Bisphenol S Promotes the Transfer of Antibiotic Resistance Genes via Transformation
Abstract
1. Introduction
2. Results
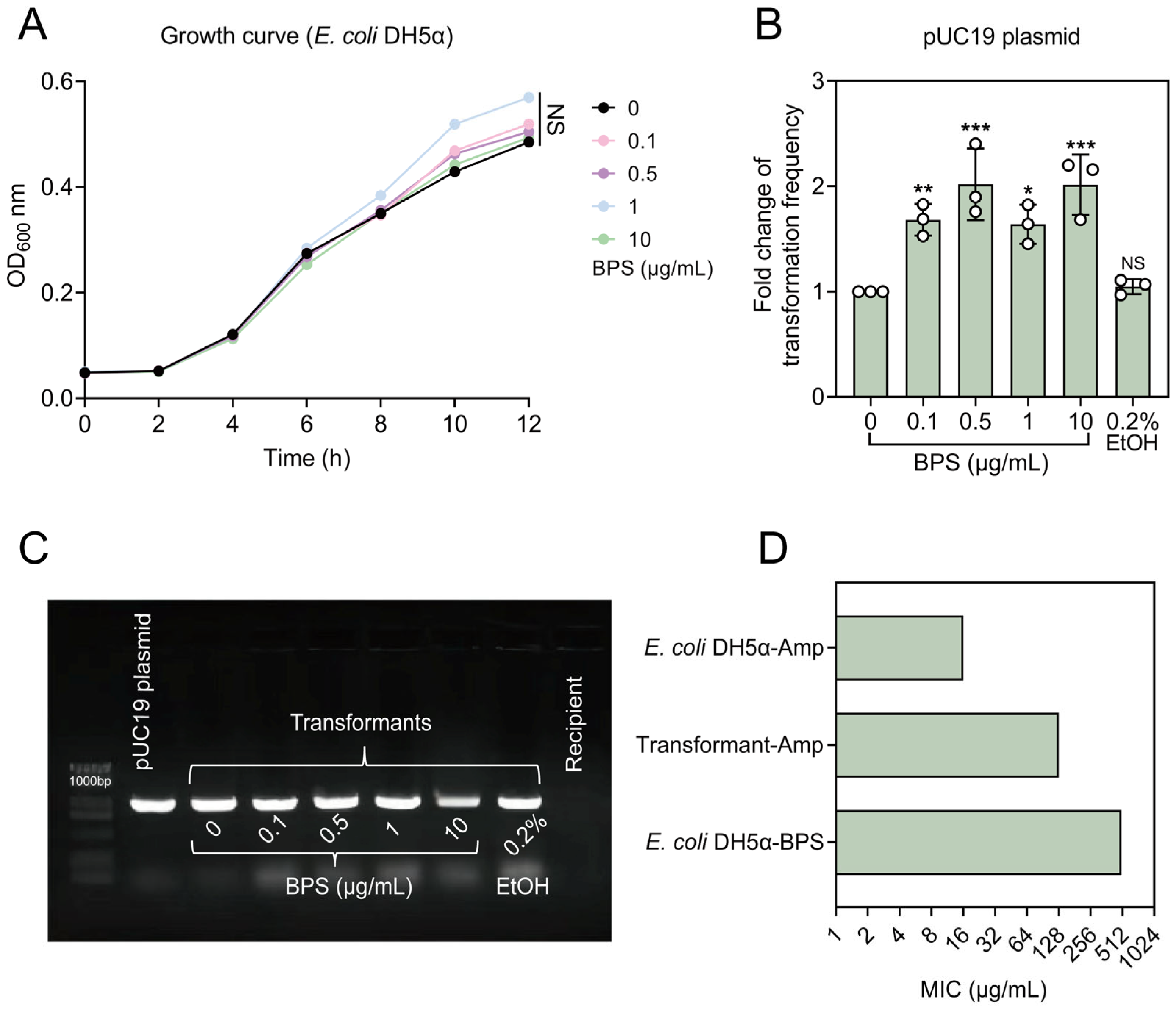
2.1. BPS Promotes the Spread of ARGs via Transformation
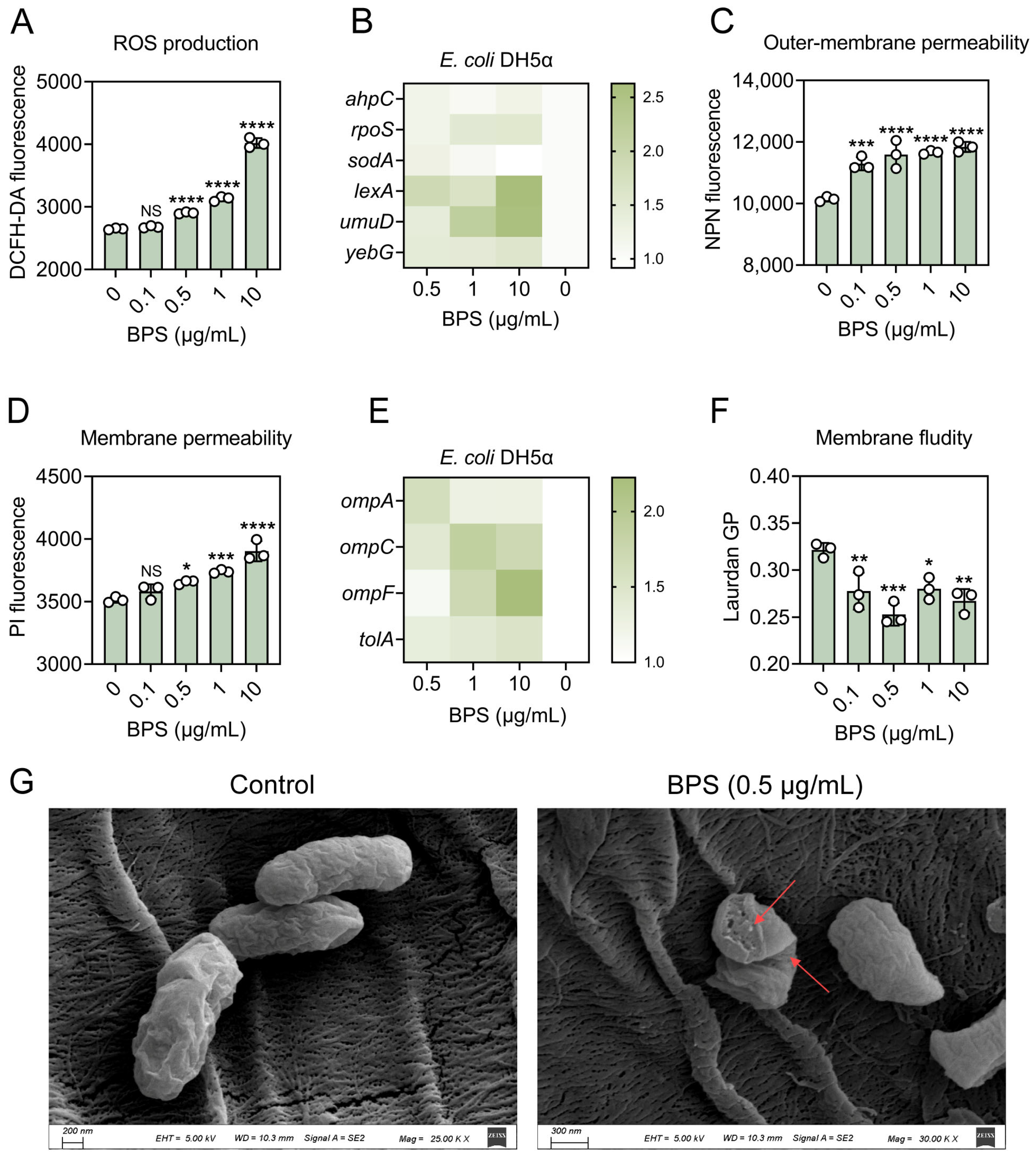
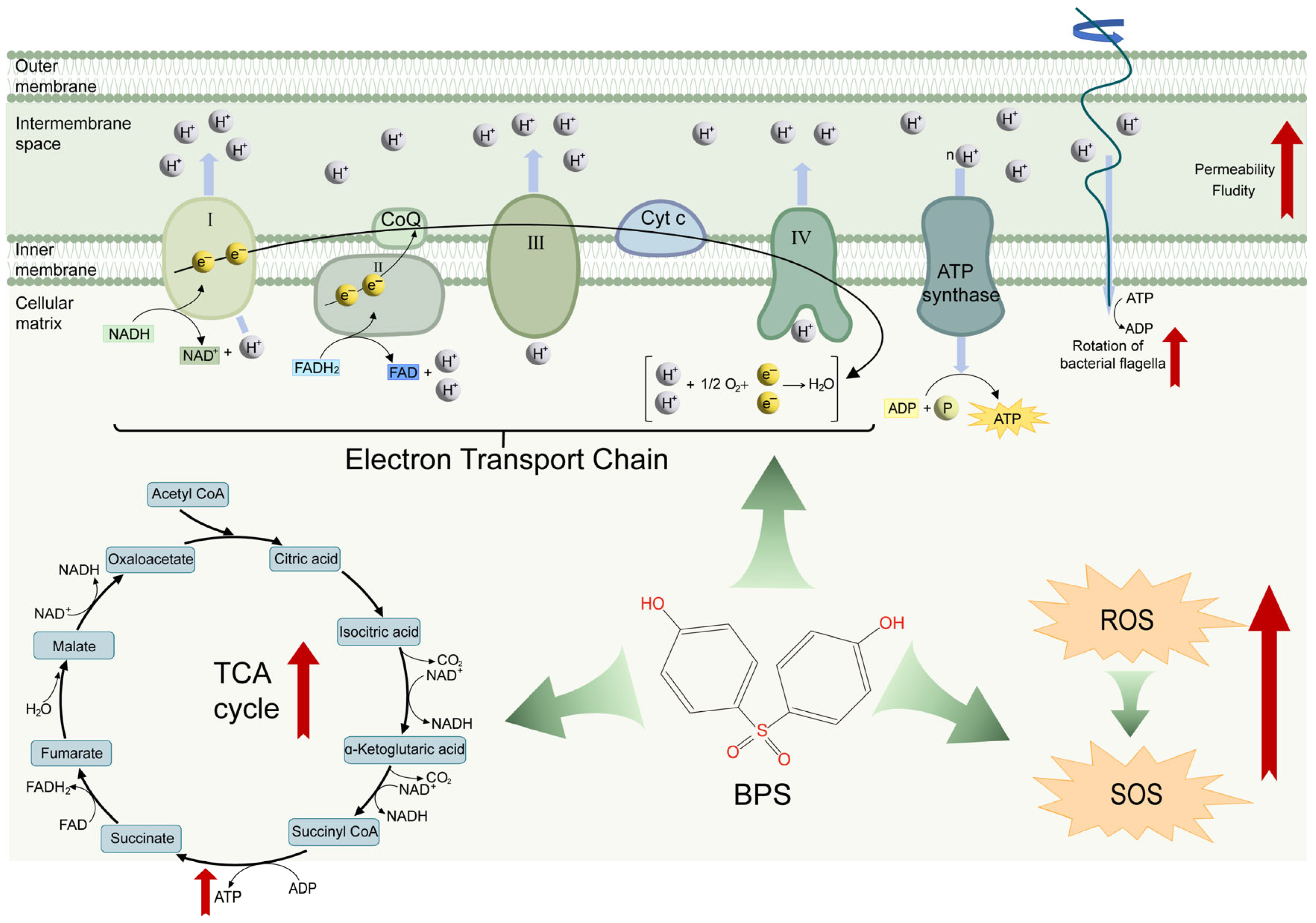
2.2. BPS Enhances Bacterial Oxidative Stress and Activates SOS Response
2.3. BPS Improves Membrane Permeability and Fluidity
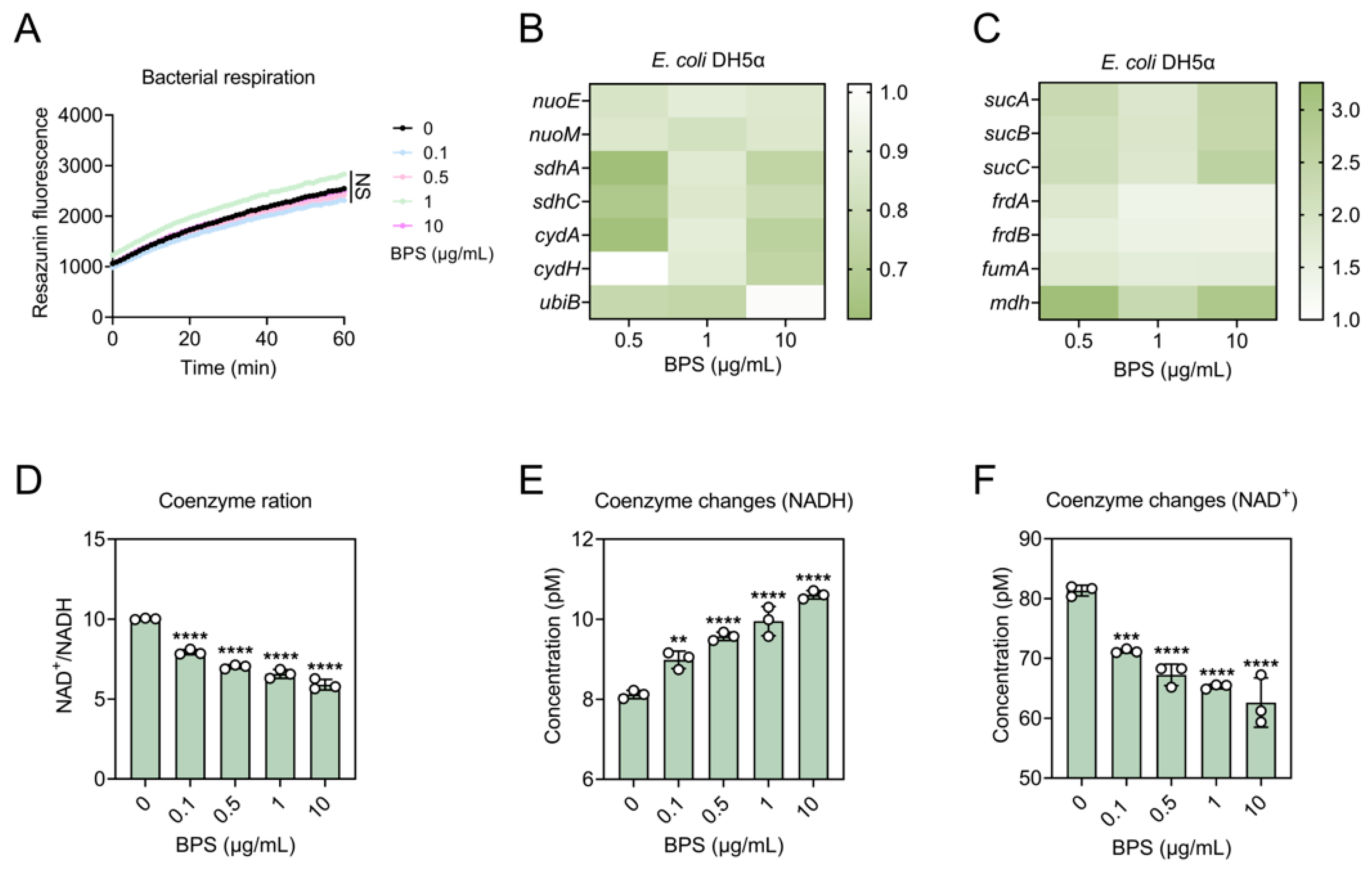
2.4. BPS Enhances Metabolic States by Accelerating TCA Cycle
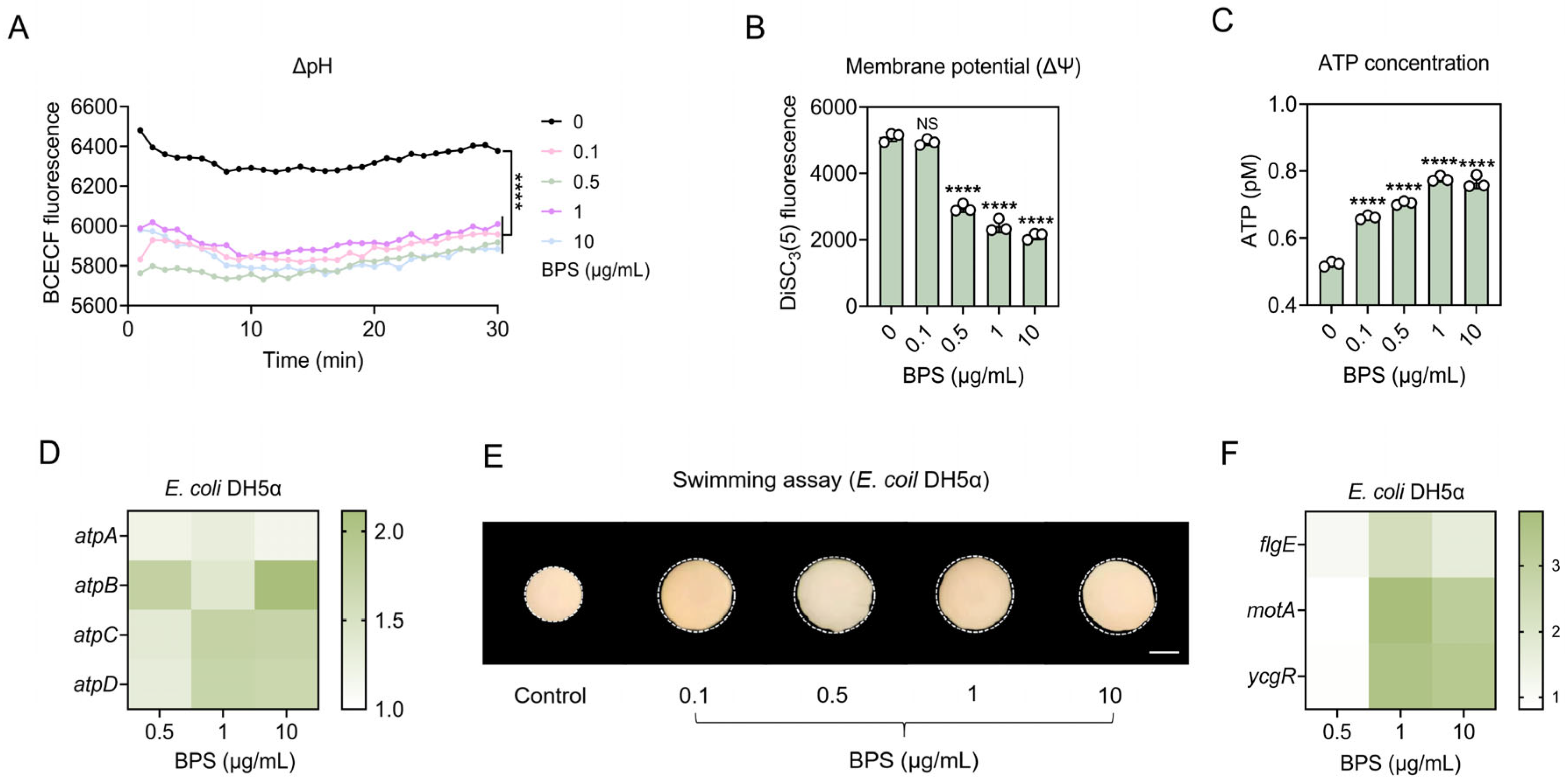
2.5. BPS Stimulates ATP Supply and Improves Flagellar Motility
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, Antibiotics, BPS, and Culture Media
4.2. Measurement of Growth Curves
4.3. Determination of Minimum Inhibitory Concentration (MIC)
4.4. Establishment of Transformation System and Validation of Transformants
4.5. Detection of Oxidative Stress Levels in Bacteria
4.6. Detection of Bacterial Membrane Permeability and Fluidity
4.7. Scanning Electron Microscope (SEM) Analysis
4.8. Detection of Bacterial NAD+/NADH
4.9. Detection of Bacterial Proton Motive Force (PMF)
4.10. Assay of Bacterial Motility
4.11. Monitoring of Bacterial Respiration Levels
4.12. Content Determination of Bacterial ATP
4.13. RNA Extraction and RT-qPCR Analysis
