Model | Species | Prevented CKM Phenotypes | Age at Measure (Weeks) | Ref. |
|---|---|---|---|---|---|---|---|
| Vitamin C | 350 mg/kg/day | Yes/No | Prenatal LPS exposure | Rat | Hypertension | 12 | [72] |
| Vitamin E | 350 mg/kg/day | Yes/No | Prenatal LPS exposure | Rat | Hypertension and kidney disease | 17 | [73] |
| Vitamin C, E, selenium and folic acid | Combined doses 1 | Yes/No | Caloric restriction | Rat | Cardiovascular disease and hypertension | 16 | [74] |
| Vitamin E | 350 mg/kg/day | Yes/No | Cholesterol-enriched diet | Rabbit | Cardiovascular disease and hypertension | 24 | [75] |
| Folic acid | 5 mg/kg/day | Yes/No | Protein restriction | Rat | Cardiovascular disease and hypertension | 15 | [76] |
| Choline | 11.7 mmol/kg in chow | Yes/No | High-fat diet | Mouse | Obesity | 9 | [77] |
| Choline | 25 mM in water | Yes/No | High-fat diet | Mouse | Fatty liver | 9 | [78] |
| Betaine | 0.1 mg/kg/day i.p. | Yes/No | Postnatal dexamethasone exposure | Rat | Dyslipidemia and fatty liver | 16 | [79] |
LPS, lipopolysaccharide. 1 alpha-tocopherol (250 mg/kg/day), ascorbic acid (150 mg/kg/day), selenium (0.3 mg/kg/day) and folic acid (4 mg/kg/day).
2.3.4. Gut Microbiota-Targeted Diet
Consuming diets abundant in plant-based ingredients, fermented foods, and high-fiber foods is associated with a more diverse and beneficial gut microbiota. These dietary patterns significantly enhance cardiovascular–kidney–metabolic health by shaping gut microbiota and the derived metabolites [129]. Similarly, the Mediterranean diet, DASH diet, and a vegetarian diet can also nourish beneficial gut bacteria [130,131,132]. Accordingly, a gut-microbiota-targeted diet, which includes probiotics, prebiotics, and postbiotics, has emerged as a reprogramming strategy to avert CKM syndrome with developmental origins (Table 4).
Probiotics and prebiotics are often discussed and implemented in clinical practice. Probiotic therapy entails the intentional introduction of beneficial microorganisms into the gut microbiota [133]. Food ingredients that promote the growth or enhance the activity of beneficial microbes are referred to as prebiotics [134]. Metabolites produced by probiotics after processing, known as postbiotics, include vitamins, secreted proteins, short-chain fatty acids (SCFAs), and secreted biosurfactants [135].
Lactobacillus casei and Lactiplantibacillus plantarum WJL, both probiotics, demonstrated reprogramming effects by enhancing gut microbiota diversity and mitigating conditions like hypertension, dyslipidemia, and insulin resistance [80,81,82,83]. Similarly, prebiotics—including inulin, oligofructose, inositol, fructooligosaccharide, and garlic oil—have been effective in protecting against high-fat diet-induced hypertension, fatty liver, obesity, and diabetes in adult offspring [80,81,82,83,84,85,86,87]. Moreover, a high-fiber diet has also been utilized as a reprogramming strategy to avert the developmental programming of obesity and diabetes [95,96].
Many foods demonstrate prebiotic activity by enhancing the growth of beneficial microbes in the gut. Abundant in polysulfides, garlic (Allium sativum) serves as a dietary source of hydrogen sulfide (H2S) donors [136]. This characteristic underpins its diverse health-promoting properties, which include cardiovascular protection, BP reduction, anti-inflammatory and antioxidant activities, prebiotic effects, and blood sugar regulation. Studies have shown that maternal supplementation with garlic oil can positively impact offspring predisposed to hypertension due to a high-fat diet. This supplementation has been associated with an increased abundance of beneficial microbes such as Bifidobacterium and Lactobacillus; higher levels of acetic acid, butyric acid, and propionic acid in plasma; and enhanced α-diversity.
SCFAs are key microbial metabolites that can function as postbiotics. Acetic acid, a plentiful SCFA, interacts with its receptors to regulate BP [137]. Previous research demonstrated that perinatal acetic acid supplementation could prevent hypertension in offspring programmed by a maternal high-fructose diet [88] or maternal minocycline exposure [89]. Another SCFA under investigation for reprogramming for CKM programming is propionic acid. Studies have revealed that propionic acid supplementation during gestation and lactation can protect adult offspring from diabetes, hypertension, and dyslipidemia [88,89,90,91]. Additionally, as a postbiotic, maternal butyric acid supplementation reversed hypertension in adult rat offspring born to dams fed a high-fructose diet [92] or a tryptophan-free diet [93]. Furthermore, butyric acid use during gestation and lactation improved diabetes outcomes in the adult offspring of nonobese diabetic mice [94].

Table 4.
Effects of maternal gut microbiota-targeted diet on offspring CKM phenotypes in animal models.
Table 4.
Effects of maternal gut microbiota-targeted diet on offspring CKM phenotypes in animal models.
