Renal Cell Carcinoma Discrimination through Attenuated Total Reflection Fourier Transform Infrared Spectroscopy of Dried Human Urine and Machine Learning Techniques
Abstract
:1. Introduction
2. Results
2.1. ATR-FTIR Spectra of Urine Samples: Comparison with Artificial Urine and Urine Components
2.2. ATR-FTIR Spectra of RCC Patients’ Urine Versus Healthy Donors
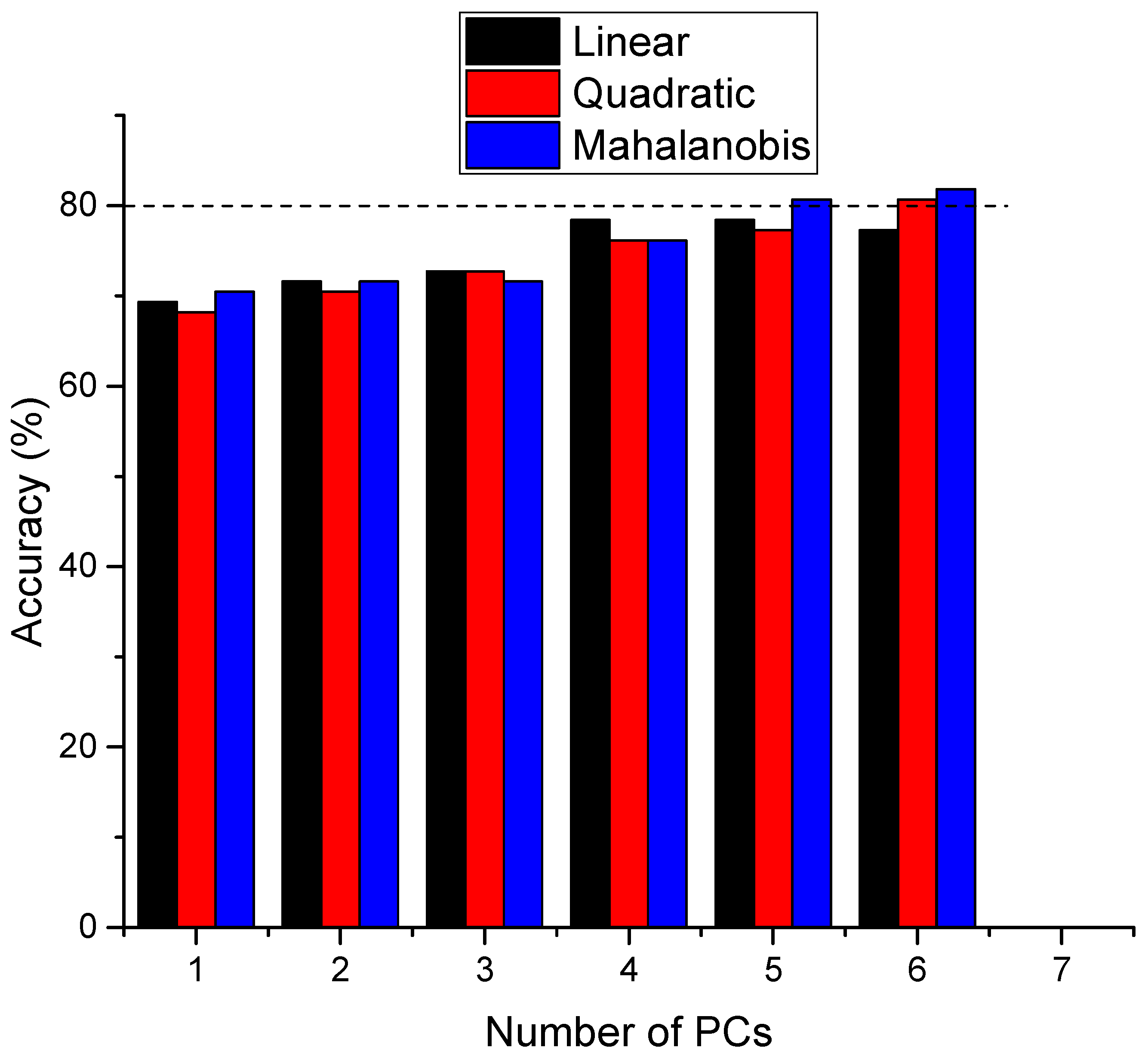
2.3. PCA–LDA Analysis
2.4. Support Vector Machine
3. Discussion
4. Materials and Methods
4.1. Research Ethics
4.2. Cohort of Patient Samples and Urine Collection
4.3. Reagents and Instrumentation
4.4. Urine Deposition and ATR-FTIR Measurements
4.5. Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Bigot, P.; Barthelemy, P.; Boissier, R.; Khene, Z.E.; Pettenati, C.; Bernhard, J.C.; Correas, J.M.; Doumerc, N.; Gimel, P.; Ingels, A.; et al. French AFU Cancer Committee Guidelines-Update 2022–2024: Management of Kidney Cancer. Prog. Urol. 2022, 32, 1195–1274. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Sun, C.Y.; Wu, I.W.; Tsai, T.L.; Sun, C.C.; Lee, C.C. Higher Risk of Malignant Neoplasms in Young Adults with End-Stage Renal Disease Receiving Haemodialysis: A Nationwide Population-Based Study. Nephrology 2019, 24, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Hora, M.; Hes, O.; Reischig, T.; Ürge, T.; Klečka, J.; Ferda, J.; Michal, M.; Eret, V. Tumours in End-Stage Kidney. Transplant. Proc. 2008, 40, 3354–3358. [Google Scholar] [CrossRef]
- Jayson, M.; Sanders, H. Increased Incidence of Serendipitously Discovered Renal Cell Carcinoma. Urology 1998, 51, 203–205. [Google Scholar] [CrossRef]
- Rai, B.P.; Luis Dominguez Escrig, J.; Vale, L.; Kuusk, T.; Capoun, O.; Soukup, V.; Bruins, H.M.; Yuan, Y.; Violette, P.D.; Santesso, N.; et al. Systematic Review of the Incidence of and Risk Factors for Urothelial Cancers and Renal Cell Carcinoma Among Patients with Haematuria. Eur. Urol. 2022, 82, 182–192. [Google Scholar] [CrossRef]
- Diana, P.; Klatte, T.; Amparore, D.; Bertolo, R.; Carbonara, U.; Erdem, S.; Ingels, A.; Kara, O.; Marandino, L.; Marchioni, M.; et al. Screening Programs for Renal Cell Carcinoma: A Systematic Review by the EAU Young Academic Urologists Renal Cancer Working Group. World J. Urol. 2023, 41, 929–940. [Google Scholar] [CrossRef]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.; Stewart, G.D. Epidemiology and Screening for Renal Cancer. World J. Urol. 2018, 36, 1341–1353. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Godoy, A.; Burge, S.W.; Burbidge, S.; Cartledge, J.; Crosbie, P.A.J.; Eckert, C.; Farquhar, F.; Hammond, D.; Hancock, N.; et al. The Yorkshire Kidney Screening Trial (YKST): Protocol for a Feasibility Study of Adding Non-Contrast Abdominal CT Scanning to Screen for Kidney Cancer and Other Abdominal Pathology within a Trial of Community-Based CT Screening for Lung Cancer. BMJ Open 2022, 12, e063018. [Google Scholar] [CrossRef]
