Molecular Mechanisms Underlying the Generation of Absence Seizures: Identification of Potential Targets for Therapeutic Intervention
Abstract
1. Introduction
2. Absence Seizures and Genetic Generalised Epilepsies
2.1. ILAE Classification and Diagnosis of Absence Seizures
2.2. Genetic Factors and the Classification of Absence Seizures
2.3. Absence Seizures in Childhood Absence Epilepsy
2.4. Changes in Brain Morphology Associated with Absence Seizures
2.5. Current Drug Treatments for Absence Seizures
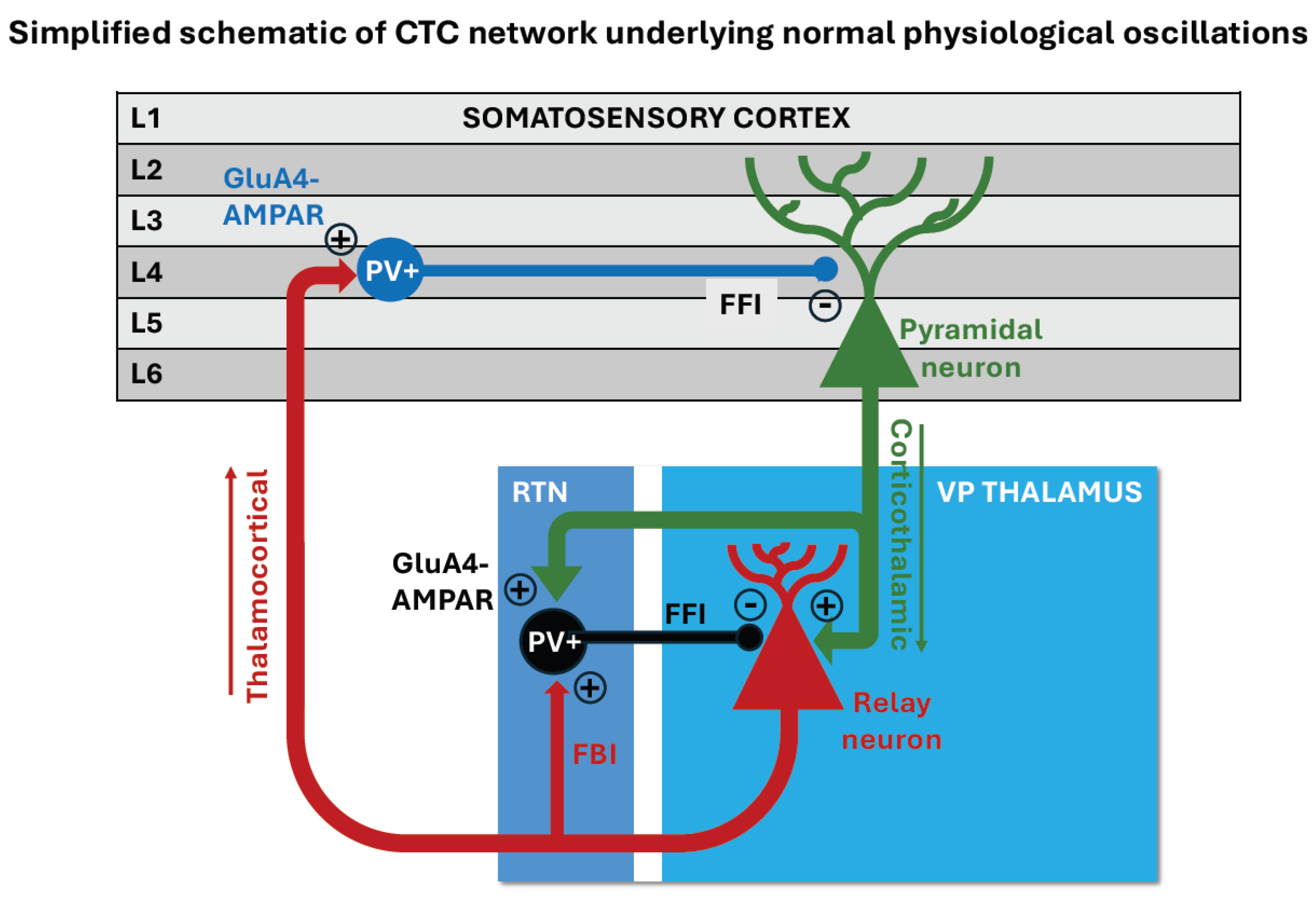
3. Absence Seizures and Cortico-Thalamo-Cortical Networks
3.1. Cortical Site of Initiation of SWDs
3.2. The Cortico-Thalamo-Cortical Network and Pathological SWD Oscillations
3.2.1. Monogenic Mouse Models of Absence Epilepsy
3.2.2. Polygenic Rat Models of Absence Epilepsy
4. The Involvement of Other Brain Regions in Absence Seizures
4.1. The Role of the Striatum in Absence Seizures
4.2. The Role of the Cerebellum in Absence Seizures
4.2.1. Evidence for Cerebellum Involvement in Epileptogenesis from Human Studies
4.2.2. Evidence for Cerebellum Involvement in Epileptogenesis from Animal Studies
4.2.3. Cerebellar Dysfunction and Ataxia in Animal Models with Absence Epilepsy
5. Identification of Causal Genetic and Molecular Mechanisms
5.1. Human Genome-Wide Association Studies
5.2. Genetic Mutations and Channelopathies Linked to Absence Epilepsy in Monogenic Mouse Models
5.2.1. Calcium Channels
5.2.2. GABA Receptor-Related Channels
5.2.3. Glutamate Receptor-Related Channels
6. Current Therapies and Future Directions
6.1. Current ASMs for Treating CAE and Their Limitations
6.2. Development of Precision Medicines to Treat CAE
6.3. Future Treatment Strategies
7. Conclusions
Funding
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Hesdorffer, D.; Logroscino, G.; Benn, E.; Katri, N.; Cascino, G.; Hauser, W. Estimating risk for developing epilepsy. Neurology 2011, 76, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Hirsch, E.; French, J.; Scheffer, I.E.; Bogacz, A.; Alsaadi, T.; Sperling, M.R.; Abdulla, F.; Zuberi, S.M.; Trinka, E.; Specchio, N.; et al. ILAE definition of the Idiopathic Generalized Epilepsy Syndromes: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1475–1499. [Google Scholar] [CrossRef]
- Unterberger, I.; Trinka, E.; Kaplan, P.W.; Walser, G.; Luef, G.; Bauer, G. Generalized nonmotor (absence) seizuresWhat do absence, generalized, and nonmotor mean? Epilepsia 2018, 59, 523–529. [Google Scholar] [CrossRef]
- Stefan, H.; Trinka, E. Generalized absence seizures: Where do we stand today? Z. Epileptol. 2022, 35, 56–72. [Google Scholar] [CrossRef]
- Panayiotopoulos, C.P. Typical absence seizures and their treatment. Arch. Dis. Child. 1999, 81, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Leresche, N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat. Rev. Neurosci. 2002, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Noebels, J.L. The Voltage-Gated Calcium Channel and Absence Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar] [PubMed]
- Crunelli, V.; Lőrincz, M.L.; McCafferty, C.; Lambert, R.C.; Leresche, N.; Di Giovanni, G.; David, F. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain 2020, 143, 2341–2368. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.M.; Malepati, S.; Manaster, L.; Rossignol, E.; Noebels, J.L. Developing a pathway to clinical trials for CACNA1A-related epilepsies: A patient organization perspective. Ther. Adv. Rare Dis. 2024, 5, 26330040241245725. [Google Scholar] [CrossRef] [PubMed]
- Vadlamudi, L.; Andermann, E.; Lombroso, C.T.; Schachter, S.C.; Milne, R.L.; Hopper, J.L.; Andermann, F.; Berkovic, S.F. Epilepsy in twins: Insights from unique historical data of William Lennox. Neurology 2004, 62, 1127–1133. [Google Scholar] [CrossRef]
- Corey, L.A.; Pellock, J.M.; Kjeldsen, M.J.; Nakken, K.O. Importance of genetic factors in the occurrence of epilepsy syndrome type: A twin study. Epilepsy Res. 2011, 97, 103–111. [Google Scholar] [CrossRef]
- Helbig, I.; Matigian, N.A.; Vadlamudi, L.; Lawrence, K.M.; Bayly, M.A.; Bain, S.M.; Diyagama, D.; Scheffer, I.E.; Mulley, J.C.; Holloway, A.J.; et al. Gene expression analysis in absence epilepsy using a monozygotic twin design. Epilepsia 2008, 49, 1546–1554. [Google Scholar] [CrossRef]
- Berkovic, S.F.; Howell, R.A.; Hay, D.A.; Hopper, J.L. Epilepsies in twins: Genetics of the major epilepsy syndromes. Ann. Neurol. 1998, 43, 435–445. [Google Scholar] [CrossRef]
- Perucca, P.; Bahlo, M.; Berkovic, S.F. The Genetics of Epilepsy. Annu. Rev. Genom. Hum. Genet. 2020, 21, 205–230. [Google Scholar] [CrossRef]
- Wallace, R.H.; Marini, C.; Petrou, S.; Harkin, L.A.; Bowser, D.N.; Panchal, R.G.; Williams, D.A.; Sutherland, G.R.; Mulley, J.C.; Scheffer, I.E.; et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001, 28, 49–52. [Google Scholar] [CrossRef]
- Cossette, P.; Liu, L.; Brisebois, K.; Dong, H.; Lortie, A.; Vanasse, M.; Saint-Hilaire, J.-M.; Carmant, L.; Verner, A.; Lu, W.-Y.; et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 2002, 31, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Suls, A.; Mullen, S.A.; Weber, Y.G.; Verhaert, K.; Ceulemans, B.; Guerrini, R.; Wuttke, T.V.; Salvo-Vargas, A.; Deprez, L.; Claes, L.R.F.; et al. Early-onset absence epilepsy caused by mutations in the glucose transporter GLUT1. Ann. Neurol. 2009, 66, 415–419. [Google Scholar] [CrossRef]