| Dietary Intervention | Dose | Periods Pregnancy/Lactation | Model | Species | Prevented CKM Phenotypes | Age at Measure (Weeks) | Ref. |
|---|---|---|---|---|---|---|---|
| Lactobacillus casei | 2 × 108 CFU/day | Yes/Yes | Maternal high-fructose diet | Rat | Hypertension | 12 | [80] |
| Lactobacillus casei | 2 × 108 CFU/day | Yes/Yes | High-fat diet | Rat | Hypertension | 16 | [81] |
| Lactiplantibacillus plantarum WJL | 1 × 109 CFU/day | Yes/Yes | Maternal high-fat/high-cholesterol diet | Rat | Hypertension, diabetes, and dyslipidemia | 13 | [82] |
| Multi-strain probiotics | Combined 1 | Yes/Yes | Maternal high-fat diet | Mouse | Diabetes | 20 | [83] |
| Long-chain inulin | 5% w/w | Yes/Yes | Maternal high-fructose diet | Rat | Hypertension | 12 | [80] |
| Long-chain inulin | 5% w/w | Yes/Yes | High-fat diet | Rat | Hypertension | 16 | [81] |
| Oligofructose | 10% w/w | Yes/Yes | Maternal high-fat/high-sucrose diet | Rat | Diabetes and fatty liver | 24 | [84] |
| Inositols | Myo-inositol/D-chiro-inositol: 7.2/0.18 mg/mL water | Yes/No | Maternal high-fat diet | Mouse | Hypertension and diabetes | 10 | [85] |
| Fructooligosaccharides | 10% w/w | Yes/No | Maternal high-fat diet | Mice | Obesity and diabetes | 12 | [86] |
| Garlic oil | 100 mg/kg/day | Yes/Yes | High-fat diet | Rat | Hypertension | 16 | [87] |
| Acetic acid | 200 mmol/L | Yes/Yes | Maternal high-fructose diet | Rat | Hypertension | 12 | [88] |
| Acetic acid | 200 mmol/L | Yes/Yes | Maternal minocycline administration | Rat | Hypertension | 12 | [89] |
| Propionic acid | 200 mmol/L | Yes/Yes | Maternal high-fructose diet | Rat | Hypertension | 12 | [88] |
| Propionic acid | 200 mmol/L | Yes/Yes | Maternal CKD | Rat | Hypertension | 12 | [90] |
| Propionic acid | 200 mmol/L | Yes/Yes | Maternal hypoxia | Mouse | Diabetes and dyslipidemia | 11 | [91] |
| Butyric acid | 400 mg/kg/day | Yes/Yes | Maternal high-fructose diet | Rat | Hypertension | 12 | [92] |
| Butyric acid | 400 mg/kg/day | Yes/Yes | Maternal tryptophan-free diet | Rat | Hypertension | 16 | [93] |
| Butyric acid | 400 mg/kg/day | Yes/Yes | NOD | Mouse | Diabetes | 16 | [94] |
| High-fiber diet | 22% chow | Yes/Yes | Maternal diabetogenic diet | Rat | Diabetes | 40 | [95] |
| High-fiber diet | NA | Yes/Yes | Maternal Western diet | Mouse | Obesity and diabetes | 8 | [96] |
CKD, chronic kidney disease; NOD, non-obese diabetic; NA, not available. 1 B. breve DM8310, L. acidophilus DM8302, L. casei DM8121 and S. thermophilus DM8309.
2.3.5. Plant Polyphenols
Most plant-based diets are rich in polyphenols, the predominant group of phytochemicals, which are natural compounds synthesized exclusively by plants [138]. These include flavanones, flavanols, isoflavones, flavones, anthocyanins, stilbenes, xanthones, lignans, and tannins [138]. Dietary polyphenols help prevent certain diseases through various mechanisms such as antioxidant activity, prebiotic effects, and epigenetic modifications [139,140]. Furthermore, polyphenols exhibit reprogramming properties and are of significant interest in disease prevention research within the DOHaD framework [141].
Resveratrol, a natural polyphenol found in grapes, is generally recognized for its antioxidant, anti-inflammatory, and prebiotic properties [142]. It has been proposed as a preventive strategy to improve cardiometabolic health [143]. Studies listed in Table 5 showed that resveratrol has beneficial actions against offspring CKM syndrome, addressing issues such as hypertension, dyslipidemia, obesity, and fatty liver [97,98,99,100].

Table 5.
Effects of maternal polyphenol supplementation on offspring CKM phenotypes in animal models.
Table 5.
Effects of maternal polyphenol supplementation on offspring CKM phenotypes in animal models.
| Dietary Intervention | Dose | Periods Pregnancy/Lactation | Model | Species | Prevented CKM Phenotypes | Age at Measure (Weeks) | Ref. |
|---|---|---|---|---|---|---|---|
| Resveratrol | 50 mg/L | Yes/Yes | Maternal CKD | Rat | Hypertension | 12 | [97] |
| Resveratrol | 147 mg/kg/day | Yes/Yes | Maternal high-fat/sucrose diet | Rat | Diabetes and obesity | 15 | [98] |
| Resveratrol | 50 mg/L | Yes/Yes | High-fat diet | Rat | Dyslipidemia, obesity, and fatty liver | 16 | [99] |
| Resveratrol | 0.2% w/w | Yes/Yes | Maternal high-fat diet | Mouse | Dyslipidemia and obesity | 14 | [100] |
| Epigallocatechin gallate | 0.1% | Yes/No | Prenatal dexamethasone exposure | Rat | Hypertension | 14 | [101] |
| Quercetin | 50 mg/kg/day | Yes/No | Maternal high-fat diet | Mouse | Hypertension | 24 | [102] |
| Grape skin extract | 200 mg/kg/day | No/Yes | Maternal high-fat diet | Rat | Hypertension | 24 | [103] |
| Curcumin | 400 mg/kg/day | Yes/Yes | Maternal hyperglycemic diet | Mouse | Obesity, diabetes, and dyslipidemia | 12 | [104] |
| Green tea | 0.24% | No/Yes | Protein restriction plus post-weaning high-fat diet | Rat | Kidney disease | 45 | [105] |
CKD, chronic kidney disease.
Another example of polyphenol flavonoids used for reprogramming in CKM programming is epigallocatechin gallate [86]. Perinatal use of epigallocatechin gallate attenuated offspring hypertension induced by antenatal dexamethasone exposure [101]. Quercetin, a polyphenol from the flavanols family, has demonstrated protective effects against offspring hypertension complicated by high-fat diets [102]. Additionally, grape skin extract, containing about 30% of total polyphenols, protected adult rat progeny from hypertension induced by maternal high-fat intake [103]. Furthermore, maternal supplementation with curcumin, a polyphenol found in turmeric, has been shown to reverse obesity, diabetes, and dyslipidemia in adult offspring primed by a maternal hyperglycemic diet [104]. Moreover, maternal intake of green tea polyphenols during lactation attenuated kidney disease in male offspring fed a high-fat diet and programmed by maternal protein restriction in rats [105]. As outlined in this review, Figure 1 illustrates the intricate associations between maternal dietary regulation, fetal programming, and offspring CKM syndrome.
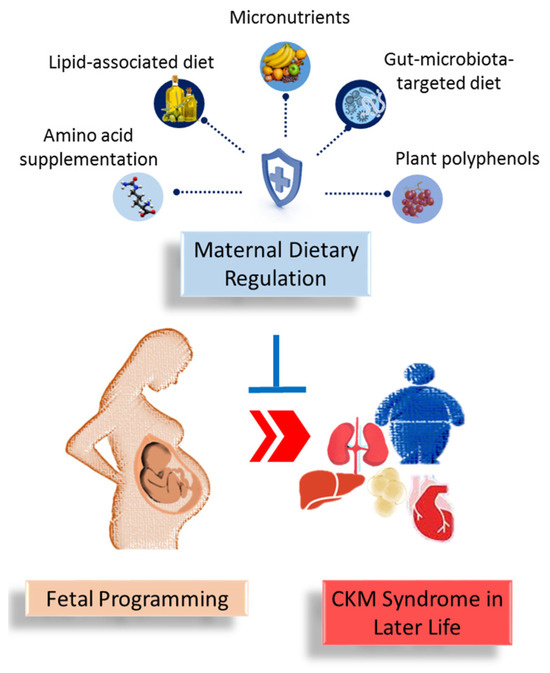
Figure 1.
