Antibody Responses in SARS-CoV-2-Exposed and/or Vaccinated Individuals Target Conserved Epitopes from Multiple CoV-2 Antigens
Abstract
:1. Introduction
2. Results
2.1. Plasma Donor Demographics
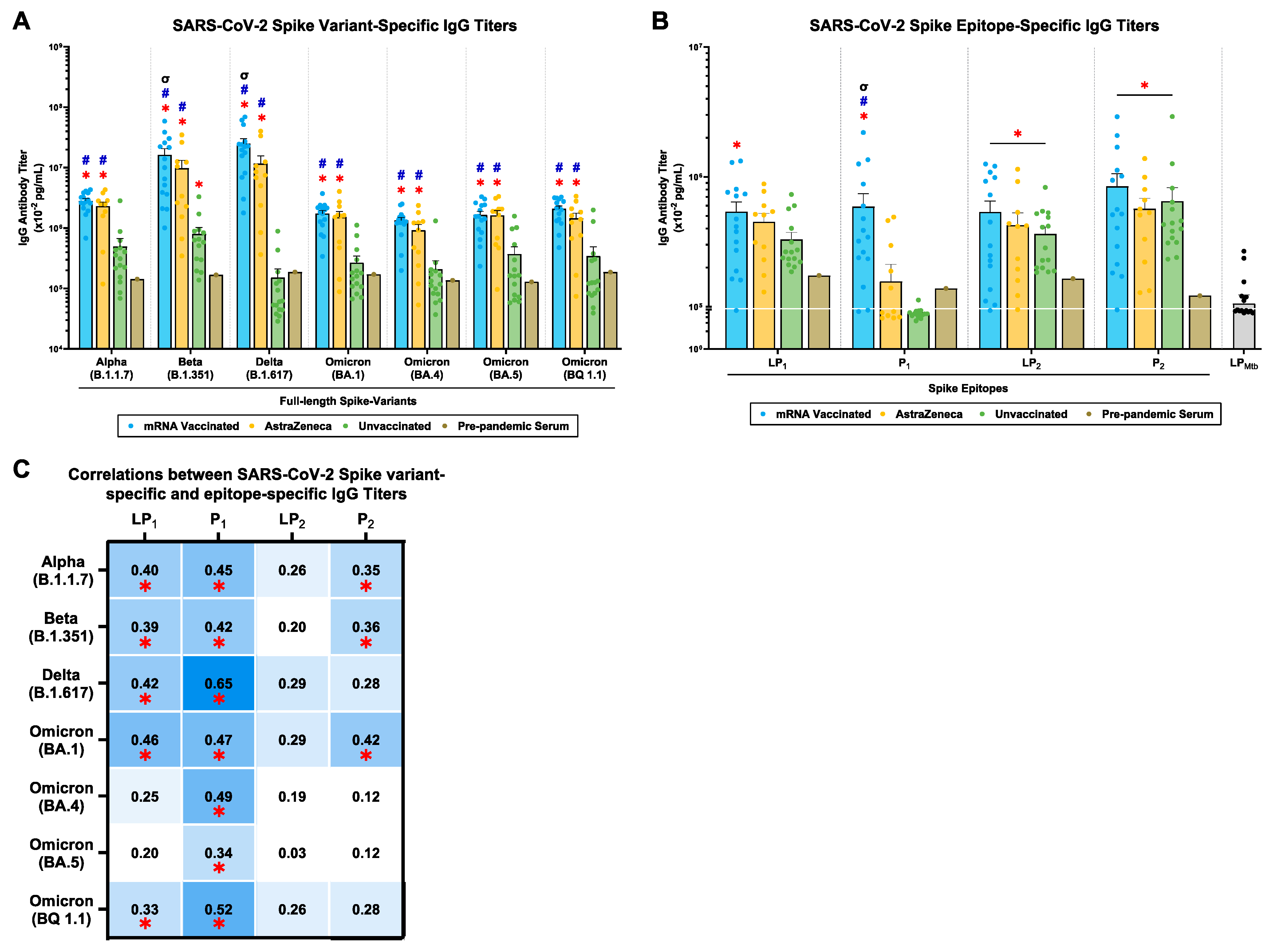
2.2. mRNA and AstraZeneca-Vaccinated Donors Generated Cross-Reactive IgG Antibody Titers against Multiple