- Charpentier, M.; Gutierrez, C.; Guillaudeux, T.; Verhoest, G.; Pedeux, R. Noninvasive Urine-Based Tests to Diagnose or Detect Recurrence of Bladder Cancer. Cancers 2021, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Nsugbe, E. A Cybernetic Framework for Predicting Preterm and Enhancing Care Strategies: A Review. Biomed. Eng. Adv. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Gajjar, K.B.; Theophilou, G.; Martin, F.L.; Martin-Hirsch, P.L. Vibrational Spectroscopy of Biofluids for Disease Screening or Diagnosis: Translation from the Laboratory to a Clinical Setting. J. Biophotonics 2014, 7, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, S.; Weber, K.; Popp, J.; Wood, B.R.; Kochan, K.; Rüther, A.; Perez-Guaita, D.; Heraud, P.; Stone, N.; Dudgeon, A.; et al. Application of Vibrational Spectroscopy and Imaging to Point-of-Care Medicine: A Review. Appl. Spectrosc. 2018, 72 (Suppl. S1), 52–84. [Google Scholar] [CrossRef]
- Gajjar, K.; Trevisan, J.; Owens, G.; Keating, P.J.; Wood, N.J.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Fourier-Transform Infrared Spectroscopy Coupled with a Classification Machine for the Analysis of Blood Plasma or Serum: A Novel Diagnostic Approach for Ovarian Cancer. Analyst 2013, 138, 3917–3926. [Google Scholar] [CrossRef]
- Hands, J.R.; Clemens, G.; Stables, R.; Ashton, K.; Brodbelt, A.; Davis, C.; Dawson, T.P.; Jenkinson, M.D.; Lea, R.W.; Walker, C.; et al. Brain Tumour Differentiation: Rapid Stratified Serum Diagnostics via Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy. J. Neurooncol 2016, 127, 463–472. [Google Scholar] [CrossRef]
- Yu, M.C.; Rich, P.; Foreman, L.; Smith, J.; Yu, M.S.; Tanna, A.; Dibbur, V.; Unwin, R.; Tam, F.W.K. Label Free Detection of Sensitive Mid-Infrared Biomarkers of Glomerulonephritis in Urine Using Fourier Transform Infrared Spectroscopy. Sci. Rep. 2017, 7, 4601. [Google Scholar] [CrossRef]
- Tamm, L.K.; Tatulian, S.A. Infrared Spectroscopy of Proteins and Peptides in Lipid Bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef] [PubMed]
- Hoşafçı, G.; Klein, O.; Oremek, G.; Mäntele, W. Clinical Chemistry without Reagents? An Infrared Spectroscopic Technique for Determination of Clinically Relevant Constituents of Body Fluids. Anal. Bioanal. Chem. 2007, 387, 1815–1822. [Google Scholar] [CrossRef]