A summary of the role of maternal dietary regulation in preventing the developmental programming of cardiovascular–kidney–metabolic (CKM) syndrome in offspring later in life.
2.4. Nutrient-Sensing Signals and CKM Programming
As mentioned above, maternal dietary intervention involves the strategic intake of various nutrients such as carbohydrates, amino acids, lipids, micronutrients, and metabolites. These nutrients trigger sensing signals that activate multiple biochemical pathways under different dietary conditions. As a result, the identification of specific molecular mechanisms underlying CKM programming and the targeting of these signaling pathways to develop ideal reprogramming interventions could offer new therapeutic opportunities. These nutrient-sensing signals include, as follows: AMP-activated protein kinase (AMPK); sirtuin (SIRT); peroxisome proliferator-activated receptors (PPARs); and PPARγ coactivator-1α (PGC-1α) [13]. To date, interventions during early life that target AMPK or PPAR signaling pathways have been documented to prevent CKM characteristics across various developmental programming models [144,145,146].
2.4.1. AMPK
Maintaining cellular metabolism within a precise range requires tight regulation of ATP levels. AMPK, a universally expressed serine/threonine protein kinase with catalytic α subunits and regulatory β and γ subunits, plays a crucial role in this regulation [147]. The activation of AMPK occurs when cellular energy levels decrease, as indicated by elevated AMP-to-ATP or ADP-to-ATP ratios. Its primary function is to restore energy balance by enhancing energy production. Polyphenol-rich foods can act as indirect AMPK activators [148]. Certain indirect AMPK activators have revealed beneficial actions on programmed hypertension, including garlic [87], resveratrol [97], epigallocatechin gallate [101], and quercetin [102]. Additionally, the activation of AMPK-PGC1α-activity protected adult rat offspring from maternal western-style-diet-induced increased adiposity and fatty liver in later life [149].
2.4.2. PPAR
Emerging evidence suggests that PPARs play a crucial role in the development of different aspects of CKM syndrome; interventions that activate them hold promise for treating these CKM-related conditions [145,150,151,152].
Nevertheless, only a few studies have assessed the influence of dietary PPAR modulators on CKM programming [145]. Certain natural PPAR agonists, such as omega-3 PUFAs and conjugated linoleic acid, have been studied in the developmental programming of hypertension, CVD, and fatty liver [69,70,71]. Due to the broad spectrum of affinity that fatty acid derivatives exhibit towards PPARs [153], determining whether their reprogramming effects are PPAR-dependent can be challenging. Another study showed that 15-Deoxy-Δ12,14-prostagandin J2 (15dPGJ2) treatment, a natural PPARγ ligand, averted programmed hypertension in maternal fructose-fed male adult rat offspring [154].
2.4.3. Sirtuin
The SIRT family comprises seven proteins (SIRT1–SIRT7) categorized as class III histone deacetylases (HDACs) [155]. These enzymes require NAD+ as a cofactor and play a crucial role in epigenetic regulation, which is fundamental to developmental programming [156]. Specifically, SIRT1 mediates the deacetylation of PGC-1α, thereby influencing the expression of PPAR target genes. Reduced renal expression of SIRT1 has been associated with hypertension in offspring complicated by a maternal methyl-deficient diet [157]. Similarly, decreased SIRT4 expression in the kidney has been linked to hypertension complicated by maternal high-fructose diet [80].
Conversely, activation of the SIRT1-AMPK α-eNOS pathway has been shown to confer benefits in alleviating hypertension [158]. The overexpression of SIRT1 was reported to reduce BP in Ang II-induced hypertension, and this action was reversed by the SIRT1 inhibitor [159]. Several polyphenols, such as resveratrol, epigallocatechin gallate, quercetin, and curcumin, are known to activate SIRTs [160]. However, the extent to which the reprogramming effects of these polyphenols, as listed in Table 1, are mediated through SIRT activation and the specific concentrations required for these effects, are unclear.
2.4.4. Others
Given that diverse maternal nutritional factors can lead to similar CKM phenotypes in adult offspring, there may be underlying mechanisms beyond nutrient-sensing signals that contribute to the pathogenesis of nutritional programming associated with CKM syndrome. Current research points to several potential mechanisms, including, as follows: oxidative stress [161,162]; epigenetic regulation [163]; gut microbiota [164]; inflammation [165]; and sex differences [166]. However, the exact effects of these mechanisms on maternal dietary interventions and their influence on the risk of CKM syndrome in offspring are not fully understood and require further investigation.
