Itaconic Acid Alleviates Perfluorooctanoic Acid-Induced Oxidative Stress and Intestinal Damage by Regulating the Keap1/Nrf2/Ho-1 Pathway and Reshaping the Gut Microbiota
Abstract
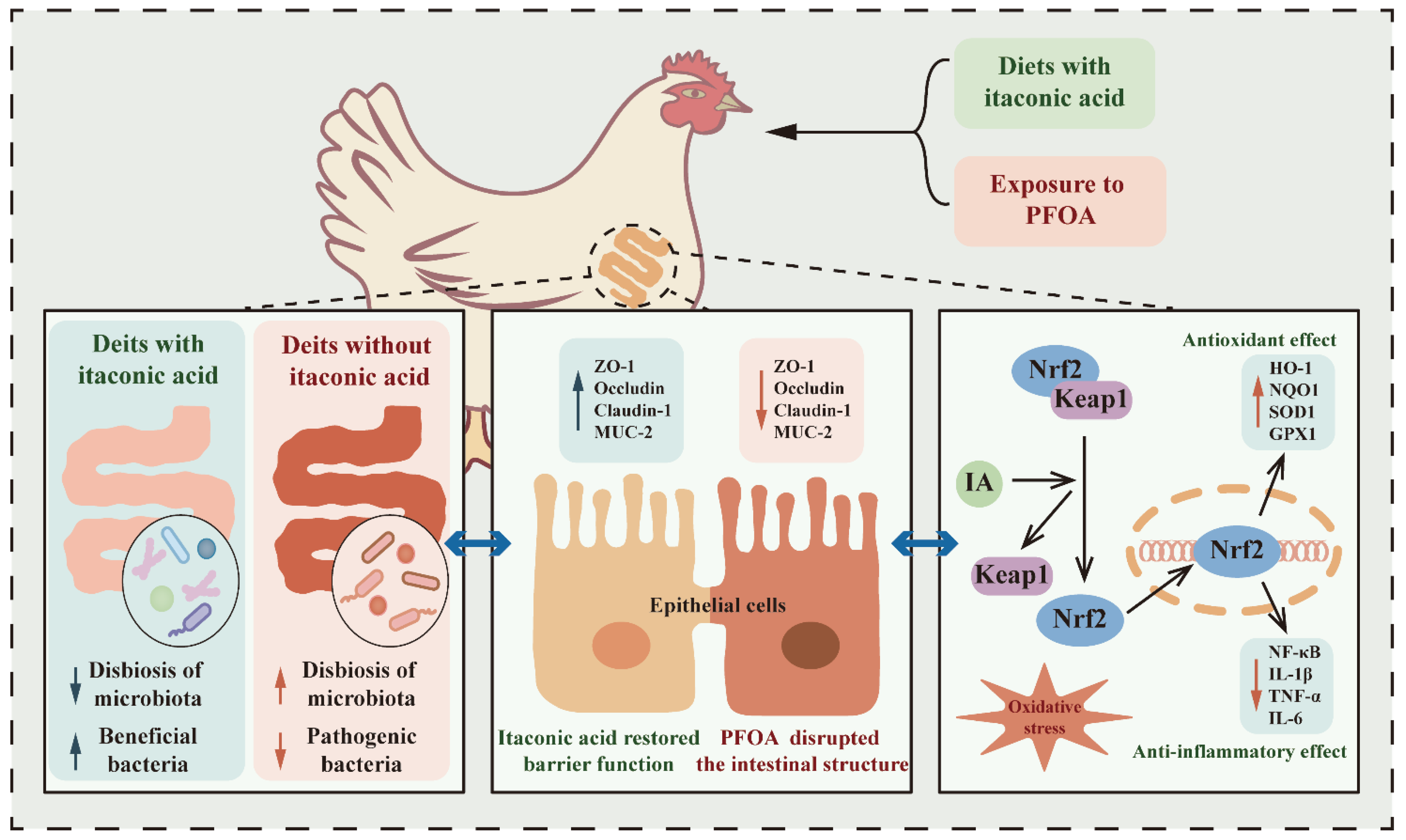
:1. Introduction
2. Results
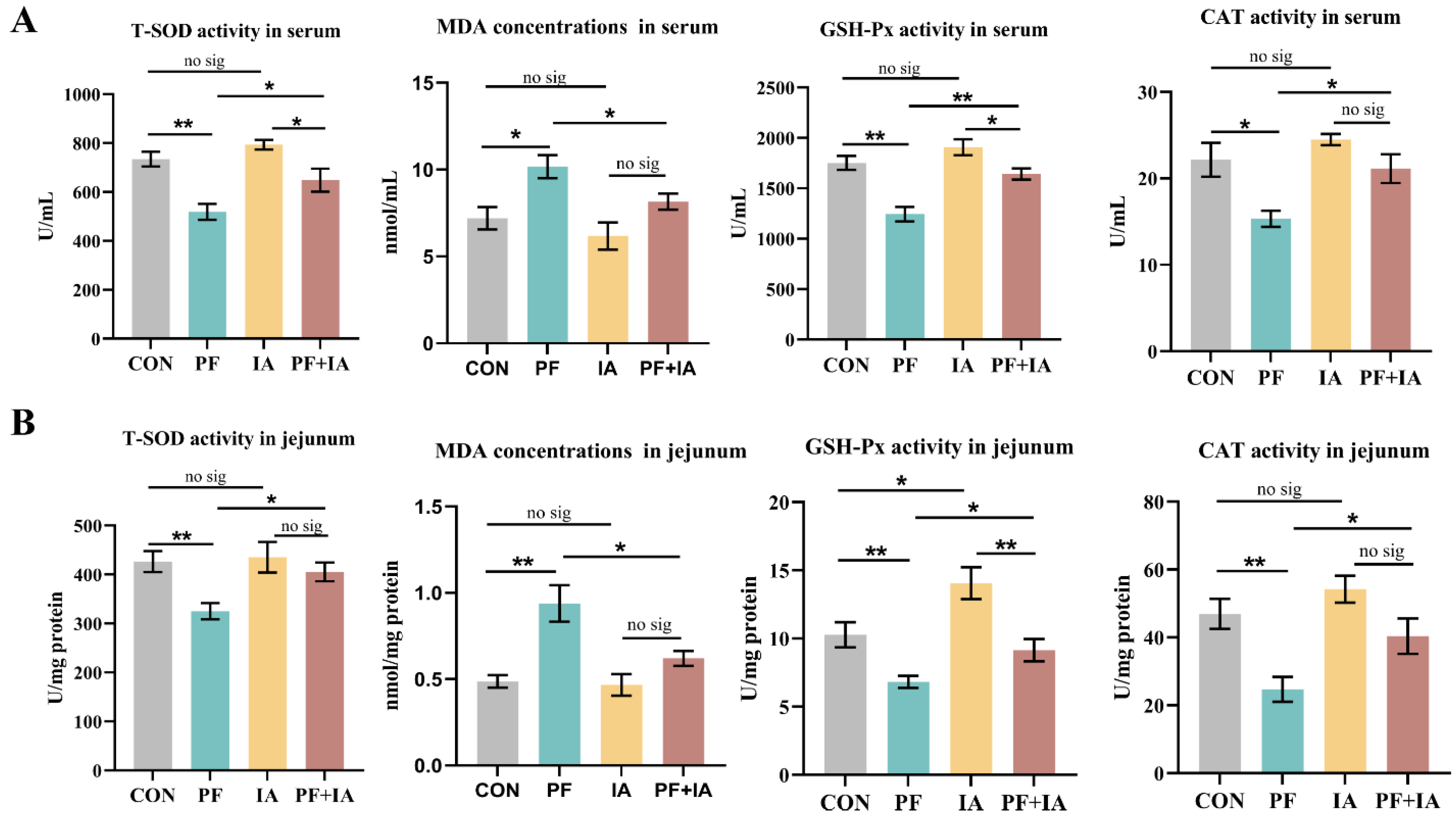
2.1. Serum and Jejunum Antioxidant Capacity
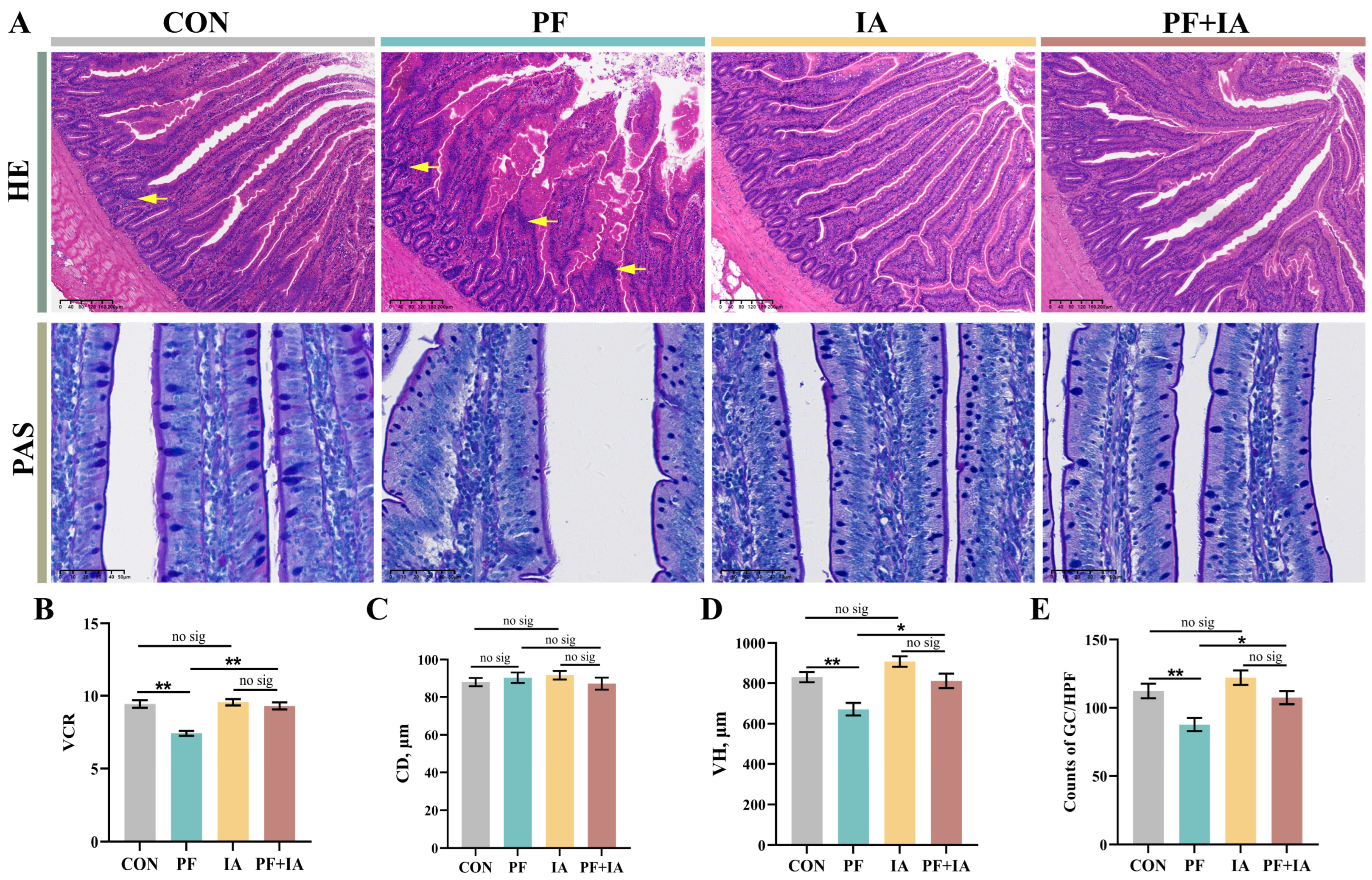
2.2. Jejunum Morphology
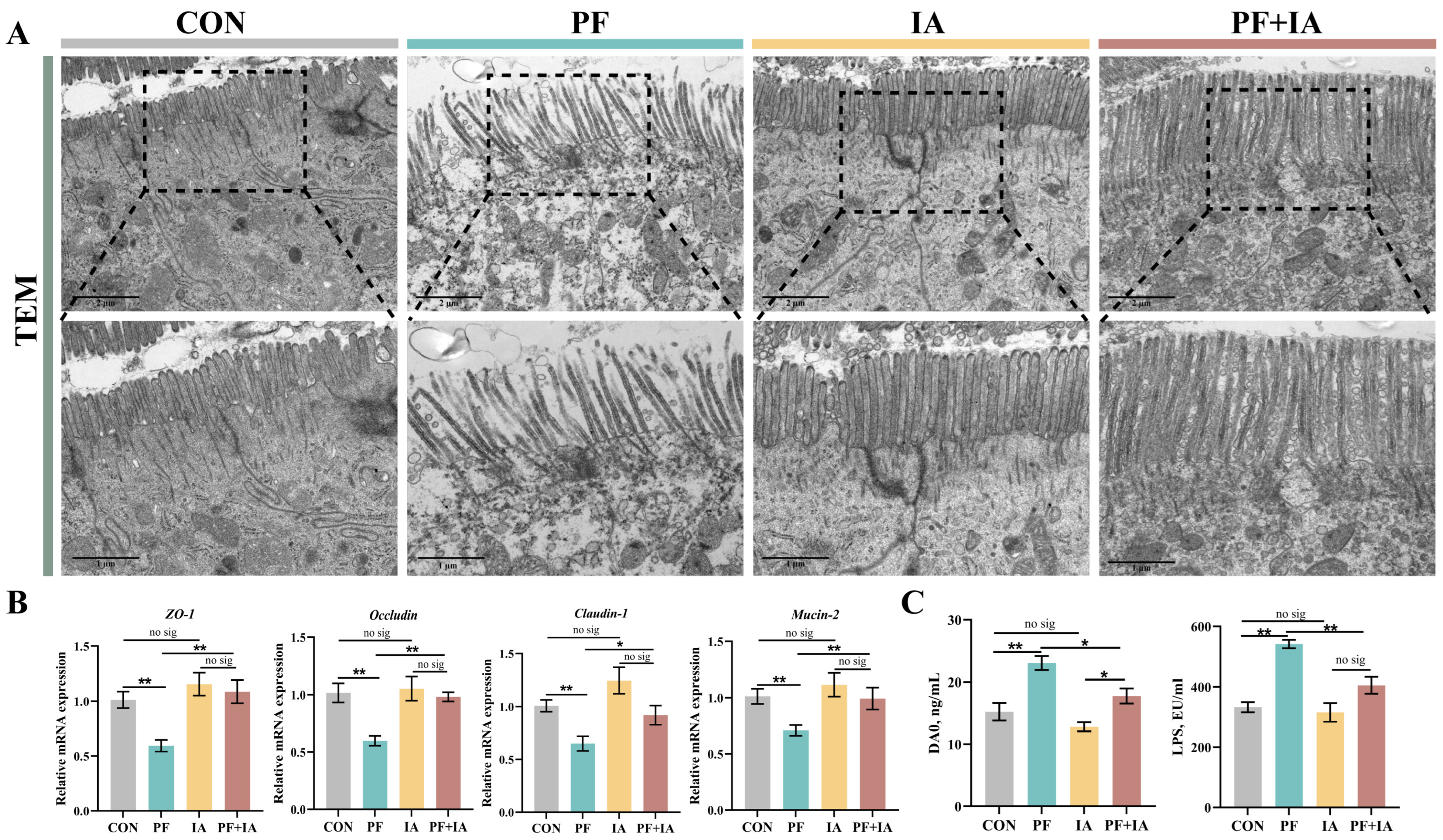
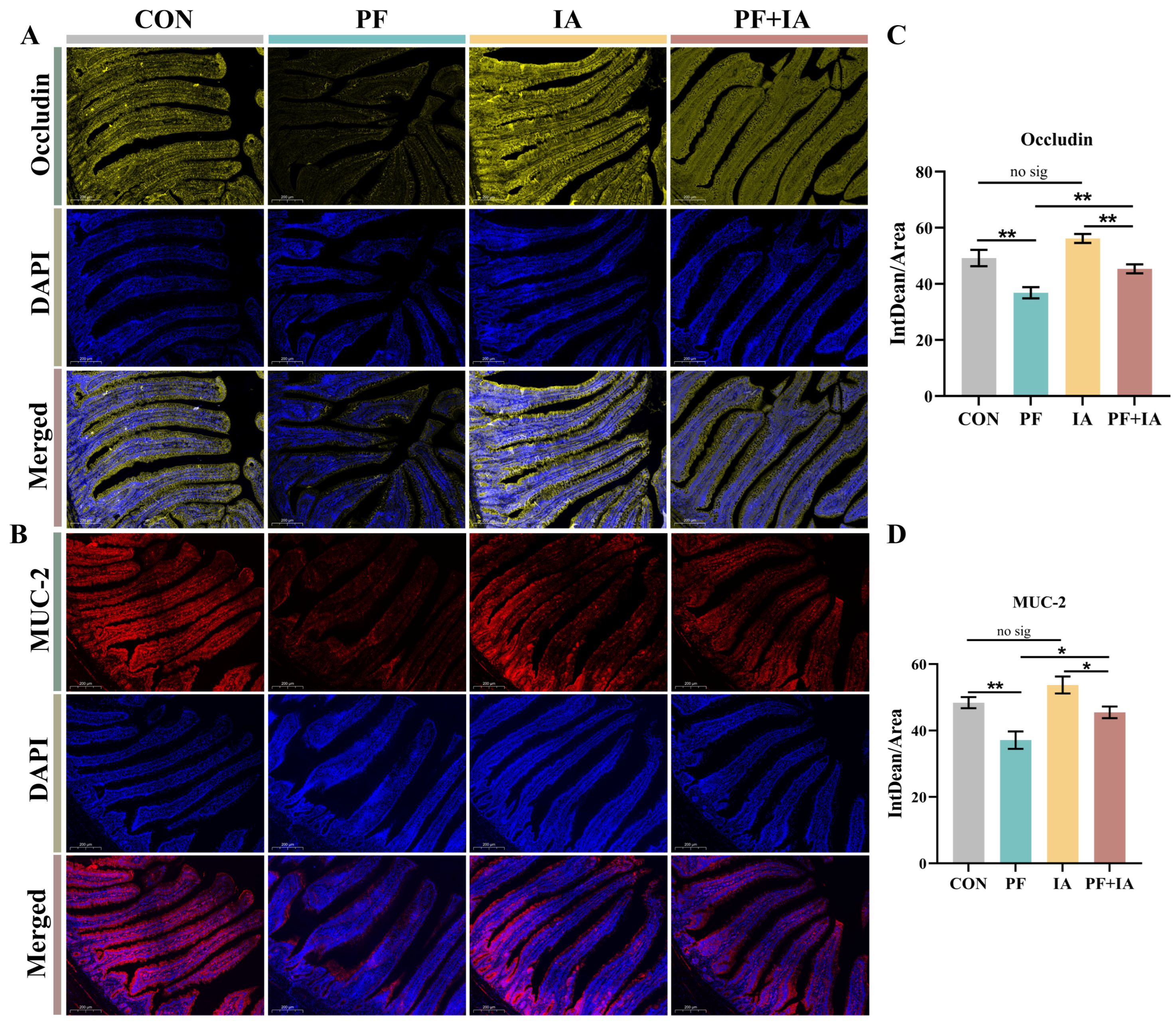
2.3. Intestinal Barrier Function
2.4. Expression of Keap1/Nrf2/HO-1 Pathway-Related Genes in Jejunum
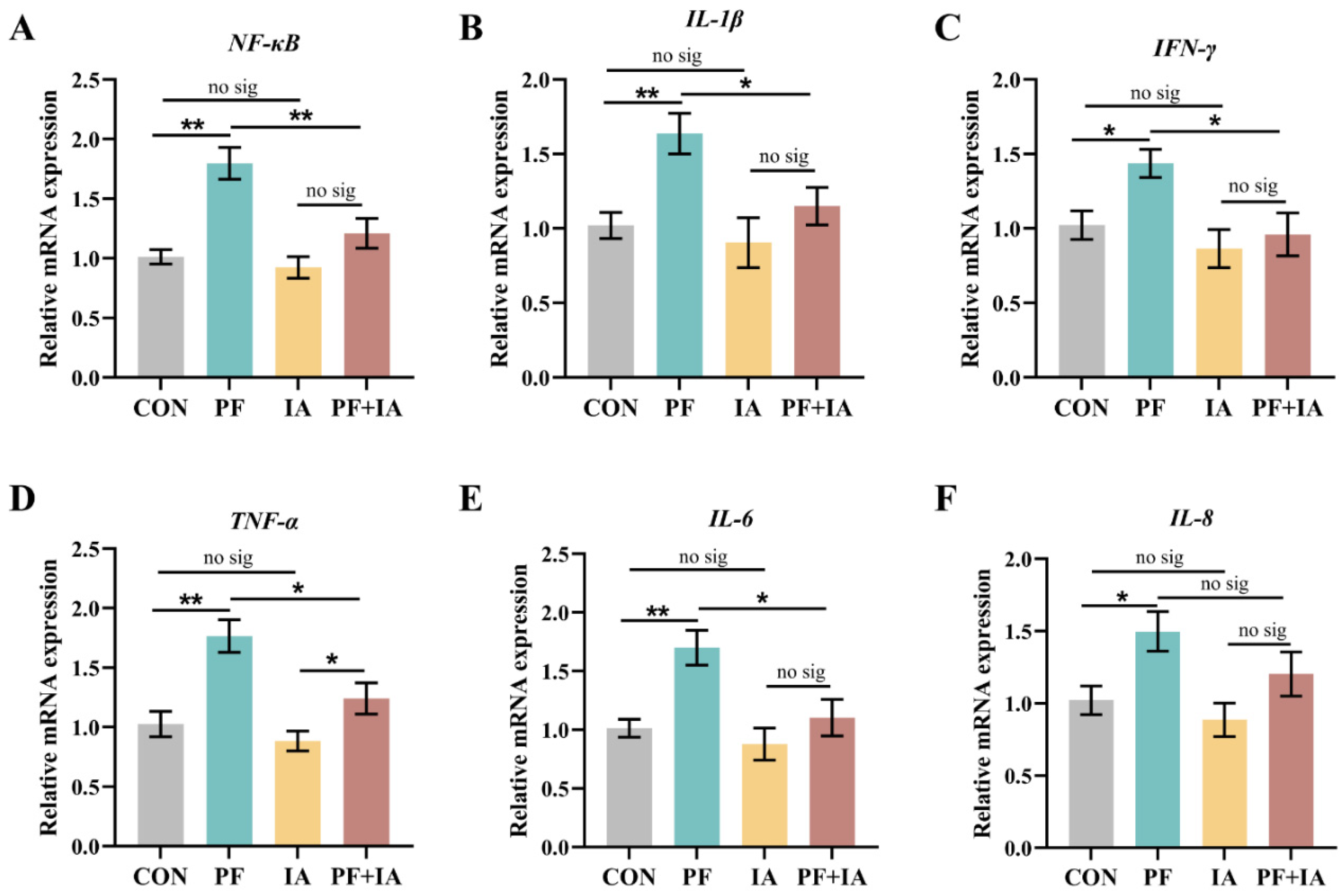
2.5. Gene Expression of Inflammatory Factors in Jejunum
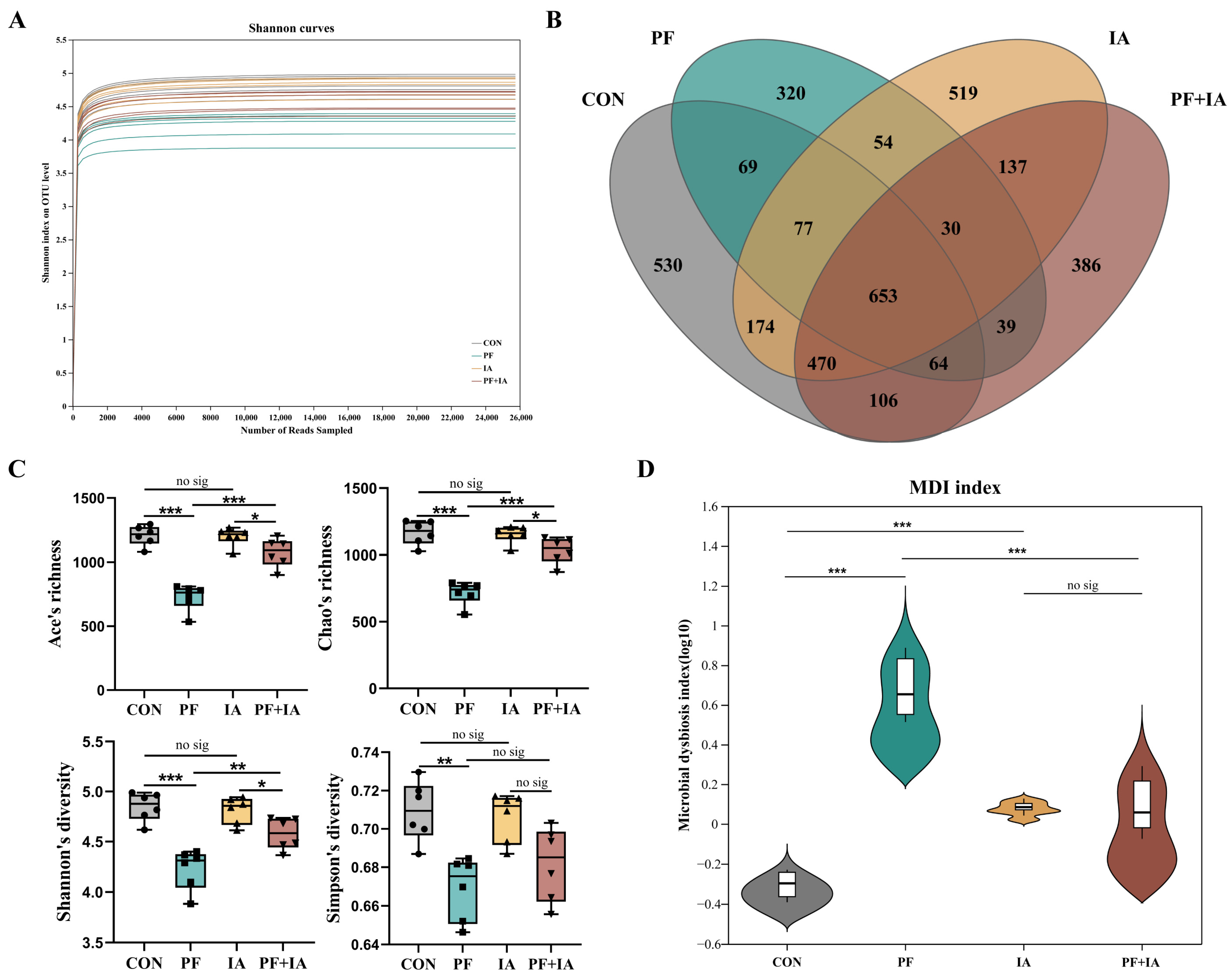
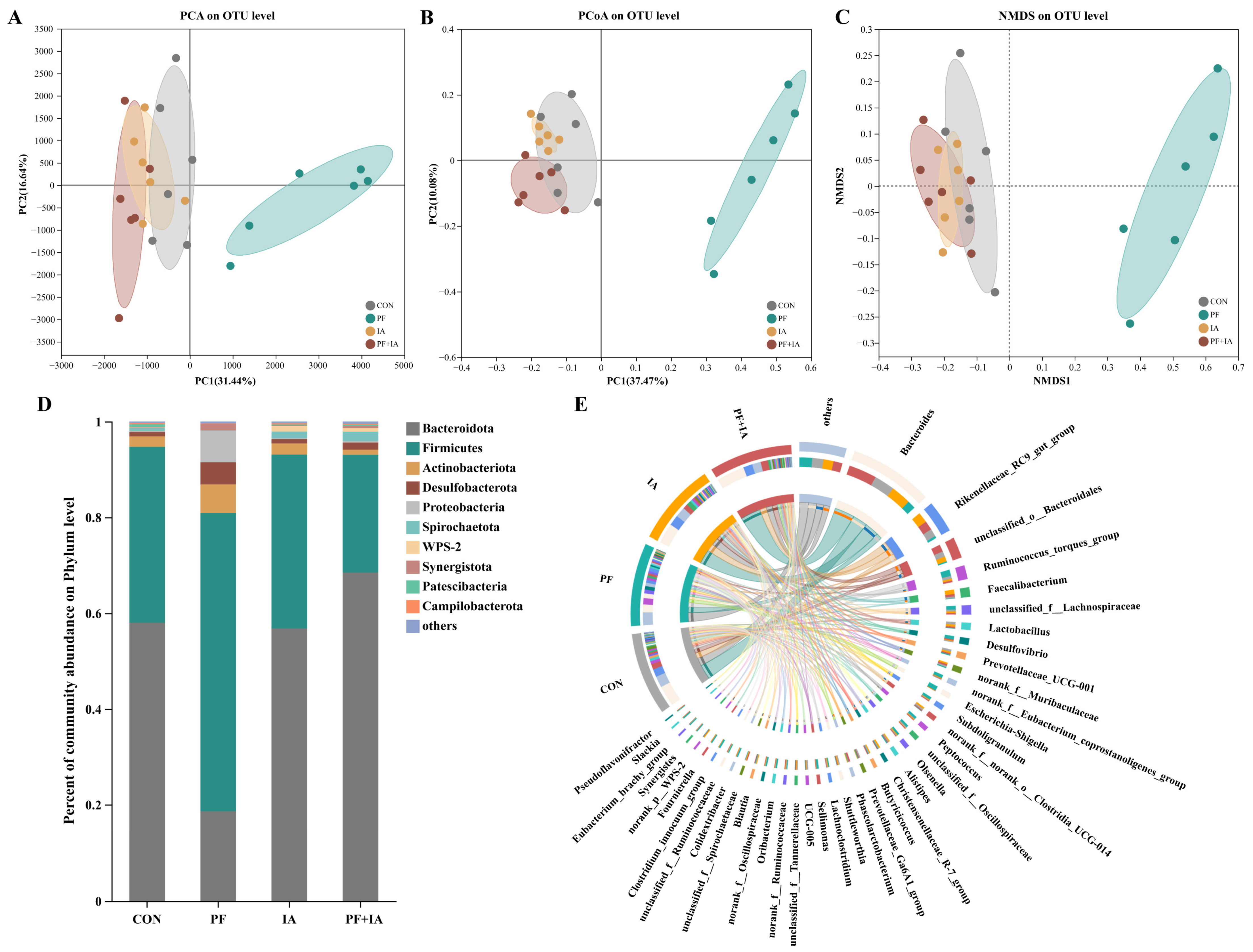
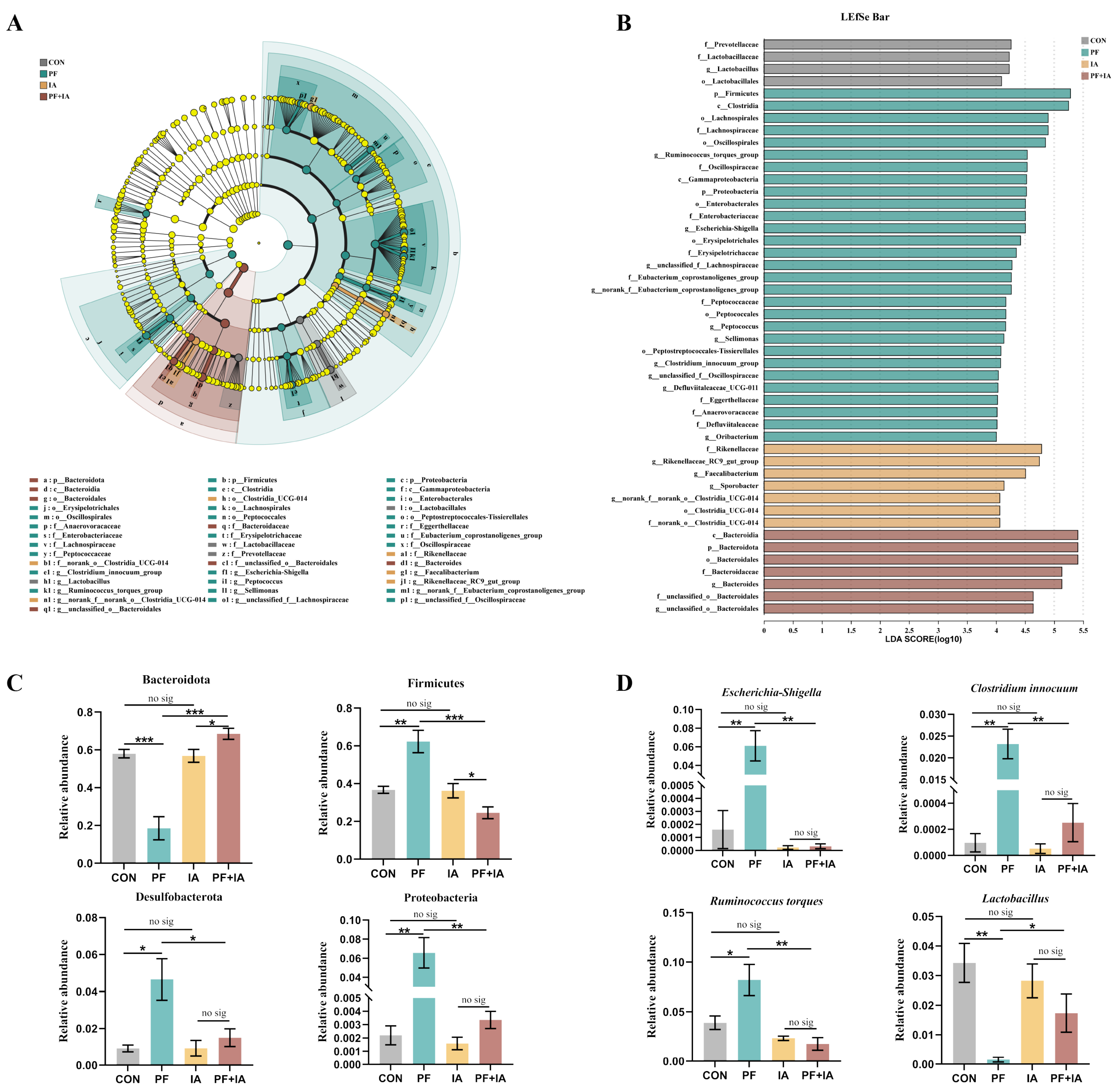
2.6. Microbial Structure and Community
2.7. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Sample Collection
4.3. Assay of Antioxidant Indices
4.4. Histological Observation
4.5. Immunofluorescence Analyses
4.6. ELISA Analysis
4.7. RT-qPCR Analysis
4.8. Analysis of Gut Microbiota
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Hu, L.; Xu, H. Dietary Exposure to Per- and Polyfluoroalkyl Substances: Potential Health Impacts on Human Liver. Sci. Total Environ. 2024, 907, 167945. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Li, R.; Li, S.-H.; Ma, X.; Liu, L.; Niu, D.; Duan, X. Perfluorooctanoic Acid (PFOA) Exposure Affects Early Embryonic Development and Offspring Oocyte Quality via Inducing Mitochondrial Dysfunction. Environ. Int. 2022, 167, 107413. [Google Scholar] [CrossRef]
- Gazzotti, T.; Sirri, F.; Ghelli, E.; Zironi, E.; Zampiga, M.; Pagliuca, G. Perfluoroalkyl Contaminants in Eggs from Backyard Chickens Reared in Italy. Food Chem. 2021, 362, 130178. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a Mixture of Legacy, Alternative, and Replacement per- and Polyfluoroalkyl Substances (PFAS) Results in Sex-Dependent Modulation of Cholesterol Metabolism and Liver Injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef]
