Identification of VvAGL Genes Reveals Their Network’s Involvement in the Modulation of Seed Abortion via Responding Multi-Hormone Signals in Grapevines
Abstract
1. Introduction
2. Results
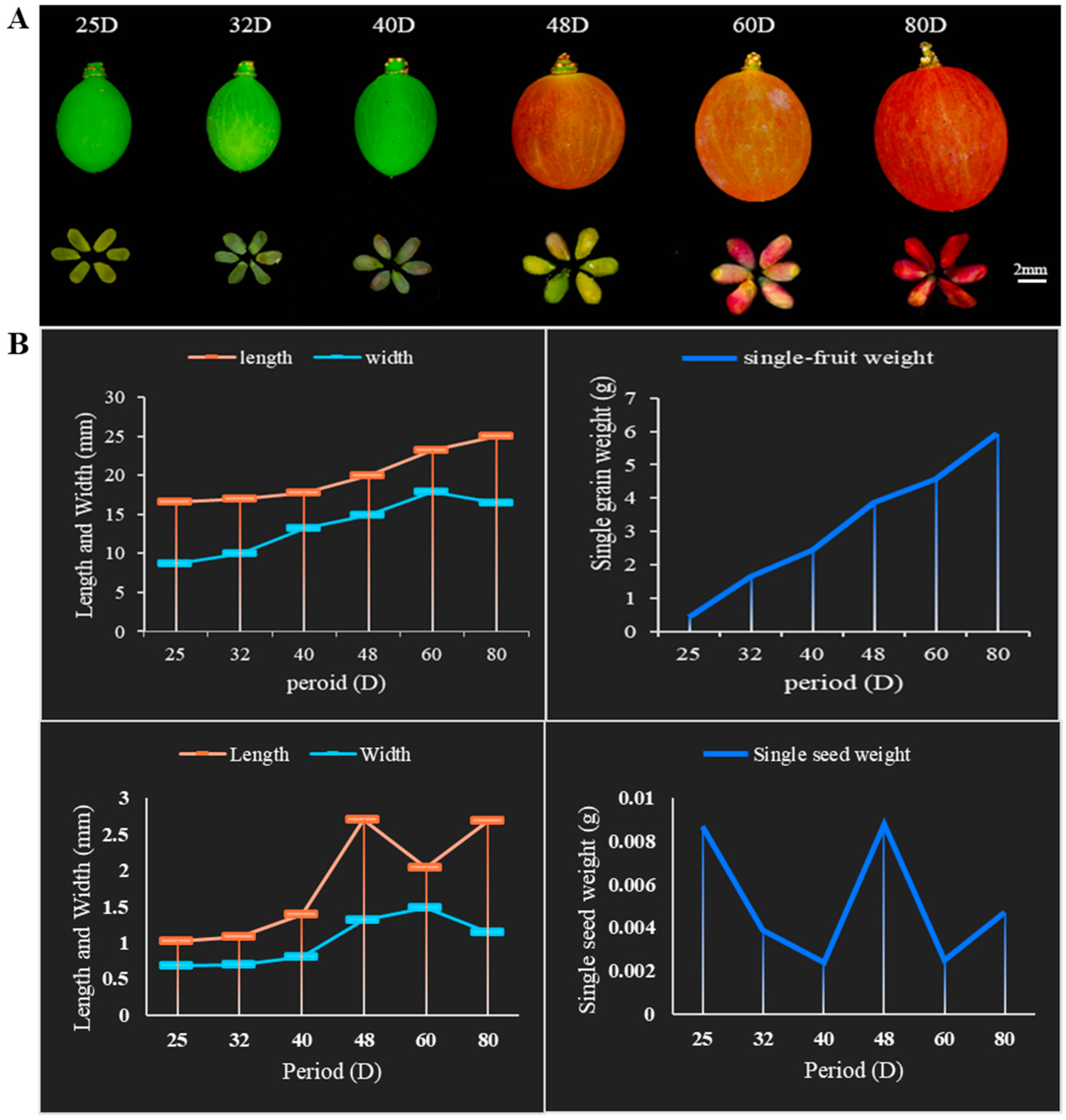
2.1. Developmental Changes in Berries during the Abortion of Grape Seeds
2.2. Identification and Characterisation of VvAGL Sub-Family Genes in Grape Seeds
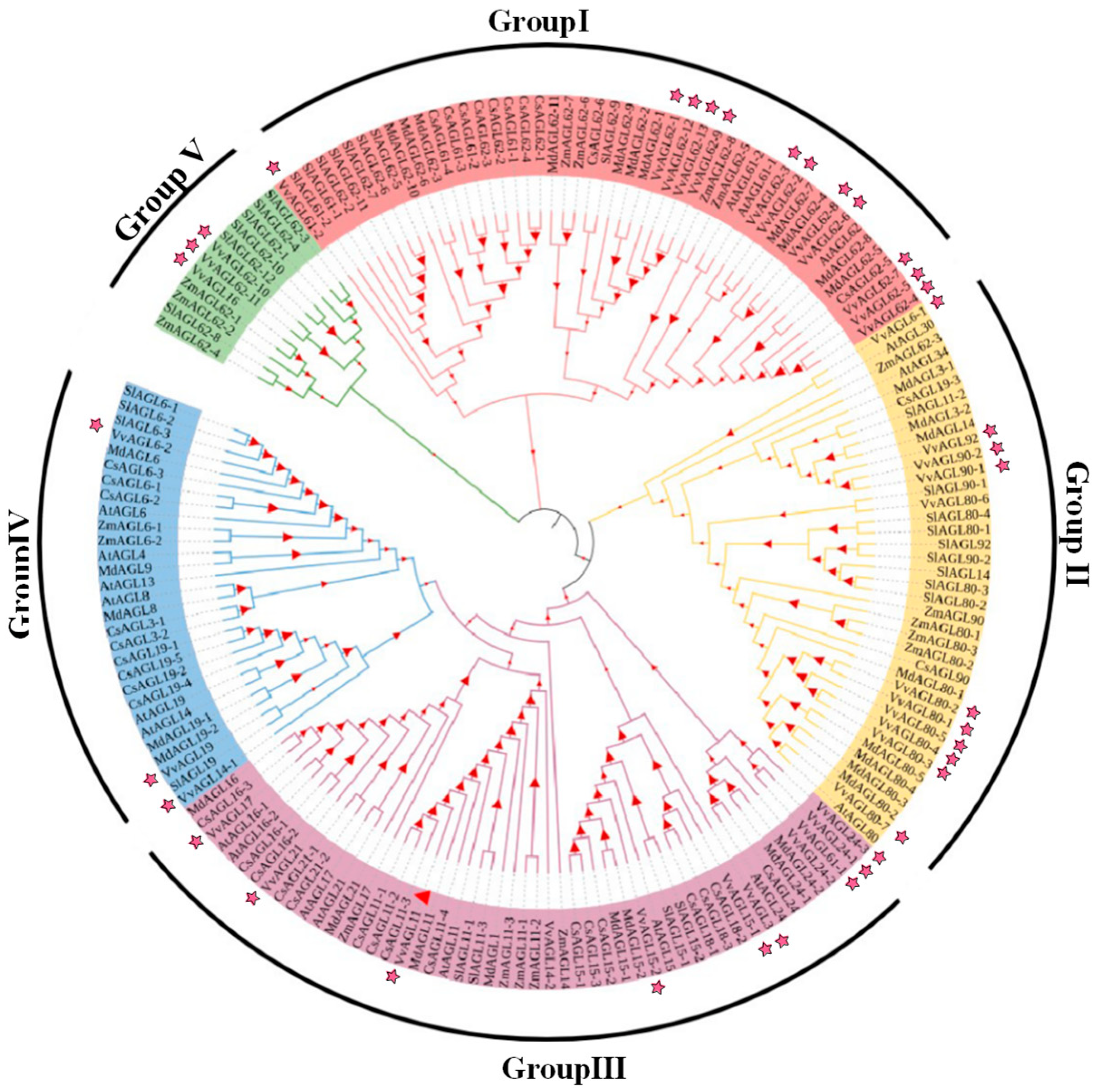
2.3. Conservative and Evolutionary Analysis of the VvAGL Family across Diverse Plant Species
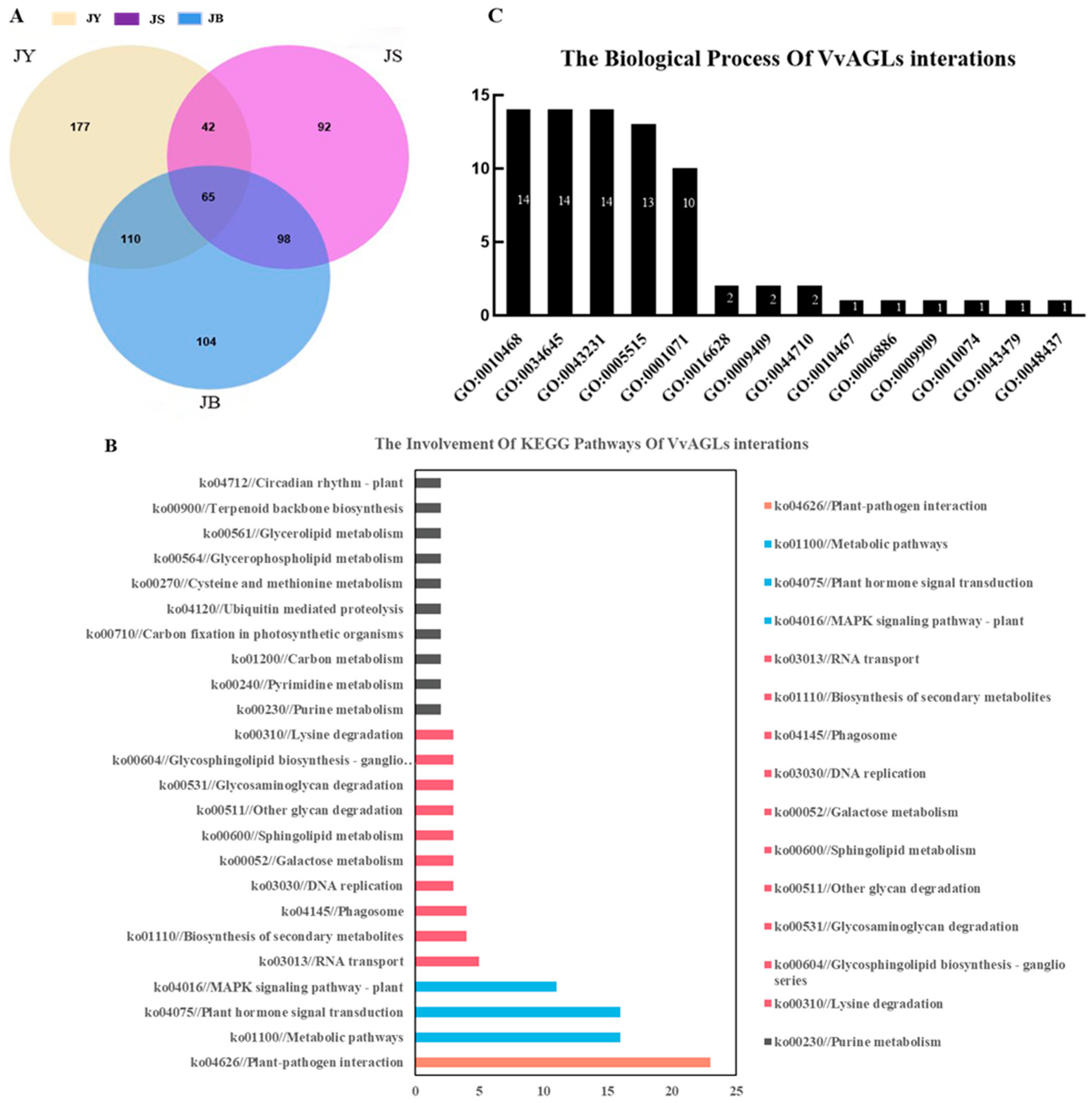
2.4. Gene Ontology and Pathway Mapping of VvAGLs
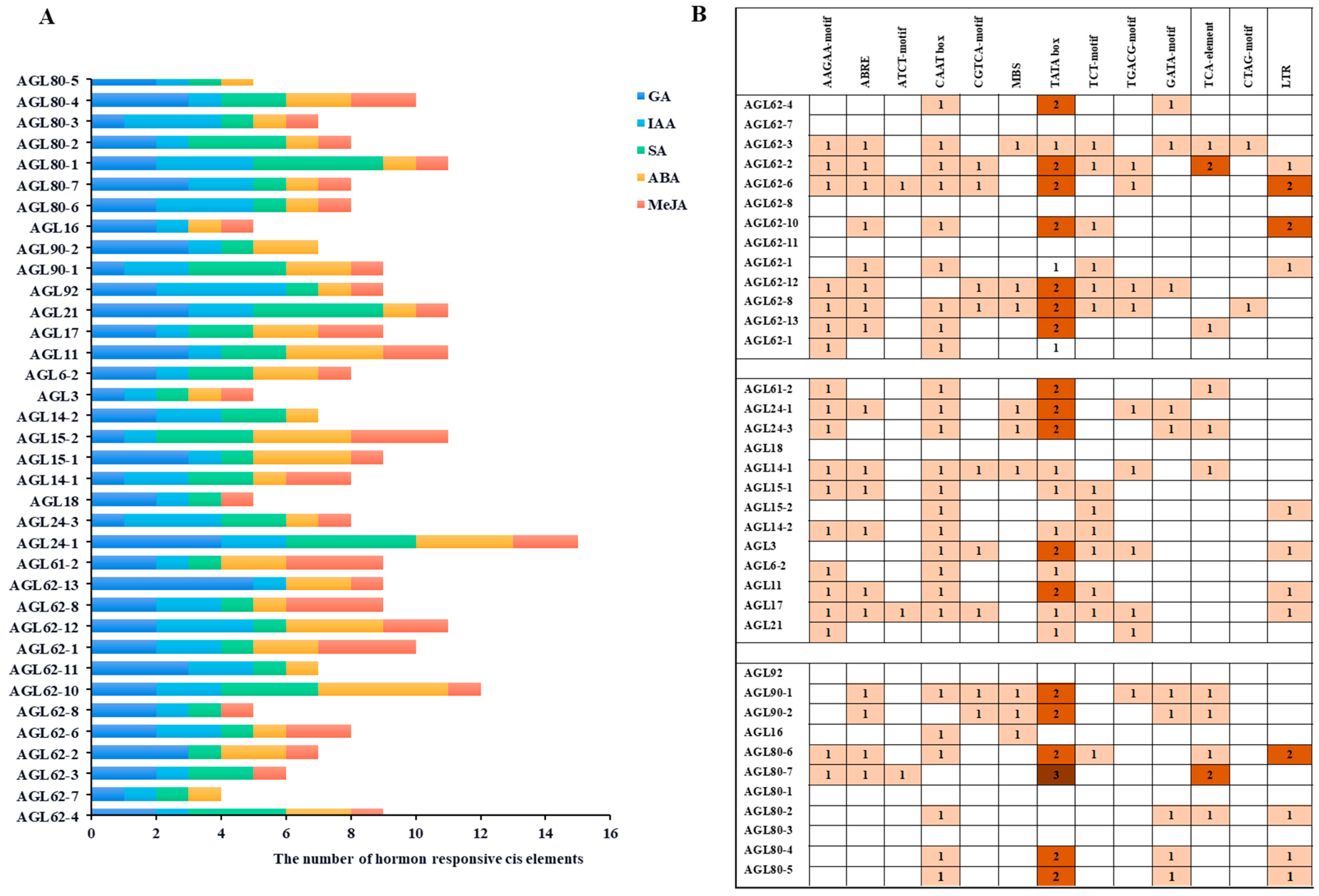
2.5. Screening of Hormone Cis-Elements in the Promoters of VvAGLs
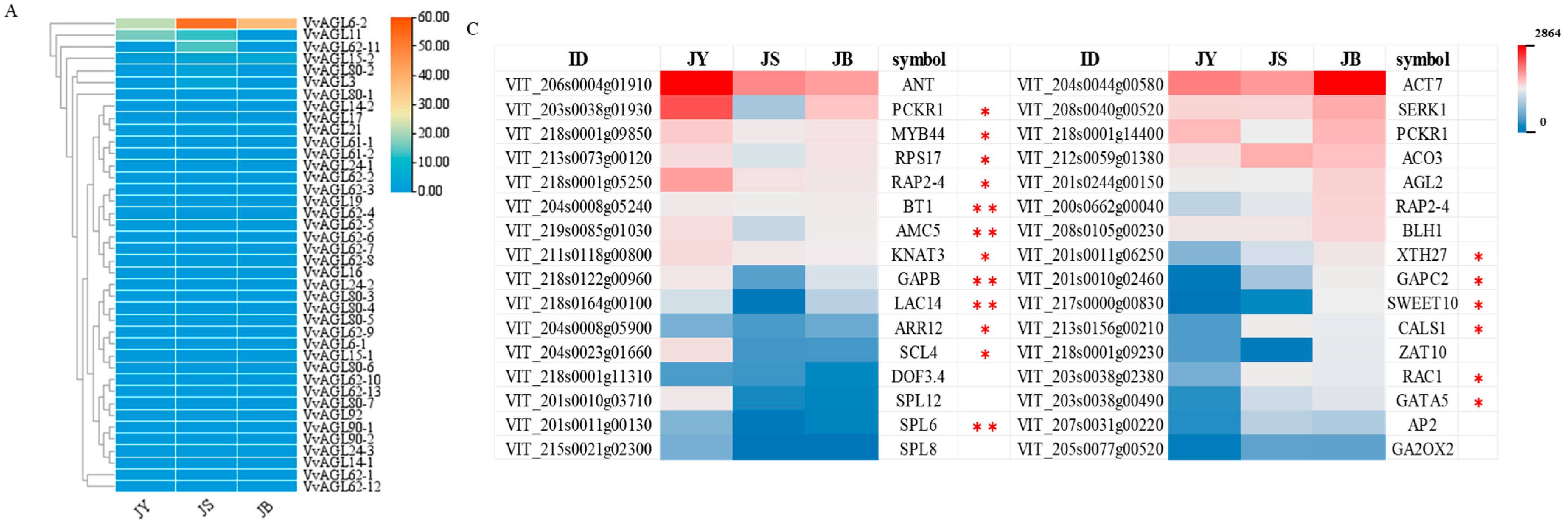
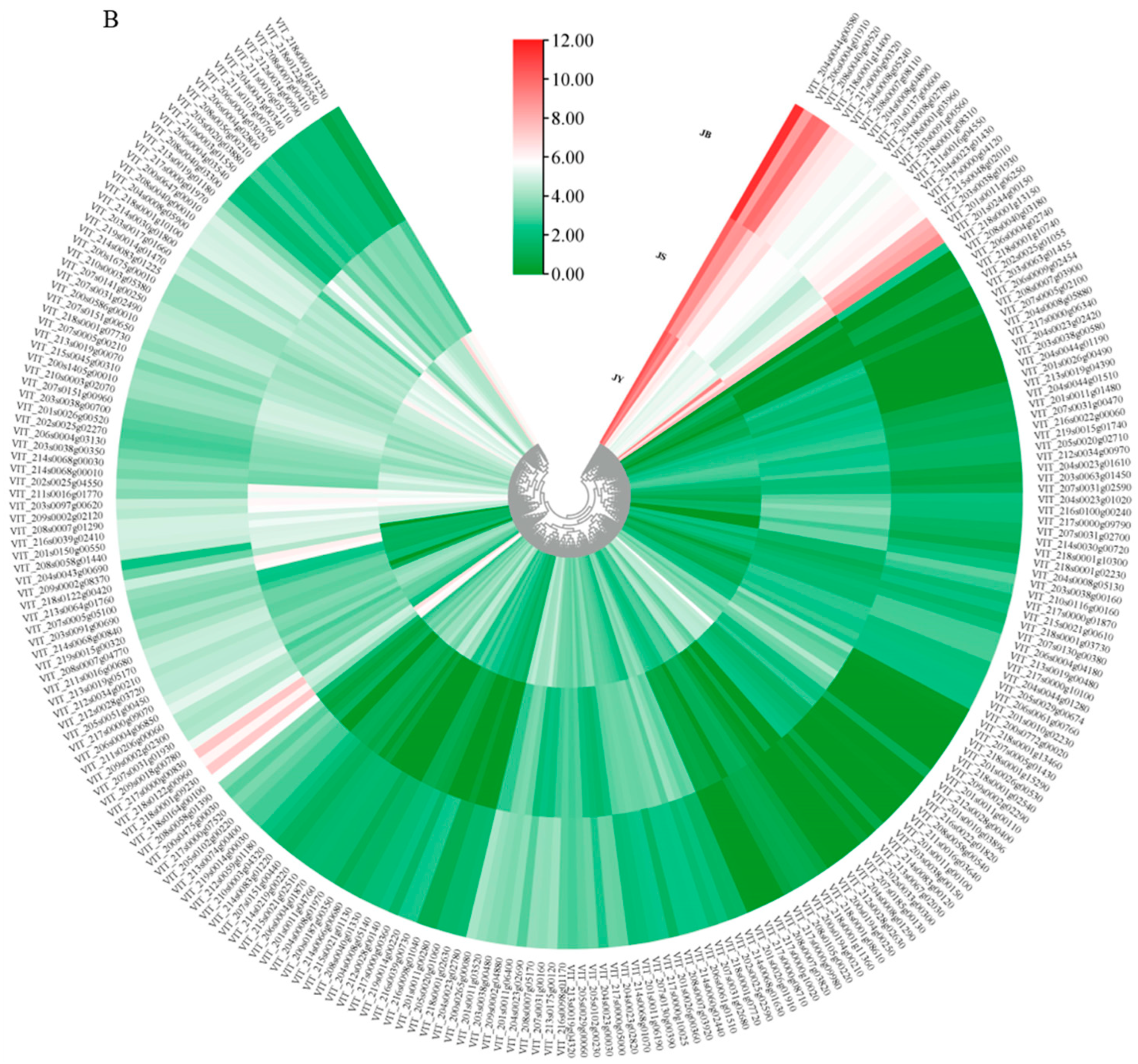
2.6. Differential Expression Profiles of VvAGLs and Their Interacting Proteins during Grape Seed Abortion Process
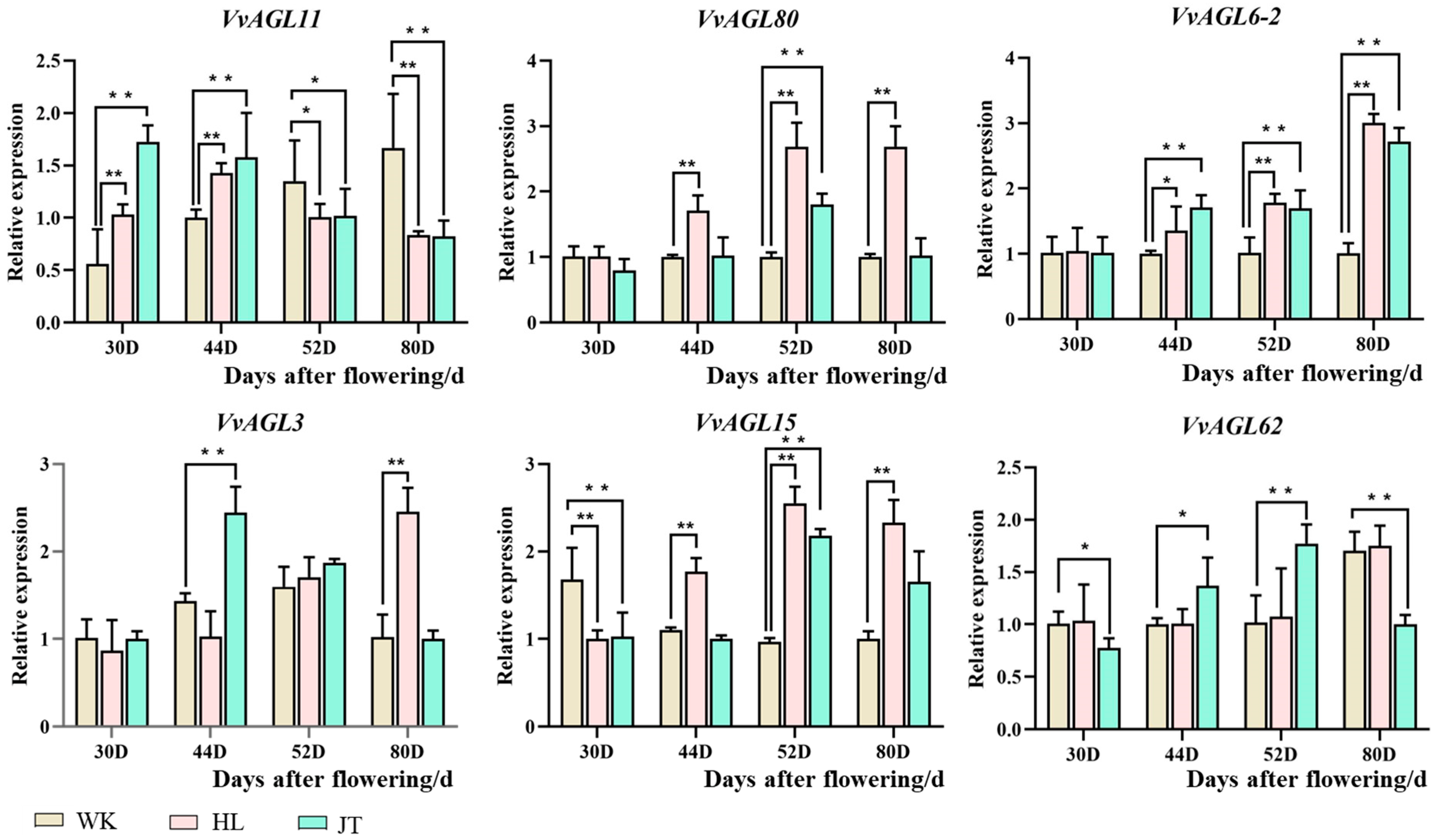
2.7. Validation of VvAGLs Expressions Using RT-qPCR Analysis
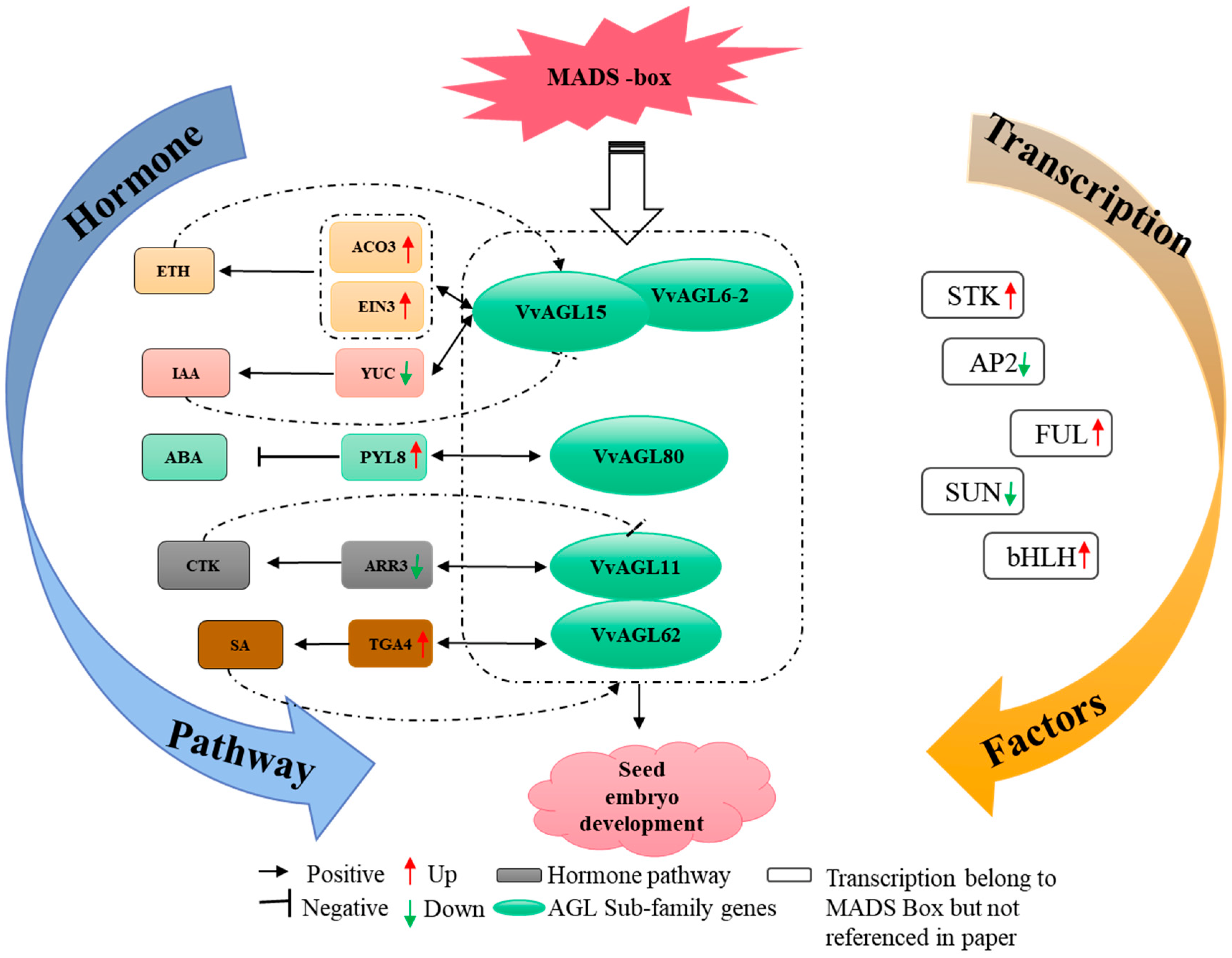
2.8. Regulatory Network of VvAGLs in Response to Multi-Hormone Signals
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Fruit Physiological Indexes
4.3. Phylogenetic Analysis
4.4. Prediction of VvAGL Gene Intron–Exon Structure
4.5. Analysis of Cis-Acting Elements in the Promoter Region of VvAGLs
4.6. RNA Extraction, Library Construction, and Sequencing
4.7. Analysis of Differentially Expressed Genes
4.8. Functional Annotation and Enrichment Analysis of VvAGL Differentially Expressed Genes
4.9. Prediction of Interacting Proteins
4.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, W.; Li, Z.-Q.; Zhou, Q.; Yao, W.-K.; Wang, Y.-J. Breeding New Seedless Grape by Means of in Vitro Embryo Rescue. Genet. Mol. Res. 2013, 12, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, J.; Lou, H.; Wang, H.; Xu, Q. Comparative Transcriptome Analysis of Dioecious, Unisexual Floral Development in Ribes diacanthum Pall. Gene 2019, 699, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Akkurt, M.; Tahmaz, H.; Veziroglu, S. Recent Developments in Seedless Grapevine Breeding. S. Afr. J. Enol. Vitic. 2019, 40, 1. [Google Scholar] [CrossRef]
- Rahman, M.A.; Balasubramani, S.P.; Basha, S.M. Molecular Characterization and Phylogenetic Analysis of MADS-Box Gene VroAGL11 Associated with Stenospermocarpic Seedlessness in Muscadine Grapes. Genes 2021, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, X.; Xuan, X.; Sadeghnezhad, E.; Liu, F.; Dong, T.; Pei, D.; Fang, J.; Wang, C. miR3633a-GA3ox2 Module Conducts Grape Seed-Embryo Abortion in Response to Gibberellin. Int. J. Mol. Sci. 2022, 23, 8767. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.D.; Kohler, C. Signalling Events Regulating Seed Coat Development. Biochem. Soc. Trans. 2014, 42, 358–363. [Google Scholar] [CrossRef]