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2022, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuca, K. Antibiotics and Antibiotic Resistance-Flipsides of the Same Coin. Curr. Pharm. Des. 2022, 28, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Lopatkin, A.J.; Huang, S.; Smith, R.P.; Srimani, J.K.; Sysoeva, T.A.; Bewick, S.; Karig, D.K.; You, L. Antibiotics as a selective driver for conjugation dynamics. Nat. Microbiol. 2016, 1, 16044. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Henderson, I.R.; Guo, J. Artificial sweeteners stimulate horizontal transfer of extracellular antibiotic resistance genes through natural transformation. ISME J. 2022, 16, 543–554. [Google Scholar] [CrossRef]
- Liao, J.; Chen, Y.; Huang, H. Effects of CO2 on the transformation of antibiotic resistance genes via increasing cell membrane channels. Environ. Pollut. 2019, 254, 113045. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, B.; Jia, Y.; Yu, F.; Wang, Z.; Liu, Y. Comprehensive analysis of disinfectants on the horizontal transfer of antibiotic resistance genes. J. Hazard. Mater. 2023, 453, 131428. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Manzan-Martins, C.; Paulesu, L. Impact of bisphenol A (BPA) on cells and tissues at the human materno-fetal interface. Tissue Cell 2021, 73, 101662. [Google Scholar] [CrossRef]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.; Tam, N.F.Y.; Zhong, M.; Wu, Q.; Liu, Z.; Huang, X.; Wei, L.; Liu, Y.; Luo, D.; Li, S. Distribution and risk assessment of antibiotic and bisphenol compounds residues in drinking water sources of Guangdong. Environ. Earth Sci. 2024, 83, 475. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, K.; Yin, J.; Lyu, B.; Zhao, Y.; Li, J.; Shao, B.; Wu, Y. Exposure to Bisphenolic Analogues in the Sixth Total Diet Study—China, 2016–2019. China CDC Wkly. 2022, 4, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.; Pu, Y.; Roberts, J.; Karthikraj, R.; Kannan, K.; Ehrhardt, R.; Veiga-Lopez, A. Gestational bisphenol S impairs placental endocrine function and the fusogenic trophoblast signaling pathway. Arch. Toxicol. 2018, 92, 1861–1876. [Google Scholar] [CrossRef]
- Sreedevi, P.R.; Suresh, K. Cold atmospheric plasma mediated cell membrane permeation and gene delivery-empirical interventions and pertinence. Adv. Colloid Interface Sci. 2023, 320, 102989. [Google Scholar] [CrossRef]
- Cao, P.; Wall, D. The Fluidity of the Bacterial Outer Membrane Is Species Specific: Bacterial Lifestyles and the Emergence of a Fluid Outer Membrane. Bioessays 2020, 42, e1900246. [Google Scholar] [CrossRef]
