A Multi-Epitope Protein for High-Performance Serodiagnosis of Chronic Chagas Disease in ELISA and Lateral Flow Platforms
Abstract
1. Introduction
2. Results
2.1. Design and Production of DxCruziV3
2.2. Performance of DxCruziV3 in ELISAs
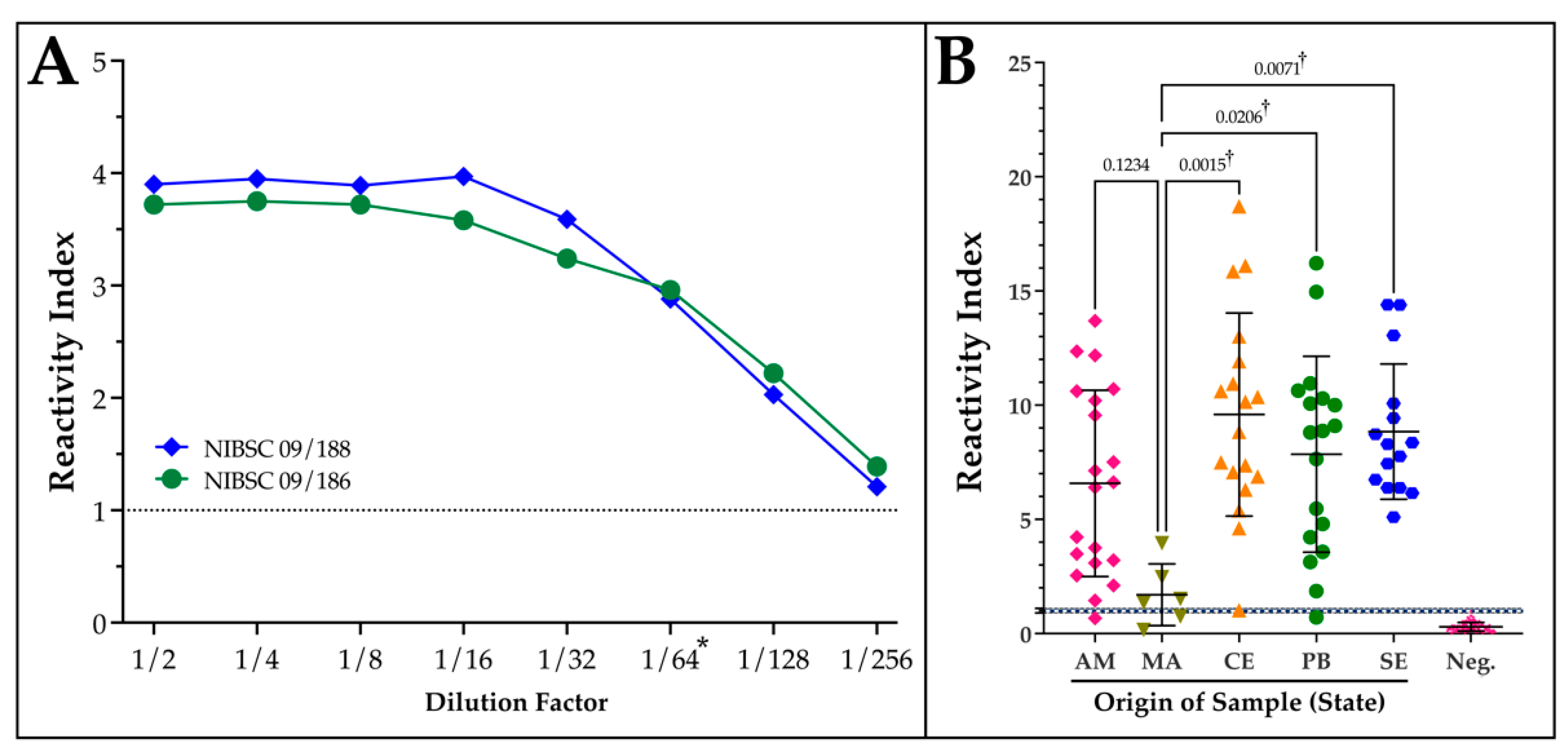
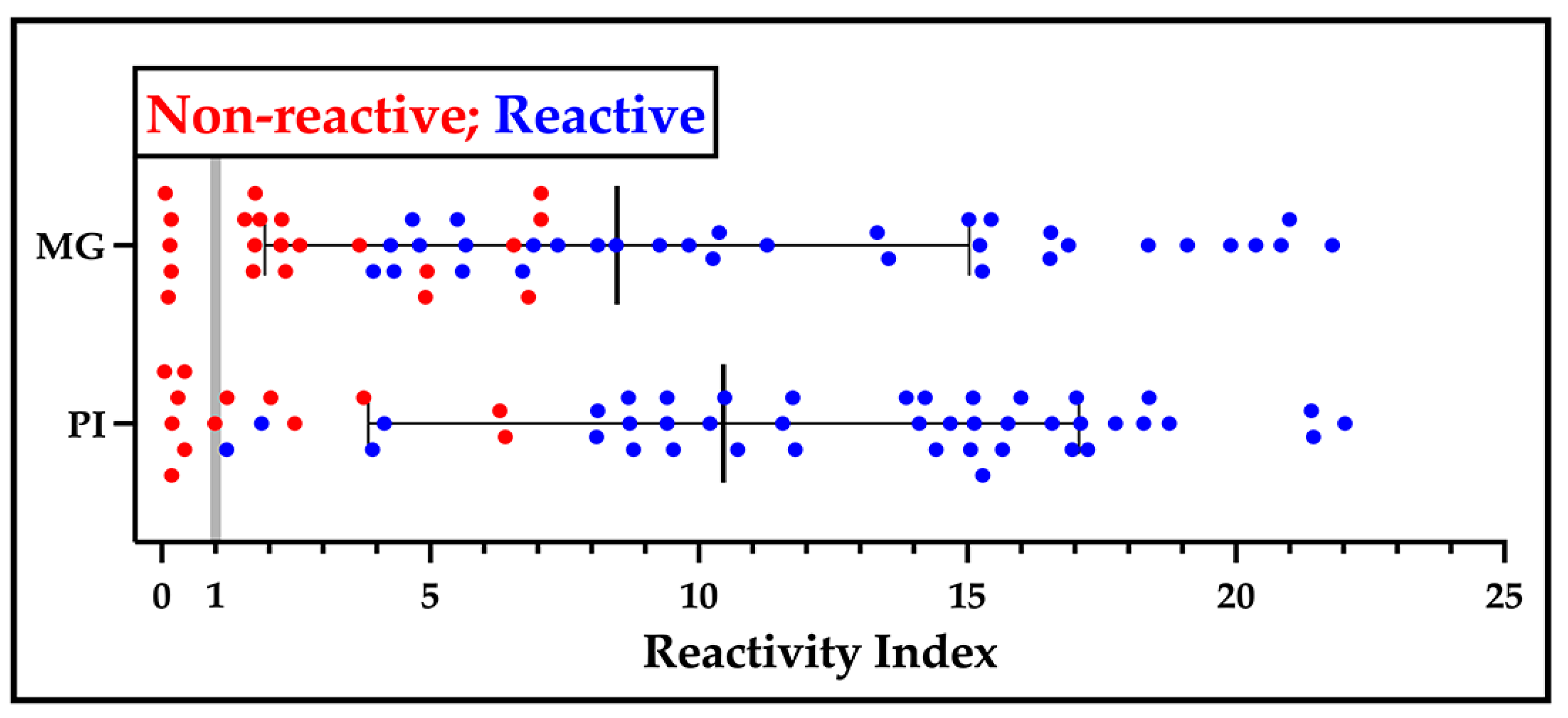
2.3. Analytical and Geographical Sensitivity of DxCruziV3
2.4. Lateral Flow Immunochromatographic Prototypes
2.5. Performance of the LFA Prototypes
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Serological Panels and Participant Profile
4.3. Cloning, Expression, and Purification of DxCruziV3
4.4. Enzyme-Linked Immunosorbent Assays
4.5. Lateral Flow Immunochromatographic Assay Preparation
4.6. Indirect Immunofluorescence
4.7. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Rincón, G.J.; Gallo-Bernal, S.; Jiménez, P.A.; Cruz-Saavedra, L.; Ramírez, J.D.; Rodríguez, M.J.; Medina-Mur, R.; Díaz-Nassif, G.; Valderrama-Achury, M.D.; Medina, H.M. Molecular and Clinical Aspects of Chronic Manifestations in Chagas Disease: A State-of-the-Art Review. Pathogens 2021, 10, 1493. [Google Scholar] [CrossRef]
- Massad, E. The Elimination of Chagas’ Disease from Brazil. Epidemiol. Infect. 2008, 136, 1153–1164. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Silva, T.P. Transmission Elimination of Chagas’ Disease by Triatoma Infestans in Brazil: An Historical Fact; Acadêmicos de Medicina da Universidade Federal do Ceará: Fortaleza, Brazil, 2006. [Google Scholar]
- Coura, J.R. The Main Sceneries of Chagas Disease Transmission. The Vectors, Blood and Oral Transmissions--a Comprehensive Review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef]
- de Souza, A.C.; Coura, J.R.; Lopes, C.M.; Junqueira, A.C.V. Eratyrus Mucronatus Stål, 1859 and Panstrongylus Rufotuberculatus (Champion, 1899) (Hemiptera, Reduviidae, Triatominae): First Records in a Riverside Community of Rio Negro, Amazonas State, Brazil. Check List 2021, 17, 905–909. [Google Scholar] [CrossRef]
- Coura, J.R.; Viñas, P.A.; Junqueira, A.C. Ecoepidemiology, Short History and Control of Chagas Disease in the Endemic Countries and the New Challenge for Non-Endemic Countries. Mem. Inst. Oswaldo Cruz 2014, 109, 856–862. [Google Scholar] [CrossRef]