- Li, S.; Liu, K.; Yu, S.; Jia, S.; Chen, S.; Fu, Y.; Sun, F.; Luo, Q.; Wang, Y. The Process of Embryo Abortion of Stenospermocarpic Grape and It Develops into Plantlet in Vitro Using Embryo Rescue. Plant Cell Tissue Organ Cult. 2020, 143, 389–409. [Google Scholar] [CrossRef]
- Favaro, R.; Pinyopich, A.; Battaglia, R.; Kooiker, M.; Borghi, L.; Ditta, G.; Yanofsky, M.F.; Kater, M.M.; Colombo, L. MADS-Box Protein Complexes Control Carpel and Ovule Development in Arabidopsis. Plant Cell 2003, 15, 2603–2611. [Google Scholar] [CrossRef]
- Diaz-Riquelme, J.; Lijavetzky, D.; Martinez-Zapater, J.M.; Jose Carmona, M. Genome-Wide Analysis of MIKCC-Type MADS Box Genes in Grapevine. Plant Physiol. 2009, 149, 354–369. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Garcia-Ponce, B.; de la Paz Sanchez, M.; Espinosa-Soto, C.; Garcia-Gomez, M.L.; Pineyro-Nelson, A.; Garay-Arroyo, A. MADS-Box Genes Underground Becoming Mainstream: Plant Root Developmental Mechanisms. New Phytol. 2019, 223, 1143–1158. [Google Scholar] [CrossRef]
- Dreni, L.; Kater, M.M. MADS Reloaded: Evolution of the AGAMOUS Subfamily Genes. New Phytol. 2014, 201, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Bennici, S.; Di Guardo, M.; Distefano, G.; La Malfa, S.; Puglisi, D.; Arcidiacono, F.; Ferlito, F.; Deng, Z.; Gentile, A.; Nicolosi, E. Influence of the Genetic Background on the Performance of Molecular Markers Linked to Seedlessness in Table Grapes. Sci. Hortic. 2019, 252, 316–323. [Google Scholar] [CrossRef]
- Mejia, N.; Soto, B.; Guerrero, M.; Casanueva, X.; Houel, C.; de los Angeles Miccono, M.; Ramos, R.; Le Cunff, L.; Boursiquot, J.-M.; Hinrichsen, P.; et al. Molecular, Genetic and Transcriptional Evidence for a Role of VvAGL11 in Stenospermocarpic Seedlessness in Grapevine. BMC Plant Biol. 2011, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, K.; Wu, J.; Wang, W.; Zhu, Y.; Li, C.; Zhao, M.; Wang, Y.; Li, C.; Zhao, L. A MADS-Box Gene Associated with Protocorm-like Body Formation in Rosa Canina Alters Floral Organ Development in Arabidopsis. Can. J. Plant Sci. 2018, 98, 309–317. [Google Scholar] [CrossRef]