- de Kovel, C.G.F.; Trucks, H.; Helbig, I.; Mefford, H.C.; Baker, C.; Leu, C.; Kluck, C.; Muhle, H.; von Spiczak, S.; Ostertag, P.; et al. Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 2009, 133 Pt 1, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xia, Y.; Li, Q.; Xue, K.; Lai, Y.; Gong, Q.; Zhou, D.; Yao, D. Diffusion and volumetry abnormalities in subcortical nuclei of patients with absence seizures. Epilepsia 2011, 52, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Daley, M.; Siddarth, P.; Levitt, J.; Loesch, I.K.; Altshuler, L.; Ly, R.; Shields, W.D.; Gurbani, S.; Caplan, R. Amygdala volumes in childhood absence epilepsy. Epilepsy Behav. 2009, 16, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Caplan, R.; Levitt, J.; Siddarth, P.; Wu, K.N.; Gurbani, S.; Sankar, R.; Shields, W.D. Frontal and temporal volumes in Childhood Absence Epilepsy. Epilepsia 2009, 50, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Pardoe, H.; Pell, G.S.; Abbott, D.F.; Berg, A.T.; Jackson, G.D. Multi-site voxel-based morphometry: Methods and a feasibility demonstration with childhood absence epilepsy. NeuroImage 2008, 42, 611–616. [Google Scholar] [CrossRef]
- Chan, C.H.; Briellmann, R.S.; Pell, G.S.; Scheffer, I.E.; Abbott, D.F.; Jackson, G.D. Thalamic Atrophy in Childhood Absence Epilepsy. Epilepsia 2006, 47, 399–405. [Google Scholar] [CrossRef]
- Betting, L.E.; Mory, S.B.; Lopes-Cendes, I.; Li, L.M.; Guerreiro, M.M.; Guerreiro, C.A.; Cendes, F. MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures. Epilepsy Behav. 2006, 8, 575–580. [Google Scholar] [CrossRef]
- Tosun, D.; Siddarth, P.; Toga, A.W.; Hermann, B.; Caplan, R. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum. Brain Mapp. 2011, 32, 580–591. [Google Scholar] [CrossRef]
- Curwood, E.K.; Pedersen, M.; Carney, P.W.; Berg, A.T.; Abbott, D.F.; Jackson, G.D. Abnormal cortical thickness connectivity persists in childhood absence epilepsy. Ann. Clin. Transl. Neurol. 2015, 2, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, B.F.; Sandhu, M.R.S.; Bertasi, R.A.O.; Bertasi, T.G.O.; Schonwald, A.; Kurup, A.; Gruenbaum, S.E.; Freedman, I.G.; Funaro, M.C.; Blumenfeld, H.; et al. Absence seizures and their relationship to depression and anxiety: Evidence for bidirectionality. Epilepsia 2021, 62, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-H.; Shim, W.-H.; Lee, J.-S.; Yoon, H.-M.; Ko, T.-S.; Yum, M.-S. Altered Structural Network in Newly Onset Childhood Absence Epilepsy. J. Clin. Neurol. 2020, 16, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Tenney, J.R.; Glauser, T.A. The Current State of Absence Epilepsy: Can We Have Your Attention? Epilepsy Curr. 2013, 13, 135–140. [Google Scholar] [CrossRef]
- Panayiotopoulos, C.P. Treatment of Typical Absence Seizures and Related Epileptic Syndromes. Pediatr. Drugs 2001, 3, 379–403. [Google Scholar] [CrossRef]
- Glauser, T.A.; Holland, K.; O’Brien, V.P.; Keddache, M.; Martin, L.J.; Clark, P.O.; Cnaan, A.; Dlugos, D.; Hirtz, D.G.; Shinnar, S.; et al. Pharmacogenetics of antiepileptic drug efficacy in childhood absence epilepsy. Ann. Neurol. 2017, 81, 444–453. [Google Scholar] [CrossRef]
- Krook-Magnuson, E.; Soltesz, I. Beyond the hammer and the scalpel: Selective circuit control for the epilepsies. Nat. Neurosci. 2015, 18, 331–338. [Google Scholar] [CrossRef]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef]
- Leitch, B. The Impact of Glutamatergic Synapse Dysfunction in the Corticothalamocortical Network on Absence Seizure Generation. Front. Mol. Neurosci. 2022, 15, 836255. [Google Scholar] [CrossRef]
- Vanluijtelaar, G.; Sitnikova, E. Global and focal aspects of absence epilepsy: The contribution of genetic models. Neurosci. Biobehav. Rev. 2006, 30, 983–1003. [Google Scholar] [CrossRef]
- Avoli, M. A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia 2012, 53, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Meeren, H.K.M.; Pijn, J.P.M.; Van Luijtelaar, E.L.J.M.; Coenen, A.M.L.; da Silva, F.H.L. Cortical Focus Drives Widespread Corticothalamic Networks during Spontaneous Absence Seizures in Rats. J. Neurosci. 2002, 22, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Meeren, H.; van Luijtelaar, G.; da Silva, F.L.; Coenen, A. Evolving Concepts on the Pathophysiology of Absence Seizures. Arch. Neurol. 2005, 62, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.-P.A.; Richards, D.A.; Bowery, N.G. Pharmacology of absence epilepsy. Trends Pharmacol. Sci. 2003, 24, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Polack, P.-O.; Guillemain, I.; Hu, E.; Deransart, C.; Depaulis, A.; Charpier, S. Deep Layer Somatosensory Cortical Neurons Initiate Spike-and-Wave Discharges in a Genetic Model of Absence Seizures. J. Neurosci. 2007, 27, 6590–6599. [Google Scholar] [CrossRef]
- Polack, P.-O.; Mahon, S.; Chavez, M.; Charpier, S. Inactivation of the Somatosensory Cortex Prevents Paroxysmal Oscillations in Cortical and Related Thalamic Neurons in a Genetic Model of Absence Epilepsy. Cereb. Cortex 2009, 19, 2078–2091. [Google Scholar] [CrossRef]
- Studer, F.; Laghouati, E.; Jarre, G.; David, O.; Pouyatos, B.; Depaulis, A. Sensory coding is impaired in rat absence epilepsy. J. Physiol. 2019, 597, 951–966. [Google Scholar] [CrossRef]
- Hillman, E.M. Coupling Mechanism and Significance of the BOLD Signal: A Status Report. Annu. Rev. Neurosci. 2014, 37, 161–181. [Google Scholar] [CrossRef]