- Coura, J.R.; Vinas, P.A. Chagas Disease: A New Worldwide Challenge. Nature 2010, 465, S6–S7. [Google Scholar] [CrossRef]
- Coura, J.R.; Dias, J.C. Epidemiology, Control and Surveillance of Chagas Disease: 100 Years after Its Discovery. Mem. Inst. Oswaldo Cruz 2009, 104 (Suppl. S1), 31–40. [Google Scholar] [CrossRef]
- WHO. Chagas Disease (American Trypanosomiasis); WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Rassi, A.; Marcondes de Rezende, J. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Dias, J.C.; Ramos, A.N.; Gontijo, E.D.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.; Almeida, E.A.; Oliveira, W.; et al. Brazilian Consensus on Chagas Disease, 2015. Epidemiol. Serv. Saude 2016, 25, 7–86. [Google Scholar] [CrossRef]
- Benziger, C.P.; do Carmo, G.A.L.; Ribeiro, A.L.P. Chagas Cardiomyopathy: Clinical Presentation and Management in the Americas. Cardiol. Clin. 2017, 35, 31–47. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverría, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef] [PubMed]
- Sabino, E.C.; Nunes, M.C.P.; Blum, J.; Molina, I.; Ribeiro, A.L.P. Cardiac Involvement in Chagas Disease and African Trypanosomiasis. Nat. Rev. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, N.R.; de Oliveira-da Silva, L.C.; Gonçalves, A.C.O.; Quintino, N.D.; Ferreira, A.M.; Bierrenbach, A.L.; Padilha da Silva, J.L.; Pereira Nunes, M.C.; Ribeiro, A.L.P.; Oliveira, C.D.L.; et al. Gastrointestinal Manifestations of Chagas Disease: A Systematic Review with Meta-Analysis. Am. J. Trop. Med. Hyg. 2024, 110, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Dantas, R.O. Management of Esophageal Dysphagia in Chagas Disease. Dysphagia 2021, 36, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, C.L.; Albajar-Viñas, P.; Wilkins, P.P.; Nieto, J.; Leiby, D.A.; Paris, L.; Scollo, K.; Flórez, C.; Guzmán-Bracho, C.; Luquetti, A.O.; et al. Comparative Evaluation of 11 Commercialized Rapid Diagnostic Tests for Detecting Trypanosoma cruzi Antibodies in Serum Banks in Areas of Endemicity and Nonendemicity. J. Clin. Microbiol. 2014, 52, 2506–2512. [Google Scholar] [CrossRef]