SARS-CoV-2 Spike Variants and P1/P2 Spike Epitopes
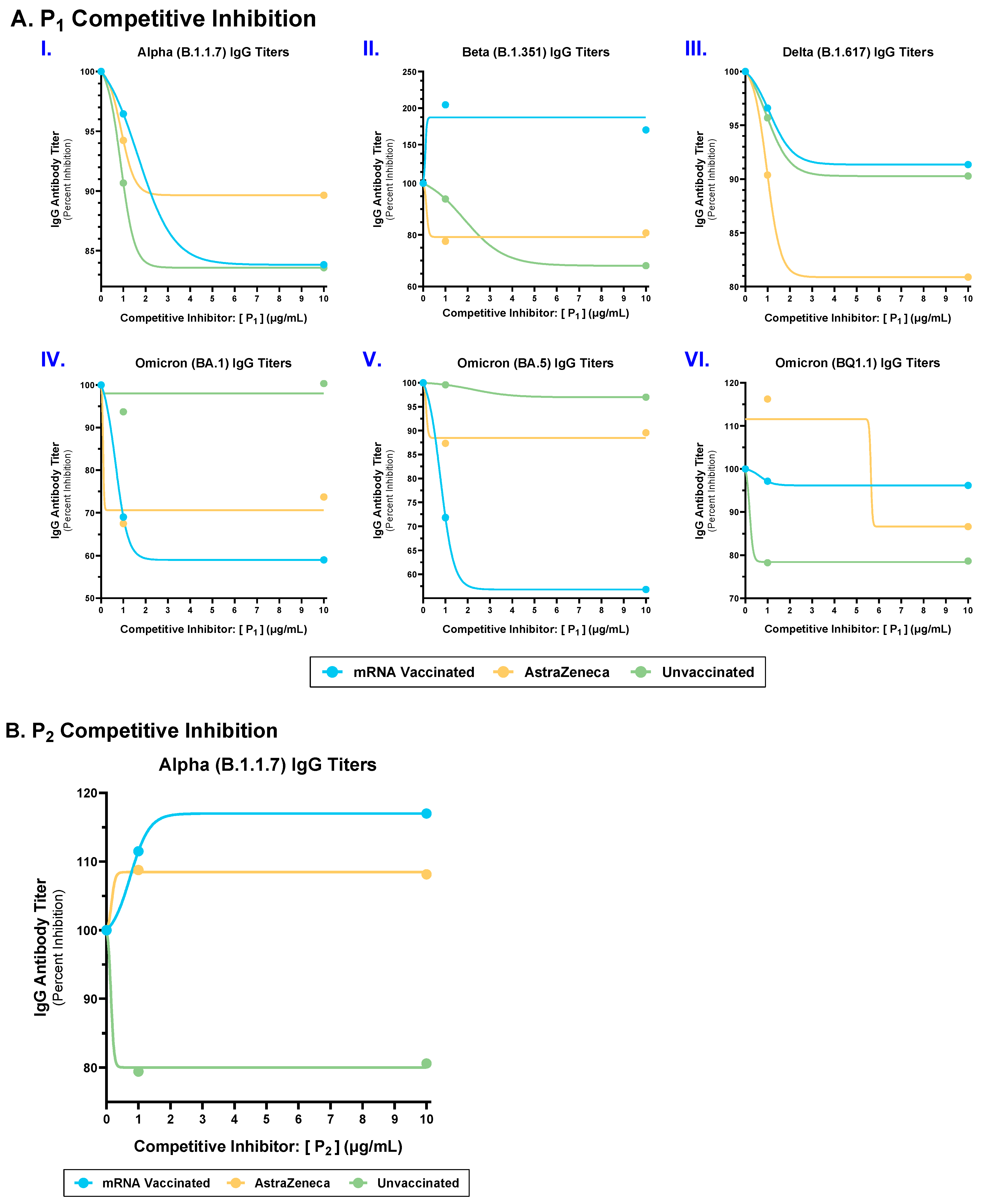
2.3. Competitive Inhibition with P1 and P2 Epitopes Reduced Cross-Reactive IgG Antibody Binding to Multiple SARS-CoV-2 Spike Variants in mRNA, AstraZeneca, and Unvaccinated Donors
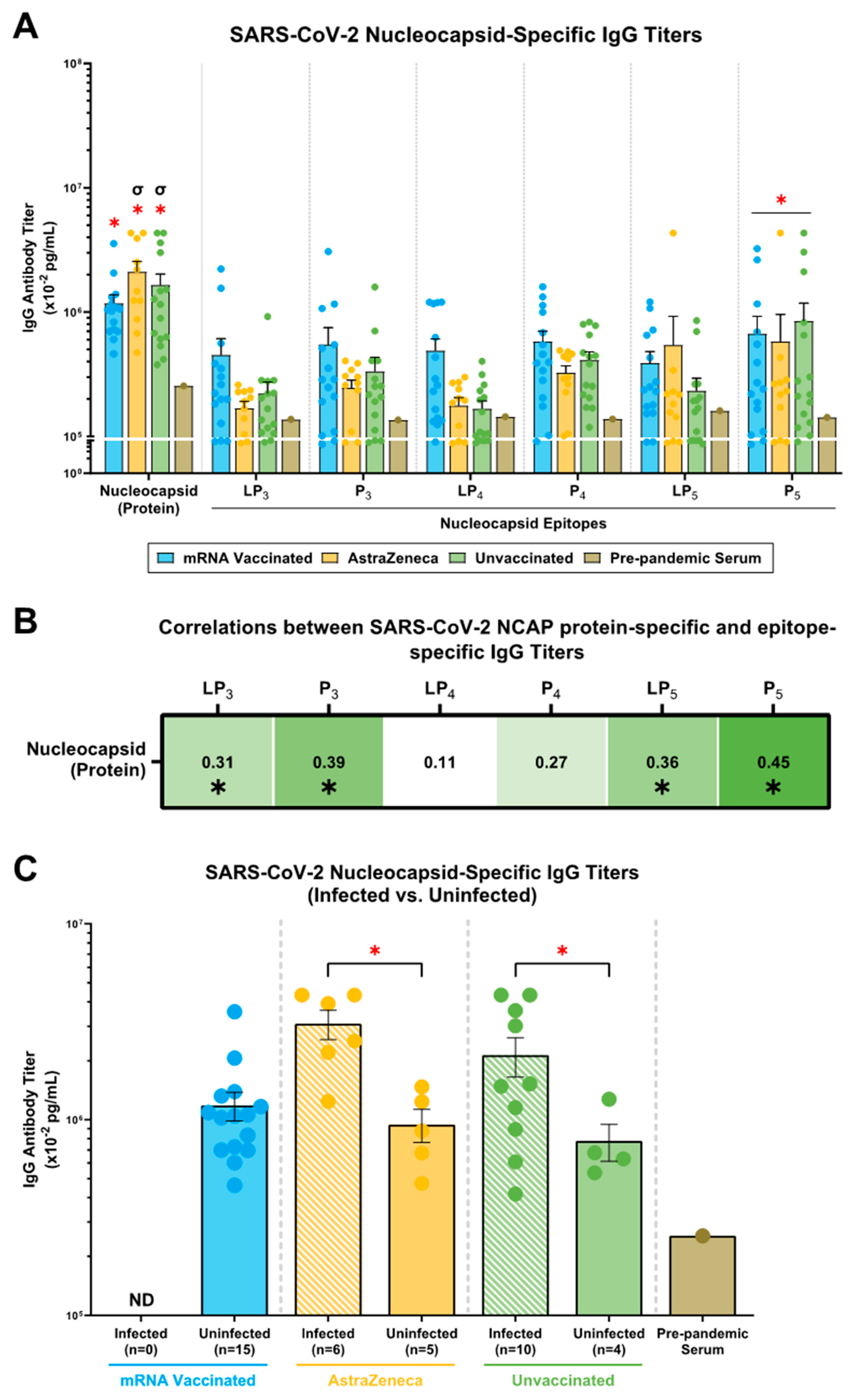