- Steenbeke, M.; De Bruyne, S.; Boelens, J.; Oyaert, M.; Glorieux, G.; Biesen, W.V.; Linjala, J.; Delanghe, J.R.; Speeckaert, M.M. Exploring the Possibilities of Infrared Spectroscopy for Urine Sediment Examination and Detection of Pathogenic Bacteria in Urinary Tract Infections. Clin. Chem. Lab. Med. 2020, 58, 1759–1767. [Google Scholar] [CrossRef]
- Oliver, K.V.; Vilasi, A.; Maréchal, A.; Moochhala, S.H.; Unwin, R.J.; Rich, P.R. Infrared Vibrational Spectroscopy: A Rapid and Novel Diagnostic and Monitoring Tool for Cystinuria. Sci. Rep. 2016, 6, 34737. [Google Scholar] [CrossRef] [PubMed]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Ashton, K.M.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Potential of Mid-Infrared Spectroscopy as a Non-Invasive Diagnostic Test in Urine for Endometrial or Ovarian Cancer. Analyst 2018, 143, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Yap, X.-L.; Ong, T.-A.; Lim, J.; Wood, B.; Lee, W.-L. Study of Prostate Cancer-Derived Extracellular Vesicles in Urine Using IR Spectroscopy. Prog. Drug Discov. Biomed. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Maitra, I.; Morais, C.L.M.; Lima, K.M.G.; Ashton, K.M.; Date, R.S.; Martin, F.L. Attenuated Total Reflection Fourier-Transform Infrared Spectral Discrimination in Human Bodily Fluids of Oesophageal Transformation to Adenocarcinoma. Analyst 2019, 144, 7447–7456. [Google Scholar] [CrossRef]
- Takamura, A.; Halamkova, L.; Ozawa, T.; Lednev, I.K. Phenotype Profiling for Forensic Purposes: Determining Donor Sex Based on Fourier Transform Infrared Spectroscopy of Urine Traces. Anal. Chem. 2019, 91, 6288–6295. [Google Scholar] [CrossRef]
- Furlan, P.; Servey, J.; Scott, S.; Peaslee, M. FTIR Analysis of Mouse Urine Urea Using IR Cards. Spectrosc. Lett. 2004, 37, 311–318. [Google Scholar] [CrossRef]
- Buhas, B.A.; Toma, V.; Beauval, J.-B.; Andras, I.; Couți, R.; Muntean, L.A.-M.; Coman, R.-T.; Maghiar, T.A.; Știufiuc, R.-I.; Lucaciu, C.M.; et al. Label-Free SERS of Urine Components: A Powerful Tool for Discriminating Renal Cell Carcinoma through Multivariate Analysis and Machine Learning Techniques. Int. J. Mol. Sci. 2024, 25, 3891. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Bax, C.; Lotesoriere, B.J.; Sironi, S.; Capelli, L. Review and Comparison of Cancer Biomarker Trends in Urine as a Basis for New Diagnostic Pathways. Cancers 2019, 11, 1244. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chen, F.-A.; Chen, C.-F.; Liu, W.