3. Materials and Methods
3.1. Search Strategy and Data Sources
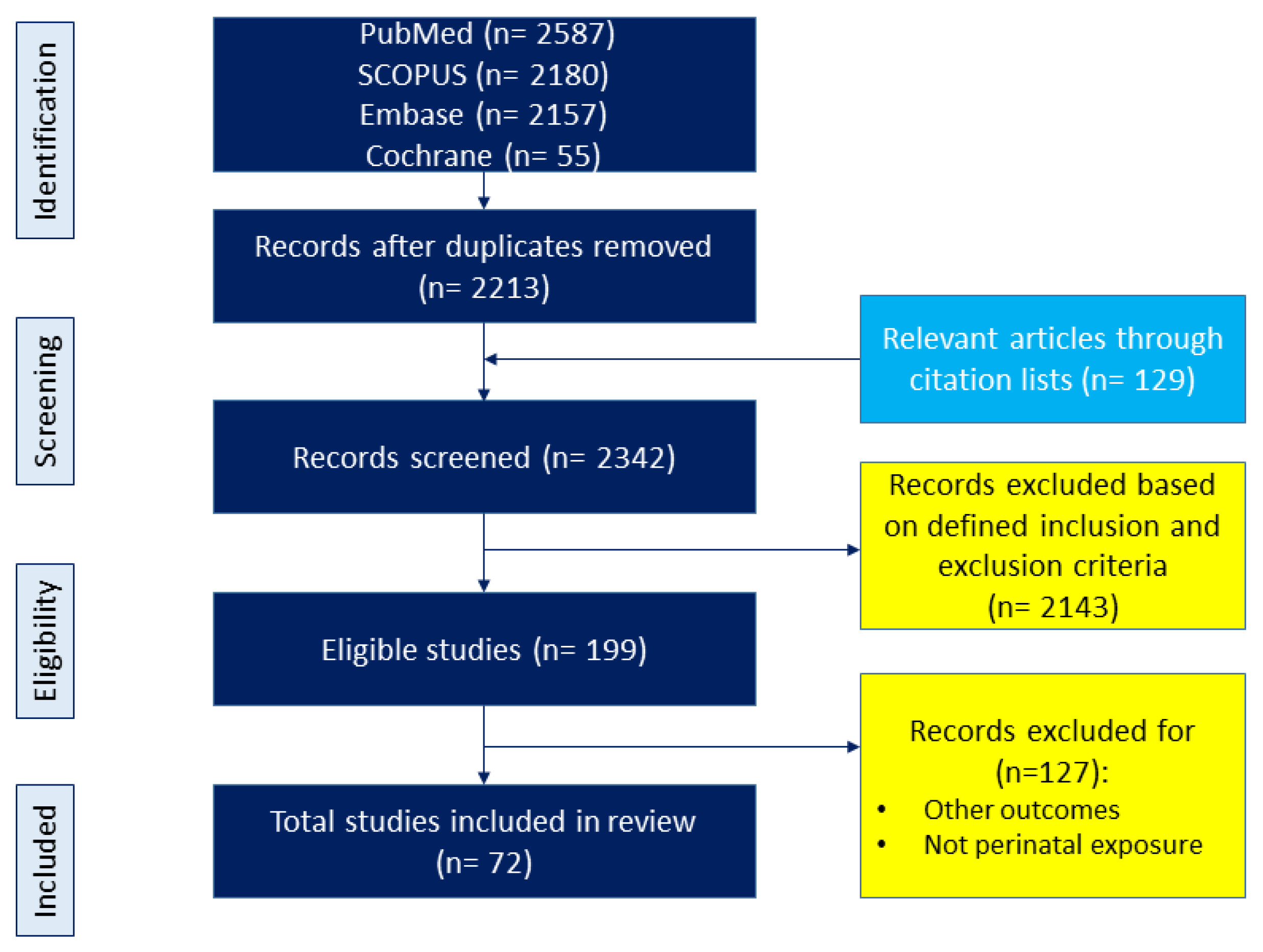
The topic was adhered to maternal diet and its relation to offspring outcomes, with a focus on CKM phenotypes. We adhered to the preferred reporting items for PRISMA Extension for Scoping Reviews (PRISMA-ScR) guidelines throughout our review process. The diet variable included nutritional content, food-based interventions, dietary pattern and quality, and other dietary-related variables; however, it did not include maternal nutritional status and non-food-based interventions. Offspring outcomes were considered as all components of CKM syndrome starting from birth. The search covered keywords and their combinations such as “developmental programming”, “DOHaD”, “offspring”, “pregnancy”, “gestation”, “lactation”, “mother”, “progeny”, “reprogramming”, “diet”, “nutrition”, “carbohydrate”, “amino acid”, “fat”, “fiber”, “micronutrient,” “protein,” “fatty acid”, “food”, “metabolic syndrome”, “hypertension”, “diabetes”, “chronic kidney disease”, “fatty liver”, “obesity”, “hyperlipidemia”, and “cardiovascular disease”. Further selections were conducted in the article identification process. The study selection process is illustrated in Figure 2.
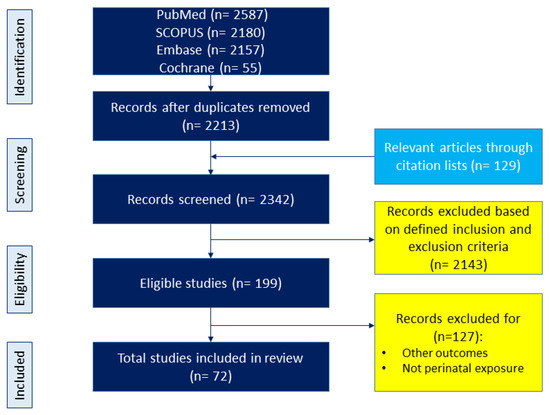
Figure 2.
Flowchart of the literature search and selection.
3.2. Article Identification
We conducted a search through scientific databases such as PubMed, SCOPUS, Embase, and the Cochrane Library. Our criteria encompassed studies published between January 2000 and April 2024, with full-text articles written in English. The entirety of our research, comprising clinical study, observational studies, clinical trials, and animal research, reached its conclusion. Inclusion criteria consisted of papers that focused on maternal dietary interventions and their impact on CKM syndrome in offspring. The exclusion criteria were, as follows: (1) papers addressing maternal nutritional status without a focus on specific dietary interventions; (2) studies involving non-food-based interventions; (3) research focusing on offspring outcomes not related to CKM syndrome; and (4) studies limited to fetal outcomes only. Editorials, letters, conference abstracts, and comments were omitted from consideration. Moreover, we scrutinized the reference lists to identify supplementary pertinent sources.
3.3. Data Extraction
A search using various keywords across different databases was conducted and yielded 6979 articles. Following the removal of duplicates, 2213 articles were initially screened for relevance to the topic. An additional 129 articles were obtained from linked research and reference lists. From these combined sources, a total of 2342 studies were screened for inclusion based on the predefined criteria. Through a secondary manual screening process, 72 articles were ultimately selected for inclusion in the present scoping review.
4. Conclusions
Currently, the significant public health impact of CKM syndrome and its associated disorders remains a major concern, largely because effective preventive interventions remain lacking [167]. Maternal dietary nutrition, along with various early-life environmental factors, plays a crucial role in determining the future risk of CKM syndrome. In recent years, DOHaD sciences have enhanced our understanding of how maternal diet and fetal programming contribute to the developmental origins of CKM syndrome, highlighting their potential as therapeutic targets for prevention. It is worth noting that dietary interventions are considered effective therapies for combating numerous human diseases and promoting health [1,2]. However, several unresolved questions remain regarding their application in the DOHaD field and clinical practice.
First, it is crucial to gather data on the long-term clinical outcomes related to CKM screening, staging, and therapeutic approaches in the pediatric population. Given that CKM syndrome was only defined in 2023, this information is urgently needed to develop effective strategies for the early identification and prevention of CKM syndrome. Second, while there is growing evidence of the beneficial effects of dietary therapies on human health, their specific impact during pregnancy needs further evaluation. These effects are likely to vary across different human populations and animal models. Third, despite advancements in the availability of various maternal dietary interventions, there has been insufficient exploration of their reprogramming effects on each element of CKM syndrome. Future animal studies should focus on improving study designs by using appropriate animal models, developing robust control measures, establishing standardized dosing protocols, and determining the optimal timing for each dietary intervention.