- Gotman, J.; Grova, C.; Bagshaw, A.; Kobayashi, E.; Aghakhani, Y.; Dubeau, F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 15236–15240. [Google Scholar] [CrossRef]
- Laufs, H.; Lengler, U.; Hamandi, K.; Kleinschmidt, A.; Krakow, K. Linking Generalized Spike-and-Wave Discharges and Resting State Brain Activity by Using EEG/fMRI in a Patient with Absence Seizures. Epilepsia 2006, 47, 444–448. [Google Scholar] [CrossRef]
- Moeller, F.; Siebner, H.R.; Wolff, S.; Muhle, H.; Granert, O.; Jansen, O.; Stephani, U.; Siniatchkin, M. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia 2008, 49, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, C.; Yang, T.; Yao, Z.; He, L.; Liu, L.; Xu, H.; Gong, Q.; Yao, D.; Zhou, D. EEG–fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009, 87, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.; Negishi, M.; Vestal, M.; Spann, M.; Chung, M.H.; Bai, X.; Purcaro, M.; Motelow, J.E.; Danielson, N.; Dix-Cooper, L.; et al. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia 2010, 51, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.; Masterton, R.; Harvey, A.; Scheffer, I.; Berkovic, S.; Jackson, G. The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology 2010, 75, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Moeller, F.; LeVan, P.; Muhle, H.; Stephani, U.; Dubeau, F.; Siniatchkin, M.; Gotman, J. Absence seizures: Individual patterns revealed by EEG-fMRI. Epilepsia 2010, 51, 2000–2010. [Google Scholar] [CrossRef]
- Bai, X.; Vestal, M.; Berman, R.; Negishi, M.; Spann, M.; Vega, C.; Desalvo, M.; Novotny, E.J.; Constable, R.T.; Blumenfeld, H. Dynamic Time Course of Typical Childhood Absence Seizures: EEG, Behavior, and Functional Magnetic Resonance Imaging. J. Neurosci. 2010, 30, 5884–5893. [Google Scholar] [CrossRef]
- Guo, J.N.; Kim, R.; Chen, Y.; Negishi, M.; Jhun, S.; Weiss, S.; Ryu, J.H.; Bai, X.; Xiao, W.; Feeney, E.; et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: A cross-sectional study. Lancet Neurol. 2016, 15, 1336–1345. [Google Scholar] [CrossRef]
- Kumar, A.; Lyzhko, E.; Hamid, L.; Srivastav, A.; Stephani, U.; Japaridze, N. Neuronal networks underlying ictal and subclinical discharges in childhood absence epilepsy. J. Neurol. 2023, 270, 1402–1415. [Google Scholar] [CrossRef]
- Benuzzi, F.; Ballotta, D.; Mirandola, L.; Ruggieri, A.; Vaudano, A.E.; Zucchelli, M.; Ferrari, E.; Nichelli, P.F.; Meletti, S. An EEG-fMRI Study on the Termination of Generalized Spike-And-Wave Discharges in Absence Epilepsy. PLoS ONE 2015, 10, e0130943. [Google Scholar] [CrossRef]
- Tangwiriyasakul, C.; Perani, S.; Centeno, M.; Yaakub, S.N.; Abela, E.; Carmichael, D.W.; Richardson, M.P. Dynamic brain network states in human generalized spike-wave discharges. Brain 2018, 141, 2981–2994. [Google Scholar] [CrossRef]
- Leitch, B. Parvalbumin Interneuron Dysfunction in Neurological Disorders: Focus on Epilepsy and Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5549. [Google Scholar] [CrossRef] [PubMed]
- Jones, E. Viewpoint: The core and matrix of thalamic organization. Neuroscience 1998, 85, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Constantinople, C.M.; Bruno, R.M. Deep Cortical Layers Are Activated Directly by Thalamus. Science 2013, 340, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Briggs, F. Structure and function of corticothalamic pathways of layer 6 neurons. In The Cerebral Cortex and Thalamus; Oxford University Press: Oxford, UK, 2024; pp. 143–151. [Google Scholar] [CrossRef]
- Paz, J.T.; Bryant, A.S.; Peng, K.; Fenno, L.; Yizhar, O.; Frankel, W.N.; Deisseroth, K.; Huguenard, J.R. A new mode of corticothalamic transmission revealed in the Gria4−/− model of absence epilepsy. Nat. Neurosci. 2011, 14, 1167–1173. [Google Scholar] [CrossRef]
- McCafferty, C.; Gruenbaum, B.F.; Tung, R.; Li, J.-J.; Zheng, X.; Salvino, P.; Vincent, P.; Kratochvil, Z.; Ryu, J.H.; Khalaf, A.; et al. Decreased but diverse activity of cortical and thalamic neurons in consciousness-impairing rodent absence seizures. Nat. Commun. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Maheshwari, A.; Noebels, J.L. Monogenic models of absence epilepsy: Windows into the complex balance between inhibition and excitation in thalamocortical microcircuits. Prog. Brain Res. 2014, 213, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Noebels, J.; Qiao, X.; Bronson, R.; Spencer, C.; Davisson, M. Stargazer: A new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990, 7, 129–135, Erratum in Epilepsy Res. 1992, 11, 72. [Google Scholar] [CrossRef]
- Letts, V.A. Stargazer—A Mouse to Seize! Epilepsy Curr. 2005, 5, 161–165. [Google Scholar] [CrossRef]
- Letts, V.A.; Felix, R.; Biddlecome, G.H.; Arikkath, J.; Mahaffey, C.L.; Valenzuela, A.; Bartlett, F.S.; Mori, Y.; Campbell, K.P.; Frankel, W.N. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nat. Genet. 1998, 19, 340–347. [Google Scholar] [CrossRef]
- Chen, L.; Chetkovich, D.M.; Petralia, R.S.; Sweeney, N.T.; Kawasaki, Y.; Wenthold, R.J.; Bredt, D.S.; Nicoll, R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408, 936–943. [Google Scholar] [CrossRef]