2.4. mRNA, AstraZeneca, and Unvaccinated Donors Showed IgG Antibody Titers against the SARS-CoV-2 N (Protein), N-Derived Lipopeptides (LP3, LP4, LP5) and Peptides (P3, P4, P5)
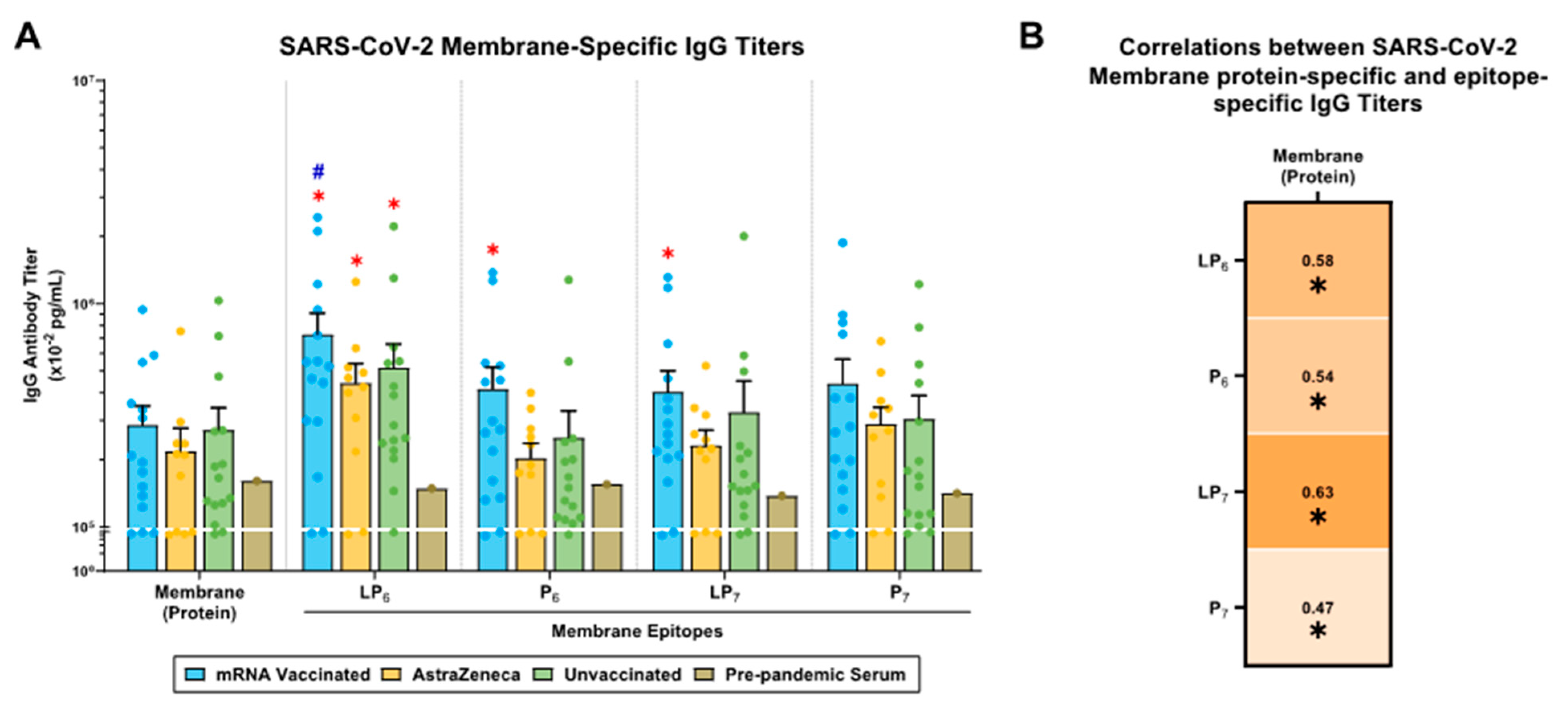
2.5. SARS-CoV-2 M-Specific IgG Antibody Responses in mRNA-Vaccinated, AstraZeneca-Vaccinated, and Unvaccinated Donors
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Human Plasma Donors
4.3. SARS-CoV-2 Proteins, Lipopeptides, and Peptides
4.4. Detection of Human IgG Antibodies and P1/P2 Competitive Antibody Assays
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization COVID-19 Cases WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=o (accessed on 23 June 2024).