Understanding the distinct mechanisms by which specific nutrient components, such as micronutrients, macronutrients, and metabolites, influence the developmental programming of CKM syndrome is crucial. There is optimism that personalized maternal diets could potentially prevent CKM syndrome and optimize offspring health outcomes.
Author Contributions
Conceptualization, Writing—original draft, Y.-L.T. and C.-N.H.; data curation, Y.-L.T. and C.-N.H.; funding acquisition, Y.-L.T. and C.-N.H.; writing—review and editing, Y.-L.T. and C.-N.H. All authors have read and agreed to the published version of the manuscript.
Funding
Work reported herein is carried out with financial support from Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan, under grants CFRPG8K0011, CMRPG8M0381, CMRPG8N0171, CMRPG8M0721, CORPG8L0551 and CORPG8P0031.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest with regard to the contents of this manuscript.
References
- Ndumele, C.E.; RAngaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, Z.J.; Yu, X.; Wang, P. Dietary regulation in health and disease. Signal Transduct. Target Ther. 2022, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C. Nutrition in early life and the programming of adult disease: A review. J. Hum. Nutr. Diet. 2015, 28 (Suppl. 1), 1–14. [Google Scholar] [CrossRef]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Double-Edged Sword Effects of Maternal Nutrition in the Developmental Programming of Hypertension. Nutrients 2018, 10, 1917. [Google Scholar] [CrossRef]
- Armitage, J.A.; Khan, I.Y.; Taylor, P.D.; Nathanielsz, P.W.; Poston, L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: How strong is the evidence from experimental models in mammals? J. Physiol. 2004, 561, 355–377. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef]
- Barker, D.J.; Eriksson, J.G.; Forsen, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Paauw, N.D.; Van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2016, 219, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.P.; Collins, F.S. Precision nutrition-the answer to “what to eat to stay healthy”. JAMA 2020, 324, 735–736. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- de la O, V.; Zazpe, I.; Ruiz-Canela, M. Effect of branched-chain amino acid supplementation, dietary intake and circulating levels in cardiometabolic diseases: An updated review. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 35–50. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef]
- Beilin, L.J.; Rouse, I.L.; Armstrong, B.K.; Margetts, B.M.; Vandongen, R. Vegetarian diet and blood pressure levels: Incidental or causal association? Am. J. Clin. Nutr. 1988, 48, 806–810. [Google Scholar] [CrossRef]
- Theodoridis, X.; Chourdakis, M.; Chrysoula, L.; Chroni, V.; Tirodimos, I.; Dipla, K.; Gkaliagkousi, E.; Triantafyllou, A. Adherence to the DASH Diet and Risk of Hypertension: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3261. [Google Scholar] [CrossRef]
- Filippou, C.; Tatakis, F.; Polyzos, D.; Manta, E.; Thomopoulos, C.; Nihoyannopoulos, P.; Tousoulis, D.; Tsioufis, K. Overview of salt restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet for blood pressure reduction. Rev. Cardiovasc. Med. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, W.; Liang, J.; Dou, J.; Guo, F.; Zhang, D.; Xu, Z.; Wang, T. Probiotics: Functional food ingredients with the potential to reduce hypertension. Front. Cell Infect. Microbiol. 2023, 13, 1220877. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Naber, T.; Purohit, S. Chronic Kidney Disease: Role of Diet for a Reduction in the Severity of the Disease. Nutrients 2021, 13, 3277. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Lakatuam, J.D.; Ma, J.Z.; Louis, T.A. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am. J. Kidney Dis. 1998, 31, 954–961. [Google Scholar] [CrossRef]
- McMahon, E.J.; Bauer, J.D.; Hawley, C.M.; Isbel, N.M.; Stowasser, M.; Johnson, D.W.; Campbell, K.L. A randomized trial of dietary sodium restriction in CKD. J. Am. Soc. Nephrol. 2013, 24, 2096–2103. [Google Scholar]
- Chan, M.; Kelly, J.; Tapsell, L. Dietary Modeling of Foods for Advanced CKD Based on General Healthy Eating Guidelines: What Should Be on the Plate? Am. J. Kidney Dis. 2017, 69, 436–450. [Google Scholar] [CrossRef]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Mathew, T.C.; Al-Zaid, N.S. Efficacy of Low-Carbohydrate Ketogenic Diet in the Treatment of Type 2 Diabetes. Med. Princ. Pract. 2021, 30, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary patterns associated with obesity outcomes in adults: An umbrella review of systematic reviews. Public Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: A randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef]
- Putnam, K.; Shoemaker, R.; Yiannikouris, F.; Cassis, L.A. The renin-Angiotensin system: A target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1219–H1230. [Google Scholar] [CrossRef]
- Vinué, Á.; Herrero-Cervera, A.; González-Navarro, H. Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models. Nutrients 2018, 10, 939. [Google Scholar] [CrossRef]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef]
- Katsiki, N.; Stoian, A.P.; Rizzo, M. Dietary patterns in non-alcoholic fatty liver disease (NAFLD): Stay on the straight and narrow path! Clin. Investig. Arterioscler. 2022, 34, S24–S31. [Google Scholar]
- Wilkins, E.; Wickramasinghe, K.; Pullar, J.; Demaio, A.R.; Roberts, N.; Perez-Blanco, K.M.; Noonan, K.; Townsend, N. Maternal nutrition and its intergenerational links to non-communicable disease metabolic risk factors: A systematic review and narrative synthesis. J. Health Popul. Nutr. 2021, 40, 20. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; van der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef]
- Wirawan, F.; Yudhantari, D.G.A.; Gayatri, A. Pre-pregnancy Diet to Maternal and Child Health Outcome: A Scoping Review of Current Evidence. J. Prev. Med. Public Health 2023, 56, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Murrin, C.M.; Heinen, M.M.; Kelleher, C.C. Are Dietary Patterns of Mothers during Pregnancy Related to Children’s Weight Status? Evidence from the Lifeways Cross- Generational Cohort Study. AIMS Public Health 2015, 2, 274–296. [Google Scholar] [CrossRef]
- Fernández-Barrés, S.; Romaguera, D.; Valvi, D.; Martínez, D.; Vioque, J.; Navarrete-Muñoz, E.; Amiano, P.; Gonzalez-Palacios, S.; Guxens, M.; Pereda, E. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: The INMA birth cohort study. Pediatr. Obes. 2016, 11, 491–499. [Google Scholar] [CrossRef]