- Sáez-Alquezar, A.; Junqueira, A.C.V.; Durans, A.M.; Guimarães, A.V.; Corrêa, J.A.; Borges-Pereira, J.; Zauza, P.L.; Cabello, P.H.; Albajar-Vinãs, P.; Provance, D.W., Jr.; et al. Geographical Origin of Chronic Chagas Disease Patients in Brazil Impacts the Performance of Commercial Tests for Anti-T. Cruzi IgG. Mem. Inst. Oswaldo Cruz 2021, 116, e210032. [Google Scholar] [CrossRef]
- Durans, A.M.; Napoleão-Pêgo, P.; Reis, F.C.G.; Dias, E.R.; Machado, L.E.S.F.; Lechuga, G.C.; Junqueira, A.C.V.; De-Simone, S.G.; Provance, D.W., Jr. Chagas Disease Diagnosis with Trypanosoma cruzi Exclusive Epitopes in GFP. Vaccines 2024, 12, 1029. [Google Scholar] [CrossRef]
- Ministry of Health PORTARIA No 57. 2018. Available online: https://www.gov.br/conitec/pt-br/midias/protocolos/pcdt_doenca_de_chagas.pdf/view (accessed on 1 June 2024).
- Edwards, M.S.; Montgomery, S.P. Congenital Chagas Disease: Progress toward Implementation of Pregnancy-Based Screening. Curr. Opin. Infect. Dis. 2021, 34, 538–545. [Google Scholar] [CrossRef]
- Close, D.W.; Paul, C.D.; Langan, P.S.; Wilce, M.C.; Traore, D.A.; Halfmann, R.; Rocha, R.C.; Waldo, G.S.; Payne, R.J.; Rucker, J.B.; et al. Thermal Green Protein, an Extremely Stable, Nonaggregating Fluorescent Protein Created by Structure-Guided Surface Engineering. Proteins 2015, 83, 1225–1237. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Brooks, J.; Yeo, M.; Carrasco, H.J.; Lewis, M.D.; Llewellyn, M.S.; Miles, M.A. Analysis of Molecular Diversity of the Trypanosoma cruzi Trypomastigote Small Surface Antigen Reveals Novel Epitopes, Evidence of Positive Selection and Potential Implications for Lineage-Specific Serology. Int. J. Parasitol. 2010, 40, 921–928. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER Server for Protein 3D Structure Prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A Unified Platform for Automated Protein Structure and Function Prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Longobardo, M.V.; Carmelo, E.; Marañón, C.; Planelles, L.; Patarroyo, M.E.; Alonso, C.; López, M.C. Mapping of the Antigenic Determinants of the T. Cruzi Kinetoplastid Membrane Protein-11. Identification of a Linear Epitope Specifically Recognized by Human Chagasic Sera. Clin. Exp. Immunol. 2001, 123, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, C.A.; Alfonso, J.; Campetella, O.; Frasch, A.C. Tandem Amino Acid Repeats from Trypanosoma cruzi Shed Antigens Increase the Half-Life of Proteins in Blood. Blood 1999, 93, 2025–2032. [Google Scholar] [CrossRef]