- Royo, C.; Torres-Perez, R.; Mauri, N.; Diestro, N.; Antonio Cabezas, J.; Marchal, C.; Lacombe, T.; Ibanez, J.; Tornel, M.; Carreno, J.; et al. The Major Origin of Seedless Grapes Is Associated with a Missense Mutation in the MADS-Box Gene VviAGL11. Plant Physiol. 2018, 177, 1234–1253. [Google Scholar] [CrossRef]
- Ocarez, N.; Mejia, N. Suppression of the D-Class MADS-Box AGL11 Gene Triggers Seedlessness in Fleshy Fruits. Plant Cell Rep. 2016, 35, 239–254. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, L.; Zeng, L.; Yang, H.; Cao, C.; Wang, R.; Zhao, Y.; Wei, W. Analysis and Characterization of MADS-Box Genes from Davidia involucrata Baill. and Regulation of Flowering Time in Arabidopsis. Russ. J. Plant Physiol. 2022, 69, 62. [Google Scholar] [CrossRef]
- Tapia-Lopez, R.; Garcia-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Perez-Ruiz, R.V.; Kim, S.-H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-Related MADS-Box Gene, XAL1 (AGL12), Regulates Root Meristem Cell Proliferation and Flowering Transition in Arabidopsis. Plant Physiol. 2008, 146, 1182–1192. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Jiao, C.; Li, Z.; Fei, Z.; Yan, X.; Liu, C.; Wang, Y.; Wang, X. Transcriptome Analyses of Seed Development in Grape Hybrids Reveals a Possible Mechanism Influencing Seed Size. BMC Genom. 2016, 17, 898. [Google Scholar] [CrossRef]
- Shirzadi, R.; Andersen, E.D.; Bjerkan, K.N.; Gloeckle, B.M.; Heese, M.; Ungru, A.; Winge, P.; Koncz, C.; Aalen, R.B.; Schnittger, A.; et al. Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin-Dependent Regulation of AGAMOUS-LIKE36. PLoS Genet. 2011, 7, e1001303. [Google Scholar] [CrossRef]
- Ezquer, I.; Mizzotti, C.; Nguema-Ona, E.; Gotte, M.; Beauzamy, L.; Viana, V.E.; Dubrulle, N.; de Oliveira, A.C.; Caporali, E.; Koroney, A.-S.; et al. The Developmental Regulator SEEDSTICK Controls Structural and Mechanical Properties of the Arabidopsis Seed Coat. Plant Cell 2016, 28, 2478–2492. [Google Scholar] [CrossRef]
- Huang, B.; Routaboul, J.-M.; Liu, M.; Deng, W.; Maza, E.; Mila, I.; Hu, G.; Zouine, M.; Frasse, P.; Vrebalov, J.T.; et al. Overexpression of the Class D MADS-Box Gene SI-AGL11 Impacts Fleshy Tissue Differentiation and Structure in Tomato Fruits. J. Exp. Bot. 2017, 68, 4869–4884. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ma, R.; Liu, Z.; Zhang, D.; Wang, S.; Guo, Y.; Chen, M. Overexpression of BnaAGL11, a MADS-Box Transcription Factor, Regulates Leaf Morphogenesis and Senescence in Brassica napus. J. Agric. Food Chem. 2022, 70, 3420–3434. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Wu, P.; Cao, Z.; Liu, Y.; Zhang, X.; Lou, J.; Liu, X.; Hu, Y.; Sun, X.; Wang, Q.; et al. A NAC Transcription Factor ZaNAC93 Confers Floral Initiation, Fruit Development, and Prickle Formation in Zanthoxylum armatum. Plant Physiol. Biochem. 2023, 201, 107813. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.-H.; Steffen, J.G.; Portereiko, M.F.; Lloyd, A.; Drews, G.N. The AGL62 MADS Domain Protein Regulates Cellularization during Endosperm Development in Arabidopsis. Plant Cell 2008, 20, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Kirkbride, R.C.; Lu, J.; Zhang, C.; Mosher, R.A.; Baulcombe, D.C.; Chen, Z.J. Maternal Small RNAs Mediate Spatial-Temporal Regulation of Gene Expression, Imprinting, and Seed Development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; He, D.; Zhou, Y.; Ni, H.; Tian, D.; Chang, G.; Jing, Y.; Lin, R.; Huang, J.; et al. AGAMOUS-LIKE67 Cooperates with the Histone Mark Reader EBS to Modulate Seed Germination under High Temperature. Plant Physiol. 2020, 184, 529–545. [Google Scholar] [CrossRef]
- Sun, R.; Gao, L.; Mi, Z.; Zheng, Y.; Li, D. CnMADS1, a MADS Transcription Factor, Positively Modulates Cell Proliferation and Lipid Metabolism in the Endosperm of Coconut (Cocos nucifera L.). Planta 2020, 252, 83. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Liu, X.; Ye, J.; Zhang, J.; Chen, Z.; Xu, F.; Zhang, W.; Liao, Y. Comparative Transcriptome Analysis Identified Differentially Expressed Genes between Male and Female Flowers of Zanthoxylum armatum Var. Novemfolius. Agronomy 2020, 10, 283. [Google Scholar] [CrossRef]
- Jose Carmona, M.; Chaib, J.; Miguel Martinez-Zapater, J.; Thomas, M.R. A Molecular Genetic Perspective of Reproductive Development in Grapevine. J. Exp. Bot. 2008, 59, 2579–2596. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, S.; Li, X.; Zhang, X.; Wang, X.; Wang, L.; Li, Z.; Wang, X. A MADS-Box Transcription Factor from Grapevine, VvMADS45, Influences Seed Development. Plant Cell Tissue Organ Cult. 2020, 141, 105–118. [Google Scholar] [CrossRef]
- Le, B.H.; Cheng, C.; Bui, A.Q.; Wagmaister, J.A.; Henry, K.F.; Pelletier, J.; Kwong, L.; Belmonte, M.; Kirkbride, R.; Horvath, S.; et al. Global Analysis of Gene Activity during Arabidopsis Seed Development and Identification of Seed-Specific Transcription Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Lovisetto, A.; Baldan, B.; Pavanello, A.; Casadoro, G. Characterization of an AGAMOUS Gene Expressed throughout Development of the Fleshy Fruit-like Structure Produced by Ginkgo biloba around Its Seeds. BMC Evol. Biol. 2015, 15, 139. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Zhao, T.; Wang, Y.; Yang, R.; Li, W.; Liu, K.; Sun, N.; Hussian, I.; Ma, X.; Yu, H.; et al. Expression Characterization of ABCDE Class MADS-Box Genes in Brassica rapa with Different Pistil Types. Plant 2023, 12, 2218. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.-H.; Jung, K.-H. Hierarchical Structures and Dissected Functions of MADS-Box Transcription Factors in Rice Development. J. Plant Biol. 2022, 65, 99–109. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Hu, Z.; Guo, X.; Tian, S.; Chen, G. Genome-Wide Analysis of the MADS-Box Transcription Factor Family in Solanum lycopersicum. Int. J. Mol. Sci. 2019, 20, 2961. [Google Scholar] [CrossRef]
- Heijmans, K.; Ament, K.; Rijpkema, A.S.; Zethof, J.; Wolters-Arts, M.; Gerats, T.; Vandenbussche, M. Redefining C and D in the Petunia ABC. Plant Cell 2012, 24, 2305–2317. [Google Scholar] [CrossRef]
- Catotti, P.; Conner, J.; Conner, P. Validation of a Molecular Marker for Seedlessness in Muscadine Grapes. Hortscience 2015, 50, S326. [Google Scholar]
- Correa, J.; Ravest, G.; Laborie, D.; Mamani, M.; Torres, E.; Munoz, C.; Pinto, M.; Hinrichsen, P. Quantitative Trait Loci for the Response to Gibberellic Acid of Berry Size and Seed Mass in Tablegrape (Vitis vinifera L.). Aust. J. Grape Wine Res. 2015, 21, 496–507. [Google Scholar] [CrossRef]
- Coen, O.; Fiume, E.; Xu, W.; De Vos, D.; Lu, J.; Pechoux, C.; Lepiniec, L.; Magnani, E. Developmental Patterning of the Sub-Epidermal Integument Cell Layer in Arabidopsis Seeds. Development 2017, 144, 1490–1497. [Google Scholar] [CrossRef]
- Liu, C.X.; Shen, S.Y.; Bao, M.Z. The Overexpression of C and D Class Genes from Petunia Exhibited Redundant and Specific Functions in Nicotiana Tabacum. In Proceedings of the III International Symposium on Germplasm of Ornamentals, Seoul, Republic of Korea, 25–28 October 2020; Kim, W.S., Lee, S.Y., Rhie, Y.H., Eds.; International Society for Horticultural Science: Leuven, Belgium; 2020; Volume 1291, pp. 277–289. [Google Scholar]
- Moschin, S.; Nigris, S.; Ezquer, I.; Masiero, S.; Cagnin, S.; Cortese, E.; Colombo, L.; Casadoro, G.; Baldan, B. Expression and Functional Analyses of Nymphaea caerulea MADS-Box Genes Contribute to Clarify the Complex Flower Patterning of Water Lilies. Front. Plant Sci. 2021, 12, 730270. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, H.; Er, H.L.; Soo, H.M.; Kumar, P.P.; Han, J.-H.; Liou, Y.C.; Yu, H. Direct Interaction of AGL24 and SOC1 Integrates Flowering Signals in Arabidopsis. Development 2008, 135, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Woo, Y.