- Chen, L.W.; Aris, I.; Bernard, J.; Tint, M.T.; Chia, A.; Colega, M.; Gluckman, P.; Shek, L.; Saw, S.M.; Chong, Y.S. Associations of maternal dietary patterns during pregnancy with offspring adiposity from birth until 54 months of age. Nutrients 2017, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Haines, J.; Liu, G.; Zhang, C.; Wang, X.; Field, A.E.; Chavarro, J.E.; Sun, Q. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: Results from two prospective cohort studies of mother-child pairs in the United States. BMJ 2018, 362, k2486. [Google Scholar] [CrossRef]
- Strohmaier, S.; Bogl, L.H.; Eliassen, A.H.; Massa, J.; Field, A.E.; Chavarro, J.E.; Ding, M.; Tamimi, R.M.; Schernhammer, E. Maternal healthful dietary patterns during peripregnancy and long-term overweight risk in their offspring. Eur. J. Epidemiol. 2020, 35, 283–293. [Google Scholar] [CrossRef]
- Jackson, A.A.; Dunn, R.L.; Marchand, M.C.; Langley-Evans, S.C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. 2002, 103, 633–639. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal citrulline supplementation prevents prenatal NG-nitro-l-arginine-methyl ester (L-NAME)-induced programmed hypertension in rats. Biol. Reprod. 2015, 92, 7. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric. Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Perinatal Use of Citrulline Rescues Hypertension in Adult Male Offspring Born to Pregnant Uremic Rats. Int. J. Mol. Sci. 2024, 25, 1612. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; de Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal Supplementation with Citrulline Increases Renal Nitric Oxide in Young Spontaneously Hypertensive Rats and Has Long-Term Antihypertensive Effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef]
- Roysommuti, S.; Lerdweeraphon, W.; Malila, P.; Jirakulsomchok, D.; Wyss, J.M. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv. Exp. Med. Biol. 2009, 643, 145–156. [Google Scholar]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Protective Role of Taurine on Rat Offspring Hypertension in the Setting of Maternal Chronic Kidney Disease. Antioxidants 2023, 12, 2059. [Google Scholar] [CrossRef] [PubMed]
- Horie, R.; Yamori, Y.; Nara, Y.; Sawamura, M.; Mano, M. Effects of sulphur amino acids on the development of hypertension and atherosclerosis in stroke-prone spontaneously hypertensive rats. J. Hypertens. Suppl. 1987, 5, S223–S225. [Google Scholar]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar]
- Thaeomor, A.; Tangnoi, C.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents the Adverse Effects of Maternal Dyslipidemia on Growth and Cardiovascular Control in Adult Rat Offspring. Adv. Exp. Med. Biol. 2019, 1155, 415–427. [Google Scholar] [PubMed]
- Li, M.; Reynolds, C.M.; Gray, C.; Patel, R.; Sloboda, D.M.; Vickers, M.H. Long-term effects of a maternal high-fat: High-fructose diet on offspring growth and metabolism and impact of maternal taurine supplementation. J. Dev. Orig. Health Dis. 2020, 11, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mensegue, M.F.; Burgueño, A.L.; Tellechea, M.L. Perinatal taurine exerts a hypotensive effect in male spontaneously hypertensive rats and down-regulates endothelial oxide nitric synthase in the aortic arch. Clin. Exp. Pharmacol. Physiol. 2020, 47, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Arany, E.; Strutt, B.; Romanus, P.; Remacle, C.; Reusens, B.; Hill, D.J. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia 2004, 47, 1831–1837. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Tiao, M.M.; Tain, Y.L. Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kwon, Y.H. Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet. Lipids Health Dis. 2023, 22, 50. [Google Scholar] [CrossRef]
- Gray, C.; Vickers, M.H.; Segovia, S.A.; Zhang, X.D.; Reynolds, C.M. A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS ONE 2015, 10, e0115994. [Google Scholar]
- Gregório, B.M.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Maternal fish oil supplementation benefits programmed offspring from rat dams fed low-protein diet. Am. J. Obstet. Gynecol. 2008, 199, e1–e7. [Google Scholar] [CrossRef]
- Sánchez-Blanco, C.; Amusquivar, E.; Bispo, K.; Herrera, E. Dietary fish oil supplementation during early pregnancy in rats on a cafeteria-diet prevents fatty liver in adult male offspring. Food Chem. Toxicol. 2019, 123, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal Inflammation-Induced Offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.S.; Santos, K.M.; Lima, N.K.S.; Cabral, E.V.; Aires, R.S.; Veras, A.C.; Paixão, A.D.; Vieira, L.D. Maternal endotoxemia induces renal collagen deposition in adult offspring: Role of NADPH oxidase/TGF-β1/MMP-2 signaling pathway. Arch. Biochem. Biophys. 2020, 684, 108306. [Google Scholar] [CrossRef] [PubMed]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Palinski, W.; D’Armiento, F.P.; Witztum, J.L.; de Nigris, F.; Casanada, F.; Condorelli, M.; Silvestre, M.; Napoli, C. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ. Res. 2001, 89, 991–996. [Google Scholar] [CrossRef]
- Torrens, C.; Brawley, L.; Anthony, F.W.; Dance, C.S.; Dunn, R.; Jackson, A.A.; Poston, L.; Hanson, M.A. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension 2006, 47, 982–987. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Edwards, K.; Dave, B.; Jack-Roberts, C.; Yu, H.; Saxena, A.; Salvador, M.; Dembitzer, M.; Phagoora, J.; Jiang, X. Prenatal Choline Supplementation during High-Fat Feeding Improves Long-Term Blood Glucose Control in Male Mouse Offspring. Nutrients 2020, 12, 144. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Kadam, I.; Reaz, A.; Bretter, R.; Saxena, A.; Johnson, C.H.; Caviglia, J.M.; Jiang, X. Prenatal Choline Supplement in a Maternal Obesity Model Modulates Offspring Hepatic Lipidomes. Nutrients 2023, 15, 965. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, S.; Jia, Y.; Sun, B.; He, B.; Zhao, R. Maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation through epigenetic modification in adult offspring rats. J. Nutr. Biochem. 2018, 54, 105–112. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.S.L.; Braga, V.A.; Noronha, S.I.S.R.; Costa, W.K.A.D.; Makki, K.; Cruz, J.C.; Brandão, L.R.; Chianca Junior, D.A.; Meugnier, E.; Leulier, F.; et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020, 11, 8939–8950. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Chen, L.; Tang, L.; Wen, S.; Liu, Y.; Yuan, J. Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. 2018, 9, 4317–4327. [Google Scholar] [CrossRef] [PubMed]