- Ibañez, C.F.; Affranchino, J.L.; Macina, R.A.; Reyes, M.B.; Leguizamon, S.; Camargo, M.E.; Aslund, L.; Pettersson, U.; Frasch, A.C. Multiple Trypanosoma cruzi Antigens Containing Tandemly Repeated Amino Acid Sequence Motifs. Mol. Biochem. Parasitol. 1988, 30, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Peralta, J.M.; Teixeira, M.G.; Shreffler, W.G.; Pereira, J.B.; Burns, J.M.; Sleath, P.R.; Reed, S.G. Serodiagnosis of Chagas’ Disease by Enzyme-Linked Immunosorbent Assay Using Two Synthetic Peptides as Antigens. J. Clin. Microbiol. 1994, 32, 971–974. [Google Scholar] [CrossRef]
- Thomas, M.C.; Fernández-Villegas, A.; Carrilero, B.; Marañón, C.; Saura, D.; Noya, O.; Segovia, M.; Alarcón de Noya, B.; Alonso, C.; López, M.C. Characterization of an Immunodominant Antigenic Epitope from Trypanosoma cruzi as a Biomarker of Chronic Chagas’ Disease Pathology. Clin. Vaccine Immunol. 2012, 19, 167–173. [Google Scholar] [CrossRef]
- Burns, J.M.; Shreffler, W.G.; Rosman, D.E.; Sleath, P.R.; March, C.J.; Reed, S.G. Identification and Synthesis of a Major Conserved Antigenic Epitope of Trypanosoma cruzi. Proc. Natl. Acad. Sci. USA 1992, 89, 1239–1243. [Google Scholar] [CrossRef]
- Houghton, R.L.; Benson, D.R.; Reynolds, L.D.; McNeill, P.D.; Sleath, P.R.; Lodes, M.J.; Skeiky, Y.A.; Leiby, D.A.; Badaro, R.; Reed, S.G. A Multi-Epitope Synthetic Peptide and Recombinant Protein for the Detection of Antibodies to Trypanosoma cruzi in Radioimmunoprecipitation-Confirmed and Consensus-Positive Sera. J. Infect. Dis. 1999, 179, 1226–1234. [Google Scholar] [CrossRef]
- Di Noia, J.M.; Buscaglia, C.A.; De Marchi, C.R.; Almeida, I.C.; Frasch, A.C. A Trypanosoma cruzi Small Surface Molecule Provides the First Immunological Evidence That Chagas’ Disease Is Due to a Single Parasite Lineage. J. Exp. Med. 2002, 195, 401–413. [Google Scholar] [CrossRef]