- Li, P.; Oyang, X.; Xie, X.; Li, Z.; Yang, H.; Xi, J.; Guo, Y.; Tian, X.; Liu, B.; Li, J.; et al. Phytotoxicity Induced by Perfluorooctanoic Acid and Perfluorooctane Sulfonate via Metabolomics. J. Hazard. Mater. 2020, 389, 121852. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zou, L.; Guo, M.; Peng, J.; Li, F.; Bi, Y.; Jiang, S.; Qin, H.; Tan, Z. Insights into the Combined Toxicity and Mechanisms of BDE-47 and PFOA in Marine Blue Mussel: An Integrated Study at the Physiochemical and Molecular Levels. Aquat. Toxicol. 2024, 273, 106999. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ren, X.; Zhang, X.; Wang, G.; Liu, H.; Wang, L. Rutin Ameliorate PFOA Induced Renal Damage by Reducing Oxidative Stress and Improving Lipid Metabolism. J. Nutr. Biochem. 2024, 123, 109501. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wan, J.; Niu, Q.; Liu, R. PFOA and PFOS Interact with Superoxide Dismutase and Induce Cytotoxicity in Mouse Primary Hepatocytes: A Combined Cellular and Molecular Methods. Environ. Res. 2019, 175, 63–70. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Zhao, B.; Li, Y.; Zhao, X.; Yuan, Z. Blueberry Anthocyanin Alleviate Perfluorooctanoic Acid-Induced Toxicity in Planarian (Dugesia japonica) by Regulating Oxidative Stress Biomarkers, ATP Contents, DNA Methylation and mRNA Expression. Environ. Pollut. 2019, 245, 957–964. [Google Scholar] [CrossRef]
- Zeng, Z.; Song, B.; Xiao, R.; Zeng, G.; Gong, J.; Chen, M.; Xu, P.; Zhang, P.; Shen, M.; Yi, H. Assessing the Human Health Risks of Perfluorooctane Sulfonate by in Vivo and in Vitro Studies. Environ. Int. 2019, 126, 598–610. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Tu, C.; Wang, Z.; Xin, W. The Protective Effect of Blueberry Anthocyanins against Perfluorooctanoic Acid-Induced Disturbance in Planarian (Dugesia japonica). Ecotoxicol. Environ. Saf. 2016, 127, 170–174. [Google Scholar] [CrossRef]