- Tomita, S.; Chen, L.; Kawasaki, Y.; Petralia, R.S.; Wenthold, R.J.; Nicoll, R.A.; Bredt, D.S. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003, 161, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Adesnik, H.; Sekiguchi, M.; Zhang, W.; Wada, K.; Howe, J.R.; Nicoll, R.A.; Bredt, D.S. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 2005, 435, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.A.; Tomita, S.; Bredt, D.S. Auxiliary Subunits Assist AMPA-Type Glutamate Receptors. Science 2006, 311, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Fukaya, M.; Qiao, X.; Sakimura, K.; Watanabe, M.; Kano, M. Impairment of AMPA Receptor Function in Cerebellar Granule Cells of Ataxic Mutant Mouse. Stargazer. J. Neurosci. 1999, 19, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, O.; Leitch, B. Selective loss of AMPA receptor subunits at inhibitory neuron synapses in the cerebellum of the ataxic stargazer mouse. Brain Res. 2012, 1427, 54–64. [Google Scholar] [CrossRef]
- Barad, Z.; Shevtsova, O.; Arbuthnott, G.; Leitch, B. Selective loss of AMPA receptors at corticothalamic synapses in the epileptic stargazer mouse. Neuroscience 2012, 217, 19–31. [Google Scholar] [CrossRef]
- Adotevi, N.K.; Leitch, B. Alterations in AMPA receptor subunit expression in cortical inhibitory interneurons in the epileptic stargazer mutant mouse. Neuroscience 2016, 339, 124–138. [Google Scholar] [CrossRef]
- Adotevi, N.K.; Leitch, B. Synaptic Changes in AMPA Receptor Subunit Expression in Cortical Parvalbumin Interneurons in the Stargazer Model of Absence Epilepsy. Front. Mol. Neurosci. 2017, 10, 434. [Google Scholar] [CrossRef]
- Adotevi, N.K.; Leitch, B. Cortical expression of AMPA receptors during postnatal development in a genetic model of absence epilepsy. Int. J. Dev. Neurosci. 2018, 73, 19–25. [Google Scholar] [CrossRef]
- Kato, A.S.; Gill, M.B.; Yu, H.; Nisenbaum, E.S.; Bredt, D.S. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010, 33, 241–248. [Google Scholar] [CrossRef]
- Everett, K.V.; Chioza, B.; Aicardi, J.; Aschauer, H.; Brouwer, O.; Callenbach, P.; Covanis, A.; Dulac, O.; Eeg-Olofsson, O.; Feucht, M.; et al. Linkage and association analysis of CACNG3 in childhood absence epilepsy. Eur. J. Hum. Genet. 2007, 15, 463–472, Erratum in Eur. J. Hum. Genet. 2008, 16, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.M.; Maclean, A.; Irfan, M.; Naeem, F.; Cass, S.; Pickard, B.S.; Muir, W.J.; Blackwood, D.H.R.; Ayub, M. Homozygosity mapping in a family presenting with schizophrenia, epilepsy and hearing impairment. Eur. J. Hum. Genet. 2008, 16, 750–758. [Google Scholar] [CrossRef]
- Beyer, B.; Deleuze, C.; Letts, V.A.; Mahaffey, C.L.; Boumil, R.M.; Lew, T.A.; Huguenard, J.R.; Frankel, W.N. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Hum. Mol. Genet. 2008, 17, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Golshani, P.; Liu, X.-B.; Jones, E.G. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.F.; Lutz, C.M.; O’Sullivan, T.; Shaughnessy, J.D., Jr.; Hawkes, R.; Frankel, W.N.; Copeland, N.G.; Jenkins, N.A. Absence Epilepsy in Tottering Mutant Mice Is Associated with Calcium Channel Defects. Cell 1996, 87, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Huda, K.; Inoue, T.; Miyata, M.; Imoto, K. Impaired Feedforward Inhibition of the Thalamocortical Projection in Epileptic Ca2+ Channel Mutant Mice, tottering. J. Neurosci. 2006, 26, 3056–3065. [Google Scholar] [CrossRef]
- Wakamori, M.; Yamazaki, K.; Matsunodaira, H.; Teramoto, T.; Tanaka, I.; Niidome, T.; Sawada, K.; Nishizawa, Y.; Sekiguchi, N.; Mori, E.; et al. Single tottering mutations responsible for the neuropathic phenotype of the P-type calcium channel. J. Biol. Chem. 1998, 273, 34857–34867. [Google Scholar] [CrossRef]
- Jouvenceau, A.; Eunson, L.H.; Spauschus, A.; Ramesh, V.; Zuberi, S.M.; Kullmann, D.M.; Hanna, M.G. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet 2001, 358, 801–807. [Google Scholar] [CrossRef]
- Panthi, S.; Leitch, B. The impact of silencing feed-forward parvalbumin-expressing inhibitory interneurons in the cortico-thalamocortical network on seizure generation and behaviour. Neurobiol. Dis. 2019, 132, 104610. [Google Scholar] [CrossRef]
- Panthi, S.; Leitch, B. Chemogenetic Activation of Feed-Forward Inhibitory Parvalbumin-Expressing Interneurons in the Cortico-Thalamocortical Network During Absence Seizures. Front. Cell. Neurosci. 2021, 15, 688905. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Onat, F.Y.; Gallagher, M.J. Animal models of absence epilepsies: What do they model and do sex and sex hormones matter? Neurobiol. Dis. 2014, 72 Pt B, 167–179. [Google Scholar] [CrossRef]
- Depaulis, A.; Charpier, S. Pathophysiology of absence epilepsy: Insights from genetic models. Neurosci. Lett. 2018, 667, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, E. Behavioral and Cognitive Comorbidities in Genetic Rat Models of Absence Epilepsy (Focusing on GAERS and WAG/Rij Rats). Biomedicines 2024, 12, 122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kole, M.H.; Bräuer, A.U.; Stuart, G.J. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J. Physiol. 2007, 578 Pt 2, 507–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cope, D.W.; Di Giovanni, G.; Fyson, S.J.; Orbán, G.; Errington, A.C.; Lorincz, M.L.; Gould, T.M.; Carter, D.A.; Crunelli, V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 2009, 15, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crunelli, V.; Leresche, N.; Cope, D.W. GABA-A Receptor Function in Typical Absence Seizures. In Jasper’s Basic Mechanisms of the Epilepsies [Internet], 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar] [PubMed]