- Gruber, A.; Zingales, B. Trypanosoma cruzi: Characterization of Two Recombinant Antigens with Potential Application in the Diagnosis of Chagas’ Disease. Exp. Parasitol. 1993, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Gomes, L.P.; Coura, J.B.; Provance, D.W.J.; De-Simone, S.G. Chagas Disease-Specific Antigens: Characterization of Epitopes in CRA/FRA by Synthetic Peptide Mapping and Evaluation by ELISA-Peptide Assay. BMC Infect. Dis. 2013, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W.; Moffatt, B.A. Use of Bacteriophage T7 RNA Polymerase to Direct Selective High-Level Expression of Cloned Genes. J. Mol. Biol. 1986, 189, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C. Nova Tripanozomiaze Humana. Mem. Inst. Oswaldo Cruz 1909, 1, 159–219. [Google Scholar] [CrossRef]
- Aufderheide, A.C.; Salo, W.; Madden, M.; Streitz, J.; Buikstra, J.; Guhl, F.; Arriaza, B.; Renier, C.; Wittmers, L.E.; Fornaciari, G.; et al. A 9,000-Year Record of Chagas’ Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2034–2039. [Google Scholar] [CrossRef]
- Guhl, F.; Jaramillo, C.; Yockteng, R.; Vallejo, G.A.; Cárdenas-Arroyo, F. Trypanosoma cruzi DNA in Human Mummies. Lancet 1997, 349, 1370. [Google Scholar] [CrossRef]
- Coura, J.R. The Discovery of Chagas Disease (1908–1909): Great Successes and Certain Misunderstandings and Challenges. Rev. Soc. Bras. Med. Trop. 2013, 46, 389–390. [Google Scholar] [CrossRef]
- Carmona-Castro, O.; Moo-Llanes, D.A.; Ramsey, J.M. Impact of Climate Change on Vector Transmission of Trypanosoma cruzi (Chagas, 1909) in North America. Med. Vet. Entomol. 2018, 32, 84–101. [Google Scholar] [CrossRef]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Porrás, A.I.; Yadon, Z.E.; Altcheh, J.; Britto, C.; Chaves, G.C.; Flevaud, L.; Martins-Filho, O.A.; Ribeiro, I.; Schijman, A.G.; Shikanai-Yasuda, M.A.; et al. Target Product Profile (TPP) for Chagas Disease Point-of-Care Diagnosis and Assessment of Response to Treatment. PLoS Negl. Trop. Dis. 2015, 9, e0003697. [Google Scholar] [CrossRef]
- Caballero, Z.C.; Sousa, O.E.; Marques, W.P.; Saez-Alquezar, A.; Umezawa, E.S. Evaluation of Serological Tests to Identify Trypanosoma cruzi Infection in Humans and Determine Cross-Reactivity with Trypanosoma rangeli and Leishmania spp. Clin. Vaccine Immunol. 2007, 14, 1045–1049. [Google Scholar] [CrossRef]