- Adedara, I.A.; Abioye, O.O.; Oyedele, G.T.; Ikeji, C.N.; Afolabi, B.A.; Rocha, J.B.T.; Farombi, E.O. Perfluorooctanoic Acid Induces Behavioral Impairment and Oxidative Injury in Nauphoeta Cinerea Nymphs. Environ. Sci. Pollut. Res. Int. 2023, 30, 110340–110351. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Fletcher, T.; Stein, C.R.; Bartell, S.M.; Darrow, L.; Lopez-Espinosa, M.-J.; Barry Ryan, P.; Savitz, D.A. Review: Evolution of Evidence on PFOA and Health Following the Assessments of the C8 Science Panel. Environ. Int. 2020, 145, 106125. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Kugathasan, S.; Barr, D.B. PFOA and Ulcerative Colitis. Environ. Res. 2018, 165, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Intestinal Homeostasis and Its Breakdown in Inflammatory Bowel Disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Xu, L.-L.; Zhong, M.-T.; Chen, Y.-K.; Lai, M.-Q.; Wang, Q.; Xie, X.-L. Gestational GenX and PFOA Exposures Induce Hepatotoxicity, Metabolic Pathway, and Microbiome Shifts in Weanling Mice. Sci. Total Environ. 2024, 907, 168059. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, F.; Meng, D.; Zhang, X.; Feng, Y.; Yin, G.; Liang, P.; Chen, S.; Liu, H. Mechanism of Action and Related Natural Regulators of Nrf2 in Nonalcoholic Fatty Liver Disease. Curr. Drug Deliv. 2024, 21, 1300–1319. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular Modulators of the NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Ji, J.-A.; Jiang, Z.-Y.; You, Q.-D. The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Zhao, Y.; Tao, L.; Liu, H.; Dong, W.; Yang, G.; Li, L. Effects of Dietary Supplementation with Itaconic Acid on the Growth Performance, Nutrient Digestibility, Slaughter Variables, Blood Biochemical Parameters, and Intestinal Morphology of Broiler Chickens. Poult. Sci. 2022, 101, 101732. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate Is an Anti-Inflammatory Metabolite That Activates Nrf2 via Alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, H.; Wei, J.; Mo, W.; Li, Q.; Lv, X. The Signaling Pathways and Therapeutic Potential of Itaconate to Alleviate Inflammation and Oxidative Stress in Inflammatory Diseases. Redox Biol. 2022, 58, 102553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Li, W.; Li, J.; Wu, W.; Kang, J.; Jiang, H.; Liu, P.; Liu, J.; Gong, W.; et al. Itaconate Inhibits SYK through Alkylation and Suppresses Inflammation against hvKP Induced Intestinal Dysbiosis. Cell Mol. Life Sci. 2023, 80, 337. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Torcianti, V.; Grasselli, F. Perfluorooctanoic Acid (PFOA) Affects Steroidogenesis and Antioxidant Defence in Granulosa Cells from Swine Ovary. Environ. Toxicol. Pharmacol. 2023, 101, 104169. [Google Scholar] [CrossRef] [PubMed]
- Shane, H.L.; Baur, R.; Lukomska, E.; Weatherly, L.; Anderson, S.E. Immunotoxicity and Allergenic Potential Induced by Topical Application of Perfluorooctanoic Acid (PFOA) in a Murine Model. Food Chem. Toxicol. 2020, 136, 111114. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zheng, J.; Yan, S.; Li, Y.; Wang, Y.; Liu, X.; Xiao, C. Exposure to Perfluorooctanoic Acid Induces Cognitive Deficits via Altering Gut Microbiota Composition, Impairing Intestinal Barrier Integrity, and Causing Inflammation in Gut and Brain. J. Agric. Food Chem. 2020, 68, 13916–13928. [Google Scholar] [CrossRef]
- Zhan, Z.; Liu, W.; Pan, L.; Bao, Y.; Yan, Z.; Hong, L. Overabundance of Veillonella Parvula Promotes Intestinal Inflammation by Activating Macrophages via LPS-TLR4 Pathway. Cell Death Discov. 2022, 8, 251. [Google Scholar] [CrossRef]