- Le, D.; Krasnopeeva, E.; Sinjab, F.; Pilizota, T.; Kim, M. Active Efflux Leads to Heterogeneous Dissipation of Proton Motive Force by Protonophores in Bacteria. mBio 2021, 12, e0067621. [Google Scholar] [CrossRef]
- You, Y.; Ye, F.; Mao, W.; Yang, H.; Lai, J.; Deng, S. An overview of the structure and function of the flagellar hook FlgE protein. World J. Microbiol. Biotechnol. 2023, 39, 126. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueievna, D.M. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zhou, H.; Niu, Y.; Li, J.; Fu, X.; Wang, S.; Xue, B.; Li, C.; Zhao, C.; et al. Bisphenols Promote the Pheromone-Responsive Plasmid-Mediated Conjugative Transfer of Antibiotic Resistance Genes in Enterococcus faecalis. Environ. Sci. Technol. 2022, 56, 17653–17662. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.L.; Zheng, Z.G.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Lu, J.; Yu, Z.; Song, H.; Bond, P.L.; Guo, J. Chlorine disinfection facilitates natural transformation through ROS-mediated oxidative stress. ISME J. 2021, 15, 2969–2985. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Martin, B.; Fichant, G.; Polard, P.; Claverys, J.P. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014, 12, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.N.; Mi, H.F.; Xue, Y.X.; Wang, D.; Zhao, X.L. The mechanism of ROS regulation of antibiotic resistance and antimicrobial lethality. Yi Chuan 2016, 38, 902–909. [Google Scholar]