- Richards, D.A.; Lemos, T.; Whitton, P.S.; Bowery, N.G. Extracellular GABA in the ventrolateral thalamus of rats exhibiting spontaneous absence epilepsy: A microdialysis study. J. Neurochem. 1995, 65, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, W.; Díez-Sampedro, A.; Richerson, G.B. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 2007, 56, 851–865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCafferty, C.; David, F.; Venzi, M.; Lőrincz, M.L.; Delicata, F.; Atherton, Z.; Recchia, G.; Orban, G.; Lambert, R.C.; Di Giovanni, G.; et al. Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat. Neurosci. 2018, 21, 744–756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallagher, M.J. Neuronal Physiology of Generalized Seizures: The 4 Horsemen of Absence Epilepsy. Epilepsy Curr. 2023, 23, 262–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, H.; Tatsukawa, T.; Shimohata, A.; Yamagata, T.; Suzuki, T.; Amano, K.; Mazaki, E.; Raveau, M.; Ogiwara, I.; Oba-Asaka, A.; et al. Impaired cortico-striatal excitatory transmission triggers epilepsy. Nat. Commun. 2019, 10, 1917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deransart, C.; Marescaux, C.; Depaulis, A. Involvement of nigral glutamatergic inputs in the control of seizures in a genetic model of absence epilepsy in the rat. Neuroscience 1996, 71, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Deransart, C.; Riban, V.; Lê, B.T.; Hechler, V.; Marescaux, C.; Depaulis, A. Evidence for the involvement of the pallidum in the modulation of seizures in a genetic model of absence epilepsy in the rat. Neurosci. Lett. 1999, 265, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Paz, J.T.; Chavez, M.; Saillet, S.; Deniau, J.M.; Charpier, S. Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J. Neurosci. 2007, 27, 929–941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kase, D.; Inoue, T.; Imoto, K. Roles of the subthalamic nucleus and subthalamic HCN channels in absence seizures. J. Neurophysiol. 2012, 107, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, T.; Mahon, S.; Charpier, S.; Leblois, A.; Hansel, D. The Role of Striatal Feedforward Inhibition in the Maintenance of Absence Seizures. J. Neurosci. 2016, 36, 9618–9632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gittis, A.H.; Leventhal, D.K.; Fensterheim, B.A.; Pettibone, J.R.; Berke, J.D.; Kreitzer, A.C. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J. Neurosci. 2011, 31, 15727–15731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, M.; Li, L.; Pittenger, C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience 2016, 324, 321–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.; Holley, S.M.; Shobe, J.L.; Chong, N.C.; Cepeda, C.; Levine, M.S.; Masmanidis, S.C. Parvalbumin Interneurons Modulate Striatal Output and Enhance Performance during Associative Learning. Neuron 2017, 93, 1451–1463.e4, Erratum in Neuron 2018, 99, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Song, I.; Keum, S.; Lee, T.; Jeong, M.J.; Kim, S.S.; McEnery, M.W.; Shin, H.S. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron 2001, 31, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kerestes, R.; Perry, A.; Vivash, L.; O’Brien, T.J.; Alvim, M.K.M.; Arienzo, D.; Aventurato, Í.K.; Ballerini, A.; Baltazar, G.F.; Bargalló, N.; et al. Patterns of subregional cerebellar atrophy across epilepsy syndromes: An ENIGMA-Epilepsy study. Epilepsia 2024, 65, 1072–1091. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, J.C.; Pakusch, J.; Mücher, B.; Brückner, A.; Depke, D.A.; Fenzl, T.; De Zeeuw, C.I.; Kros, L.; Hoebeek, F.E.; Mark, M.D. Controlling absence seizures from the cerebellar nuclei via activation of the Gq signaling pathway. Cell. Mol. Life Sci. 2022, 79, 197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beckinghausen, J.; Ortiz-Guzman, J.; Lin, T.; Bachman, B.; Leon, L.E.S.; Liu, Y.; Heck, D.H.; Arenkiel, B.R.; Sillitoe, R.V. The cerebellum contributes to generalized seizures by altering activity in the ventral posteromedial nucleus. Commun. Biol. 2023, 6, 1–17, Erratum in Commun. Biol. 2024, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Rajjoub, R.K.; Wood, J.H.; VAN Buren, J.M. Significance of Purkinje cell density in seizure suppression by chronic cerebellar stimulation. Neurology 1976, 26, 645. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Gemignani, F.; Marchese, M. The involvement of Purkinje cells in progressive myoclonic epilepsy: Focus on neuronal ceroid lipofuscinosis. Neurobiol. Dis. 2023, 185, 106258. [Google Scholar] [CrossRef] [PubMed]
- Dam, M.; Bolwig, T.; Hertz, M.; Bajorec, J.; Lomax, P.; Dam, A.M. Does Seizure Activity Produce Purkinje Cell Loss? Epilepsia 1984, 25, 747–751. [Google Scholar] [CrossRef]
- Roostaei, T.; Nazeri, A.; Sahraian, M.A.; Minagar, A. The Human Cerebellum: A review of physiologic neuroanatomy. Neurol. Clin. 2014, 32, 859–869. [Google Scholar] [CrossRef]
- Ming, X.; Prasad, N.; Thulasi, V.; Elkins, K.; Shivamurthy, V.K.N. Possible contribution of cerebellar disinhibition in epilepsy. Epilepsy Behav. 2021, 118, 107944. [Google Scholar] [CrossRef]
- Moeller, F.; Maneshi, M.; Pittau, F.; Gholipour, T.; Bellec, P.; Dubeau, F.; Grova, C.; Gotman, J. Functional connectivity in patients with idiopathic generalized epilepsy. Epilepsia 2011, 52, 515–522. [Google Scholar] [CrossRef]
- Niedermeyer, E.; Uematsu, S. Electroencephalographic recordings from deep cerebellar structures in patients with uncontrolled epileptic seizures. Electroencephalogr. Clin. Neurophysiol. 1974, 37, 355–365. [Google Scholar] [CrossRef]