-S.; Shih, C.-J.; Ou, S.-M.; Yang, W.-C.; Lin, C.-C.; Yang, A.-H. Diagnostic Accuracy of Urine Protein/Creatinine Ratio Is Influenced by Urine Concentration. PLoS ONE 2015, 10, e0137460. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Ahmed, S.; Syed, I.A.; Ansari, S.A.; Ahmad, A. Rheological and Infrared Spectroscopic Investigations of Normal and Chronic Kidney Disease Urine. Orient. J. Chem. 2016, 32, 1421–1431. [Google Scholar] [CrossRef]
- Sarigul, N.; Kurultak, İ.; Uslu Gökceoğlu, A.; Korkmaz, F. Urine Analysis Using FTIR Spectroscopy: A Study on Healthy Adults and Children. J. Biophotonics 2021, 14, e202100009. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Zezell, D.M. Diabetes Monitoring through Urine Analysis Using ATR-FTIR Spectroscopy and Machine Learning. Chemosensors 2023, 11, 565. [Google Scholar] [CrossRef]
- Elkins, K.M. Rapid Presumptive “Fingerprinting” of Body Fluids and Materials by ATR FT-IR Spectroscopy. J. Forensic Sci. 2011, 56, 1580–1587. [Google Scholar] [CrossRef]
- Takamura, A.; Watanabe, K.; Akutsu, T.; Ozawa, T. Soft and Robust Identification of Body Fluid Using Fourier Transform Infrared Spectroscopy and Chemometric Strategies for Forensic Analysis. Sci. Rep. 2018, 8, 8459. [Google Scholar] [CrossRef]
- Orphanou, C.M. The Detection and Discrimination of Human Body Fluids Using ATR FT-IR Spectroscopy. Forensic Sci. Int. 2015, 252, e10–e16. [Google Scholar] [CrossRef]
- De Bruyne, S.; Speeckaert, M.M.; Delanghe, J.R. Applications of Mid-Infrared Spectroscopy in the Clinical Laboratory Setting. Crit. Rev. Clin. Lab. Sci. 2018, 55, 1–20. [Google Scholar] [CrossRef]
- Yousif, E.S.; Abdulkareem, D.T.; Alboaisa, N.S.E.; Mohammad, E.J. Detection of Urinary Bladder Cancer By (ATR-FTIR) Spectroscopy. Syst. Rev. Pharm. 2020, 11, 1932–1937. Available online: https://openurl.ebsco.com/EPDB%3Agcd%3A4%3A7222692/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A156331639&crl=c (accessed on 12 March 2024).
- Das, T.; Harshey, A.; Srivastava, A.; Nigam, K.; Yadav, V.K.; Sharma, K.; Sharma, A. Analysis of the Ex-Vivo Transformation of Semen, Saliva and Urine as They Dry out Using ATR-FTIR Spectroscopy and Chemometric Approach. Sci. Rep. 2021, 11, 11855. [Google Scholar] [CrossRef]
- Schreiber, J.B. Issues and Recommendations for Exploratory Factor Analysis and Principal Component Analysis. Res. Social. Adm. Pharm. 2021, 17, 1004–1011. [Google Scholar] [CrossRef]
- LIBSVM—A Library for Support Vector Machines. Available online: https://www.csie.ntu.edu.tw/~cjlin/libsvm/index.html (accessed on 6 September 2024).