- Patel, R.S.; Agrawal, B. Heterologous Immunity Induced by 1st Generation COVID-19 Vaccines and Its Role in Developing a Pan-Coronavirus Vaccine. Front. Immunol. 2022, 13, 952229. [Google Scholar] [CrossRef] [PubMed]
- Echaide, M.; Chocarro de Erauso, L.; Bocanegra, A.; Blanco, E.; Kochan, G.; Escors, D. MRNA Vaccines against SARS-CoV-2: Advantages and Caveats. Int. J. Mol. Sci. 2023, 24, 5944. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune Imprinting, Breadth of Variant Recognition, and Germinal Center Response in Human SARS-CoV-2 Infection and Vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Johnston, T.S.; Li, S.H.; Painter, M.M.; Atkinson, R.K.; Douek, N.R.; Reeg, D.B.; Douek, D.C.; Wherry, E.J.; Hensley, S.E. Immunological Imprinting Shapes the Specificity of Human Antibody Responses against SARS-CoV-2 Variants. Immunity 2024, 57, 912–925.e4. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bretones, M.; Fouchier, R.A.M.; Koopmans, M.P.G.; van Nierop, G.P. Impact of Antigenic Evolution and Original Antigenic Sin on SARS-CoV-2 Immunity. J. Clin. Investig. 2023, 133, e162192. [Google Scholar] [CrossRef]
- Agrawal, B. Heterologous Immunity: Role in Natural and Vaccine-Induced Resistance to Infections. Front. Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of Protection against SARS-CoV-2 Infection and COVID-19 Disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Lopez-Munoz, A.D.; Kosik, I.; Holly, J.; Yewdell, J.W. Cell Surface SARS-CoV-2 Nucleocapsid Protein Modulates Innate and Adaptive Immunity. Sci. Adv. 2022, 8, eabp9770. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Li, P.; Li, L.; Yi, Y.; Ma, Q.; Cao, C. Profile of Antibodies to the Nucleocapsid Protein of the Severe Acute Respiratory Syndrome (SARS)-Associated Coronavirus in Probable SARS Patients. Clin. Diagn. Lab. Immunol. 2004, 11, 227–228. [Google Scholar] [CrossRef]
- Peng, B.; Ming, Y.; Yang, C. Regulatory B Cells: The Cutting Edge of Immune Tolerance in Kidney Transplantation Review-Article. Cell Death Dis. 2018, 9, 109. [Google Scholar] [CrossRef]
- Heide, J.; Schulte, S.; Kohsar, M.; Brehm, T.T.; Herrmann, M.; Karsten, H.; Marget, M.; Peine, S.; Johansson, A.M.; Sette, A.; et al. Broadly Directed SARS-CoV-2-Specific CD4+ T Cell Response Includes Frequently Detected Peptide Specificities within the Membrane and Nucleoprotein in Patients with Acute and Resolved COVID-19. PLoS Pathog. 2021, 17, e1009842. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhao, M.; Liu, K.; Xu, K.; Wong, G.; Tan, W.; Gao, G.F. T-Cell Immunity of SARS-CoV: Implications for Vaccine Development against MERS-CoV. Antiviral Res. 2017, 137, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yang, L.; Wang, L.; Li, J.; Huang, J.; Lu, Z.Q.; Koup, R.A.; Bailer, R.T.; Wu, C. you Long-Lived Memory T Lymphocyte Responses against SARS Coronavirus Nucleocapsid Protein in SARS-Recovered Patients. Virology 2006, 351, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and