- Kandel, A.; Buzsáki, G. Cerebellar neuronal activity correlates with spike and wave EEG patterns in the rat. Epilepsy Res. 1993, 16, 1–9. [Google Scholar] [CrossRef]
- Mark, M.D.; Maejima, T.; Kuckelsberg, D.; Yoo, J.W.; Hyde, R.A.; Shah, V.; Gutierrez, D.; Moreno, R.L.; Kruse, W.; Noebels, J.L.; et al. Delayed Postnatal Loss of P/Q-Type Calcium Channels Recapitulates the Absence Epilepsy, Dyskinesia, and Ataxia Phenotypes of Genomic Cacna1A Mutations. J. Neurosci. 2011, 31, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Maejima, T.; Wollenweber, P.; Teusner, L.U.; Noebels, J.L.; Herlitze, S.; Mark, M.D. Postnatal Loss of P/Q-Type Channels Confined to Rhombic-Lip-Derived Neurons Alters Synaptic Transmission at the Parallel Fiber to Purkinje Cell Synapse and Replicates Genomic Cacna1a Mutation Phenotype of Ataxia and Seizures in Mice. J. Neurosci. 2013, 33, 5162–5174. [Google Scholar] [CrossRef] [PubMed]
- Kros, L.; Rooda, O.H.J.E.; Spanke, J.K.; Alva, P.; van Dongen, M.N.; Karapatis, A.; Tolner, E.A.; Strydis, C.; Davey, N.; Winkelman, B.H.J.; et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann. Neurol. 2015, 77, 1027–1049. [Google Scholar] [CrossRef] [PubMed]
- Kros, L.; Lindeman, S.; Rooda, O.H.J.E.; Murugesan, P.; Bina, L.; Bosman, L.W.J.; De Zeeuw, C.I.; Hoebeek, F.E. Synchronicity and Rhythmicity of Purkinje Cell Firing during Generalized Spike-and-Wave Discharges in a Natural Mouse Model of Absence Epilepsy. Front. Cell. Neurosci. 2017, 11, 346, Erratum in Front. Cell. Neurosci. 2017, 11, 369. [Google Scholar] [CrossRef]
- Streng, M.L.; Froula, J.M.; Krook-Magnuson, E. The cerebellum’s understated role and influences in the epilepsies. Neurobiol. Dis. 2023, 183, 106160. [Google Scholar] [CrossRef]
- Streng, M.L.; Krook-Magnuson, E. The cerebellum and epilepsy. Epilepsy Behav. 2020, 121 Pt B, 106909. [Google Scholar] [CrossRef]
- Richardson, C.A.; Leitch, B. Cerebellar Golgi, Purkinje, and basket cells have reduced γ-aminobutyric acid immunoreactivity in stargazer mutant mice. J. Comp. Neurol. 2002, 453, 85–99. [Google Scholar] [CrossRef]
- Richardson, C.A.; Leitch, B. Phenotype of cerebellar glutamatergic neurons is altered in stargazer mutant mice lacking brain-derived neurotrophic factor mRNA expression. J. Comp. Neurol. 2004, 481, 145–159. [Google Scholar] [CrossRef]
- Leitch, B.; Shevtsova, O.; Guévremont, D.; Williams, J. Loss of calcium channels in the cerebellum of the ataxic and epileptic stargazer mutant mouse. Brain Res. 2009, 1279, 156–167. [Google Scholar] [CrossRef]
- Trotman, M.; Barad, Z.; Guévremont, D.; Williams, J.; Leitch, B. Changes in the GRIP 1&2 scaffolding proteins in the cerebellum of the ataxic stargazer mouse. Brain Res. 2014, 1546, 53–62. [Google Scholar] [CrossRef]
- Marcián, V.; Filip, P.; Bareš, M.; Brázdil, M. Cerebellar Dysfunction and Ataxia in Patients with Epilepsy: Coincidence, Consequence, or Cause? Tremor Other Hyperkinet. Mov. (N. Y.) 2016, 6, 376, Erratum in Tremor Other Hyperkinet. Mov. (N. Y.) 2016, 6, 416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EPICURE Consortium; Steffens, M.; Leu, C.; Ruppert, A.-K.; Zara, F.; Striano, P.; Robbiano, A.; Capovilla, G.; Tinuper, P.; Gambardella, A.; et al. Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum. Mol. Genet. 2012, 21, 5359–5372. [Google Scholar] [CrossRef] [PubMed]
- The International League against Epilepsy Consortium on Complex Epilepsies; Anney, R.J.L.; Avbersek, A.; Balding, D.; Baum, L.; Becker, F.; Berkovic, S.F.; Bradfield, J.P.; Brody, L.C.; Buono, R.J.; et al. Genetic determinants of common epilepsies: A meta-analysis of genome-wide association studies. Lancet Neurol. 2014, 13, 893–903. [Google Scholar] [CrossRef]
- The International League against Epilepsy Consortium on Complex Epilepsies; Abou-Khalil, B.; Auce, P.; Avbersek, A.; Bahlo, M.; Balding, D.J.; Bast, T.; Baum, L.; Becker, A.J.; Becker, F.; et al. Genome-wide mega-analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Feng, Y.-C.A.; Howrigan, D.P.; Abbott, L.E.; Tashman, K.; Cerrato, F.; Singh, T.; Heyne, H.; Byrnes, A.; Churchhouse, C.; Watts, N.; et al. Ultra-Rare Genetic Variation in the Epilepsies: A Whole-Exome Sequencing Study of 17,606 Individuals. Am. J. Hum. Genet. 2019, 105, 267–282. [Google Scholar] [CrossRef]
- Rossignol, E.; Kruglikov, I.; Van Den Maagdenberg, A.M.; Rudy, B.; Fishell, G. CaV2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann. Neurol. 2013, 74, 209–222. [Google Scholar] [CrossRef]
- Ernst, W.L.; Zhang, Y.; Yoo, J.W.; Ernst, S.J.; Noebels, J.L. Genetic Enhancement of Thalamocortical Network Activity by Elevating α1G-Mediated Low-Voltage-Activated Calcium Current Induces Pure Absence Epilepsy. J. Neurosci. 2009, 29, 1615–1625. [Google Scholar] [CrossRef]
- Song, I.; Kim, D.; Choi, S.; Sun, M.; Kim, Y.; Shin, H.-S. Role of the α1G T-Type Calcium Channel in Spontaneous Absence Seizures in Mutant Mice. J. Neurosci. 2004, 24, 5249–5257. [Google Scholar] [CrossRef]
- Miao, Q.-L.; Herlitze, S.; Mark, M.D.; Noebels, J.L. Adult loss of Cacna1a in mice recapitulates childhood absence epilepsy by distinct thalamic bursting mechanisms. Brain 2019, 143, 161–174, Erratum in Brain 2020, 143, e24. [Google Scholar] [CrossRef]