- Pan, L.; Ma, M.; Wang, Y.; Dai, W.; Fu, T.; Wang, L.; Shang, Q.; Yu, G. Polyguluronate Alleviates Ulcerative Colitis by Targeting the Gut Commensal Lactobacillus Murinus and Its Anti-Inflammatory Metabolites. Int. J. Biol. Macromol. 2024, 257, 128592. [Google Scholar] [CrossRef]
- Capuzzi, E.; Ossola, P.; Caldiroli, A.; Auxilia, A.M.; Buoli, M. Malondialdehyde as a Candidate Biomarker for Bipolar Disorder: A Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110469. [Google Scholar] [CrossRef]
- Miranda, A.F.; Trestrail, C.; Lekamge, S.; Nugegoda, D. Effects of Perfluorooctanoic Acid (PFOA) on the Thyroid Status, Vitellogenin, and Oxidant-Antioxidant Balance in the Murray River Rainbowfish. Ecotoxicology 2020, 29, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Zhong, M.-T.; Gi, M.; Chen, Y.-K.; Lai, M.-Q.; Liu, J.-Y.; Liu, Y.-M.; Wang, Q.; Xie, X.-L. Inulin Alleviates Perfluorooctanoic Acid-Induced Intestinal Injury in Mice by Modulating the PI3K/AKT/mTOR Signaling Pathway. Environ. Pollut. 2024, 342, 123090. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, J.; Zhang, H.; Yu, J.; Zhou, Y.; Wang, Y.; Li, T.; Yan, M.; Li, B.; Chen, Y.; et al. Atractylodes Macrocephala Koidz Polysaccharide Improves Glycolipid Metabolism Disorders through Activation of Aryl Hydrocarbon Receptor by Gut Flora-Produced Tryptophan Metabolites. Int. J. Biol. Macromol. 2023, 253, 126987. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the Seeds of Plantago asiatica L. Alleviates Nonylphenol Induced Intestinal Barrier Injury by Regulating Tight Junctions in Human Caco-2 Cell Line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Wang, A.; Guan, C.; Wang, T.; Mu, G.; Tuo, Y. Lactiplantibacillus Plantarum-Derived Indole-3-Lactic Acid Ameliorates Intestinal Barrier Integrity through the AhR/Nrf2/NF-κB Axis. J. Agric. Food Chem. 2024, 72, 9236–9246. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.-T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.-N. Nrf2-Deficient Mice Have an Increased Susceptibility to Dextran Sulfate Sodium-Induced Colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Jin, G.; Gao, K.; Wang, S.; Zhang, X.; Zhou, K.; Cai, Y.; Zhou, X.; Zhao, Z. Network Pharmacology-Based Study on the Mechanism of ShenKang Injection in Diabetic Kidney Disease through Keap1/Nrf2/Ho-1 Signaling Pathway. Phytomedicine 2023, 118, 154915. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol Attenuates Ferroptosis against LPS-Induced Acute Lung Injury in Mice by Keap1-Nrf2/HO-1 Pathway. J. Transl. Med. 2021, 19, 96. [Google Scholar] [CrossRef]
- Liu, W.; Yang, B.; Wu, L.; Zou, W.; Pan, X.; Zou, T.; Liu, F.; Xia, L.; Wang, X.; Zhang, D. Involvement of NRF2 in Perfluorooctanoic Acid-Induced Testicular Damage in Male Mice. Biol. Reprod. 2015, 93, 41. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Deng, L.; Luo, D.; Ma, R.; Lu, Q. Oxyberberine Ameliorates TNBS-Induced Colitis in Rats through Suppressing Inflammation and Oxidative Stress via Keap1/Nrf2/NF-κB Signaling Pathways. Phytomedicine 2023, 116, 154899. [Google Scholar] [CrossRef]
- Liu, X.; Quan, S.; Han, Q.; Li, J.; Gao, X.; Zhang, J.; Liu, D. Effectiveness of the Fruit of Rosa odorata Sweet var. gigantea (Coll. et Hemsl.) Rehd. et Wils in the Protection and the Healing of Ethanol-Induced Rat Gastric Mucosa Ulcer Based on Nrf2/NF-κB Pathway Regulation. J. Ethnopharmacol. 2022, 282, 114626. [Google Scholar] [CrossRef]
- Liu, M.; Ma, J.; Xu, J.; Huangfu, W.; Zhang, Y.; Ali, Q.; Liu, B.; Li, D.; Cui, Y.; Wang, Z.; et al. Fecal Microbiota Transplantation Alleviates Intestinal Inflammatory Diarrhea Caused by Oxidative Stress and Pyroptosis via Reducing Gut Microbiota-Derived Lipopolysaccharides. Int. J. Biol. Macromol. 