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [CrossRef] [PubMed]
- Jordaens, S.; Zwaenepoel, K.; Tjalma, W.; Deben, C.; Beyers, K.; Vankerckhoven, V.; Pauwels, P.; Vorsters, A. Urine Biomarkers in Cancer Detection: A Systematic Review of Preanalytical Parameters and Applied Methods. Int. J. Cancer 2023, 152, 2186–2205. [Google Scholar] [CrossRef] [PubMed]
- Bifarin, O.O.; Gaul, D.A.; Sah, S.; Arnold, R.S.; Ogan, K.; Master, V.A.; Roberts, D.L.; Bergquist, S.H.; Petros, J.A.; Edison, A.S.; et al. Urine-Based Metabolomics and Machine Learning Reveals Metabolites Associated with Renal Cell Carcinoma Stage. Cancers 2021, 13, 6253. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Bedke, J.; Bex, A.; Capitanio, U.; Giles, R.H.; Hora, M.; Klatte, T.; Marconi, L.; Powles, T.; et al. EAU Guidelines on Renal Cell Carcinoma. Available online: https://uroweb.org/guidelines/renal-cell-carcinoma (accessed on 8 September 2024).
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Sarigul, N.; Bozatli, L.; Kurultak, I.; Korkmaz, F. Using Urine FTIR Spectra to Screen Autism Spectrum Disorder. Sci. Rep. 2023, 13, 19466. [Google Scholar] [CrossRef]
- Nsugbe, E.; Ser, H.L.; Ong, H.F.; Ming, L.C.; Goh, K.W.; Goh, B.H.; Lee, W.L. On an Affordable Approach towards the Diagnosis and Care for Prostate Cancer Patients Using Urine, FTIR and Prediction Machines. Diagnostics 2022, 12, 2099. [Google Scholar] [CrossRef]
- Buhas, B.A.; Toma, V.; Crisan, N.; Ploussard, G.; Maghiar, T.A.; Știufiuc, R.I.; Lucaciu, C.M. High-Accuracy Renal Cell Carcinoma Discrimination through Label-Free SERS of Blood Serum and Multivariate Analysis. Biosensors 2023, 13, 813. [Google Scholar] [CrossRef]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-Free Surface-Enhanced Raman Spectroscopy of Biofluids: Fundamental Aspects and Diagnostic Applications. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef]