Strong Memory CD4+ and CD8+ T Cells Induced by SARS-CoV-2 in UK Convalescent Individuals Following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.M.; Gutiérrez, A.H.; Boyle, C.M.; Terry, F.; McGonnigal, B.G.; Salazar, A.; Princiotta, M.F.; Martin, W.D.; De Groot, A.S.; Moise, L. Highly Conserved, Non-Human-like, and Cross-Reactive SARS-CoV-2 T Cell Epitopes for COVID-19 Vaccine Design and Validation. NPJ Vaccines 2021, 6, 71. [Google Scholar] [CrossRef]
- Vashi, Y.; Jagrit, V.; Kumar, S. Understanding the B and T Cell Epitopes of Spike Protein of Severe Acute Respiratory Syndrome Coronavirus-2: A Computational Way to Predict the Immunogens. Infect. Genet. Evol. 2020, 84, 104382. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 Variants on the Total CD4+ and CD8+ T Cell Reactivity in Infected or Vaccinated Individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Li, C.K.; Wu, H.; Yan, H.; Ma, S.; Wang, L.; Zhang, M.; Tang, X.; Temperton, N.J.; Weiss, R.A.; Brenchley, J.M.; et al. T Cell Responses to Whole SARS Coronavirus in Humans. J. Immunol. 2008, 181, 5490–5500. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680.e2. [Google Scholar] [CrossRef]
- Wang, J.; Wen, J.; Li, J.; Yin, J.; Zhu, Q.; Wang, H.; Yang, Y.; Qin, E.; You, B.; Li, W.; et al. Assessment of Immunoreactive Synthetic Peptides from the Structural Proteins of Severe Acute Respiratory Syndrome Coronavirus. Clin. Chem. 2003, 49, 1989–1996. [Google Scholar] [CrossRef]
- Patel, R.S.; Agrawal, B. Mucosal Immunization with Lipopeptides Derived from Conserved Regions of SARS-CoV-2 Antigens Induce Robust Cellular and Cross-Variant Humoral Immune Responses in Mice. Front. Immunol. 2023, 14, 1178523. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, E.; Pierros, V.; Stravopodis, D.J.; Tsangaris, G.T. Prediction of SARS-CoV-2 Omicron Variant Immunogenicity, Immune Escape and Pathogenicity, through the Analysis of Spike Protein-Specific Core Unique Peptides. Vaccines 2022, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, A.; Jędruchniewicz, N.; Torelli, A.; Kaczmarzyk-Radka, A.; Coluccio, R.; Kłak, M.; Konieczny, A.; Ferenc, S.; Witkiewicz, W.; Montomoli, E.; et al. Antibodies Specific to SARS-CoV-2 Proteins N, S and E in Covid-19 Patients in the Normal Population and in Historical Samples. J. Gen. Virol. 2021, 102, 001692. [Google Scholar] [CrossRef] [PubMed]
- Den Hartog, G.; Schepp, R.M.; Kuijer, M.; Geurtsvankessel, C.; Van Beek, J.; Rots, N.; Koopmans, M.P.G.; Van Der Klis, F.R.M.; Van Binnendijk, R.S. SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J. Infect. Dis. 2020, 222, 1452–1461. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Y.; Hou, H.; Wang, F.; Ouyang, Z.Q.; Zhang, Y.; Lai, D.Y.; Banga Ndzouboukou, J.L.; Xu, Z.W.; Zhang, B.; et al. Antibody Dynamics to SARS-CoV-2 in Asymptomatic COVID-19 Infections. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 551–561. [Google Scholar] [CrossRef]