- Powell, K.L.; Cain, S.M.; Ng, C.; Sirdesai, S.; David, L.S.; Kyi, M.; Garcia, E.; Tyson, J.R.; Reid, C.A.; Bahlo, M.; et al. A Cav3.2 T-Type Calcium Channel Point Mutation Has Splice-Variant-Specific Effects on Function and Segregates with Seizure Expression in a Polygenic Rat Model of Absence Epilepsy. J. Neurosci. 2009, 29, 371–380. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, J.; Pan, H.; Zhang, Y.; Wu, H.; Xu, K.; Liu, X.; Jiang, Y.; Bao, X.; Yao, Z.; et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann. Neurol. 2003, 54, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Eckle, V.; Shcheglovitov, A.; Vitko, I.; Dey, D.; Yap, C.C.; Winckler, B.; Perez-Reyes, E. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J. Physiol. 2014, 592, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Monteil, A.; Bidaud, I.; Sugimoto, Y.; Suzuki, T.; Hamano, S.-I.; Oguni, H.; Osawa, M.; Alonso, M.E.; Delgado-Escueta, A.V.; et al. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Mutation in brief #962. Online. Hum. Mutat. 2007, 28, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Zamponi, G.W. Genetic T-type calcium channelopathies. J. Med. Genet. 2019, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.; Mcpike, A. Ducky, a Neurological Mutation in Mice Characterized by Deficiency of Cerebrosides. Exp. Med. Surg. 1970, 28, 256–269. [Google Scholar]
- Burgess, D.L.; Jones, J.M.; Meisler, M.H.; Noebels, J.L. Mutation of the Ca2+ Channel β Subunit Gene Cchb4 Is Associated with Ataxia and Seizures in the Lethargic (lh) Mouse. Cell 1997, 88, 385–392. [Google Scholar] [CrossRef]
- Elsen, F.P.; Liljelund, P.; Werner, D.F.; Olsen, R.W.; Homanics, G.E.; Harrison, N.L. GABAA-R α1 subunit knockin mutation leads to abnormal EEG and anesthetic-induced seizure-like activity in mice. Brain Res. 2006, 1078, 60–70. [Google Scholar] [CrossRef]
- Arain, F.M.; Boyd, K.L.; Gallagher, M.J. Decreased viability and absence-like epilepsy in mice lacking or deficient in the GABAA receptor α1 subunit. Epilepsia 2012, 53, e161–e165. [Google Scholar] [CrossRef]
- DeLorey, T.M.; Handforth, A.; Anagnostaras, S.G.; Homanics, G.E.; Minassian, B.A.; Asatourian, A.; Fanselow, M.S.; Delgado-Escueta, A.; Ellison, G.D.; Olsen, R.W. Mice Lacking the β3Subunit of the GABAAReceptor Have the Epilepsy Phenotype and Many of the Behavioral Characteristics of Angelman Syndrome. J. Neurosci. 1998, 18, 8505–8514. [Google Scholar] [CrossRef]
- Frugier, G.; Coussen, F.; Giraud, M.-F.; Odessa, M.-F.; Emerit, M.B.; Boué-Grabot, E.; Garret, M. A γ2(R43Q) Mutation, Linked to Epilepsy in Humans, Alters GABAA Receptor Assembly and Modifies Subunit Composition on the Cell Surface. J. Biol. Chem. 2007, 282, 3819–3828. [Google Scholar] [CrossRef]
- Maljevic, S.; Krampfl, K.; Cobilanschi, J.; Tilgen, N.; Beyer, S.; Weber, Y.G.; Schlesinger, F.; Ursu, D.; Melzer, W.; Cossette, P.; et al. A mutation in the GABAA receptor α1-subunit is associated with absence epilepsy. Ann. Neurol. 2006, 59, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Urak, L.; Feucht, M.; Fathi, N.; Hornik, K.; Fuchs, K. A GABRB3 promoter haplotype associated with childhood absence epilepsy impairs transcriptional activity. Hum. Mol. Genet. 2006, 15, 2533–2541, Erratum in Hum. Mol. Genet. 2006, 15, 3272. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Z.; Ding, L.; Deel, M.E.; Arain, F.M.; Murray, C.R.; Patel, R.S.; Flanagan, C.D.; Gallagher, M.J. Altered Cortical GABAA Receptor Composition, Physiology, and Endocytosis in a Mouse Model of a Human Genetic Absence Epilepsy Syndrome. J. Biol. Chem. 2013, 288, 21458–21472. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Z.; Ning, G.; Guo, Y.; Ali, R.; Macdonald, R.L.; De Blas, A.L.; Luscher, B.; Chen, G. γ-Aminobutyric Acid Type A (GABAA) Receptor α Subunits Play a Direct Role in Synaptic Versus Extrasynaptic Targeting. J. Biol. Chem. 2012, 287, 27417–27430. [Google Scholar] [CrossRef] [PubMed]
- Pirker, S.; Schwarzer, C.; Wieselthaler, A.; Sieghart, W.; Sperk, G. GABAA receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 2000, 101, 815–850. [Google Scholar] [CrossRef]
- Huntsman, M.M.; Porcello, D.M.; Homanics, G.E.; DeLorey, T.M.; Huguenard, J.R. Reciprocal Inhibitory Connections and Network Synchrony in the Mammalian Thalamus. Science 1999, 283, 541–543. [Google Scholar] [CrossRef]
- Seo, S.; Leitch, B. Altered thalamic GABAA-receptor subunit expression in the stargazer mouse model of absence epilepsy. Epilepsia 2014, 55, 224–232. [Google Scholar] [CrossRef]
- Seo, S.; Leitch, B. Synaptic changes in GABAA receptor expression in the thalamus of the stargazer mouse model of absence epilepsy. Neuroscience 2015, 306, 28–38. [Google Scholar] [CrossRef]
- Seo, S.; Leitch, B. Postnatal expression of thalamic GABAA receptor subunits in the stargazer mouse model of absence epilepsy. NeuroReport 2017, 28, 1255–1260. [Google Scholar] [CrossRef]
- Hassan, M.; Adotevi, N.K.; Leitch, B. Altered GABAA Receptor Expression in the Primary Somatosensory Cortex of a Mouse Model of Genetic Absence Epilepsy. Int. J. Mol. Sci. 2022, 23, 15685. [Google Scholar] [CrossRef]