- Esposito, A.; Bonifacio, A.; Sergo, V.; Fornasaro, S. Label-Free Surface Enhanced Raman Scattering (SERS) on Centrifugal Silver Plasmonic Paper (CSPP): A Novel Methodology for Unprocessed Biofluids Sampling and Analysis. Biosensors 2021, 11, 467. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Vigo, F.; Tozzi, A.; Disler, M.; Gisi, A.; Kavvadias, V.; Kavvadias, T. Vibrational Spectroscopy in Urine Samples as a Medical Tool: Review and Overview on the Current State-of-the-Art. Diagnostics 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef] [PubMed]
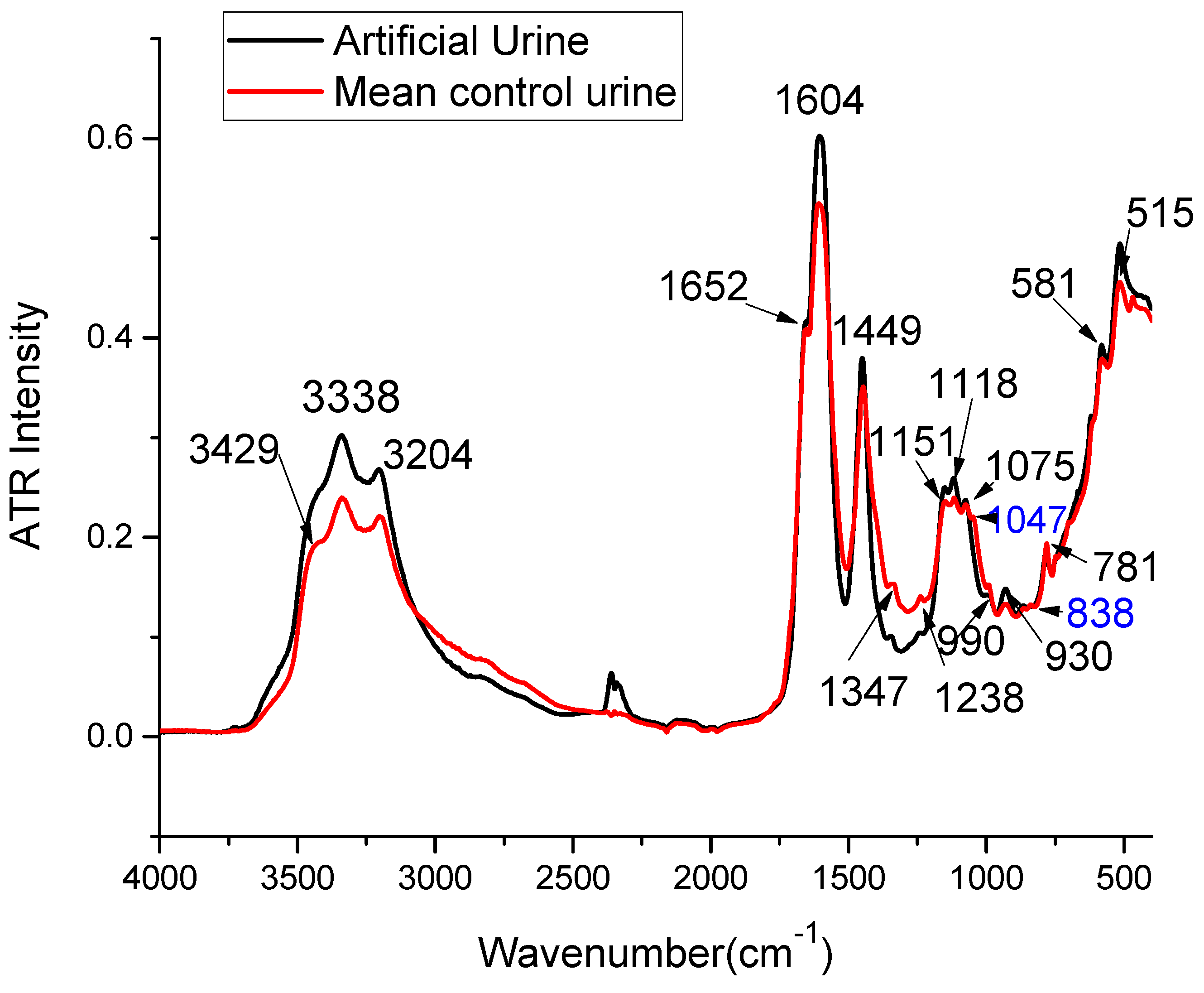
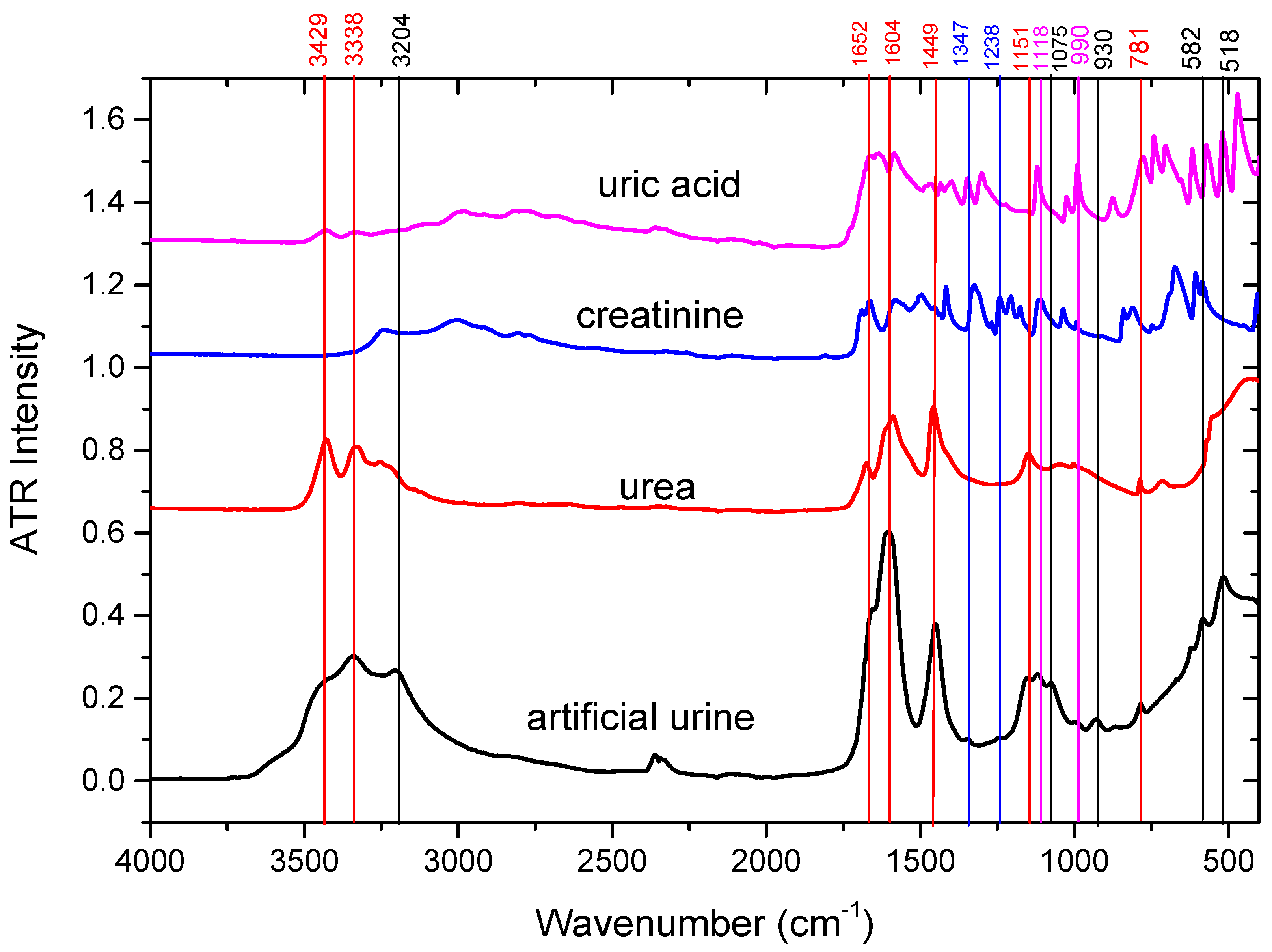



| Wavenumber Measured [cm−1] | Wavenumber Reported [cm−1] | Assignments in the Literature | Tentative Assignments in this Study |
|---|---|---|---|
| 513 | 517 | CO deformation vibration (Calcium oxalate) [20] | Glucose |
| 581 | 571–593 | deformation vibration of the PO4 group (Magnesium ammonium phosphate) [20] OH libration vibrations (Calcium oxalate) [20] Strong C-H deformation [32] | Phosphate |
| 781 | 756–783 | CO deformation (Calcium oxalate) [20] deformation vibration of the PO4 group (Magnesium ammonium phosphate) [20] C–O–C stretching in Esters (Cholesterol) [20] ω N-H, C-H (ring) δ (Urea, uric acid) [33] | Urea, Uric acid |
| 930 | 929–947 | OH libration vibrations (Calcium oxalate) [20], ν S-O, ν P-OH (Phosphate, sulfate, nucleic acids) [33] | Phosphate |
| 990 | 978–1015 | ν PO4 (Magnesium ammonium phosphate) [20], νas (C-C), β-sheet of proteins δ(=CH) of lipids [34], ν(C-O), ν(C-C), δ(OCH) [34] | Uric acid |
| 1075 | 1060–1084 | P-O, NH2, S=O stretching (Urea, sulfate, phosphate, nucleic acids) [33] P-O, NH2, S=O stretching [34] CH2OH groups, C–O stretching and COH groups, symmetric Glycosylated proteins, PO2—stretching (Glycosylated proteins) [35,36,37] νs CO–O–C (Carbohydrates) [38] C–O–C stretching in Esters (Cholesterol) [20] Very Weak, Sugar ring vibration [32] | Phosphate |