- Paul, H.A.; Collins, K.H.; Nicolucci, A.C.; Urbanski, S.J.; Hart, D.A.; Vogel, H.J.; Reimer, R.A. Maternal prebiotic supplementation reduces fatty liver development in offspring through altered microbial and metabolomic profiles in rats. FASEB J. 2019, 33, 5153–5167. [Google Scholar] [CrossRef]
- Longo, M.; Alrais, M.; Tamayo, E.H.; Ferrari, F.; Facchinetti, F.; Refuerzo, J.S.; Blackwell, S.C.; Sibai, B.M. Vascular and metabolic profiles in offspring born to pregnant mice with metabolic syndrome treated with inositols. Am. J. Obstet. Gynecol. 2019, 220, 279.e1–279.e9. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ando, Y.; Nishida, A.; Yamano, M.; Suzuki, S.; Takada, H.; Kimura, I. Fructooligosaccharides Intake during Pregnancy Improves Metabolic Phenotype of Offspring in High Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2024, 68, e2300758. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Garlic Oil Supplementation Prevents High-Fat Diet-Induced Hypertension in Adult Rat Offspring: Implications of H2S-Generating Pathway in the Gut and Kidneys. Mol. Nutr. Food Res. 2021, 65, e2001116. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on gut microbial metabolite trimethylamine-N-Oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yu, H.R.; Chan, J.Y.H.; Lee, W.C.; Wu, K.L.H.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Acetate Supplementation Reverses Blood Pressure Increase in Male Offspring Induced by Exposure to Minocycline during Pregnancy and Lactation. Int. J. Mol. Sci. 2022, 23, 7924. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tzeng, H.T.; Lee, W.C.; Wu, K.L.H.; Yu, H.R.; Chan, J.Y.H.; Hsu, C.N. Reprogramming Effects of Postbiotic Butyrate and Propionate on Maternal High-Fructose Diet-Induced Offspring Hypertension. Nutrients 2023, 15, 1682. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.F.; Hsu, C.N. Perinatal Propionate Supplementation Protects Adult Male Offspring from Maternal Chronic Kidney Disease-Induced Hypertension. Nutrients 2022, 14, 3435. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Y.Y.; Li, S.P.; Zhao, H.M.; Jiang, F.J.; Wu, Y.X.; Tong, Y.; Pang, Q.F. Maternal propionate supplementation ameliorates glucose and lipid metabolic disturbance in hypoxia-induced fetal growth restriction. Food Funct. 2022, 13, 10724–10736. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yu, H.R.; Lin, I.C.; Tiao, M.M.; Huang, L.T.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Cao, M.; Chen, H.; Zhang, M.; Dong, X.; Ren, Z.; Sun, J.; Pan, L.L. Butyrate Ameliorates Antibiotic-Driven Type 1 Diabetes in the Female Offspring of Nonobese Diabetic Mice. J. Agric. Food Chem. 2020, 68, 3112–3120. [Google Scholar] [CrossRef]
- Toh, H.; Thomson, J.A.; Jiang, P. Maternal High-Fiber Diet Protects Offspring against Type 2 Diabetes. Nutrients 2020, 13, 94. [Google Scholar] [CrossRef]
- Herzl, E.; Schmitt, E.E.; Shearrer, G.; Keith, J.F. The Effects of a Western Diet vs. a High-Fiber Unprocessed Diet on Health Outcomes in Mice Offspring. Nutrients 2023, 15, 2858. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Brawerman, G.M.; Kereliuk, S.M.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal resveratrol administration protects against gestational diabetes-induced glucose intolerance and islet dysfunction in the rat offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Yu, H.R.; Tsai, C.C.; Huang, L.T.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lai, Y.J.; et al. Resveratrol intake during pregnancy and lactation re-programs adiposity and ameliorates leptin resistance in male progeny induced by maternal high-fat/high sucrose plus postnatal high-fat/high sucrose diets via fat metabolism regulation. Lipids Health Dis. 2020, 19, 174. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Oest, M.E.; Prater, M.R. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Resende, A.C.; Emiliano, A.F.; Cordeiro, V.S.; de Bem, G.F.; de Cavalho, L.C.; de Oliveira, P.R.; Neto, M.L.; Costa, C.A.; Boaventura, G.T.; de Moura, R.S. Grape skin extract protects against programmed changes in the adult rat offspring caused by maternal high-fat diet during lactation. J. Nutr. Biochem. 2013, 24, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.C.; Amaro, L.B.R.; Batista Jorge, A.H.; Lelis, S.F.; Lelis, D.F.; Guimarães, A.L.S.; Santos, S.H.S.; Andrade, J.M.O. Curcumin improves metabolic response and increases expression of thermogenesis-associated markers in adipose tissue of male offspring from obese dams. Mol. Cell Endocrinol. 2023, 563, 111840. [Google Scholar] [CrossRef]
- Kataoka, S.; Norikura, T.; Sato, S. Maternal green tea polyphenol intake during lactation attenuates kidney injury in high-fat-diet-fed male offspring programmed by maternal protein restriction in rats. J. Nutr. Biochem. 2018, 56, 99–108. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Pollock, C.A.; Saad, S. Nutrition and Developmental Origins of Kidney Disease. Nutrients 2023, 15, 4207. [Google Scholar] [CrossRef] [PubMed]
- Fabbiano, S.; Suárez-Zamorano, N.; Rigo, D.; Veyrat-Durebex, C.; Stevanovic Dokic, A.; Colin, D.J.; Trajkovski, M. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab. 2016, 24, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.T.; Treviño-Villarreal, J.H.; Mejia, P.; Grondin, Y.; Harputlugil, E.; Hine, C.; Vargas, D.; Zheng, H.; Ozaki, C.K.; Kristal, B.S.; et al. Protein and Calorie Restriction Contribute Additively to Protection from Renal Ischemia Reperfusion Injury Partly via Leptin Reduction in Male Mice. J. Nutr. 2015, 145, 1717–1727. [Google Scholar] [CrossRef]
- Sloan, C.; Tuinei, J.; Nemetz, K.; Frandsen, J.; Soto, J.; Wride, N.; Sempokuya, T.; Alegria, L.; Bugger, H.; Abel, E.D. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes 2011, 60, 1424–1434. [Google Scholar] [CrossRef]
- Vickers, M.H.; Reddy, S.; Ikenasio, B.A.; Breier, B.H. Dysregulation of the adipoinsular axis—A mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J. Endocrinol. 2001, 170, 323–332. [Google Scholar] [CrossRef][Green Version]