-M.; Ryu, S.-I.; Shin, Y.-D.; Kim, W.T.; Park, K.Y.; Lee, I.-J.; An, G. Further Characterization of a Rice AGL12 Group MADS-Box Gene, OsMADS26. Plant Physiol. 2008, 147, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Meynard, D.; Khong, G.N.; Pauluzzi, G.; Guiderdoni, E.; Gantet, P. Analysis of the Expression of the AGL17-like Clade of MADS-Box Transcription Factors in Rice. Gene Expr. Patterns 2013, 13, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Masiero, S.; Vanzulli, S.; Lardelli, P.; Kater, M.M.; Colombo, L. AGL23, a Type I MADS-Box Gene That Controls Female Gametophyte and Embryo Development in Arabidopsis. Plant J. 2008, 54, 1037–1048. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. miR156/SPL10 Modulates Lateral Root Development, Branching and Leaf Morphology in Arabidopsis by Silencing AGAMOUS-LIKE 79. Front. Plant Sci. 2018, 8, 2226, Corrigendum in Front. Plant Sci. 2019, 10, 515. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, J.; Wei, D.; Wang, Z.; Tang, Q. Flowering Signal Integrator AGL24 Interacts with K Domain of AGL18 in Brassica juncea. Biochem. Biophys. Res. Commun. 2019, 518, 148–153. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Dong, Y.; Ma, X.; Zhou, W.; Wang, Z.; Fan, Y.; Wei, D.; Tang, Q. AGL19 Directly Interacts with Floral Signal Integrator AGL24 in Flowering Time Control of Brassica juncea. Acta Physiol. Plant. 2020, 42, 175. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, J.; Sun, Z.; Xing, Y.; Zhang, Q.; Fang, K.; Cao, Q.; Qin, L. The MADS-Box Transcription Factor CmAGL11 Modulates Somatic Embryogenesis in Chinese Chestnut (Castanea mollissima Blume). J. Integr. Agric. 2020, 19, 1033–1043. [Google Scholar] [CrossRef]
- Xu, G.; Huang, J.; Lei, S.; Sun, X.; Li, X. Comparative Gene Expression Profile Analysis of Ovules Provides Insights into Jatropha curcas L. Ovule Development. Sci. Rep. 2019, 9, 15973. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, X.; Singer, S.D.; Li, J.; Zhang, H.; Gao, M.; Wang, L.; Song, J.; Wang, X. Effect of GA3 Treatment on Seed Development and Seed-Related Gene Expression in Grape. PLoS ONE 2013, 8, e80044. [Google Scholar] [CrossRef]
- Zhang, W.; Abdelrahman, M.; Jiu, S.; Guan, L.; Han, J.; Zheng, T.; Jia, H.; Song, C.; Fang, J.; Wang, C. VvmiR160s/VvARFs Interaction and Their Spatio-Temporal Expression/Cleavage Products during GA-Induced Grape Parthenocarpy. BMC Plant Biol. 2019, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Ravest, G.; Mamani, M.; Giacomelli, L.; Moser, C.; Pastenes, C.; Hinrichsen, P. Bioactive Gibberellins Show Differential Abundance at Key Phenological Stages for Berry Growth in Table Grapes. Am. J. Enol. Vitic. 2017, 68, 478–484. [Google Scholar] [CrossRef]