- Zhang, S.; Yang, M.J.; Peng, B.; Peng, X.X.; Li, H. Reduced ROS-mediated antibiotic resistance and its reverting by glucose in Vibrio alginolyticus. Environ. Microbiol. 2020, 22, 4367–4380. [Google Scholar] [CrossRef]
- Mantilla-Calderon, D.; Plewa, M.J.; Michoud, G.; Fodelianakis, S.; Daffonchio, D.; Hong, P.Y. Water Disinfection Byproducts Increase Natural Transformation Rates of Environmental DNA in Acinetobacter baylyi ADP1. Environ. Sci. Technol. 2019, 53, 6520–6528. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, A.Z.; He, M.; Li, D.; Chen, J. Subinhibitory concentrations of disinfectants promote the horizontal transfer of multidrug resistance genes within and across genera. Environ. Sci. Technol. 2017, 51, 570–580. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Lu, J.; Bond, P.L.; Guo, J. Nonnutritive sweeteners can promote the dissemination of antibiotic resistance through conjugative gene transfer. ISME J. 2021, 15, 2117–2130. [Google Scholar] [CrossRef]
- Simon, S.M.; Sousa, F.J.; Mohana-Borges, R.; Walker, G.C. Regulation of Escherichia coli SOS mutagenesis by dimeric intrinsically disordered umuD gene products. Proc. Natl. Acad. Sci. USA 2008, 105, 1152–1157. [Google Scholar] [CrossRef]