- Hassan, M.; Grattan, D.R.; Leitch, B. Developmental Inhibitory Changes in the Primary Somatosensory Cortex of the Stargazer Mouse Model of Absence Epilepsy. Biomolecules 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, V.E.; Di Cara, G.; Mencaroni, E.; Verrotti, A. Therapeutic Options for Childhood Absence Epilepsy. Pediatr. Rep. 2021, 13, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.K.; McGinnis, E. A Practical Guide to Treatment of Childhood Absence Epilepsy. Pediatr. Drugs 2019, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.A.; Cnaan, A.; Shinnar, S.; Hirtz, D.G.; Dlugos, D.; Masur, D.; Clark, P.O.; Capparelli, E.V.; Adamson, P.C. Ethosuximide, Valproic Acid, and Lamotrigine in Childhood Absence Epilepsy. N. Engl. J. Med. 2010, 362, 790–799. [Google Scholar] [CrossRef]
- Glauser, T.A.; Cnaan, A.; Shinnar, S.; Hirtz, D.G.; Dlugos, D.; Masur, D.; Clark, P.O.; Adamson, P.C.; Childhood Absence Epilepsy Study Team. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: Initial monotherapy outcomes at 12 months. Epilepsia 2012, 54, 141–155. [Google Scholar] [CrossRef]
- Cnaan, A.; Shinnar, S.; Arya, R.; Adamson, P.C.; Clark, P.O.; Dlugos, D.; Hirtz, D.G.; Masur, D.; Glauser, T.A.; Abram, H.; et al. Second monotherapy in childhood absence epilepsy. Neurology 2017, 88, 182–190. [Google Scholar] [CrossRef]
- Crunelli, V.; Leresche, L. Block of Thalamic T-Type Ca2+ Channels by Ethosuximide Is Not the Whole Story. Epilepsy Curr. 2002, 2, 53–56. [Google Scholar] [CrossRef]
- Johnston, D. Valproic Acid: Update on Its Mechanisms of Action. Epilepsia 1984, 25, S1–S4. [Google Scholar] [CrossRef]
- Tomson, T.; Marson, A.; Boon, P.; Canevini, M.P.; Covanis, A.; Gaily, E.; Kälviäinen, R.; Trinka, E. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia 2015, 56, 1006–1019. [Google Scholar] [CrossRef]
- Verrotti, A.; Striano, P.; Iapadre, G.; Zagaroli, L.; Bonanni, P.; Coppola, G.; Elia, M.; Mecarelli, O.; Franzoni, E.; De Liso, P.; et al. The pharmacological management of Lennox-Gastaut syndrome and critical literature review. Seizure 2018, 63, 17–25. [Google Scholar] [CrossRef]
- Catterall, W.A. Sodium Channels, Inherited Epilepsy, and Antiepileptic Drugs. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.C.; Camfield, C.S.; Camfield, P.R.; Gordon, K.E.; Dooley, J.M. Long-term prognosis of typical childhood absence epilepsy: Remission or progression to juvenile myoclonic epilepsy. Neurology 1996, 47, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Trinka, E.; Baumgartner, S.; Unterberger, I.; Unterrainer, J.; Luef, G.; Haberlandt, E.; Bauer, G. Long-term prognosis for childhood and juvenile absence epilepsy. J. Neurol. 2004, 251, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Grosso, S.; Galimberti, D.; Vezzosi, P.; Farnetani, M.; Di Bartolo, R.M.; Bazzotti, S.; Morgese, G.; Balestri, P. Childhood Absence Epilepsy: Evolution and Prognostic Factors. Epilepsia 2005, 46, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Valentin, A.; Hindocha, N.; Osei-Lah, A.; Fisniku, L.; McCormick, D.; Asherson, P.; Moran, N.; Makoff, A.; Nashef, L. Idiopathic Generalized Epilepsy with Absences: Syndrome Classification. Epilepsia 2007, 48, 2187–2190. [Google Scholar] [CrossRef]
- Callenbach, P.M.; Bouma, P.A.; Geerts, A.T.; Arts, W.F.M.; Stroink, H.; Peeters, E.A.; van Donselaar, C.A.; Peters, A.B.; Brouwer, O.F. Long-term outcome of childhood absence epilepsy: Dutch Study of Epilepsy in Childhood. Epilepsy Res. 2009, 83, 249–256. [Google Scholar] [CrossRef]
- Morse, E.; Giblin, K.; Chung, M.H.; Dohle, C.; Berg, A.T.; Blumenfeld, H. Historical trend toward improved long-term outcome in childhood absence epilepsy. Epilepsy Res. 2019, 152, 7–10. [Google Scholar] [CrossRef]
- Byrne, S.; Enright, N.; Delanty, N. Precision therapy in the genetic epilepsies of childhood. Dev. Med. Child Neurol. 2021, 63, 1276–1282. [Google Scholar] [CrossRef]
- Berg, A.T.; Levy, S.R.; Testa, F.M.; Blumenfeld, H. Long-term seizure remission in childhood absence epilepsy: Might initial treatment matter? Epilepsia 2014, 55, 551–557. [Google Scholar] [CrossRef]
- Dezsi, G.; Ozturk, E.; Stanic, D.; Powell, K.L.; Blumenfeld, H.; O’Brien, T.J.; Jones, N.C. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia 2013, 54, 635–643. [Google Scholar] [CrossRef]
- Koch, N.A.; Sonnenberg, L.; Hedrich, U.B.S.; Lauxmann, S.; Benda, J. Loss or gain of function? Effects of ion channel mutations on neuronal firing depend on the neuron type. Front. Neurol. 2023, 14, 1194811. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.-H.; Chahine, M.; Scantlebury, M.H.; Appendino, J.P. Channelopathies in epilepsy: An overview of clinical presentations, pathogenic mechanisms, and therapeutic insights. J. Neurol. 2024, 271, 3063–3094. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitch, B. Molecular Mechanisms Underlying the Generation of Absence Seizures: Identification of Potential Targets for Therapeutic Intervention. Int. J. Mol. Sci. 2024, 25, 9821. https://doi.org/10.3390/ijms25189821
Leitch B. Molecular Mechanisms Underlying the Generation of Absence Seizures: Identification of Potential Targets for Therapeutic Intervention. International Journal of Molecular Sciences. 2024; 25(18):9821. https://doi.org/10.3390/ijms25189821
Chicago/Turabian StyleLeitch, Beulah. 2024. "Molecular Mechanisms Underlying the Generation of Absence Seizures: Identification of Potential Targets for Therapeutic Intervention" International Journal of Molecular Sciences 25, no. 18: 9821. https://doi.org/10.3390/ijms25189821
APA StyleLeitch, B. (2024). Molecular Mechanisms Underlying the Generation of Absence Seizures: Identification of Potential Targets for Therapeutic Intervention. International Journal of Molecular Sciences, 25(18), 9821. https://doi.org/10.3390/ijms25189821