- Cicaloni, V.; Costanti, F.; Pasqui, A.; Bianchini, M.; Niccolai, N.; Bongini, P. A Bioinformatics Approach to Investigate Structural and Non-Structural Proteins in Human Coronaviruses. Front. Genet. 2022, 13, 891418. [Google Scholar] [CrossRef]
- Kumar, A.; Asghar, A.; Singh, H.N.; Faiq, M.A.; Kumar, S.; Narayan, R.K.; Kumar, G.; Dwivedi, P.; Sahni, C.; Jha, R.K.; et al. SARS-CoV-2 Omicron Variant Genomic Sequences and Their Epidemiological Correlates Regarding the End of the Pandemic: In Silico Analysis. JMIR Bioinform. Biotechmol. 2023, 4, e42700. [Google Scholar] [CrossRef]
- Sajini, A.A.; Alkayyal, A.A.; Mubaraki, F.A. The Recombination Potential between SARS-CoV-2 and MERS-CoV from Cross-Species Spill-over Infections. J. Epidemiol. Glob. Health 2021, 11, 155–159. [Google Scholar] [CrossRef]
- Tamminen, K.; Salminen, M.; Blazevic, V. Seroprevalence and SARS-CoV-2 Cross-Reactivity of Endemic Coronavirus OC43 and 229E Antibodies in Finnish Children and Adults. Clin. Immunol. 2021, 229, 108782. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, D.; Han, Y.; Yan, H.; Chong, H.; Ren, L.; Wang, J.; Li, T.; He, Y. Cross-Reactive Neutralization of SARS-CoV-2 by Serum Antibodies from Recovered SARS Patients and Immunized Animals. Sci. Adv. 2020, 6, eabc9999. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Le Sage, V.; Bolton, M.J.; Eilola, T.; Jones, J.E.; Kormuth, K.A.; Nturibi, E.; Balmaseda, A.; Gordon, A.; Lakdawala, S.S.; et al. Original Antigenic Sin Priming of Influenza Virus Hemagglutinin Stalk Antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 17221–17227. [Google Scholar] [CrossRef] [PubMed]
- Petráš, M.; Králová Lesná, I. SARS-CoV-2 Vaccination in the Context of Original Antigenic Sin. Hum. Vaccin. Immunother. 2022, 18, 1949953. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Addetia, A.; Seo, A.J.; Brown, J.; Sprouse, K.R.; Logue, J.; Clark, E.; Franko, N.; Chu, H.; Veesler, D. Persistent Immune Imprinting after XBB.1.5 COVID Vaccination in Humans. bioRxiv 2023. [Google Scholar] [CrossRef]
- Blankson, J.N. Bivalent COVID-19 Vaccines: Can the Original Antigenic Sin Be Forgiven? J. Infect. Dis. 2023, 227, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Mandhane, A.; Tripathy, C.S.; Behera, S.K. Peptide and Protein in Vaccine Delivery. In Peptide and Protein Drug Delivery Using Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Grobben, M.; Van Der Straten, K.; Brouwer, P.J.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Appelman, B.; Ayesha Lavell, A.H.; Van Vught, L.A.; Burger, J.A.; et al. Cross-Reactive Antibodies after Sars-Cov-2 Infection and Vaccination. Elife 2021, 10, e70330. [Google Scholar] [CrossRef]
- Song, G.; He, W.; Callaghan, S.; Anzanello, F.; Huang, D.; Ricketts, J.; Torres, J.L.; Beutler, N.; Peng, L.; Vargas, S.; et al. Cross-Reactive Serum and Memory B-Cell Responses to Spike Protein in SARS-CoV-2 and Endemic Coronavirus Infection. Nat. Commun. 2021, 12, 2938. [Google Scholar] [CrossRef]