- Kulbacka, J.; Choromanska, A.; Rossowska, J.; Wezgowiec, J.; Saczko, J.; Rols, M.P. Cell Membrane Transport Mechanisms: Ion Channels and Electrical Properties of Cell Membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [PubMed]
- Lu, J.; Wang, Y.; Zhang, S.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmental concentrations can enhance the spread of extracellular antibiotic resistance genes through transformation. Sci. Total Environ. 2020, 713, 136621. [Google Scholar] [CrossRef]
- Henry, R.; Crane, B.; Powell, D.; Deveson Lucas, D.; Li, Z.; Aranda, J.; Harrison, P.; Nation, R.L.; Adler, B.; Harper, M.; et al. The transcriptomic response of Acinetobacter baumannii to colistin and doripenem alone and in combination in an in vitro pharmacokinetics/pharmacodynamics model. J. Antimicrob. Chemother. 2015, 70, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yong, Y.; Zhu, C.; Yang, H.; Fang, B. Exogenous D-ribose promotes gentamicin treatment of several drug-resistant Salmonella. Front. Microbiol. 2022, 13, 1053330. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, I.; Arana, L.; Ugarte-Uribe, B.; Gomez-Rubio, E.; Martin-Santamaria, S.; Garbisu, C.; Alkorta, I. Type IV Coupling Proteins as Potential Targets to Control the Dissemination of Antibiotic Resistance. Front. Mol. Biosci. 2020, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.V.; MacNeill, S.A. ATP-dependent DNA ligases. Genome Biol. 2002, 3, REVIEWS3005. [Google Scholar] [CrossRef]