- Sun, X.; Shantharaj, D.; Kang, X.; Ni, M. Transcriptional and Hormonal Signaling Control of Arabidopsis Seed Development. Curr. Opin. Plant Biol. 2010, 13, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Geng, X.; Chen, S.; Liu, K.; Yu, S.; Wang, X.; Zhang, C.; Zhang, J.; Wen, Y.; Luo, Q.; et al. The Co-Expression of Genes Involved in Seed Coat and Endosperm Development Promotes Seed Abortion in Grapevine. Planta 2021, 254, 87. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Kitamura, Y.; Numaguchi, K.; Itai, A. Characterization of Transcriptomic Response in Ovules Derived from Inter-Subgeneric Hybridization in Prunus (Rosaceae) Species. Plant Reprod. 2021, 34, 255–266. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A Rapid and Effective Method for RNA Extraction from Different Tissues of Grapevine and Other Woody Plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]



| Gene ID | Symbol | Pathway | K-ID | Go Process |
|---|---|---|---|---|
| VIT_200s0250g00085 | AGL61-1 | ko04075//Plant hormone signal transduction | K14486 | GO:0010468//regulation of gene expression; GO:0034645//cellular macromolecule biosynthetic processGO:0010467//gene expression |
| VIT_207s0129g00650 | AGL6-1 | |||
| VIT_200s1450g00005 | AGL62-1 | |||
| VIT_201s0010g01500 | AGL62-2 | |||
| VIT_203s0088g00510 | AGL62-4 | |||
| VIT_203s0088g00550 | AGL62-5 | |||
| VIT_203s0088g00590 | AGL62-6 | |||
| VIT_203s0088g00600 | AGL62-7 | |||
| VIT_203s0088g00610 | AGL62-8 | |||
| VIT_207s0005g06590 | AGL62-9 | |||
| VIT_210s0003g03015 | AGL62-10 | |||
| VIT_210s0003g03020 | AGL62-11 | |||
| VIT_210s0003g03025 | AGL62-12 | |||
| VIT_210s0003g03970 | AGL62-13 | |||
| VIT_200s0211g00110 | AGL17 | ko04075//Plant hormone signal transduction | K14486 | GO:0010468//regulation of gene expression;GO:0034645//cellular macromolecule biosynthetic processGO:0044710//single-organism metabolic processGO:0006886//intracellular protein transport;GO:0009409//response to cold;GO:0009909//regulation of flower development;GO:0010074//maintenance of meristem identityGO:0043479//pigment accumulation in tissues in response to UV light;GO:0048437//floral organ developmentGO:0000904//cell morphogenesis involved in differentiation;GO:0009664//plant-type cell wall organization;GO:0009888//tissue developmentGO:0048580//regulation of post-embryonic development;GO:0080154//regulation of fertilization |