2024, 261, 129696. [Google Scholar] [CrossRef] [PubMed]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Sen, P.; Fan, Y.; Schlezinger, J.J.; Ehrlich, S.D.; Webster, T.F.; Hyötyläinen, T.; Pedersen, O.; Orešič, M. Exposure to Environmental Toxicants Is Associated with Gut Microbiome Dysbiosis, Insulin Resistance and Obesity. Environ. Int. 2024, 186, 108569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Bai, D.; Zhong, J.; Hu, X.; Zhang, R.; Zhen, W.; Ito, K.; Zhang, B.; Yang, Y.; et al. Effect of Dietary Aspirin Eugenol Ester on the Growth Performance, Antioxidant Capacity, Intestinal Inflammation, and Cecal Microbiota of Broilers under High Stocking Density. Poult. Sci. 2024, 103, 103825. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut Microbiota-Derived Inosine from Dietary Barley Leaf Supplementation Attenuates Colitis through PPARγ Signaling Activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Liu, H.; Yang, G.; Li, L. Microbiome-Metabolomics Analysis Reveals Abatement Effects of Itaconic Acid on Odorous Compound Production in Arbor Acre Broilers. BMC Microbiol. 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Le, P.-H.; Chiu, C.-T.; Yeh, P.-J.; Pan, Y.-B.; Chiu, C.-H. Clostridium Innocuum Infection in Hospitalised Patients with Inflammatory Bowel Disease. J. Infect. 2022, 84, 337–342. [Google Scholar] [CrossRef]
- Raygoza Garay, J.A.; Turpin, W.; Lee, S.-H.; Smith, M.I.; Goethel, A.; Griffiths, A.M.; Moayyedi, P.; Espin-Garcia, O.; Abreu, M.; Aumais, G.L.; et al. Gut Microbiome Composition Is Associated with Future Onset of Crohn’s Disease in Healthy First-Degree Relatives. Gastroenterology 2023, 165, 670–681. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main Active Components of Jiawei Gegen Qinlian Decoction Protects against Ulcerative Colitis under Different Dietary Environments in a Gut Microbiota-Dependent Manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta Americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia Muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, D.; Ge, C.; Luo, X.; Du, L.; Zhang, X.; Hui, C. Combined Effects of Microplastics and Chlortetracycline on the Intestinal Barrier, Gut Microbiota, and Antibiotic Resistome of Muscovy Ducks (Cairina moschata). Sci. Total Environ. 2023, 887, 164050. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Z.; Lv, Y.; Ge, C.; Luo, X.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Hericium Erinaceus Polysaccharides Ameliorate Nonalcoholic Fatty Liver Disease via Gut Microbiota and Tryptophan Metabolism Regulation in an Aged Laying Hen Model. Int. J. Biol. Macromol. 2024, 273, 132735. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Hu, Z.; Luo, X.; Ge, C.; Lv, Y.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Itaconic Acid Alleviates Perfluorooctanoic Acid-Induced Oxidative Stress and Intestinal Damage by Regulating the Keap1/Nrf2/Ho-1 Pathway and Reshaping the Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 9826. https://doi.org/10.3390/ijms25189826
Wu L, Hu Z, Luo X, Ge C, Lv Y, Zhan S, Huang W, Shen X, Yu D, Liu B. Itaconic Acid Alleviates Perfluorooctanoic Acid-Induced Oxidative Stress and Intestinal Damage by Regulating the Keap1/Nrf2/Ho-1 Pathway and Reshaping the Gut Microbiota. International Journal of Molecular Sciences. 2024; 25(18):9826. https://doi.org/10.3390/ijms25189826
Chicago/Turabian StyleWu, Lianchi, Zhaoying Hu, Xinyu Luo, Chaoyue Ge, Yujie Lv, Shenao Zhan, Weichen Huang, Xinyu Shen, Dongyou Yu, and Bing Liu. 2024. "Itaconic Acid Alleviates Perfluorooctanoic Acid-Induced Oxidative Stress and Intestinal Damage by Regulating the Keap1/Nrf2/Ho-1 Pathway and Reshaping the Gut Microbiota" International Journal of Molecular Sciences 25, no. 18: 9826. https://doi.org/10.3390/ijms25189826