- Mehring, A.; Erdmann, N.; Walther, J.; Stiefelmaier, J.; Strieth, D.; Ulber, R. A simple and low-cost resazurin assay for vitality assessment across species. J. Biotechnol. 2021, 333, 63–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhu, S.; Sun, J.; Liu, Y. Bisphenol S Promotes the Transfer of Antibiotic Resistance Genes via Transformation. Int. J. Mol. Sci. 2024, 25, 9819. https://doi.org/10.3390/ijms25189819
Zhang J, Zhu S, Sun J, Liu Y. Bisphenol S Promotes the Transfer of Antibiotic Resistance Genes via Transformation. International Journal of Molecular Sciences. 2024; 25(18):9819. https://doi.org/10.3390/ijms25189819
Chicago/Turabian StyleZhang, Jiayi, Shuyao Zhu, Jingyi Sun, and Yuan Liu. 2024. "Bisphenol S Promotes the Transfer of Antibiotic Resistance Genes via Transformation" International Journal of Molecular Sciences 25, no. 18: 9819. https://doi.org/10.3390/ijms25189819
APA StyleZhang, J., Zhu, S., Sun, J., & Liu, Y. (2024). Bisphenol S Promotes the Transfer of Antibiotic Resistance Genes via Transformation. International Journal of Molecular Sciences, 25(18), 9819. https://doi.org/10.3390/ijms25189819