| VIT_200s0211g00180 | AGL21 | |||
| VIT_200s0250g00085 | AGL61-2 | |||
| VIT_200s0729g00010 | AGL24-1 | |||
| VIT_202s0025g04650 | AGL19 | |||
| VIT_203s0167g00100 | AGL24-2 | |||
| VIT_208s0007g08790 | AGL15-1 | |||
| VIT_213s0158g00100 | AGL15-2 | |||
| VIT_214s0068g01800 | AGL3 | |||
| VIT_215s0024g02000 | AGL24-3 | |||
| VIT_215s0048g01240 | AGL14-1 | |||
| VIT_215s0048g01270 | AGL6-2 | |||
| VIT_216s0022g02400 | AGL14-2 | |||
| VIT_218s0041g01880 | AGL11 | |||
| VIT_202s0109g00382 | AGL80-6 | ko04075//Plant hormone signal transduction | K14486 | GO:0010468//regulation of gene expression; GO:0034645//cellular macromolecule biosynthetic process |
| VIT_202s0109g00384 | AGL80-7 | |||
| VIT_203s0097g00192 | AGL16 | |||
| VIT_205s0020g01043 | AGL80-1 | |||
| VIT_205s0020g01046 | AGL80-2 | |||
| VIT_205s0020g01055 | AGL80-3 | |||
| VIT_208s0032g00974 | AGL80-4 | |||
| VIT_214s0060g00300 | AGL80-5 | |||
| VIT_215s0021g00560 | AGL92 | |||
| VIT_215s0021g02220 | AGL90-1 | |||
| VIT_215s0021g02250 | AGL90-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Aziz, R.B.; Wang, Y.; Xuan, X.; Yu, M.; Qi, Z.; Chen, X.; Wu, Q.; Qu, Z.; Dong, T.; et al. Identification of VvAGL Genes Reveals Their Network’s Involvement in the Modulation of Seed Abortion via Responding Multi-Hormone Signals in Grapevines. Int. J. Mol. Sci. 2024, 25, 9849. https://doi.org/10.3390/ijms25189849
Liu F, Aziz RB, Wang Y, Xuan X, Yu M, Qi Z, Chen X, Wu Q, Qu Z, Dong T, et al. Identification of VvAGL Genes Reveals Their Network’s Involvement in the Modulation of Seed Abortion via Responding Multi-Hormone Signals in Grapevines. International Journal of Molecular Sciences. 2024; 25(18):9849. https://doi.org/10.3390/ijms25189849
Chicago/Turabian StyleLiu, Fei, Rana Badar Aziz, Yumiao Wang, Xuxian Xuan, Mucheng Yu, Ziyang Qi, Xinpeng Chen, Qiqi Wu, Ziyang Qu, Tianyu Dong, and et al. 2024. "Identification of VvAGL Genes Reveals Their Network’s Involvement in the Modulation of Seed Abortion via Responding Multi-Hormone Signals in Grapevines" International Journal of Molecular Sciences 25, no. 18: 9849. https://doi.org/10.3390/ijms25189849
APA StyleLiu, F., Aziz, R. B., Wang, Y., Xuan, X., Yu, M., Qi, Z., Chen, X., Wu, Q., Qu, Z., Dong, T., Li, S., Fang, J., & Wang, C. (2024). Identification of VvAGL Genes Reveals Their Network’s Involvement in the Modulation of Seed Abortion via Responding Multi-Hormone Signals in Grapevines. International Journal of Molecular Sciences, 25(18), 9849. https://doi.org/10.3390/ijms25189849