- Daltro, R.T.; Leony, L.M.; Freitas, N.E.M.; Silva, Â.A.O.; Santos, E.F.; Del-Rei, R.P.; Brito, M.E.F.; Brandão-Filho, S.P.; Gomes, Y.M.; Silva, M.S.; et al. Cross-Reactivity Using Chimeric Trypanosoma cruzi Antigens: Diagnostic Performance in Settings Where Chagas Disease and American Cutaneous or Visceral Leishmaniasis Are Coendemic. J. Clin. Microbiol. 2019, 57, e00762-19. [Google Scholar] [CrossRef] [PubMed]
- Otani, M.; Hockley, J.; Bracho, C.G.; Rijpkema, S.; Luguetti, A.O.; Duncan, R.; Rigsby, P.; Albajar-viñas, P.; Padilla, A. Evaluation of Two International Reference Standards for Antibodies to Trypanosoma cruzi in a WHO Collaborative Study; WHO Technical Report; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Saez-Alquezar, A.; Junqueira, A.C.; Durans, A.M.; Guimarães, A.V.; Corrêa, J.A.; Provance, D.W., Jr.; Cabello, P.H.; Coura, J.R.; Viñas, P.A. Application of WHO International Biological Standards to Evaluate Commercial Serological Tests for Chronic Chagas Disease. Mem. Inst. Oswaldo Cruz 2020, 115, e200214. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.D.; Bracco, L.; Salas-Sarduy, E.; Ramsey, J.M.; Nolan, M.S.; Lynn, M.K.; Altcheh, J.; Ballering, G.E.; Torrico, F.; Kesper, N.; et al. The Trypanosoma cruzi Antigen and Epitope Atlas: Antibody Specificities in Chagas Disease Patients across the Americas. Nat. Commun. 2023, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; Junqueira, A.C.; Ferreira, J.M.B. Surveillance of Seroepidemiology and Morbidity of Chagas Disease in the Negro River, Brazilian Amazon. Mem. Inst. Oswaldo Cruz 2018, 113, 17–23. [Google Scholar] [CrossRef]
- William Provance, D.; da Matta Durans, A.; Lechuga, G.C.; da Rocha Dias, E.; Morel, C.M.; De Simone, S.G. Surpassing the Natural Limits of Serological Diagnostic Tests. hLife, 2024; in press. [Google Scholar] [CrossRef]
- Ramos, L.G.; de Souza, K.R.; Júnior, P.A.S.; Câmara, C.C.; Castelo-Branco, F.S.; Boechat, N.; Carvalho, S.A. Tackling the Challenges of Human Chagas Disease: A Comprehensive Review of Treatment Strategies in the Chronic Phase and Emerging Therapeutic Approaches. Acta Trop. 2024, 256, 107264. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; López, M.C.; Esteva, M.; Zrein, M.; Casellas, A.; Gómez, I.; Granjon, E.; Méndez, S.; Benítez, C.; Ruiz, A.M.; et al. Serological Reactivity against T. Cruzi-Derived Antigens: Evaluation of Their Suitability for the Assessment of Response to Treatment in Chronic Chagas Disease. Acta Trop. 2021, 221, 105990. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; Abril, M.; Alarcón de Noya, B.; Almeida, I.C.; Angheben, A.; Araujo Jorge, T.; Chatelain, E.; Esteva, M.; Gascón, J.; Grijalva, M.J.; et al. Target Product Profile for a Test for the Early Assessment of Treatment Efficacy in Chagas Disease Patients: An Expert Consensus. PLoS Negl. Trop. Dis. 2020, 14, e0008035. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Biological Standardization; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2011; pp. 1–244. [Google Scholar]
- Coura, J.R.; Junqueira, A.C. Ecological Diversity of Trypanosoma cruzi Transmission in the Amazon Basin. The Main Scenaries in the Brazilian Amazon. Acta Trop. 2015, 151, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Saravia, S.A.M.; Montero, I.F.; Linhares, B.M.; Santos, R.A.; Marcia, J.A.F. Mineralogical Composition and Bioactive Molecules in the Pulp and Seed of Patauá (Oenocarpus Bataua Mart.): A Palm from the Amazon. Int. J. Plant Soil. Sci. 2020, 31, 1–7. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
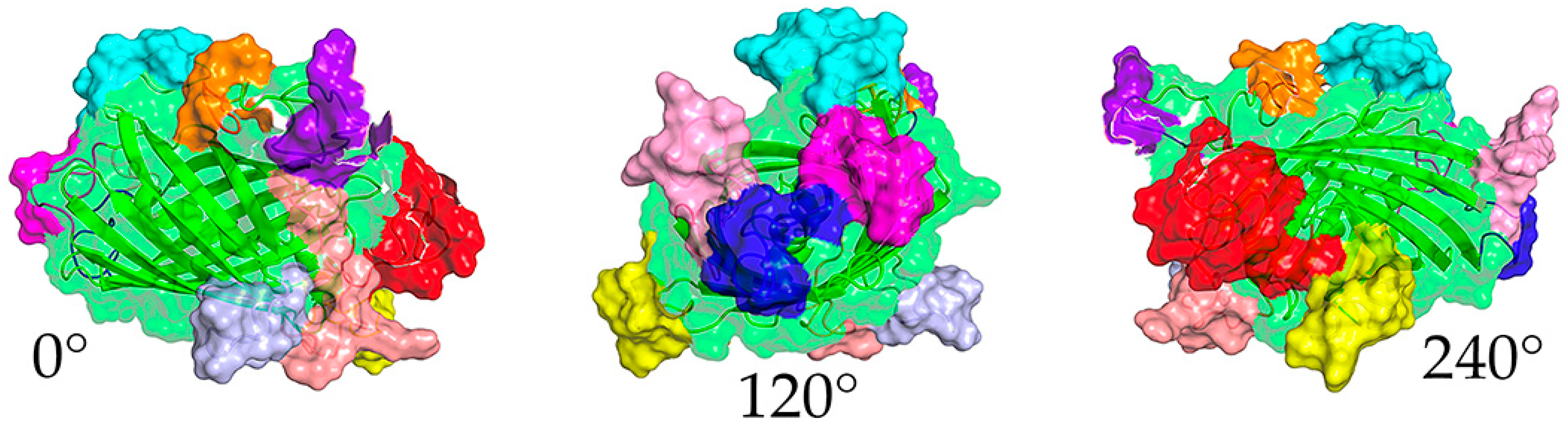

| Epitope | Sequence | Insertion Site 1 | Color 2 | Protein Origin | Reference |
|---|---|---|---|---|---|
| 1 | KFAELLEQQKNAQFPGK | N-term | Red | KMP11 | [26] |
| 2 | DSSAHSTPSTPA | 50 | Blue | SAPA | [27] |
| 3 | GDKPSPFGQAAAADK | 114 | Yellow | PEP-2 | [28,29] |
| 4 | FGQAAAGDKPS | 127 | Magenta | TcCA-2 | [30] |
| 5 | AEPKPAEPKS | 153 | Lt. Blue | TcD-2 | [31,32] |
| 6 | TSSTPPSGTENKPAT | 167 | Cyan | TSSA | [28,33] |
| 7 | GTSEEGSRGGSSMPS | 183 | Salmon | TcLo1.2 | [32] |
| 8 | SPFGQAAAGDK | 244 | Pink | B13 | [29,34] |
| 9 | KAAIAPA | C-term | Purple | TcE | [32] |
| 10 | KQRAAETK | C-term | Orange | CRA | [35] |
| Assay | Repetitions 1 | Volume | Reactive | Non-Reactive |
|---|---|---|---|---|
| V3ib-LFA Field 2 | 1 | 10 µL | 43 | 124 |
| sV3-LFA Field 2 | 1 | 10 µL | 43 | 124 |
| V3ib-LFA Lab 3 | 0–1 | 5 µL | 45 | 25 |
| sV3-LFA Lab (Lot#1) 3 | 0–1 | 5 µL | 44 | 123 |
| sV3-LFA Lab (Lot #2) 4 | 1 | 5 µL | 36 | 131 |
| V3ib ELISA (in-house) | 3–8 | 0.5–2 µL | 39 | 128 |
| ELISA Wiener | 2–3 | 10 µL | 32 | 135 |