- Sealy, R.E.; Hurwitz, J.L. Cross-Reactive Immune Responses toward the Common Cold Human Coronaviruses and Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2): Mini-Review and a Murine Study. Microorganisms 2021, 9, 1643. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Fernández-Soto, D.; Bueno, P.; Garaigorta, U.; Gastaminza, P.; Bueno, J.L.; Duarte, R.F.; Jara, R.; Valés-Gómez, M.; Reyburn, H.T. SARS-CoV-2 Membrane Protein-Specific Antibodies from Critically Ill SARS-CoV-2–Infected Individuals Interact with Fc Receptor–Expressing Cells but Do Not Neutralize the Virus. J. Leukoc. Biol. 2024, 115, 985–991. [Google Scholar] [CrossRef]
| Pathogen | Protein | Location | Code | Amino Acid Sequence | Epitope Characteristics [10,11,12,13,14,15,16,17,18,19,20,21] |
|---|---|---|---|---|---|
| SARS-CoV-2 | S1 | 492–505 | LP1 | LQSYGFQPTNGVGYK(Palmitoyl)G |
|
| P1 | LQSYGFQPTNGVGY | ||||
| S2 | 814–826 | LP2 | KRSFIEDLLFNKVK(Palmitoyl)G |
| |
| P2 | KRSFIEDLLFNKV | ||||
| N | 358–372 | LP3 | IDAYKTFPPTEPKKDK(Palmitoyl)G |
| |
| P3 | IDAYKTFPPTEPKKD | ||||
| 317–331 | LP4 | MSRIGMEVTPSGTWLK(Palmitoyl)G |
| ||
| P4 | MSRIGMEVTPSGTWL | ||||
| 158–172 | LP5 | VLQLPQGTTLPKGFYK(Palmitoyl)G |
| ||
| P5 | VLQLPQGTTLPKGFY | ||||
| M | 98–112 | LP6 | ASFRLFARTRSMWSFK(Palmitoyl)G |
| |
| P6 | ASFRLFARTRSMWSF | ||||
| 34–48 | LP7 | LLQFAYANRNRFLYIK(Palmitoyl)G |
| ||
| P7 | LLQFAYANRNRFLYI |
| mRNA Vaccinated (N = 15) | AstraZeneca Vaccinated (N = 11) | Unvaccinated (N = 15) | ||
|---|---|---|---|---|
| Sample Collection Timeline | ||||
| July 2021–Aug 2021 | Apr 2021–Jan 2022 | July 2020–July 2021 | ||
| Sex, n (%) | ||||
| Females | 11 (73.3%) | 6 (54.5%) | 6 (40.0%) | |
| Males | 4 (26.7%) | 5 (45.5%) | 9 (60.0%) | |
| Age, median (range) | ||||
| 25 (19–65) | 58 (28–63) | 42 (20–94) | ||
| Reported COVID-19 Infection, n (%) | ||||
| - | 6 (54.5%) | 10 (66.6%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, D.; Patel, R.S.; Lam, A.; Glover, Q.; Srinivasan, C.; Herchen, A.; Ritchie, B.; Agrawal, B. Antibody Responses in SARS-CoV-2-Exposed and/or Vaccinated Individuals Target Conserved Epitopes from Multiple CoV-2 Antigens. Int. J. Mol. Sci. 2024, 25, 9814. https://doi.org/10.3390/ijms25189814
Yao D, Patel RS, Lam A, Glover Q, Srinivasan C, Herchen A, Ritchie B, Agrawal B. Antibody Responses in SARS-CoV-2-Exposed and/or Vaccinated Individuals Target Conserved Epitopes from Multiple CoV-2 Antigens. International Journal of Molecular Sciences. 2024; 25(18):9814. https://doi.org/10.3390/ijms25189814
Chicago/Turabian StyleYao, David, Raj S. Patel, Adrien Lam, Quarshie Glover, Cindy Srinivasan, Alex Herchen, Bruce Ritchie, and Babita Agrawal. 2024. "Antibody Responses in SARS-CoV-2-Exposed and/or Vaccinated Individuals Target Conserved Epitopes from Multiple CoV-2 Antigens" International Journal of Molecular Sciences 25, no. 18: 9814. https://doi.org/10.3390/ijms25189814