| 1118 | 1113–1115 | C-H, C-N-C stretching (Creatinine) [33] ν (C-O), ν(C-C), δ(OCH) [34] | Creatinine |
| 1151 | 1143–1157 | C-NH2, C-O, S-O stretching (Urea, uric acid, citrate, sulfate) [33] NH2 deformation (Urea) [26,35], νs PO2− [38] | Urea |
| 1238 | 1230–1238 | ν C-N, CH2 rocking (Creatinine, uric acid, citrate) [33] asymmetrical PO− (nucleic acids) [38] | Creatinine |
| 1347 | 1316–1399 | ω CH3CH2 [39], ν C-N (Uric acid, creatinine) [33] νs COO− (proteins) [20,38] νs CO (Calcium oxalate) [20], Amide III (Cholesterol) [20] Lactate, carboxylic acids and derivatives [32] | Creatinine, Uric acid |
| 1449 | 1447–1468 | δ CH2 (lipids) [20,34,38], νas (C-N) [34] δ C-H (Urea, uric acid, creatinine) [33] νas C–N (Urea) [26,35,36] CH3CH2 deformation mode [39] CH2 vibrations of lipids [23] ammonium ion (Magnesium ammonium phosphate) [20] δ CH2, δ CH3 ν(N=O) symmetrical deformation [32] | Urea |
| 1604 | 1604–1620 | N-H deformation, C-N-H vibrations (Urea) [33] νas (C-N) bending [34] νas CO group (Cystine) [20] νas (Calcium oxalate) [20] OH deformation vibration of water of crystallization (Magnesium ammonium phosphate) [20] | Urea |
| 1652 | 1641–1685 | Amide I [20,32,34,35,39] Random coil [35], α helix [35] 3–10 helix, π helix, type III turn [35] C=O stretching, C=N stretching (Urea, uric acid, creatinine, proteins) [33] β sheet & β turn [35] | Urea, Creatinine, Uric acid |
| 3204 | 3196–3240 | O–H stretching [36,37,40], Symmetric N–H stretching of Amide A [36,37,40], C–H stretch (Cholesterol) [20] | Urea, Creatinine |
| 3338 | 3280–3346 | νas H–O–H [35,36,40], ν N-H (Urea, creatinine) [33] | Urea |
| 3429 | 3406–3461 | NH group and/or OH (Cystine) [20] H2O or N-H [32] N-H stretching (Urea) [33] | Urea |