- Mossa, F.; Carter, F.; Walsh, S.W.; Kenny, D.A.; Smith, G.W.; Ireland, J.L.; Hildebrandt, T.B.; Lonergan, P.; Ireland, J.J.; Evans, A.C. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 2013, 88, 92. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.S.; Lang, A.L.; Grant, A.R.; Nijland, M.J. Maternal nutrient restriction in sheep: Hypertension and decreased nephron number in offspring at 9 months of age. J. Physiol. 2005, 565, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.M.; Barão, M.A.; Odo, L.N.; Nascimento Gomes, G.; do Carmo Franco, M.; Nigro, D.; Lucas, S.R.; Laurindo, F.R.; Brandizzi, L.I.; Zaladek Gil, F. L-Arginine effects on blood pressure and renal function of intrauterine restricted rats. Pediatr. Nephrol. 2002, 17, 856–862. [Google Scholar] [CrossRef]
- Carvalho, D.S.; Diniz, M.M.; Haidar, A.A.; Cavanal, M.F.; da Silva Alves, E.; Carpinelli, A.R.; Gil, F.Z.; Hirata, A.E. L-Arginine supplementation improves insulin sensitivity and beta cell function in the offspring of diabetic rats through AKT and PDX-1 activation. Eur. J. Pharmacol. 2016, 791, 780–787. [Google Scholar] [CrossRef]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- Boucknooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Physician 2009, 80, 345–350. [Google Scholar]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee on Nutrition. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed]
- Vahdaninia, M.; Mackenzie, H.; Dean, T.; Helps, S. The effectiveness of ω-3 polyunsaturated fatty acid interventions during pregnancy on obesity measures in the offspring: An up-to-date systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 2597–2613. [Google Scholar] [CrossRef]
- Zhang, F.F.; Barr, S.I.; McNulty, H.; Li, D.; Blumberg, J.B. Health effects of vitamin and mineral supplements. BMJ 2020, 369, 2511. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Nexo, E. Gastrointestinal Handling of Water-Soluble Vitamins. Compr. Physiol. 2018, 8, 1291–1311. [Google Scholar] [PubMed]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Napoli, C. The fetal origins of atherosclerosis: Maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J. 2002, 16, 1348–1360. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Viswanathan, M.; Treiman, K.A.; Kish-Doto, J.; Middleton, J.C.; Coker-Schwimmer, E.J.; Nicholson, W.K. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 317, 190–203. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Maternal methyl donor supplementation: A potential therapy for metabolic disorder in offspring. J. Nutr. Biochem. 2024, 124, 109533. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Raheli´c, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Lange, B.; Frick, J.S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, S.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. In Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, ON, Canada, 2002. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Amp. Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecien, I.; Wlodek, L. Biological properties of garlic and garlic-derived Organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary Phenolic Compounds: Biochemistry, Metabolism and Significance in Animal and Human Health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Novel Insights on Dietary Polyphenols for Prevention in Early-Life Origins of Hypertension: A Review Focusing on Preclinical Animal Models. Int. J. Mol. Sci. 2022, 23, 6620. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Staneviciene, I.; Mongirdiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y.H. PPARs Link Early Life Nutritional Insults to Later Programmed Hypertension and Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, Á.; Deutsch, M.J.; Plösch, T.; Ensenauer, R. The peroxisome proliferator-activated receptors under epigenetic control in placental metabolism and fetal development. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E797–E810. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Kasper, P.; Breuer, S.; Hoffmann, T.; Vohlen, C.; Janoschek, R.; Schmitz, L.; Appel, S.; Fink, G.; Hünseler, C.; Quaas, A.; et al. Maternal Exercise Mediates Hepatic Metabolic Programming via Activation of AMPK-PGC1α Axis in the Offspring of Obese Mothers. Cells 2021, 10, 1247. [Google Scholar] [CrossRef]
- Azhar, S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010, 6, 657–691. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Ruan, X.; Zheng, F.; Guan, Y. PPARs and the kidney in metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2008, 294, F1032–F1047. [Google Scholar] [CrossRef] [PubMed]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016, 38, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Mautone, N.; Zwergel, C.; Mai, A.; Rotili, D. Sirtuin modulators: Where are we now? A review of patents from 2015 to 2019. Expert Opin. Ther. Pat. 2020, 30, 389–407. [Google Scholar] [CrossRef]
- Linnér, A.; Almgren, M. Epigenetic programming-The important first 1000 days. Acta Paediatr. 2020, 109, 443–452. [Google Scholar] [CrossRef]
- Sugden, M.C.; Caton, P.W.; Holness, M.J. PPAR control: It’s SIRTainly as easy as PGC. J. Endocrinol. 2010, 204, 93–104. [Google Scholar] [CrossRef]
- Feng, R.; Ullah, M.; Chen, K.; Ali, Q.; Lin, Y.; Sun, Z. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J. Extracell. Vesicles 2020, 9, 1783869. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, H.; Liu, Y.; Yang, Z.; Yao, H.; Liu, T.; Gou, T.; Wang, L.; Zhang, J.; Tian, Y.; et al. Novel role of the SIRT1 in endocrine and metabolic diseases. Int. J. Biol. Sci. 2023, 19, 484–501. [Google Scholar] [CrossRef]
- Wiciński, M.; Erdmann, J.; Nowacka, A.; Kuźmiński, O.; Michalak, K.; Janowski, K.; Ohla, J.; Biernaciak, A.; Szambelan, M.; Zabrzyński, J. Natural Phytochemicals as SIRT Activators-Focus on Potential Biochemical Mechanisms. Nutrients 2023, 15, 3578. [Google Scholar] [CrossRef]
- Avila, J.G.; Echeverri, I.; de Plata, C.A.; Castillo, A. Impact of oxidative stress during pregnancy on fetal epigenetic patterns and early origin of vascular diseases. Nutr. Rev. 2015, 73, 12–21. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Interplay between maternal nutrition and epigenetic programming on offspring hypertension. J. Nutr. Biochem. 2024, 127, 109604. [Google Scholar] [CrossRef] [PubMed]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Gallagher, K.; Beck, C.; Kumar, R.; Gernand, A.D. Maternal-Fetal Inflammation in the Placenta and the Developmental Origins of Health and Disease. Front. Immunol. 2020, 11, 531543. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, N.B.; Intapad, S.; Alexander, B.T. Sex differences in the developmental programming of hypertension. Acta Physiol. 2014, 210, 307–316. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).