| ELISA Bioclin | 2 | 10 µL | 61 | 106 |
| Immunofluorescence Indirect | 1–2 | 5 µL | 48 | 119 |
| Assay | Non-Divergent (n = 150) | Final Call (n = 167) | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Field V3ib LFA | 100% | 100% | 95.2% | 97.6% |
| Field sV3 LFA | 100% | 100% | 90.5% | 96.0% |
| V3ib-ELISA | 87.5% | 98.3% | 87.5% | 97.6% |
| ELISA Wiener | 71.9% | 97.5% | 64.3% | 96.0% |
| ELISA Bioclin | 90.6% | 79.7% | 83.3% | 79.2% |
| Immunofluorescence | 93.8% | 88.1% | 76.2% | 87.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, E.R.; Durans, A.M.; Succar, B.B.; Pinto, L.A.L.T.; Lechuga, G.C.; Miguez, M.G.; Figueira-Mansur, J.; Argondizzo, A.P.C.; Bernardo, A.R.; Diniz, R.L.; et al. A Multi-Epitope Protein for High-Performance Serodiagnosis of Chronic Chagas Disease in ELISA and Lateral Flow Platforms. Int. J. Mol. Sci. 2024, 25, 9811. https://doi.org/10.3390/ijms25189811
Dias ER, Durans AM, Succar BB, Pinto LALT, Lechuga GC, Miguez MG, Figueira-Mansur J, Argondizzo APC, Bernardo AR, Diniz RL, et al. A Multi-Epitope Protein for High-Performance Serodiagnosis of Chronic Chagas Disease in ELISA and Lateral Flow Platforms. International Journal of Molecular Sciences. 2024; 25(18):9811. https://doi.org/10.3390/ijms25189811
Chicago/Turabian StyleDias, Evandro R., Andressa M. Durans, Barbara B. Succar, Luiz André L. T. Pinto, Guilherme C. Lechuga, Mariana G. Miguez, Janaina Figueira-Mansur, Ana P. C. Argondizzo, Aline R. Bernardo, Rafaela L. Diniz, and et al. 2024. "A Multi-Epitope Protein for High-Performance Serodiagnosis of Chronic Chagas Disease in ELISA and Lateral Flow Platforms" International Journal of Molecular Sciences 25, no. 18: 9811. https://doi.org/10.3390/ijms25189811
APA StyleDias, E. R., Durans, A. M., Succar, B. B., Pinto, L. A. L. T., Lechuga, G. C., Miguez, M. G., Figueira-Mansur, J., Argondizzo, A. P. C., Bernardo, A. R., Diniz, R. L., Esteves, G. S., Silva, E. D., Morel, C. M., Borges-Pereira, J., De-Simone, S. G., Junqueira, A. C. V., & Provance, D. W., Jr. (2024). A Multi-Epitope Protein for High-Performance Serodiagnosis of Chronic Chagas Disease in ELISA and Lateral Flow Platforms. International Journal of Molecular Sciences, 25(18), 9811. https://doi.org/10.3390/ijms25189811