| Wavenumber (cm−1) | 3429 | 3338 | 3204 | 1652 | 1604 | 1449 | 1347 | 1238 | 1151 | 1075 | 1047 | 930 | 838 | 771 | 581 | 515 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | 0.112 | 0.176 | 0.086 | 0.034 | 0.121 | 0.289 | 0.008 | 0.039 | 0.782 | 0.463 | 0.306 | 0.485 | 0.053 | 0.058 | 0.098 | 0.189 |
| No. PC | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | 0.174 | 0.178 | 0.350 | <0.001 | 0.560 | 0.159 | 0.026 | 0.75 | 0.199 | 0.516 | 0.103 | 0.281 | 0.941 | 0.822 | 0.693 |
| Kernel | Linear | Polynomial | RBF | Sigmoid |
|---|---|---|---|---|
| Training accuracy (%) | 88.6 | 88.6 | 90.9 | 73.9 |
| Validation accuracy (%) | 76.1 | 76.1 | 71.6 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buhas, B.A.; Muntean, L.A.-M.; Ploussard, G.; Feciche, B.O.; Andras, I.; Toma, V.; Maghiar, T.A.; Crișan, N.; Știufiuc, R.-I.; Lucaciu, C.M. Renal Cell Carcinoma Discrimination through Attenuated Total Reflection Fourier Transform Infrared Spectroscopy of Dried Human Urine and Machine Learning Techniques. Int. J. Mol. Sci. 2024, 25, 9830. https://doi.org/10.3390/ijms25189830
Buhas BA, Muntean LA-M, Ploussard G, Feciche BO, Andras I, Toma V, Maghiar TA, Crișan N, Știufiuc R-I, Lucaciu CM. Renal Cell Carcinoma Discrimination through Attenuated Total Reflection Fourier Transform Infrared Spectroscopy of Dried Human Urine and Machine Learning Techniques. International Journal of Molecular Sciences. 2024; 25(18):9830. https://doi.org/10.3390/ijms25189830
Chicago/Turabian StyleBuhas, Bogdan Adrian, Lucia Ana-Maria Muntean, Guillaume Ploussard, Bogdan Ovidiu Feciche, Iulia Andras, Valentin Toma, Teodor Andrei Maghiar, Nicolae Crișan, Rareș-Ionuț Știufiuc, and Constantin Mihai Lucaciu. 2024. "Renal Cell Carcinoma Discrimination through Attenuated Total Reflection Fourier Transform Infrared Spectroscopy of Dried Human Urine and Machine Learning Techniques" International Journal of Molecular Sciences 25, no. 18: 9830. https://doi.org/10.3390/ijms25189830







